Graphical abstract
Keywords: Food contact materials, Differential scanning calorimetry, Degree of crystallinity, Migration of additives, Pre-heating
Highlights
-
•
Pre-heating of polypropylene food cups lead to changes in the degree of crystallinity.
-
•
Changes starting with treatments as low as at 40 °C.
-
•
The variation in crystallinity affect the specific migration of several substances.
-
•
Changes in migrated amounts can be crucial if occurring close to legislative limits.
Abstract
Laboratories unexpectedly carried out pre-heating of polypropylene beverage cups prior to performing a migration test in a proficiency test. Principal component analysis of the data collected showed that the preheating temperature of the cups contributed to an increased variance of the data and distinguishing pre-heating and non-pre-heating groups. This triggered to study the effect of applying such pre-heating on the physical structure of the material and on the migration of additives to food simulant D1 (ethanol 50% v/v). Several cups were pre-heated at selected temperatures and either analyzed with differential scanning calorimetry to establish the degree of crystallinity or used for the migration test. Six target additives from Regulation (EU) No 10/2011 were quantified in the food simulant using HPLC-FLD and LC–MS. Results show that pre-heating of the beverage cups led to a significant change in the degree of crystallinity, resulting in a change of analyte migration in comparison to the migration results from non-pre-heated cups.
1. Introduction
The migration of substances from food contact materials (FCMs) to food is mainly a physico-chemical process. On the FCM side, factors like the type of material (e.g. plastic, ceramics, metal, paper), the material thickness, the degree of crystallinity, the number and type of layers (including combinations of different FCMs like plastic and paper), and the amount and chemical nature of substances present in the material are the main characteristics to be taken into account when studying potential migration of substances to food. On the food side, the type of food (liquid, solid, viscous), the nature of the food (acidic, fatty), the duration of the contact with the FCM and the temperature at which the contact between FCM and food occurs, can have an influence on the level of migration of a certain substance (Silva, García, Cooper, Franz, & Losada, 2006; Simoneau, 2008). The migration process in plastics has been mathematically described (Hoekstra et al., 2015).
As temperature is one of the key factors influencing the migration process, it needs to be well controlled (Simoneau, 2008). The pre-heating of FCM prior to migration testing could be one way of maintaining the temperature in the migration test during the contact time within a predefined temperature range. Two inter-laboratory exercises (ILCs) (Tsochatzis, Mieth, Simoneau, & Hoekstra, 2016; Tsochatzis, Alberto Lopes, Robouch, & Hoekstra, 2018) were organised to assess the competence of National Reference Laboratories (NRLs) for FCMs in monitoring the migration of FCM No. 500 (2,5-bis(5-tert-butyl-2-benzoxazolyl)thiophene) from polypropylene (PP) food contact cups into official food simulant D1 (ethanol 50% v/v) (Regulation (EU) No 10/2011). Unexpectedly some of the NRLs pre-heated the cups prior to migration testing. Principal component analysis of the results showed that pre-heating of the cups had increased the variance of the migration results (Tsochatzis et al., 2018).
Several studies have shown that structural changes of polymers due to heat treatment can happen at temperatures below the melting point, also at short time exposure (Abdel-Mohti, Garbash, Almagahwi, & Shen, 2015; Andersson, Hakkarainen, & Albertsson, 2012; Howell & Smith, 2006). Alin et al. observed an increase of the specific migration of antioxidants from different polypropylene (PP) articles with different degrees of crystallinity to food simulants and solvents during microwave heating (Alin & Hakkarainen, 2010). Very few studies looked at the effect of pre-heating, degree of crystallinity and migration. An increase in the degree of crystallinity of PP packaging was evidenced after migration experiments with several food simulants during microwave heating (Alin & Hakkarainen, 2011). Song et al. (2016) presented a study on the thermal aging of polyethylene terephthalate (PET) in which they pre-heated PET before measuring the migration of antimony into a 4% acetic acid. While changes in the degree of crystallinity of PET were observed, no influence of pre-heating on the migration of antimony was mentioned.
Our findings of the proficiency test and the little information available were our reasons to investigate the effect of pre-heating of polypropylene cups prior to the performance of a migration test on the physical structure and on the migration of selected additives into food simulant D1.
2. Materials and methods
2.1. Chemicals
Chromasolv grade purity Ethanol (EtOH; CAS: 64-17-5), methanol (MeOH; CAS: 67-56-1), acetonitrile (ACN; CAS: 75-05-8) and chloroform (Chl; CAS:67-66-3) and formic acid (≥96%) were purchased from Sigma Aldrich (Steinheim, Germany). Ultrapure water (18.2 MΩ) was obtained from a Milli-Q system (Millipore, Bedford, USA). PTFE, membrane filters (17 mm diameter, 0.2 μm) were supplied from CPS Analitica (Milan, Italy). The analytical standards of 1palmitoyl glycerol, 2-palmitoy glycerol, 1-stearoyl glycerol and 2-stearoyl glycerol were purchased from TRC Chemicals (Toronto, Canada), while 2,5-bis(5-tert-butyl-2-benzoxazolyl)thiophene (99%) was purchased from Sigma Aldrich (Buchs, Switzerland). Bis(4-propylbenzylidene) propylsorbitol (not available on the market) was provided by the official collection of the European Union Reference Laboratory for FCM (EURL-FCM)Chemical and legislative information of the analysed migrants are presented in Table 1 (Section 2.7).
Table 1.
Chemical and legislative information of quantified migrants.
| Chemical name | Chemical Formula | Chemical structure | CAS No. | FCM No. |
|---|---|---|---|---|
| 1-palmitoyl glycerol | C19H38O4 |  |
26657-96-5 | Part of mixture 50 |
| 2-palmitoyl glycerol |  |
23470-00-0 | ||
| 1-stearoyl glycerol | C21H42O4 | 22610-61-3 | Part of mixture 53 | |
| 2-stearoyl glycerol |  |
621-61-4 | ||
| 2,5-bis(5-tert-butyl-2-benzoxazolyl)thiophene | C26H26N2O2S |  |
7128-64-5 | 500 |
| bis(4-propylbenzylidene) propylsorbitol | C29H40O6 |  |
882073-43-0 | 808 |
2.2. Preparation of standard solutions
Stock solutions containing 100 μg mL−1 of each of the target analytes were prepared in chloroform. Appropriate concentrations of the working solutions were then prepared gravimetrically by diluting the stock solutions with methanol. Stock solutions were stored at -20 °C, while the working solutions were kept at 4 °C. Fresh working solutions were produced every week. Both stock and working solutions have been prepared in amber vials
2.3. Food contact PP cups
Food PP cups were bought from a selected supplier. They were intended for single-use and for use with alcoholic and/or hot beverages. The volume of each cup was 0.3 L. For the identification and characterization of the polymers, additional cups labelled as PP-R (random copolymer) and PP-C (heterophasic copolymer) were bought from a local market store. A PP homopolymer (PP-H) cup already present in the laboratory was also used in this study for identification and characterization.
2.4. Instrumentation
2.4.1. Differential scanning calorimetry (DSC) and thermogravimetric analysis (TGA)
The DSC analyses were performed using a TA Instruments Model Q100 (Newcastle, DE, USA) equipped with an autosampler. After sample introduction and temperature equilibration the sample was heated from 0 to 300 °C at 20 °C/min (1st cycle), cooled to 0 °C at 20 °C/min (2nd cycle), then heated up to 300 °C at 20 °C/min. The heating scans were performed under constant nitrogen gas flow (50 mL/min). The following equation was used for the calculation of the degree of crystallinity (Xc) of the PP cups:
| Xc (%) = 100 * ΔHm /ΔHm°, |
Where ΔHm is the melting enthalpy of the sample determined from the surface of melting peak area from the thermogram and ΔHm° is the melting enthalpy of a 100% crystalline polymer sample [later set to 207 J/g (Wunderlich, 1990)].
The Thermal analyzer (model Q500) from TA Instruments (Newcastle, DE, USA) was used for the TGA analysis. Samples of 2–6 mg were heated in a platinum pan using two types of temperature programs: from 20 to 1000 °C at 10 °C/min to obtain the degradation curves, and at 40, 60, 70, 75, 80 or 90 °C for 24 h to investigate the stability of the material.
2.4.2. Fourier-transform infrared spectroscopy (FTIR)
A diamond crystal Attenuated Total Reflectance (ATR) FTIR spectrometer (Perkin Elmer spectrum 2000) was used to acquire the spectra ranging from 4000 to 530 cm−1, with a resolution of 4.00 cm−1. A total of 8 scans were acquired for each PP sample, and averaged to eliminate background noise. PP samples were analyzed as such without any preparation, and the obtained spectra were compared with a known “reference” spectrum.
2.4.3. Optical microscopy
A Zeiss microscope (model Axioskop 2 Mat) equipped with an Axiocam camera was used to detect any macroscopic changes in the PP cups due to pre-heating. The analysis was performed using transmitted light and 3 different magnifications (×100, 200 or 500).
2.4.4. Liquid chromatography systems
The identification and quantification of the target substances migrating from the PP cups was performed using an ultra-high-pressure liquid chromatograph coupled to a quadrupole time-of-flight mass spectrometer (UHPLC-qTOF-MS) and an high-performance liquid chromatograph with fluorescence detection (HPLC-FLD) (Alberto Lopes, Tsochatzis, Emons, & Hoekstra, 2018; Tsochatzis et al., 2018).
The HPLC-FLD set-up consisted of an Agilent Technologies 1200 series HPLC system (Waldbronn, Germany), equipped with a thermostatic column compartment, an autosampler, a Diode Array Detector (DAD) and a FLD. The Thermo Scientific HyPURITY C18 chromatographic column (150 × 3.0 mm, 5 μm particle size) of Thermo Fisher Scientific Inc., (USA) was thermostated at 40 °C. A linear gradient elution program using a mixture of acetonitrile (A) and ultrapure water (B) was used, starting with an isocratic step of 30% B, from 0 to 23 min, followed by a gradient decrease of B to 5% (at 27 min) and an equilibration post-time of 6 min to find back the initial conditions prior to the next injection. The injection volume was set to 25 μL and the target analyte was measured using emission and excitation wavelengths of 480 nm and 365 nm, respectively.
An Agilent 1290 system was connected to a qTOF-MS detector (Agilent 6540 UHD Accurate-Mass, Agilent, Germany), using an electrospray interface (ESI) operated in the positive or negative ionization modes. The temperature of the source was set to 325 °C and a nitrogen flow (from a nitrogen generator) was used as drying gas (2.8 bar) as well as nebulizing gas at a flow of 10 L min−1. The TOF-MS detector was set to acquire MS data over an m/z range of 100 to 1600 amu. The ESI capillary voltage was 4 kV or 3 kV in the positive or negative modes, respectively. The analysis was performed using a Kinetex C18 chromatographic column (50 × 2.1 mm, 1.7 μm particle size) of Phenomenex (USA) with a flow rate of 200 μL min−1. The column was thermostated at 40 °C. The mobile phase consisted of water with 0.1% formic acid (A) and methanol with 0.1% formic acid (B). A gradient program was applied, starting from 50% B and changing linearly to 95% B in 30 min, followed by an isocratic elution for 12 min. The chromatographic column was equilibrated to reach initial conditions in two minutes. The injection volume was 5 μL. Both methods have been previously single-laboratory validated by the EURL-FCM (Alberto Lopes et al., 2018; Tsochatzis et al., 2018).
2.5. Pre-heating of food contact PP cups
Laboratories appeared during the proficiency test to pre-heat FCM articles prior to migration testing in order to maintain the temperature in the food simulant in the required temperature range, e.g. for testing at 70 °C, articles were pre-heated in the range of 70–90 °C up to 3 h (Tsochatzis et al., 2018). In addition FCM articles may be exposed to extreme temperatures (40–60 °C) during transport of several days before use. Sets of two PP cups were heat-treated at 40, 60, 70, 75, 80 and 90 °C from 10 min to 5 days (7200 min) in ventilated ovens. One cup was then analyzed using DSC, while the other one was used for the migration experiment after cooling down to room temperature. A total of 30 pairs of PP cups have been heat-treated.
2.6. Migration experiments
The migration conditions investigated in this work derived from the intended use of the FCM, taking into consideration the worst foreseeable conditions of use. It was therefore assumed that the investigated PP cups could be filled with a hot alcoholic beverage, for which Regulation (EU) No 10/2011 requires migration experiments to be performed at 70 ± 2 °C from 0 to 2 h using food simulant D1 (50% v/v ethanol).
A protocol for the migration to be carried within the requested temperature range has been developed and optimized in-house based on the outcome of the two proficiency tests organized by the EURL-FCM on this topic (Tsochatzis et al., 2016, 2018). A 108 L thermostatic oven (Memmert, Schwabach, Germany) has been used operating with fan at the highest speed and, with a temperature set at 71 °C (±0.1 °C).
Food simulant D1 was prepared 300 mL (±2.5 mL) was transferred into glass Schott bottles with PTFE insulated caps. The filled bottles were preheated in another oven at 75 °C (±0.1 °C) for 12–14 h before use. The food contact cups were filled-up outside and close (<40 cm) to the thermostatic oven and immediately placed inside the oven. The filling procedure had to take less than 15 s, in order to avoid temperature drop. Temperatures of both oven and food simulant have been under constant control throughout the experiment, using exclusively calibrated data loggers.
In order to minimize food simulant losses during the migration, the studied cups have been covered with clock glasses with inert O-rings on top as to apply pressure and seal the test specimens as much as possible. The volume of the food simulant was measured after migration as to evaluate losses. All observed losses were lower than 1% of the initial food simulant volume.
2.7. Quantification of migrants
Table 1 presents the chemical and legislative information related to the target substances analyzed in food simulant D1 after the migration experiment. HPLC-FLD was used for the quantification of FCM No. 500, while the other substances were quantified using LC–MS. These substances were studied because of their detectable presence in the PP cups and the structural difference. FCM No. 500 and 808 have a specific migration limit of 0.6 and 5 mg/kg food, respectively.
2.8. Data processing and statistical analysis
All the statistical data treatment, including Principal Component Analysis (PCA), was performed using Minitab 18.0, a statistical software from Minitab Inc.
3. Results and discussion
3.1. DSC and TGA analysis
3.1.1. Identification of the type of PP cups used
At first the DSC measurements on the PP cup resulted in a melting temperature of ca. 145 °C, indicating the presence of PP copolymers (Alin & Hakkarainen, 2010; Bertoldo, Ciardelli, Ferrara, & Scoponi, 2003), despite the claim of the producer having delivered PP homopolymer with a typical melting temperature of ca. 160 °C. Further DSC and TGA tests were carried out using two cups labelled as PP copolymers (PP-R and PP-C), and a cup known to be made of PP homopolymer (PP-H) to confirm the cup composition.
Significantly different melting curves are visible in Fig. 1a, with two distinct minima of the heat flow: around 148 °C (for the PP-R and PP cups), and at 164 °C (PP-C and PP-H cups). Similarly, the crystallization curves (Fig. 1b) show three maxima of the heat flow: at 110 °C (for the PP cups), 117 °C (PP-R and PP-C) and 125 °C (PP-H). These results indicate that the PP cups are probably made of a different PP copolymer.
Fig. 1.
DSC thermograms of PP sample cups: (a) melting curves and (b) crystallization curves.
Consequently, two types of experiments were performed for the TGA analysis to monitor mass loss: a) the typical thermal decomposition analysis and b) an isothermal analysis. The isothermal analysis showed no significant mass loss (less than 0.3% after 24 h) during the heating at 40 °C–90 °C (thermograms not presented).
The results of the thermal decomposition analysis are shown in Fig. 2. The PP-C, PP-R, and the PP cups present the same decomposition behavior, starting at around 320 °C until full degradation at 490 °C. PP-H revealed a different degradation pattern, starting at 280 °C until full degradation around 470 °C. These results further confirm the difference between the PP cups studied here and the PP-H cups.
Fig. 2.
GA thermograms of PP cups: overlay of the thermal decomposition curves.
3.1.2. Study of the degree of the crystallinity
The melting temperature and the calculated degree of crystallinity of the PP cups (1st and 2nd heat-cycle) that were heat-treated at 40 and 90 °C have been compared to those of non-heat-treated cups (Table 2). The full set of results for all the heat-treatment temperatures used is presented as Supplementary material (Table S1).
Table 2.
Melting point temperature (Tm) and degree of crystallinity (XC) of the PP cups in the first and second DSC heating cycle.
| Heat treatment |
Tm (°C) |
XC (%) |
|||
|---|---|---|---|---|---|
| Temperature (°C) | Duration (min) | 1st heating | 2nd heating | 1st heating | 2nd heating |
| 40 | 10 | 146.6 | 145.8 | 31.3 | 34.4 |
| 30 | 145.3 | 144.9 | 31.5 | 35.7 | |
| 60 | 144.4 | 144.2 | 30.6 | 34.3 | |
| 120 | 145.2 | 145.6 | 29.6 | 34.0 | |
| 180 | 146.9 | 147.1 | 29.0 | 33.5 | |
| 1440 (1 day) | 144.7 | 144.7 | 28.0 | 34.2 | |
| 7200 (5 days) | 146.4 | 146.1 | 28.9 | 31.1 | |
| 90 | 10 | 144.9 | 146.4 | 29.8 | 32.9 |
| 30 | 146.4 | 147.5 | 29.6 | 32.8 | |
| 60 | 146.0 | 147.2 | 26.6 | 30.1 | |
| 120 | 145.0 | 146.2 | 32.0 | 34.9 | |
| 180 | 145.6 | 146.6 | 30.6 | 34.5 | |
| 1440 (1 day) | 145.2 | 146.4 | 29.4 | 31.5 | |
| 7200 (5 days) | 145.1 | 146.2 | 29.3 | 31.3 | |
| Non-preheated cups | Average (n = 5) | 145.1 | 145.8 | 31.5 | 34.5 |
| SD | 0.6 | 0.3 | 0.2 | 0.3 | |
Table 2 shows that the temperature and the duration of the pre-heating may have a significant effect on the degree of crystallinity (Xc, %) of the material, calculated on the results of the first heating cycle. Additionally, the increase of the Xc observed in the second DSC heating cycle is significant, due to cancelling of the thermal history of the polymer that takes place during this cycle. However, the results also showed that this cancellation was not entirely efficient (Alin & Hakkarainen, 2010; Bertoldo et al., 2003; Ferrara, Bertoldo, Scoponi, & Ciardelli, 2001).
Fig. 3 presents the measured degree of crystallinity (Xc, %) as a function of the duration of heat treatment (log scale used for the x-axis). The upper line represents the Xc of the non-heat-treated samples (31.5%). Overall, Xc decreases significantly for the all the investigated temperatures (40–90 °C) starting at 31.5% to reach a final value around 28.5% (lower line). However, a significantly decrease in Xc is observed already after 10 min at 80 and 90 °C. An anomalous drop in crystallinity is observed at 60 min (at 60 °C and above), which could be attributed to a disarrangement of the crystal net due to relaxation processes of the amorphous regions of the polymer under thermic stress. The system seems to rearrange to recover some crystallinity, resulting in an equilibrium state after a few days. Similar behaviour has been observed in other works, although with either a different polymer or items (Farhoodi et al., 2012; Song et al., 2016).)
Fig. 3.
Degree of crystallinity vs. the duration of the heat treatment at different temperatures.
The fact that Xc is always below 41% indicates that the PP cups studied here were not made of a homopolymer but were a copolymer, as already seen in Section 3.1.1 (Alin & Hakkarainen, 2010; Bertoldo et al., 2003; Ferrara et al., 2001). Hence, it is very likely that the producer mislabeled his product.
3.2. FT-IR and optical microscopy analysis
PP cups were analyzed using FT-IR spectroscopy to confirm the composition of the cups and to evaluate any potential effects due to heat-treatment. The polymer of the studied cups was identified as PP, but could not be further specified as PP-H, PP-R or PP-C.
The FT-IR spectra of the heat-treated samples were similar to those of non-heat-treated samples, indicating that no chemical changes occurred. This was further confirmed by the TGA thermograms of the isothermic analysis, where only insignificant mass losses (less than 0.3%) were observed.
The images of the material surface obtained by optical microscopy (Fig. 4a–c) show that the PP cup samples express some surface differences after heat treatment compared with non-heat-treated PP samples. A similar effect was observed by Andersson et al. for PLA materials subjected to heat treatment simulating accelerated aging (Andersson et al., 2012). This was attributed to structural modifications (cf. crystallinity) and to the migration/evaporation of low molecular mass additives during the heat treatment, thus creating holes on the surface of the material. Similar holes were observed on the surface of the heat-treated PP cups studied here (see dark dots in Fig. 4).
Fig. 4.
Optical microscopy images (500×) of the surface of PP cups after heat treatment: (a) no heating; (b) at 40 °C for 1 day; (c) at 75 ° C for 1 day.
3.3. Quantification of migrants from the heat-treated PP cups
The identification and quantification of the migrating substances in food simulant D1 were performed using HPLC-FLD (due to its higher sensitivity) for additive FCM No. 500 and UHPLC-qTOF-MS for FCM No. 808 and glycerol esters. Fig. 5 shows a typical LC–MS chromatogram of the food simulant after the migration experiment with the PP cups at 70 °C ± 2 °C for 2 h.
Fig. 5.
Typical total ion chromatogram of food simulant D1 after the migration experiment with the PP cups.
The amounts migrating from non-heat-treated PP cups are presented in Table 3. The two substances with established SMLs were found to migrate at levels below those defined in Regulation (EU) No 10/2011. The additive with the highest specific migration was found to be 1-palmitoyl glycerol, with mass fractions already in the mg/kg range and ten times higher than the second highest migrating substance.
Table 3.
Results of the migration experiments on the non-pre-heated PP cups by article filling at 70 °C for 2 h in food simulant D1.
| Substances | SML, μg/kgd | Specific migration (μg/kg ± SD) |
|---|---|---|
| 1-palmitoyl glycerola | – | 7470 ± 810e |
| 2-palmitoyl glycerola | – | 860 ± 100e |
| 1-stearoyl glycerolb | – | 430 ± 70e |
| 2-stearoyl glycerolb | – | 930 ± 80e |
| 2,5-bis(5-tert-butyl-2-benzoxazolyl)thiophene (FCM No. 500) | 600 | 4.80 ± 0.35c |
| bis(4-propylbenzylidene) propylsorbitol (FCM No. 808) | 5000 | 480 ± 30e |
SD: standard deviation.
Part of mixture FCM No. 50.
Part of mixture FCM No. 53.
Quantification by HPLC-FLD.
From Reg. (EU) No. 10/2011.
Quantification by LC–MS.
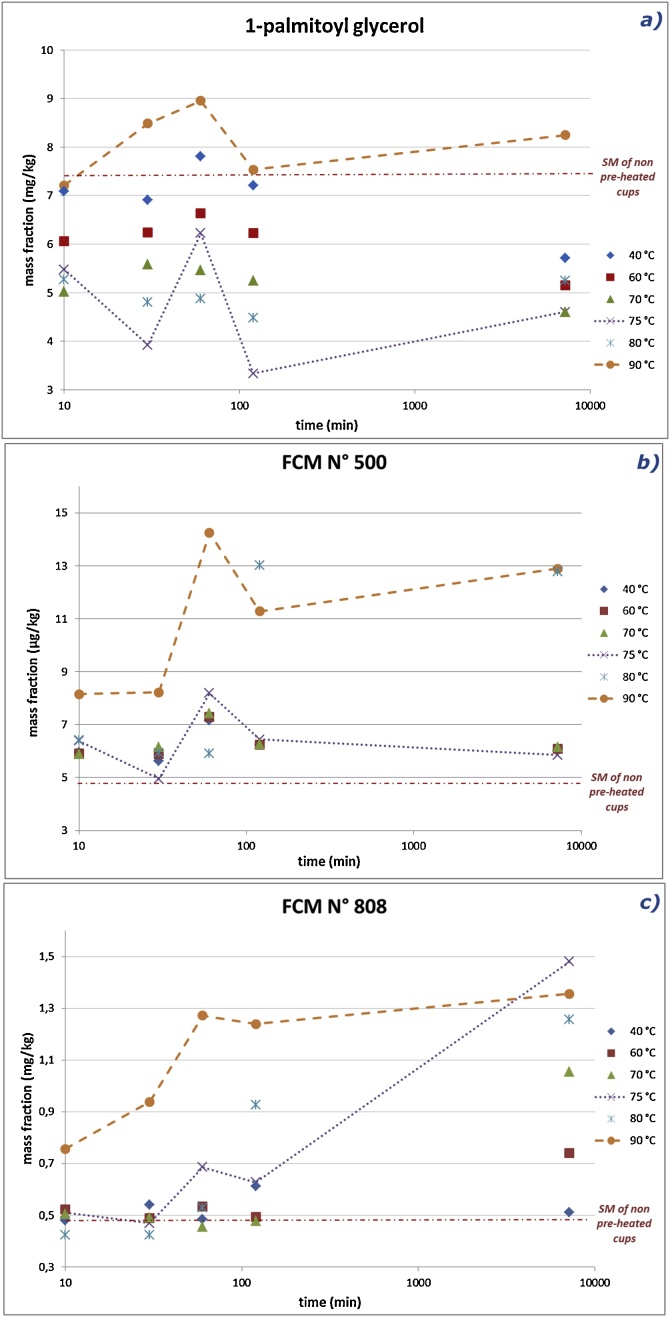
The effect of (prior) pre-heating the PP cups on the specific migration of 1-palmitoyl glycerol, FCM No. 500 and FCM No. 808 is presented in Fig. 6. The variation of the specific migration of the remaining additives was less significant and the corresponding data are provided as Supplementary material (Table S2).
Fig. 6.
Specific migration (SM) of 1-palmitoyl glycerol (a, in mg/kg), FCM No. 500 (b, in μg/kg) and FCM No. 808 (c, in mg/kg) after pre-heating the PP cups, at different temperatures, followed by migration at 70 °C for 2 h with food simulant D1 as a function of the pre-heating contact time (presented as log-time scale).
Unlike cups pre-heated at 90 °C, in Fig. 6a it can be seen that the migrated amount of 1-palmitoyl glycerol in the pre-heating contact temperature range of 40–80 °C was always below the one determined for non-pre-heated PP cups (7.5 mg/kg). Increasing the pre-heating contact time caused an overall decrease in the migrated amount (averages of 6 or 5 mg/kg at 60 or 70 °C, respectively). The contact temperature of 75 °C results in a major change in the migration behavior characterized by the lowest mass fractions detected (around 4 mg/kg) and a sharp increase at 60 min (up to 6 mg/kg). Consequently, the migration behavior seems inverted, gradually increasing from 5 mg/kg (at 80 °C) to fractions ranging from 7 to 9 mg/kg (for 90 °C).
Fig. 6b presents a different migration profile from pre-heated PP cups for FCM No. 500: (i) all migrated amounts are higher than the one of non-pre-heated cups (around 5 μg/kg); (ii) no significant increase in migrated amounts (around 6 μg/kg) is observed for pre-heating temperatures up to 75 °C; (iii) an abrupt increase in migration is again observed at 60 min (for 75 °C), followed by a drastic change in the migration profile at higher temperatures (80 or 90 °C) characterized by a significant increase around 60 min to reach a migration plateau of 13 μg/kg after 2 h (up to 5 days).
Fig. 6c presents the migration profiles from pre-heated PP cups for FCM No. 808. The migration of the pre-heated PP cups for temperatures in the range of 40–80 °C was similar to that of the non-pre-heated cups below 60 min of pre-heating contact time, whereas that for a temperature of 90 °C was higher. For temperatures in the range of 60–90 °C, the migration increased with the pre-heating contact time.
The migration level of 1-palmitoyl was significantly higher (mg/kg range) when compared with the remaining glycerols (2-palmitoyl glycerol, 1-steroyl glycerol and 2-stearoyl glycerol), which had migration levels in the μg/kg range. These glycerols showed a migration similar to 1-palmitoyl glycerol, with a generally decreasing trend to lower amounts than the initial migration. After a pre-heating of 5 days, the only exceptions to this trend are results at 80 °C and 90 °C, which represent higher amounts than the initial ones (1-steroyl glycerol and 2-stearoyl glycerol) or the same (2-palmitoyl glycerol). The observed changes in migration behavior seem to be correlated with the chemical structure of the additives. While an increase of the migration rate with prolongation of the duration of the pre-heating was observed for the higher molecular weight and bulkier additives (FCM No. 500 and 808), a decrease was observed for the lower molecular weight and linear structure ones (glycerols).
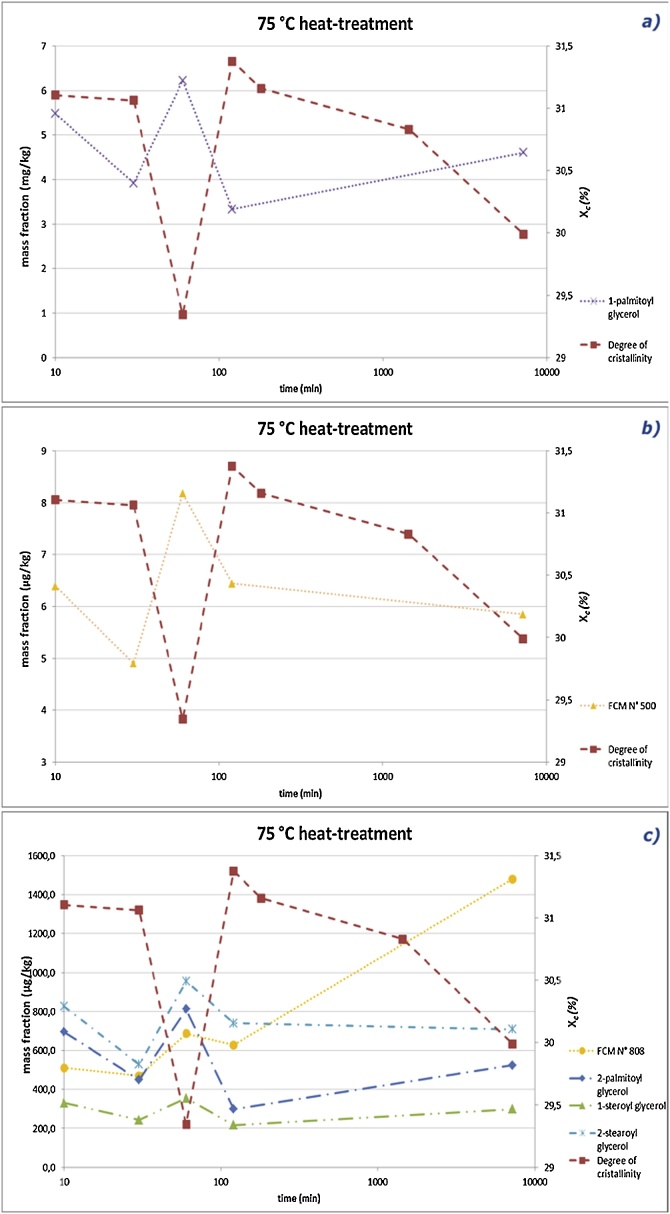
Fig. 7 shows that for all the substances investigated a significant increase in migration from pre-heated cups for 1 h relates to a drastic decrease in the degree of crystallinity observed.
Fig. 7.
Degree of crystallinity and specific migrations for pre-heated cups at 75 °C as a function of the pre-heating contact time (presented as log-time scale) for (a) 1-palmitoyl glycerol, (b) FCM N° 500 and (c) FCM N° 808 and remaining glycerols.
3.4. Multivariate analysis of the results
The EURL-FCM organized two inter-laboratory comparisons dealing with temperature control during migration experiments (at 70 °C for 2 h) (Tsochatzis et al., 2016, 2018). A principal component analysis (PCA) of the data from participants indicated that laboratories having heated the cups prior to the migration experiment obtained biased results and contributed negatively to the variance of the dataset (Tsochatzis et al., 2018). A PCA of the data obtained in the current work is presented in Fig. 8.
Fig. 8.
PCA score plot (a) and loading plot (b), for the five studied parameters. Data points in the blue dashed circle are the non-pre-heated data and data points in the red dashed circle are the pre-heated data. The colored dots represent: green, FCM No. 808; blue, 2-palmitoyl glycerol; brown, 2-stearoyl glycerol; light blue, 1-stearoyl glycerol; red, 1-palmitoyl glycerol; purple, FCM No. 500. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article).
Two main clusters are clearly observed in the PCA score plot (Fig. 8 – a), distinguishing the non-pre-heated PP cups (blue dashed circled data) and the pre-heated cups (red dashed circle data). Even a 10 min heat-treatment at 40 °C was sufficient to place all substances in a different cluster than the non-pre-heated cups results. The scattering of the values within the red dashed circle cluster relates to the effect of heat-treatment on Xc and the migration levels of the additives. The two additives that show the most disperse scattering are FCM No. 500 and 1-palmitoyl glycerol, as they were the ones presenting the most drastic variations.
It can be concluded from the loading plot (Fig. 8 – b), that the pre-heating temperature and time are two parameters highly correlated and balanced by the degree of crystallinity. Similarly, the six target analytes and their migration to the food simulant are correlated. The PCA shows that for 2-palmitoyl glycerol, 1-stearoyl glycerol, 2-stearoyl glycerol and FCM No. 808 the variance of the migration results is mainly determined by the degree of crystallinity, the pre-heating time and the temperature of the PP. For 1-palmitoyl glycerol and FCM No. 500, the two additives with the highest (above 4 mg/kg range) and lowest (few μg/kg range) migration levels, respectively, both the migration (expressed as mass fraction) and the type of substance seem to have contributed to the data variance.
4. Conclusions
This work has shown that the heat-treatment of semi-crystalline polymeric food contact cups can lead to changes in the degree of crystallinity, starting as low as at 40 °C. Physical changes have also been observed on the surface of the material. The variation in crystallinity affected the specific migration of several substances. Sample handing before the migration test can be designed in such way that pre-heating of FCM articles before the migration test is not necessary. Therefore, it is recommended to refrain from any pre-heating of FCM articles prior to migration experiments to avoid any unwanted and uncontrolled temperature-induced effect on the migration of a substance and to harmonise migration test approaches among official control laboratories. These potential effects would be of particular importance for decision-making when the migrating amounts are close to SMLs.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Disclaimer
Certain commercial equipment, instruments, and materials are identified in this paper/report to specify adequately the experimental procedure. In no case does such identification imply recommendation or endorsement by the European Commission, nor does it imply that the material or equipment is necessarily the best available for the purpose.
Acknowledgments
The authors would like to acknowledge both Hendrik Emons and Hugues Crutzen for their support on this work.
Footnotes
Supplementary material related to this article can be found, in the online version, at doi:https://doi.org/10.1016/j.fpsl.2019.100305.
Appendix A. Supplementary data
The following is Supplementary data to this article:
References
- Abdel-Mohti A., Garbash A.N., Almagahwi S., Shen H. Effect of layer and film thickness and temperature on the mechanical property of micro- and nano-layered PC/PMMA films subjected to thermal aging. Materials. 2015;8:2062–2075. [Google Scholar]
- Alberto Lopes J.F., Tsochatzis E.D., Emons H., Hoekstra E. Development and validation of an HPLC method with fluorescence detection for the determination of fluorescent whitening agents migrating from plastic beverage cups. Food Additives & Contaminants Part A. 2018;35:1438–1446. doi: 10.1080/19440049.2018.1459053. [DOI] [PubMed] [Google Scholar]
- Alin J., Hakkarainen M. Type of polypropylene material significantly influences the migration of antioxidants from polymer packaging to food simulants during microwave heating. Journal of Applied Polymer Science. 2010;118:1084–1093. [Google Scholar]
- Alin J., Hakkarainen M. Microwave heating causes rapid degradation of antioxidants in polypropylene packaging, leading to greatly increased specific migration to food simulants as shown by ESI-MS and GC-MS. Journal of Agricultural and Food Chemistry. 2011;59:5418–5427. doi: 10.1021/jf1048639. [DOI] [PubMed] [Google Scholar]
- Andersson S.R., Hakkarainen M., Albertsson A.C. Long-term properties and migration of low molecular mass compounds from modified PLLA materials during accelerated ageing. Polymer Degradation and Stability. 2012;97:914–920. [Google Scholar]
- Bertoldo M., Ciardelli F., Ferrara G., Scoponi M. Effect of the structure of reactor poly(propyleneco-ethylene) blends on the diffusion coefficient and activation energy of a conventional antioxidant. Macromolecular Chemistry and Physics. 2003;204:1869–1875. [Google Scholar]
- European Commission Regulation (EU) No 10/2011 of 14 January 2011 on plastic materials and articles intended to come into contact with food. https://eur-lex.europa.eu/legal-content/EN/TXT/HTML/?uri=CELEX:32011R0010&from=EN (Accessed 10 June 2018).
- Farhoodi M., Mousavi S.M., Sotudeh-Gharebagh R., Emam-Djomeh Z., Oromiehie A., Mansour H. A study on physical aging of semicrystalline polyethylene terephthalate below the glass transition point. Journal of Applied Research and Technology. 2012;10:698–702. [Google Scholar]
- Ferrara G., Bertoldo M., Scoponi M., Ciardelli F. Diffusion coefficient and activation energy of Irganox 1010 in poly(propylene-co-ethylene) copolymers. Polymer Degradation and Stability. 2001;73:411–416. [Google Scholar]
- Hoekstra E.J., Brandsch R., Dequatre C., Mercea P., Milana M.R., Störmer A.…Simoneau C. EUR 27529 EN. Publications Office of the European Union; Luxembourg: 2015. Practical guidelines on the application of migration modelling for the estimation of specific migration. [Google Scholar]
- Howell B.A., Smith P.B. Thermal degradation of vilylidene chloride/4-vinylpyridine copolymers. Journal of Thermal Analysis and Calorimetry. 2006;83:71–73. [Google Scholar]
- Silva A.S., García R.S., Cooper I., Franz R., Losada P.P. Compilation of analytical methods and guidelines for the determination of selected model migrants from plastic packaging. Trends in Food Science & Technology. 2006;17:535–546. [Google Scholar]
- Simoneau C. Food contact materials. Comprehensive Analytical Chemistry. 2008;51:733–773. [Google Scholar]
- Song X., Sun Z., Li C., Gao J., Ma J., Guo B.…Gao J. Effect of thermal aging on migration characteristics of heavy metal in PET. Mater. Sci. Forum. 2016;859:142–147. [Google Scholar]
- Tsochatzis E., Mieth A., Simoneau C., Hoekstra E. Publications Office of the European Union; Luxembourg: 2016. Report of an inter-laboratory comparison from the European Union Reference Laboratory for Food Contact Materials. ILC01 2015 – Temperature control during migration tests by article filling. European Commission, Joint Research Centre, EUR 27826 EN (2016) [Google Scholar]
- Tsochatzis E., Alberto Lopes J.F., Robouch P., Hoekstra E. European Commission, Joint Research Centre, EUR 29121 EN. Publications Office of the European Union; Luxembourg: 2018. EURL-FCM-02-2016 proficiency test report: Temperature control during migration and quantification of migrated FCM No 500 by article filling. [Google Scholar]
- Wunderlich B. Thermal Analysis. Academic Press Limited; London, United Kingdom: 1990. ATHAS table of thermal properties of linear macromolecules; pp. 417–431. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.