Heterogeneous neuroimaging findings in Parkinson’s dementia localise to a common brain network centred on the hippocampus. This result is symptom and disease-stage specific, as visual hallucinations and mild cognitive impairment map to different networks. These results make sense of an otherwise discordant neuroimaging literature, lending insight into Parkinson’s dementia.
Keywords: Parkinson’s disease, dementia, mild cognitive impairment, visual hallucinations, imaging
Abstract
Dementia is a common and devastating symptom of Parkinson’s disease but the anatomical substrate remains unclear. Some evidence points towards hippocampal involvement but neuroimaging abnormalities have been reported throughout the brain and are largely inconsistent across studies. Here, we test whether these disparate neuroimaging findings for Parkinson’s disease dementia localize to a common brain network. We used a literature search to identify studies reporting neuroimaging correlates of Parkinson’s dementia (11 studies, 385 patients). We restricted our search to studies of brain atrophy and hypometabolism that compared Parkinson’s patients with dementia to those without cognitive involvement. We used a standard coordinate-based activation likelihood estimation meta-analysis to assess for consistency in the neuroimaging findings. We then used a new approach, coordinate-based network mapping, to test whether neuroimaging findings localized to a common brain network. This approach uses resting-state functional connectivity from a large cohort of normative subjects (n = 1000) to identify the network of regions connected to a reported neuroimaging coordinate. Activation likelihood estimation meta-analysis failed to identify any brain regions consistently associated with Parkinson’s dementia, showing major heterogeneity across studies. In contrast, coordinate-based network mapping found that these heterogeneous neuroimaging findings localized to a specific brain network centred on the hippocampus. Next, we tested whether this network showed symptom specificity and stage specificity by performing two further analyses. We tested symptom specificity by examining studies of Parkinson’s hallucinations (9 studies, 402 patients) that are frequently co-morbid with Parkinson’s dementia. We tested for stage specificity by using studies of mild cognitive impairment in Parkinson’s disease (15 studies, 844 patients). Coordinate-based network mapping revealed that correlates of visual hallucinations fell within a network centred on bilateral lateral geniculate nucleus and correlates of mild cognitive impairment in Parkinson’s disease fell within a network centred on posterior default mode network. In both cases, the identified networks were distinct from the hippocampal network of Parkinson’s dementia. Our results link heterogeneous neuroimaging findings in Parkinson’s dementia to a common network centred on the hippocampus. This finding was symptom and stage-specific, with implications for understanding Parkinson’s dementia and heterogeneity of neuroimaging findings in general.
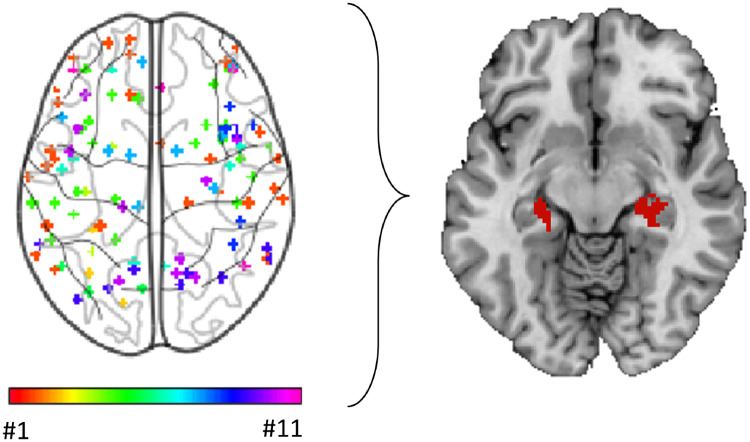
Graphical Abstract
Graphical Abstract.
Introduction
Dementia is a common and debilitating aspect of Parkinson’s disease: 50% of patients will develop dementia within 10 years of diagnosis (Williams-Gray et al., 2013), and it carries significant societal and economic burden (Spottke et al., 2005; Leroi et al., 2012) with high levels of frailty and nursing home admissions (Fredericks et al., 2017; Weir et al., 2018). Identifying the neuroanatomical substrate of Parkinson’s disease with dementia (PD dementia) could aid prognosis and treatment development. Unfortunately, this neuroanatomical substrate remains unclear.
One possibility is that PD dementia stems from the hippocampus, a region known to play a critical role in memory and in other forms of dementia (Fox et al., 1996; Seeley et al., 2009; Darby et al., 2019). Memory problems are frequently the first subjective cognitive complaint in Parkinson’s disease (Noe et al., 2004) and are a prominent component of PD dementia (Whittington et al., 2000; Bronnick et al., 2007; Muslimovic et al., 2007; Reid et al., 2011; Wang et al., 2015), forming part of the diagnostic criteria for PD dementia (Emre et al., 2007). In patients with PD dementia the hippocampus shows a higher density of Lewy pathology (Harding and Halliday, 2001; Apaydin et al., 2002; Arnold et al., 2013; Hall et al., 2014), reduction in cholinergic activity (Hall et al., 2014) and progressive atrophy with disease progression (Aybek et al., 2009; Weintraub et al., 2011, 2012; Morales et al., 2013; Kandiah et al., 2014; Mak et al., 2015; Gee et al., 2017; Mihaescu et al., 2018).
However, the role of the hippocampus in PD dementia remains uncertain for several reasons. First, although memory problems are an early subjective complaint (Noe et al., 2004), objective testing usually shows early deficits in visuospatial and executive function (Janvin et al., 2006a; Williams-Gray et al., 2013; Kalbe et al., 2016). At this stage, patients are often considered as having PD with mild cognitive impairment (PD-MCI) (Emre et al., 2007; Kehagia et al., 2013). Although 90% of these patients will eventually progress to PD dementia, worse visuospatial deficits, not memory deficits, are associated with rapid progression (Williams-Gray et al., 2013; Weil et al., 2017). Second, most PD dementia patients have co-morbid symptoms such as visual hallucinations, whose neural substrate is also unclear but is unlikely to localize to the hippocampus (Fenelon et al., 2000; Gallagher et al., 2011). Finally, PD dementia is associated with Lewy pathology and atrophy throughout nearly the entire brain (Hurtig et al., 2000; Braak et al., 2005; Irwin et al., 2012). Neuroimaging studies of PD dementia have been particularly heterogeneous (Lanskey et al., 2018), with atrophy or hypometabolism reported in frontal (Song et al., 2011; Melzer et al., 2012), temporal (Melzer et al., 2012; Pagonabarraga et al., 2013), parietal (Melzer et al., 2012; Pereira et al., 2014), occipital (Melzer et al., 2012) and insular cortices (Mak et al., 2014) as well as numerous subcortical regions (Melzer et al., 2012; Foo et al., 2017; Schneider et al., 2017). Different meta-analyses of the coordinates reported by these studies have also been inconsistent (Minkova et al., 2017; Mihaescu et al., 2018).
An assumption underlying many conventional neuroimaging studies is that abnormalities should localize to specific brain regions in order to explain specific symptoms (Eickhoff et al., 2009). However, some symptoms may localize better to brain networks, rather than specific brain regions (Fox et al., 2005; Dickerson and Sperling, 2009; Seeley et al., 2009). We have used this approach to link lesions found in disparate brain regions that produce similar symptoms to a common brain network, a technique known as lesion network mapping (Boes et al., 2015; Fox, 2018; Joutsa et al., 2018a). Recently, we validated an extension of lesion network mapping termed coordinate-based network mapping (Darby et al., 2019). We showed that heterogeneous neuroimaging findings in Alzheimer’s disease map to a common brain network, centred on the hippocampus (Darby et al., 2019). This result was specific compared to neurodegenerative diseases that are not characterized by memory decline.
Here, we apply this technique to PD dementia. We hypothesize that: (i) coordinate network mapping will reveal a common network involved in PD dementia centred on the hippocampus; (ii) this network will be specific compared with the highly co-morbid symptom of visual hallucinations; (iii) this network will be specific compared to PD-MCI which is an earlier stage of PD dementia more commonly characterized by visuospatial or executive dysfunction.
Materials and methods
Search strategy
We identified studies reporting neuroimaging abnormalities in patients with Parkinson’s disease dementia and with mild cognitive impairment by performing a search of the PubMed databases for papers published between 1 January 1985 and 4 June 2018. Four sets of keywords were used: Parkinson or Parkinson’s; dement*, dementia, mild cognitive impairment or MCI; MRI or magnetic resonance imaging combined with voxel-based morphometry, VBM or struct*; and PET, fludeoxyglucose (FDG)-PET or single photon emission computed tomography (SPECT), restricted to human studies. A similar search was performed to identify relevant studies on visual hallucinations in Parkinson’s disease and included hallucinations, Parkinson’s disease, MRI and FDG-PET or SPECT, as above. The reference lists of relevant review articles were then hand searched for potential missed studies.
Inclusion and exclusion criteria
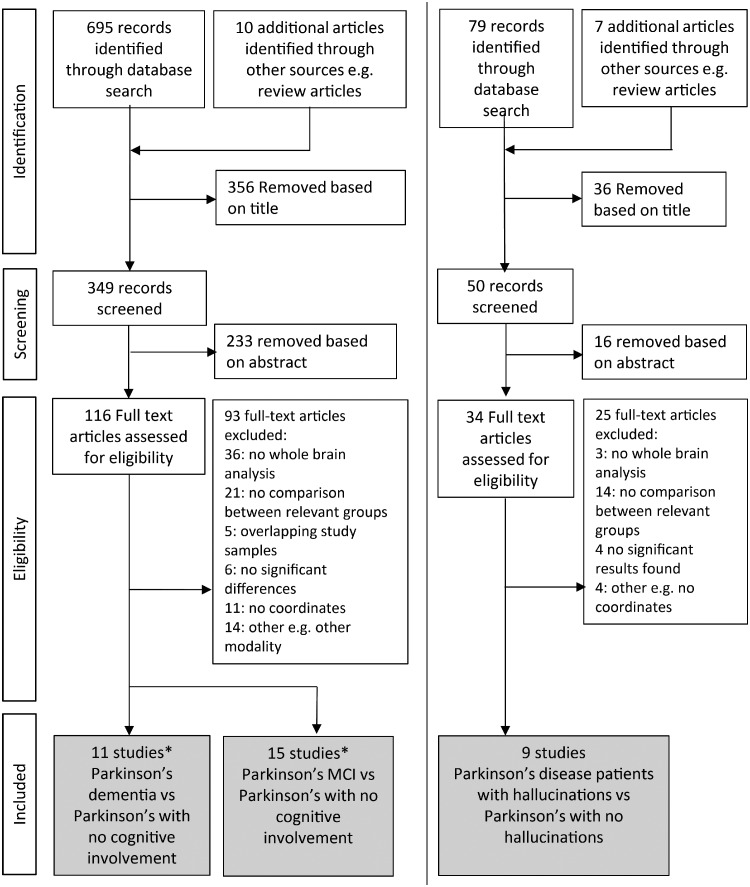
The meta-analyses included only articles that (i) involved patients with Parkinson’s disease and dementia (or hallucinations), with PD dementia defined as a dementia syndrome that developed in the context of established Parkinson’s disease (Emre et al., 2007); (ii) reported coordinates for atrophy (using VBM or cortical thickness measures) or hypometabolism (FDG-PET or SPECT) between the relevant patient groups; (iii) used comparisons between the symptom in question and Parkinson’s patients without that symptom; (iv) reported whole-brain results for these changes; (v) coordinates were reported in stereotactic space (montreal neurological institute (MNI) or Talairach). We excluded the following: (i) studies exclusively reporting changes in dementia with Lewy Bodies; (ii) studies without direct comparisons between patient groups (e.g. brain regions correlating with cognitive scores); (iii) non-original or duplicate studies; (iv) studies that confined their search within specific regions of interest; (v) studies that reported no differences between patient groups; (vi) case reports; (vii) studies that did not report coordinates or where reported coordinates diverged significantly from reported locations. (See Fig. 1 for flow diagrams for the searches and Tables 1–3 for included studies for each of the searches.)
Figure 1.
Systematic literature search and study selection. Neuroimaging studies of Parkinson’s disease (PD) dementia, PD with visual hallucinations and PD-MCI were selected in accordance with PRISMA guidelines. *Three studies included both PD dementia and PD-MCI comparisons.
Table 1.
Clinical characteristics and scanning modalities of studies of Parkinson’s dementia (PDD) versus Parkinson’s without cognitive involvement (PD)
| First author | Modality | N | N | Age | Age PD | MMSE PDD | MMSE PD | H&Y PDD | H&Y PD | UPDRS PDD | UPDRS PD |
|---|---|---|---|---|---|---|---|---|---|---|---|
| PDD | PD | PDD | |||||||||
| Total n = 175 | Total n = 210 | ||||||||||
| (1) Beyerb,c | VBM | 16 | 20 | 73.5 | 72.5 | 19.4 | 28.2 | 3 | 2.4 | ||
| (2) Burtonb | VBM | 26 | 31 | 72.3 | 75.2 | 18.9 | 26.4 | 36.4 | 25.8 | ||
| (3) Geec | VBM | 23 | 10 | 71.6 | 69.4 | 27.3 | 28.9 | 14.4 | 15.3 | ||
| (4) Goldmanb | VBM | 24 | 26 | ||||||||
| (5) Kleinc | FDG-PET | 8 | 9 | 62 | 67 | 21 | 28.4 | 2 | 3 | 24 | 25 |
| (6) Leeb | VBM | 16 | 16 | 69.9 | 68.3 | 19.6 | 27.3 | 2.6 | 1.7 | ||
| (7) Nagano-Saitob,c | VBM | 9 | 17 | 67.3 | 65.4 | 16.1 | 27.9 | 3.3 | 3.1 | ||
| (8) Songb | VBM | 18 | 23 | 72 | 69.1 | 18.1 | 28.6 | 32.1 | 16.9 | ||
| (9) Tangb,c | FDG-PET | 10 | 30 | 61.4 | 61.9 | 23.2 | 28.5 | 2.5 | 1.8 | 30.7 | 23 |
| (10) Xia | VBM | 12 | 12 | 69.3 | 65.6 | 23.4 | 28.1 | 3 | 1.8 | 44 | 14.3 |
| (11) Yongb | FDG-PET | 13 | 16 | 73.4 | 64.2 | 15.4 | 27.3 | 3.2 | 2.1 | ||
| Summary (mean(SD)) | 16 (6) | 19 (8) | 69.3 (4) | 67.8 (4) | 20.2 (4)a | 28.0 (0.8)a | 2.8 (0.5) | 2.3 (0.6) | 30.3 (10) | 20.1 (5) |
Wilcox test shows significant difference between groups (Other comparisons are not significantly different).
Indicates established criteria were used to define PD dementia. Extended neuropsychological testing was used in the remaining studies.
Indicates the study matched PDD and PD groups for motor stage.
FDG-PET, fluorodeoxyglucose positron emission tomography; H&Y, Hoehn and Yahr; MMSE, mini-mental state examination; PDD, Parkinson’s disease dementia; SPECT, single photon emission computed tomography; UPDRS, Unified Parkinson’s disease rating scale score (part III, motor); VBM, voxel-based morphometry.
Data extraction and demographics
Data were extracted from each of the identified studies using a predefined data extraction form, to include information on author, publication year, sample size, demographics, clinical information, modality and coordinates. Talairach coordinates were converted into MNI coordinates using the automated transformation provided with GingerALE (http://www.brainmap.org/ale/), unless the study specified that the original analysis was conducted in MNI space and converted post hoc into Talairach space, in which case we used the conversion provided by MNI to Talairach converter programme (http://sprout022.sprout.yale.edu/mni2tal/mni2tal.html).
We tested for significant demographic differences such as age and Hoehn and Yahr using two-tailed Welch’s t-tests or Mann-Whitney-Wilcoxon tests for non-normally distributed data. P < 0.05 was accepted as threshold for statistical significance. Analyses were performed in R (https://www.r-project.org/).
Activation likelihood estimation meta-analyses
We used GingerALE 2.3.6 (http://brainmap.org/ale) to perform an activation likelihood estimation meta-analysis for Parkinson’s dementia compared with Parkinson’s without cognitive involvement using standard methods (Eickhoff et al., 2009, 2012). In brief, a 3D Gaussian probability distribution is generated centred on each individual study coordinate, and modified by the sample size from each study. This enabled us to estimate the uncertainty surrounding each coordinate. These distributions were then combined across all the studies for the relevant comparison to produce activation likelihood estimate maps. We used the threshold of P < 0.05 false discovery rate (FDR)-corrected to determine significance and also tested convergence against a null distribution of 1000 simulated datasets with identical numbers of foci experiments and subjects with the foci randomly distributed. For these meta-analyses, cluster-forming threshold was set at P < 0.001 and cluster-level inference threshold at P < 0.05. The same approach was used to perform separate activation likelihood estimation meta-analyses for Parkinson’s with and without visual hallucinations; and Parkinson’s-MCI (PD-MCI) compared with Parkinson’s without cognitive involvement. We also directly compared studies of PD dementia to those of PD hallucinations and PD-MCI, using the same statistical methods described above.
Coordinate-based network mapping
Next, we used a recently validated technique termed coordinate-based network mapping (Darby et al., 2019) to test the hypothesis that neuroimaging findings from studies of PD dementia would localize to a common brain network. This technique is modified from lesion network mapping, a technique used to test whether brain lesions map to a common brain network (Darby et al., 2017; Horn et al., 2017). For each neuroimaging study, we created 4 mm spherical seeds at the reported coordinates. We added these seeds together to produce one combined seed for each study (Eickhoff et al., 2009; Yarkoni et al., 2011; Darby et al., 2019). We then identified the network of brain regions functionally connected to the seed using a connectome database from 1000 normal subjects (Yeo et al., 2011; Holmes et al., 2015). We thresholded each connectivity map at t ≥ 7 (corresponding to family-wise error (FWE) voxel-based correction P < 10−6) (Joutsa et al., 2018b) to derive a network map for each study. These binarized maps were then overlapped to identify network connections common to the greatest number of studies of PD dementia. We performed this analysis across the entire brain, as well as for an a priori region of interest (ROI) in the hippocampus, defined using the publically available SPM anatomical toolbox (http://www.fz-juelich.de/inm/inm-1/DE/Forschung/_docs/SPMAnatomyToolbox/SPMAnatomyToolbox_node.html) (Amunts et al., 2005). We also computed functional connectivity between each study’s coordinates and this a priori hippocampal ROI using our 1000 subject normative connectome. Pearson’s correlations coefficients were converted to a normal distribution using Fisher’s r to z transform then averaged across our 1000 subjects. We tested for significance of this connection across studies using permutation testing in R (one-sample, two tailed, P < 0.05). We used one-tailed significance testing for this ROI analysis given our a priori hypothesis that coordinates from PD dementia studies should be positively connected to the hippocampus.
Specificity of network localization for Parkinson’s disease dementia
To test for symptom specificity, we repeated the above analyses using studies of PD visual hallucinations, defined as the presence of visual hallucinations in the context of Parkinson’s disease, where patients with visual hallucinations were directly compared with patients with PD without hallucinations. To test for stage specificity, we repeated the above analyses using studies of PD-MCI. PD-MCI was defined as cognitive deficits in the context of established Parkinson’s disease not of sufficient severity to impair functional independence (Petersen et al., 2001; Winblad et al., 2004; Litvan et al., 2012). Studies were selected that directly compared patients with PD-MCI with Parkinson’s and no cognitive involvement.
Network connectivity maps from studies of PD hallucinations or studies of PD-MCI were statistically compared to network maps from studies of PD dementia on a voxel-wise basis using permutation testing within FSL PALM (two-tailed, voxel-based FWE correction P < 0.05). Permutation testing with voxel-based FWE correction for multiple comparisons reduces the risk of false positives (Eklund et al., 2016) and is consistent with best-practice recommendations for neuroimaging (Poldrack et al., 2017). To maximize sensitivity, this voxel-wise analysis was restricted to a mask defined by our a priori hippocampal ROI. Functional connectivity between study coordinates and our a priori hippocampal ROI was also computed and compared using permutation testing within R (https://www.r-project.org/) (two sample, one tailed, P < 0.05). We used one-tailed significance testing for this ROI analysis given our a priori hypothesis that coordinates from PD dementia studies should be more connected to the hippocampus than studies of PD visual hallucinations or studies of PD-MCI.
In a post hoc analysis, we also tested for specificity of our PD visual hallucination findings to the lateral geniculate nucleus (LGN). For this analysis, an LGN ROI was generated using 18 mm spheres centred on previously described coordinates (Burgel et al., 2006). Note that unlike our hippocampus ROI, our LGN ROI was not specified a priori, but selected post hoc based on the results of our whole-brain network mapping of PD visual hallucinations.
Data availability
The data on which this study is based were all obtained from published and publically available reports (see Tables 1–3 for details).
Results
Heterogeneous neuroimaging findings for Parkinson’s dementia are linked to a common network centred in the hippocampus
We identified 11 studies that reported neuroimaging abnormalities in patients with PD dementia (total n = 175) compared to PD without cognitive impairment (total n = 210, Table 1). All studies used established criteria to diagnose Parkinson’s diease (Calne et al., 1992; Hughes et al., 1992; Larsen et al., 1994), and the majority (8 out of 11) used established criteria to define PD dementia (American Psychiatric Association, 1996; Emre et al., 2007). Between groups, there was no significant difference in age (t(18) = 0.8, P = 0.46), disease stage (H&Y, t(11) = −1.9, P = 0.085), or motor function (unified Parkinson's disease rating scale (UPDRS) III, t(−2.2) = 7, P = 0.063), but a large difference in cognition as expected [mini-mental state examination, W = 98, P = 0.00032]. Cognitive scores in the PD dementia group were similar across studies (mean mini-mental state examination 20.2, SD 3.6).
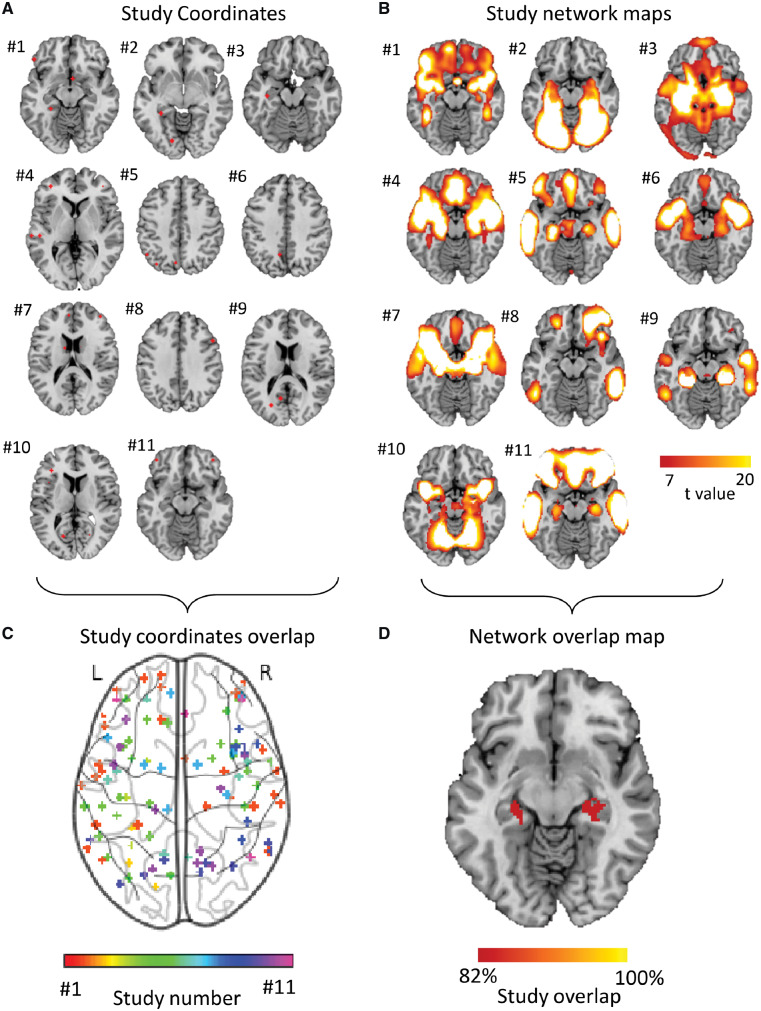
Neuroimaging findings from these studies were highly heterogeneous (Fig. 2A and C). Using standard meta-analytic methods, no voxels or clusters appeared more often than expected by chance. Only 4/11 studies (36%) contributed to the most consistent finding, which was in the right insula (MNI coordinates 41.5, 6.8, −17.7).
Figure 2.
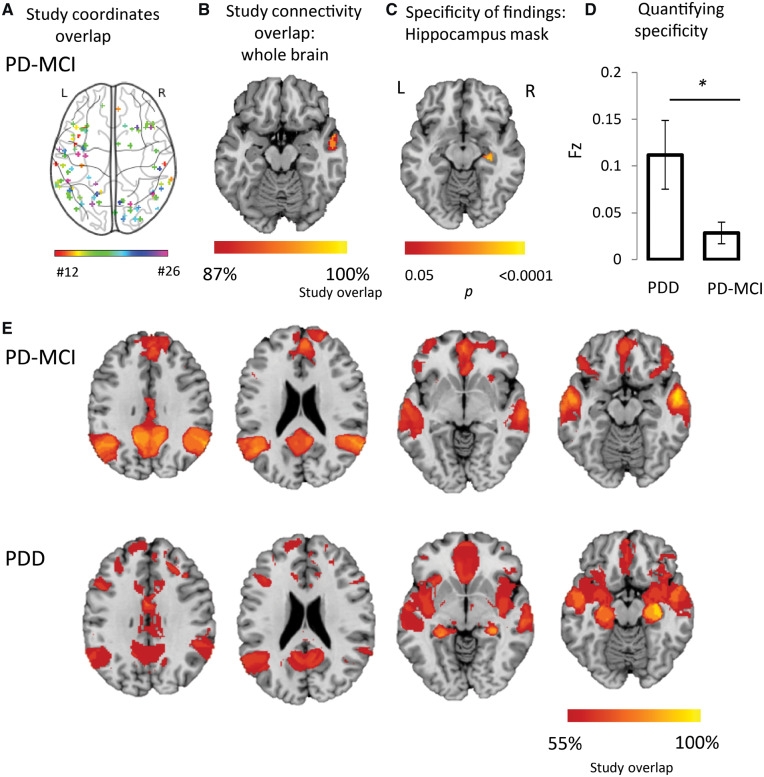
Heterogeneous neuroimaging findings in Parkinson’s disease dementia are part of a common brain network centred on the hippocampus. (A) Study coordinates. Location of coordinates for each study of Parkinson’s dementia compared with Parkinson’s without cognitive involvement. Spherical seeds were generated at each reported significant coordinate for each study of PD dementia, then added together to create one map of neuroimaging findings for each study. Numbers refer to the study number as listed in Table 1. (B) Study network maps. Regions significantly connected to each study’s neuroimaging findings were calculated using a large (n = 1000) normative connectome, creating a network map for each study (FWE-corrected P < 10−6). Locations of network connectivity for each study of Parkinson’s dementia compared with Parkinson’s without cognitive involvement. (C) Study coordinates overlap. Combined location of all coordinates across all studies of PD dementia shows pronounced heterogeneity. Each study is represented by a different colour. (D) Network overlap map. Network maps from each study were overlaid to identify functional connections common to the greatest number of studies in a whole-brain analysis. Over 80% of studies were functionally connected to the bilateral hippocampus. Section at z = −16 is shown.
Next, we tested whether these heterogeneous neuroimaging findings localized to a common brain network. For each study, we generated a 4-mm sphere at each reported coordinate to obtain a study-specific map of abnormalities related to Parkinson’s dementia. We then identified the network of brain regions functionally connected to each study-specific map using a large (n = 1000) normative connectome. Each network map was thresholded (t ≥ 7, FWE P < 10−6), binarized, then overlapped to identify regions common to all or most studies (Fig. 2D). Applying this approach to the 11 studies of PD dementia in a whole-brain analysis, we found over 90% reproducibility, with peak network overlap in the right hippocampus (Fig. 2D). The second highest peak was in the left hippocampus (>80% of studies). Both areas of peak overlap fell within our a priori hippocampal ROI, and connectivity to this ROI was significant across studies of PD dementia (t = 3.0, P = 0.01). In summary, although neuroimaging studies of atrophy and hypometabolism in PD dementia reported heterogeneous coordinates, these coordinates were part of a common brain network centred on the hippocampus.
Specificity of network localization compared to visual hallucinations in Parkinson’s disease
To determine whether network localization to the hippocampus was specific to the symptom of dementia in PD, we performed a separate meta-analysis of hallucinations. We identified nine studies reporting atrophy or hypometabolism in patients with PD hallucinations (total n = 168) compared with PD without hallucinations (total n = 234, Table 2). All studies used UK Brain Bank criteria to define Parkinson’s disease (Hughes et al., 1992) and 7 of the 9 studies used established methods to define the presence of visual hallucinations (Cummings, 1997; Ravina et al., 2007; Goetz et al., 2008). All except two studies (Ramirez-Ruiz et al., 2007; Lee et al., 2017) controlled for cognition. Between groups there was no significant difference in age (t(14) = 0.59, P = 0.56), disease stage (H&Y, t(10) = 1.0, P = 0.34), or cognition (mini-mental state examination, t(12) = −0.7, P = 0.48).
Table 2.
Clinical characteristics and scanning modalities of included studies of Parkinson’s hallucinations
| First author | Modality | N PDVH | N PD | Age PDVH | Age PD | MMSE PDVH | MMSE PD | H&Y PDVH | H&Y PD | UPDRS PDVH | UPDRS PD |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Total n = 168 | Total n = 234 | ||||||||||
| (27) Boeckerb | FDG-PET | 8 | 11 | 72.88 | 70.56 | 25.75 | 26.82 | 46.25 | 32.73 | ||
| (28) Gasca-Salasb | FDG-PET | 9 | 12 | 70.7 | 70.8 | 27 | 25.9 | 16.1 | 17 | ||
| (29) Goldmanb | VBM | 25 | 25 | 75.4 | 74.8 | 25.1 | 23.9 | 3 | 3 | 43.5 | 39 |
| (30) Leeb | VBM | 10 | 21 | 69.4 | 66.2 | 27.6 | 28.2 | 2.2 | 1.8 | 22.5 | 16.4 |
| (31) Oishi | SPECT | 24 | 41 | 69.5 | 68.6 | 25.1 | 26.5 | 3.3 | 3 | ||
| (32) Pagonobarragab | VBM | 15 | 27 | 64.1 | 66.3 | 135a | 136a | 1.9 | 1.9 | 21.7 | 18.6 |
| (33) Ramirez-Ruis | VBM | 18 | 20 | 27 | 29.1 | 3.2 | 2.5 | 29.3 | 24.5 | ||
| (34) Shinb | VBM | 46 | 64 | 71.3 | 70.7 | 25.2 | 25.7 | 24.1 | 21.6 | ||
| (35) Watanabeb | VBM | 13 | 13 | 66.6 | 63.6 | 27.9 | 90 | 2.9 | 2.4 | 23.4 | 28.6 |
| Summary (mean (SD)) | 19 (12) | 26 (17) | 70.0 (4) | 68.9 (3.5) | 26.3 (1) | 26.9 (2) | 2.8 (0.6) | 2.4 (0.5) | 28.8 (11) | 24.8 (8) |
Matis dementia rating supplied.
Indicates established criteria were used to define PD hallucinations.
FDG-PET, fluorodeoxyglucose positron emission tomography; MMSE, mini-mental state examination; PDVH, Parkinson’s disease with visual hallucinations; SPECT, single photon emission computed tomography; UPDRS, unified parkinson's disease rating scale score (part III, motor); VBM, voxel-based morphometry.
No significant differences between groups for any of these comparisons.
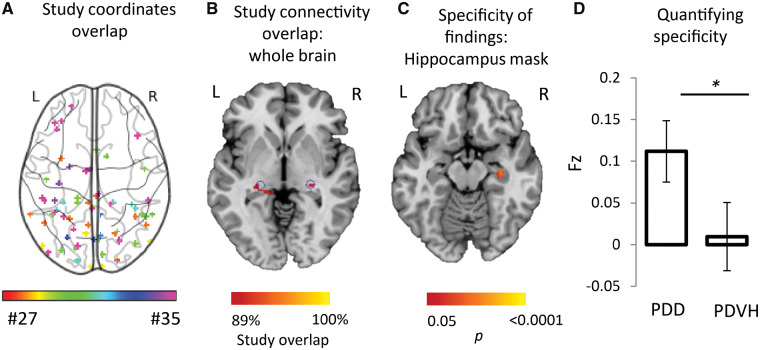
We found that using standard methods for meta-analysis, neuroimaging results were heterogeneous (Fig. 3A). No consistent clusters were found, either across studies of PD hallucinations or when comparing studies of PD hallucinations with studies of PD dementia.
Figure 3.
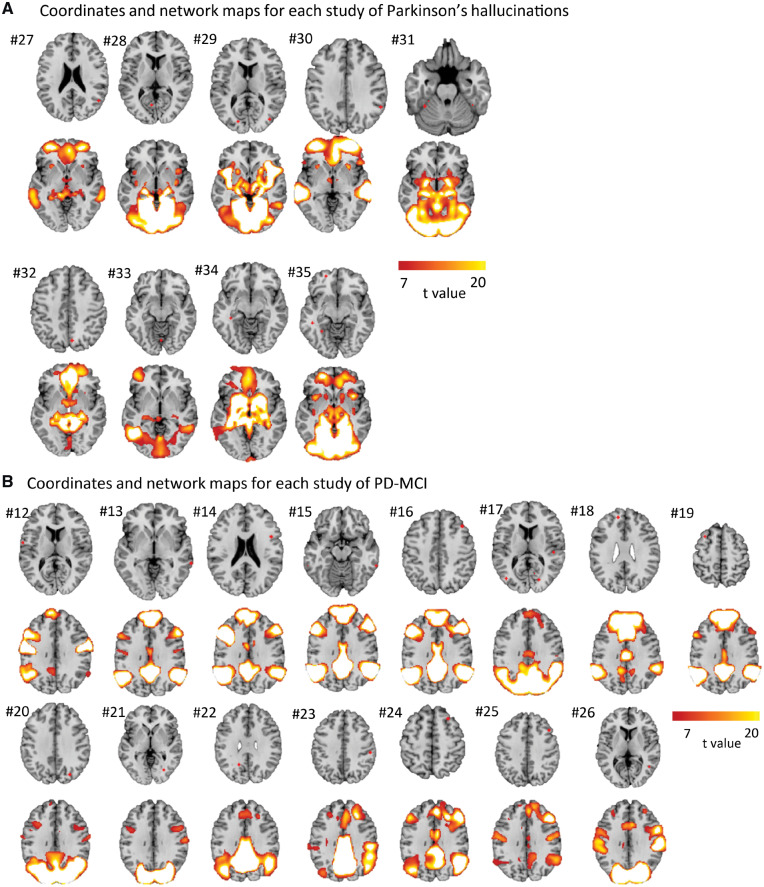
Heterogeneous neuroimaging findings in Parkinson’s disease hallucinations are part of a different brain network than PD dementia, centred on the lateral geniculate nucleus. (A) Combined location of all coordinates across all studies of Parkinson’s with visual hallucinations shows pronounced heterogeneity. Each study is represented by a different colour. (B) Connectivity maps (across the whole brain) for each study of PD hallucinations were generated and overlaid, showing network overlap in the lateral geniculate nuclei bilaterally. Section shown is at z = −4. Blue circles indicate location of lateral geniculate nucleus based on published coordinates (Burgel et al., 2006). (C) Direct comparison of network maps generated from studies of PD dementia and PD hallucinations shows specificity of hippocampal connectivity to studies of PD dementia. Map is masked to the hippocampi and FWE-corrected P < 0.05. Section shown is at z = −16. (D) Connectivity to our a priori ROI in the hippocampus was significantly stronger for studies of PD dementia compared to studies of PD hallucinations. Coordinate and network maps for all studies can be viewed in Fig. 5. * P < 0.05; PDD, Parkinson’s disease dementia; PDVH, Parkinson’s disease with visual hallucinations.
When we applied the same coordinate network mapping approach we used for dementia to studies of PD hallucinations, we again found that the vast majority of studies (89%) mapped to a common brain network. However, this time the peak network overlap was the lateral geniculate nuclei (LGN) in the thalamus, not the hippocampus (Fig. 3B). Directly testing for specificity of hippocampal connectivity for PD dementia, we found that coordinates from studies of PD dementia were more connected to voxels in the right hippocampus (Fig. 3C, P < 0.05 FWE-corrected) and more connected to our a priori hippocampal ROI (z = −1.75, P = 0.04, one-tailed permutation test, Fig. 3D) compared to studies of PD hallucinations.
A post hoc analysis tests for specificity of LGN connectivity for PD hallucinations (versus studies of PD dementia) found that coordinates from studies of PD hallucinations were more connected to voxels in the right LGN (P < 0.05 FWE-corrected) and to an anatomically defined ROI in bilateral LGN (z = 1.91, P = 0.028, one-tailed permutation test).
Network localization reveals separate networks involved at milder stages of cognitive involvement in Parkinson’s disease
Next, we examined whether network localization would reveal separate networks according to stage of cognitive involvement in Parkinson’s disease. We identified 15 studies examining differences in atrophy or hypometabolism between people with PD-MCI (total n = 355) and those with Parkinson’s disease and no cognitive impairment (total n = 489, Table 3). Thirteen out of 15 studies used established criteria for Parkinson’s disease diagnosis (Hughes et al., 1992; Larsen et al., 1994; Gelb et al., 1999) and 13 out of 15 studies used recent (Litvan et al., 2012) or previous (Petersen et al., 2001) criteria for PD-MCI. Despite these different methods, there was relatively little variability in mini-mental state examination scores across the studies (PD-MCI 27.3 (SD 1.0), Parkinson’s disease without cognitive involvement 28.8 (SD 0.5)). Between groups, PD-MCI patients were older (t(28) = 2.5, P = 0.018)) and showed worse cognition (t(18)=4.7, P < 0.001), but did not differ in terms of disease stage (H&Y, t(22) = 0.8, P = 0.42) or motor disability (UPDRS III, t(22) = 1.6, P = 0.12; Table 3).
Table 3.
Clinical characteristics and scanning modalities of included studies of PDMCI versus Parkinson's disease with no cognitive involvement
| First author | Modality | N PD-MCI | N PD | Age PD-MCI | Age PD | MMSE PD-MCI | MMSE PD | H&Y PD-MCI | H&Y PD | UPDRS PD-MCI | UPDRS PD |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Total n = 355 | Total n = 489 | ||||||||||
| (12) Beyerc | VBM | 8 | 12 | 77.4 | 69 | 25.9 | 29.4 | 2.6 | 2.3 | ||
| (13) Dantia | Freesurfer | 18 | 18 | 66.5 | 60.6 | 26.4 | 28.7 | 1.6 | 1.3 | ||
| (14) Garcia-Garciaa | FDG-PET | 28 | 21 | 71.5 | 67 | 28 | 29.5 | 2.9 | 2.6 | 17.7 | 16.4 |
| (15) Hosokaid | FDG-PET | 13 | 27 | 67.6 | 65.7 | 27.1 | 27.9 | 2.7 | 2.5 | 22.4 | 18.5 |
| (16) Huangc | FDG-PET | 18 | 18 | 62.4 | 59 | 27.1 | 28.2 | 3.6 | 3.1 | 34.9 | 29.2 |
| (17) Lyooc | FDG-PET | 18 | 20 | 65.5 | 62 | 27 | 29 | 2.3 | 2.3 | 25.5 | 22 |
| (18) Maka | VBM | 24 | 66 | 68.99 | 63.48 | 26.91 | 28.36 | 1.81 | 1.91 | 19.96 | 17.44 |
| (19) Maka | Cortical thickness | 39 | 66 | 69.4 | 62.9 | 28.1 | 29.1 | 2.1 | 1.9 | 29 | 25.3 |
| (20) Nobilic | SPECT | 15 | 15 | 71.5 | 70.8 | 27.3 | 28.7 | 22.9 | 15.3 | ||
| (21) Pagonobarragad | Freesurfer | 26 | 26 | 73.3 | 71.5 | 128e | 134e | 2 | 2.2 | 21 | 24 |
| (22) Pereirab | Freesurfer | 33 | 90 | 63.4 | 59.4 | 25.7f | 28.1f | 2 | 2 | 21.5 | 19.6 |
| (23) Seguraa | Freesurfer | 47 | 43 | 67.72 | 60.77 | 28.68 | 29.47 | 17.79 | 13.16 | ||
| (24) Songc | VBM | 27 | 23 | 71.3 | 69.1 | 25.8 | 28.6 | 18.6 | 16.9 | ||
| (25) Tanga | FDG-PET | 20 | 30 | 61.9 | 61.9 | 28.4 | 28.5 | 2.1 | 1.8 | 30 | 23 |
| (26) Zhangc | VBM | 21 | 14 | 63.8 | 58.5 | 28.85 | 29.07 | 1.77 | 1.42 | ||
| Summary (mean (SD)) | 24 (10) | 33 (23) | 68.1 (4)* | 64.1 (4)* | 27.3 (1)* | 28.8 (0.5)* | 2.3 (0.6) | 2.1 (0.5) | 23.4 (5) | 20.1 (5) |
Significant difference between groups. (Other comparisons are not significantly different).
Established criteria used to define PD-MCI (Litvan et al., 2012).
Close approximation of established criteria used to define PD-MCI.
Previous criteria used to define PD-MCI (Petersen et al., 2001).
Alternative method used to define PD-MCI (Clinical Dementia Rating score of 0.5).
Matis dementia rating supplied.
MOCA supplied.
FDG-PET, fluorodeoxyglucose positron emission tomography; H&Y, Hoehn and Yahr; MMSE, mini-mental state examination; PD-MCI, Parkinson’s disease with mild cognitive impairment; SPECT, single photon emission computed tomography; UPDRS, Unified Parkinson’s disease rating scale score (part III, motor); VBM, voxel-based morphometry.
Standard meta-analysis techniques again revealed heterogeneity of neuroimaging findings across studies (Figs 4A and 5), with no significant coordinates at either FDR or cluster inference levels of correction. Only 3/15 studies (20%) contributed to the most consistent clusters.
Figure 4.
Heterogeneous neuroimaging findings in Parkinson’s disease MCI are part of a network centred on posterior nodes of the default mode network. (A) Combined location of all coordinates across all studies of Parkinson’s mild cognitive impairment (PD-MCI) shows pronounced heterogeneity. Each study is represented by a different colour. (B) Connectivity maps for each study of PD-MCI were generated and overlaid, showing peak network overlap in the lateral temporal cortex. Section shown is at z = −18. (C) Direct comparison of network maps generated from studies of PD dementia and PD-MCI shows specificity of hippocampal connectivity to studies of PD dementia. Map is masked to the hippocampi and FWE-corrected P < 0.05. Section shown is at z = −14. (D) Connectivity to our a priori ROI in the hippocampus was significantly stronger for studies of PD dementia compared to studies of PD-MCI. (E) At lower network overlap thresholds, there are similarities between PD-MCI and PD dementia. This suggests that posterior nodes of the DMN are affected in both PD-MCI and PD dementia, and that at later stages, once PD dementia takes hold, hippocampal networks are affected. Sections shown are at z = 30, z = 21, z = −7 and z = −16. Coordinate and network maps for all studies can be viewed in Fig. 5. * P < 0.05; PDD, Parkinson’s disease dementia; PD-MCI, Parkinson’s disease with mild cognitive impairment.
Figure 5.
Coordinate maps and network maps for Parkinson’s Hallucinations and for PD-MCI. (A) Location of coordinates and of network connectivity for each study of Parkinson’s with hallucinations compared with Parkinson’s without hallucinations. (B) Location of coordinates and of network connectivity for each study of PD-MCI compared with Parkinson’s without cognitive involvement. Numbers refer to number of study in Tables 2 and 3.
Using coordinate network mapping, over 80% of studies showed connectivity to posterior nodes of the default mode network (DMN), with peak overlap in lateral temporal cortex (Fig. 4B). Directly testing for specificity of hippocampal connectivity for PD dementia versus PD-MCI, we found that coordinates from studies of PD dementia were more connected to voxels in the right hippocampus (P < 0.05, FWE-corrected, Fig. 4C) and more connected to our a priori hippocampal ROI (z = −2.2, P = 0.013, one-tailed permutation test, Fig. 4D) compared to studies of PD-MCI.
Importantly, PD-MCI and PD dementia are a spectrum of cognitive involvement, and therefore, as well as finding differences between studies of PD-MCI and PD dementia in hippocampal regions, we would expect to see similarities in other networks. We therefore examined our network maps for PD dementia and PD-MCI at lower thresholds and found many similarities, with both PD dementia and PD-MCI showing network overlap in posterior nodes of the DMN (Fig. 4E). As such, while the peak network overlap was different, and significantly different in the hippocampus, at lower thresholds similar networks were apparent.
Discussion
We show that neuroimaging findings in Parkinson’s dementia are heterogeneous across different studies, but are part of a common brain network centred on the hippocampi. This result was symptom-specific, as visual hallucinations mapped to a different network centred on the lateral geniculate nucleus. This finding was also stage-specific, as neuroimaging findings in PD-MCI mapped to a network centred on the lateral temporal cortex and posterior brain regions.
Network localization of heterogeneous neuroimaging findings
Our finding that neuroimaging abnormalities in Parkinson’s dementia localize to a connected brain network, rather than one specific brain region, is consistent with an accumulating literature on network localization of neuropsychiatric symptoms (Boes et al., 2015; Fox, 2018; Joutsa et al., 2018a) and neurodegenerative diseases (Seeley et al., 2009; Zhou et al., 2012). Recently, we validated a new method for testing whether heterogeneous neuroimaging coordinates across different studies localize to a connected brain network (Darby et al., 2019). The current study further validates and extends this method, showing specificity for highly co-morbid symptoms (PD dementia versus PD hallucinations) and specificity for disease stage (PD dementia versus PD-MCI). These results suggest that coordinate-based network mapping may help address a variety of neuroimaging questions that have proven difficult to address with conventional methods.
Parkinson’s dementia and hippocampal networks
If heterogeneous neuroimaging findings in PD dementia were going to localize to any brain network, a network centred on the hippocampus makes sense. Clinically, memory is always affected in patients with PD dementia (Bronnick et al., 2007; Muslimovic et al., 2007; Reid et al., 2011), and memory tests best distinguish PD dementia from Parkinson’s disease (Kiesmann et al., 2013). Pathologically, the hippocampus shows more Lewy bodies (Harding and Halliday, 2001; Apaydin et al., 2002; Arnold et al., 2013; Hall et al., 2014) reduced cholinergic activity (Hall et al., 2014), and progressive volume loss in PD dementia (Hwang et al., 2013; Pagonabarraga et al., 2013; Zarei et al., 2013; Rektorova et al., 2014).
Despite this evidence, the importance of the hippocampus in PD dementia has been a source of debate (Emre et al., 2007; Irwin et al., 2012; Hall et al., 2014; Aarsland et al., 2017). In particular, neuroimaging studies have reported abnormalities in numerous brain regions outside the hippocampus (Burton et al., 2004; Klein et al., 2010; Song et al., 2011; Melzer et al., 2012). The current results help reconcile this debate, showing that these heterogeneous neuroimaging abnormalities are part of a common brain network, centred on the hippocampus.
Parkinson’s dementia, Alzheimer’s disease and hippocampal networks
Our results implicating a hippocampal network in PD dementia aligns with other studies implicating a hippocampal network in Alzheimer’s dementia (Seeley et al., 2009; Crossley et al., 2014; Darby et al., 2019). There are several possibilities for this convergence. One possibility is that the patients with PD dementia included in the above neuroimaging studies have co-morbid Alzheimer’s dementia, leading to a similar network localization. PD dementia is thought to be clinically and neuropathologically distinct from Alzheimer’s disease (Aarsland et al., 2005; Farlow and Cummings, 2008; Irwin et al., 2017). However, in later disease stages the amnestic component can be similar (Janvin et al., 2006b; Bronnick et al., 2007; Emre et al., 2007), and PD dementia is characterized by amyloid and tau-related pathology in addition to alpha-synuclein (Compta et al., 2011; Irwin et al., 2017). As such this possibility cannot be excluded.
A second possibility, and the one we favour, is that PD dementia and Alzheimer’s disease are distinct disorders with distinct pathologies, but either pathology can involve the hippocampal network and cause dementia. In Alzheimer’s disease, hippocampal networks are affected early, leading to early amnestic symptoms followed by dementia. In contrast, Parkinson’s disease affects other networks first (especially posterior nodes of the DMN (Tessitore et al., 2012; Hou et al., 2016)), but once hippocampal networks are affected, the patient develops memory impairment and dementia. Our finding of higher specificity for the right hippocampus, which is linked with spatial rather than verbal memory (Ezzati et al., 2016), would also be consistent with the higher propensity for spatial rather than verbal memory changes in PD dementia (Noe et al., 2004).
Finally, it is important to consider whether our convergent localization in Alzheimer’s disease and PD dementia could be an artefact of our network mapping technique. Our technique will be biased towards identification of network hubs connected to the greatest number of other brain regions. However, this is unlikely to explain the current results as other nodes in the DMN are equally if not more connected to other brain regions compared to the hippocampus (Buckner et al., 2009). Second, network localization to the hippocampus is specific to disorders of memory impairment, including Alzheimer’s disease and PD dementia. Neuroimaging coordinates from studies of other neurodegenerative diseases (Darby et al., 2019), co-morbid symptoms in PD dementia such as visual hallucinations (Fig. 3), or even PD-MCI, an earlier stage of PD dementia which involves minimal memory impairment (Fig. 4), fail to show network overlap in the hippocampus.
Network localization of Parkinson’s hallucinations to the lateral geniculate nuclei
Although initially included as a control for PD dementia, our findings in PD hallucinations are important in their own right. Heterogeneous neuroimaging abnormalities in PD hallucinations were part of a common network centred on the LGN. A central role of the LGN in visual hallucinations in PD has been hypothesized since the 1930s (de Morsier, 1936, 1938; Carter and Ffytche, 2015) and supported by more recent evidence (Diederich et al., 2014). Intriguingly, our previous work on brainstem lesions causing visual hallucinations also implicated the LGN (Boes et al., 2015), suggesting a common network localization for visual hallucinations independent of the underlying aetiology (stroke versus Parkinson’s disease).
A potential mechanistic model for the role of the LGN in hallucinations centres on two modes of signalling: a tonic mode, and a burst mode (Jones, 2009). During tonic mode, LGN cells are relatively depolarized and the LGN acts as a relay between retina and visual cortex. During burst mode, thalamic cells become hyperpolarized and are more likely to be enhanced by feedback from higher cortical regions. Intriguingly, this state occurs during drowsy inattentiveness, the same state linked with hallucinations (Llinas and Steriade, 2006).
Note that our results implicating the LGN do not preclude the involvement of other brain regions in PD hallucinations, such as the DMN (Shine et al., 2014; Yao et al., 2014; Shine et al., 2015). In fact, one recent theory suggests that thalamic denervation to regions such as the LGN may reduce DMN inhibition resulting in visual hallucinations (Xuereb et al., 1991; Ricciardi et al., 2015; Onofrj et al., 2017).
Although PD-associated visual hallucinations are associated with cognitive impairment (Barnes and David, 2001), almost all studies of PD hallucinations controlled for cognition, and across studies, cognition was not poorer in PD patients with visual hallucinations. Recent reports also reveal that hallucinations can be seen at earlier stages of PD in the absence of cognitive involvement (Pagonabarraga et al., 2016), consistent with separate underlying processes or neuroanatomical substrates.
Network localization reveals involvement of posterior nodes of the default mode network in Parkinson’s mild cognitive impairment
Our finding that neuroimaging abnormalities in PD-MCI localize to a network centred on posterior nodes of the DMN is consistent with clinical evidence that the earliest cognitive deficits in PD involve visuospatial processing (Williams-Gray et al., 2013; Weil et al., 2017). Lewy-related pathology in posterior brain regions increases the risk of dementia in PD (Toledo et al., 2016), loss of connectivity in the DMN correlates with cognitive performance in Parkinson’s disease (Tessitore et al., 2012; Karunanayaka et al., 2016) and reduced DMN functional connectivity is seen in lateral temporal nodes as well as posterior brain regions in patients with PD-MCI (Hou et al., 2016). Importantly, posterior DMN involvement was seen in both PD-MCI and PD dementia, consistent with the notion that these are part of the same spectrum of disease. Our findings are consistent with a model of cognitive impairment in PD that starts in posterior nodes of the DMN, causing early visuospatial deficits and MCI that eventually progresses to involve the hippocampal network as well, causing memory impairment and PD dementia. Such a model is consistent with the network propagation theory of neurodegenerative disease (Seeley et al., 2009) and prion-like spread of alpha-synuclein (Zhou et al., 2012).
Our findings are also consistent with the PD-related cognitive pattern identified by Eidelberg and colleagues using functional imaging (Huang et al., 2007; Hirano et al., 2012). This pattern of metabolic activity correlates with cognitive function in non-demented people with PD (Mattis et al., 2011), with reduced activity in prefrontal and parietal regions. This pattern bears strong resemblance to the network we identified in PD-MCI (see Fig. 4).
Limitations
There are several limitations to consider in our study. The numbers of studies included for each analysis are relatively small, particularly for PD dementia (n = 11) and Parkinson’s hallucinations (n = 9). These low study numbers undoubtedly contribute to the negative results of our activation likelihood estimation meta-analyses and those of other groups (Minkova et al., 2017). Similarly, we used only one activation likelihood estimation meta-analysis technique. Other meta-analysis techniques have been applied in PD dementia and could produce different results (Pan et al., 2013; Mihaescu et al., 2018). Finally, due to these low study numbers, we used liberal one-tailed statistics for some analyses (e.g. connectivity of PD dementia coordinates versus PD hallucination coordinates to our a priori hippocampal ROI). We believe this was justified given clear a priori hypotheses regarding the direction of the finding; however, this result should be interpreted with caution until replicated. Note that the majority of our findings, including our voxel-wise analyses of hippocampal connectivity, were significant using standard two-tailed statistics. It is also important to note that studies with different numbers of coordinates did not dominate the analysis, as the coordinates for each study were used as a single (multi-location) seed.
A second limitation is clinical and study heterogeneity. For example, PD hallucinations can co-occur with cognitive impairment (Barnes and David, 2001) and PD-MCI can involve a range of cognitive domains (Litvan et al., 2012). However, key clinical factors were controlled across studies: studies of PD dementia and PD-MCI controlled for motor impairment and disease stage, while studies of PD hallucinations controlled for cognition. Moreover, any heterogeneity across studies should bias us against the current findings of common network localization.
Finally, we used a normative connectome to link heterogeneous neuroimaging findings, similar to prior work from our lab (Darby et al., 2018, 2019; Fox, 2018). However, one could argue that a connectome derived from Parkinson’s disease patients should work better for linking neuroimaging findings in Parkinson’s disease patients. Although intuitive, prior studies suggest that using disease-specific connectomes has minimal effect on network mapping results, and if anything weakens results due to worse signal to noise inherent in patient-based connectomes (Horn et al., 2017; Weigand et al., 2018).
Funding
R.S.W. is supported by a Wellcome Clinical Research Career Development Fellowship (201567/Z/16/Z). M.D.F. is supported by funding from the Sidney R. Baer, Jr. Foundation, the NIH (R01MH113929), the Nancy Lurie Marks Foundation and the G. Harold and Leila Y. Mathers Charitable Foundation. R.R.D. is supported by funding from the Alzheimer’s foundation, Brightfocus foundation, Sidney Baer, Jr. Foundation and Vanderbilt faculty research scholars program.
Competing interests
R.W. reports personal fees from General Electric.
Glossary
Abbreviations
- ALE
activation likelihood estimation
- DMN
default mode network
- H&Y
Hoehn and Yahr
- LGN
lateral geniculate nucleus
- MCI
mild cognitive impairment
- MMSE
mini-mental state examination
- PD dementia
Parkinson’s disease with dementia
- PD-PMCI
mild cognitive impairment associated with Parkinson’s disease
- PDNC
Parkinson’s disease with normal cognition
- PDVH
PD with visual hallucinations
References
- Aarsland D, Creese B, Politis M, Chaudhuri KR, Ffytche DH, Weintraub D, et al. Cognitive decline in Parkinson disease. Nat Rev Neurol 2017; 13: 217–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aarsland D, Perry R, Brown A, Larsen JP, Ballard C.. Neuropathology of dementia in Parkinson's disease: a prospective, community-based study. Ann Neurol 2005; 58: 773–6. [DOI] [PubMed] [Google Scholar]
- American Psychiatric Association. Diagnosis and statistical manual of mental disorders, DSM IV. Washington DC: APA; 1996. [Google Scholar]
- Amunts K, Kedo O, Kindler M, Pieperhoff P, Mohlberg H, Shah NJ, et al. Cytoarchitectonic mapping of the human amygdala, hippocampal region and entorhinal cortex: intersubject variability and probability maps. Anat Embryol 2005; 210: 343–52. [DOI] [PubMed] [Google Scholar]
- Apaydin H, Ahlskog JE, Parisi JE, Boeve BF, Dickson DW.. Parkinson disease neuropathology: later-developing dementia and loss of the levodopa response. Arch Neurol 2002; 59: 102–12. [DOI] [PubMed] [Google Scholar]
- Arnold SE, Toledo JB, Appleby DH, Xie SX, Wang L-S, Baek Y, et al. Comparative survey of the topographical distribution of signature molecular lesions in major neurodegenerative diseases. J Comp Neurol 2013; 521: 4339–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aybek S, Lazeyras F, Gronchi-Perrin A, Burkhard PR, Villemure JG, Vingerhoets FJ.. Hippocampal atrophy predicts conversion to dementia after STN-DBS in Parkinson's disease. Parkinsonism Relat Disord 2009; 15: 521–4. [DOI] [PubMed] [Google Scholar]
- Barnes J, David AS.. Visual hallucinations in Parkinson's disease: a review and phenomenological survey. J Neurol Neurosurg Psychiatry 2001; 70: 727–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boes AD, Prasad S, Liu H, Liu Q, Pascual-Leone A, Caviness VS, et al. Network localization of neurological symptoms from focal brain lesions. Brain 2015; 138: 3061–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braak H, Rub U, Jansen Steur EN, Del TK, de Vos RA.. Cognitive status correlates with neuropathologic stage in Parkinson disease. Neurology 2005; 64: 1404–10. [DOI] [PubMed] [Google Scholar]
- Bronnick K, Emre M, Lane R, Tekin S, Aarsland D.. Profile of cognitive impairment in dementia associated with Parkinson's disease compared with Alzheimer's disease. J Neurol Neurosurg Psychiatry 2007; 78: 1064–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckner RL, Sepulcre J, Talukdar T, Krienen FM, Liu H, Hedden T, et al. Cortical hubs revealed by intrinsic functional connectivity: mapping, assessment of stability, and relation to Alzheimer's disease. J Neurosci 2009; 29: 1860–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burgel U, Amunts K, Hoemke L, Mohlberg H, Gilsbach JM, Zilles K.. White matter fiber tracts of the human brain: three-dimensional mapping at microscopic resolution, topography and intersubject variability. Neuroimage 2006; 29: 1092–105. [DOI] [PubMed] [Google Scholar]
- Burton EJ, McKeith IG, Burn DJ, Williams ED, O'Brien JT.. Cerebral atrophy in Parkinson's disease with and without dementia: a comparison with Alzheimer's disease, dementia with Lewy bodies and controls. Brain 2004; 127: 791–800. [DOI] [PubMed] [Google Scholar]
- Calne DB, Snow BJ, Lee C.. Criteria for diagnosing Parkinson's disease. Ann Neurol 1992; 32 Suppl: S125–S127. [DOI] [PubMed] [Google Scholar]
- Carter R, Ffytche DH.. On visual hallucinations and cortical networks: a trans-diagnostic review. J Neurol 2015; 262: 1780–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Compta Y, Parkkinen L, O'Sullivan SS, Vandrovcova J, Holton JL, Collins C, et al. Lewy- and Alzheimer-type pathologies in Parkinson's disease dementia: which is more important? Brain 2011; 134: 1493–505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crossley NA, Mechelli A, Scott J, Carletti F, Fox PT, McGuire P, et al. The hubs of the human connectome are generally implicated in the anatomy of brain disorders. Brain 2014; 137: 2382–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cummings JL. The neuropsychiatric inventory: assessing psychopathology in dementia patients. Neurology 1997; 48: 10S–S16. [DOI] [PubMed] [Google Scholar]
- Darby RR, Joutsa J, Burke MJ, Fox MD.. Lesion network localization of free will. Proc Natl Acad Sci USA 2018; 115: 10792–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darby RR, Joutsa J, Fox MD.. Network localization of heterogeneous neuroimaging findings. Brain 2019; 142: 70–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darby RR, Laganiere S, Pascual-Leone A, Prasad S, Fox MD.. Finding the imposter: brain connectivity of lesions causing delusional misidentifications. Brain 2017; 140: 497–507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Morsier G. Les automatismes visuels. (Hallucinations visuelles retrochiasmatiques). Schweizerische Medizinische Wochenschrift 1936; 66: 700–3. [Google Scholar]
- de Morsier G. Les hallucinations: etude Oto-neuro-ophtalmologique. Rev Oto-Neuro-Ophtalmol 1938; 16: 244–352. [Google Scholar]
- Dickerson BC, Sperling RA.. Large-scale functional brain network abnormalities in Alzheimer's disease: insights from functional neuroimaging. Behav Neurol 2009; 21: 63–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diederich NJ, Stebbins G, Schiltz C, Goetz CG.. Are patients with Parkinson's disease blind to blindsight? Brain 2014; 137: 1838–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eickhoff SB, Bzdok D, Laird AR, Kurth F, Fox PT.. Activation likelihood estimation meta-analysis revisited. Neuroimage 2012; 59: 2349–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eickhoff SB, Laird AR, Grefkes C, Wang LE, Zilles K, Fox PT.. Coordinate-based activation likelihood estimation meta-analysis of neuroimaging data: a random-effects approach based on empirical estimates of spatial uncertainty. Hum Brain Mapp 2009; 30: 2907–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eklund A, Nichols TE, Knutsson H.. Cluster failure: Why fMRI inferences for spatial extent have inflated false-positive rates. Proc Natl Acad Sci USA 2016; 113: 7900–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emre M, Aarsland D, Brown R, Burn DJ, Duyckaerts C, Mizuno Y, et al. Clinical diagnostic criteria for dementia associated with Parkinson's disease. Mov Disord 2007; 22: 1689–707. [DOI] [PubMed] [Google Scholar]
- Ezzati A, Katz MJ, Zammit AR, Lipton ML, Zimmerman ME, Sliwinski MJ, et al. Differential association of left and right hippocampal volumes with verbal episodic and spatial memory in older adults. Neuropsychologia 2016; 93: 380–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farlow MR, Cummings J.. A modern hypothesis: the distinct pathologies of dementia associated with Parkinson's disease versus Alzheimer's disease. Dement Geriatr Cogn Disord 2008; 25: 301–8. [DOI] [PubMed] [Google Scholar]
- Fenelon G, Mahieux F, Huon R, Ziegler M.. Hallucinations in Parkinson's disease: prevalence, phenomenology and risk factors. Brain 2000; 123 (Pt 4): 733–45. [DOI] [PubMed] [Google Scholar]
- Foo H, Mak E, Yong TT, Wen MC, Chander RJ, Au WL, et al. Progression of subcortical atrophy in mild Parkinson's disease and its impact on cognition. Eur J Neurol 2017; 24: 341–8. [DOI] [PubMed] [Google Scholar]
- Fox MD. Mapping symptoms to brain networks with the human connectome. N Engl J Med 2018; 379: 2237–45. [DOI] [PubMed] [Google Scholar]
- Fox MD, Snyder AZ, Vincent JL, Corbetta M, Van Essen DC, Raichle ME.. The human brain is intrinsically organized into dynamic, anticorrelated functional networks. Proc Natl Acad Sci USA 2005; 102: 9673–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox NC, Warrington EK, Freeborough PA, et al. Presymptomatic hippocampal atrophy in Alzheimer's disease. A longitudinal MRI study. Brain 1996; 119 (Pt 6): 2001–7. [DOI] [PubMed] [Google Scholar]
- Fredericks D, Norton JC, Atchison C, Schoenhaus R, Pill MW.. Parkinson’s disease and Parkinson's disease psychosis: a perspective on the challenges, treatments, and economic burden. Am J Manag Care 2017; 23: S83–S92. [PubMed] [Google Scholar]
- Gallagher DA, Parkkinen L, O'Sullivan SS, Spratt A, Shah A, Davey CC, et al. Testing an aetiological model of visual hallucinations in Parkinson’s disease. Brain 2011; 134: 3299–309. [DOI] [PubMed] [Google Scholar]
- Gee M, Dukart J, Draganski B, Wayne Martin WR, Emery D, Camicioli R.. Regional volumetric change in Parkinson's disease with cognitive decline. J Neurol Sci 2017; 373: 88–94. [DOI] [PubMed] [Google Scholar]
- Gelb DJ, Oliver E, Gilman S.. Diagnostic criteria for Parkinson disease. Arch Neurol 1999; 56: 33–9. [DOI] [PubMed] [Google Scholar]
- Goetz CG, Tilley BC, Shaftman SR, Stebbins GT, Fahn S, Martinez-Martin P, et al. Movement Disorder Society-sponsored revision of the Unified Parkinson's Disease Rating Scale (MDS-UPDRS): scale presentation and clinimetric testing results. Mov Disord 2008; 23: 2129–70. [DOI] [PubMed] [Google Scholar]
- Hall H, Reyes S, Landeck N, Bye C, Leanza G, Double K, et al. Hippocampal Lewy pathology and cholinergic dysfunction are associated with dementia in Parkinson's disease. Brain 2014; 137: 2493–508. [DOI] [PubMed] [Google Scholar]
- Harding AJ, Halliday GM.. Cortical Lewy body pathology in the diagnosis of dementia. Acta Neuropathol 2001; 102: 355–63. [DOI] [PubMed] [Google Scholar]
- Hirano S, Shinotoh H, Eidelberg D.. Functional brain imaging of cognitive dysfunction in Parkinson’s disease. J Neurol Neurosurg Psychiatry 2012; 83: 963–9. [DOI] [PubMed] [Google Scholar]
- Holmes AJ, Hollinshead MO, O’Keefe TM, Petrov VI, Fariello GR, Wald LL, et al. Brain Genomics Superstruct Project initial data release with structural, functional, and behavioral measures. Sci Data 2015; 2: 150031.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horn A, Reich M, Vorwerk J, Li N, Wenzel G, Fang Q, et al. Connectivity Predicts deep brain stimulation outcome in Parkinson disease. Ann Neurol 2017; 82: 67–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hou Y, Yang J, Luo C, et al. Dysfunction of the default mode network in drug-naive Parkinson's disease with mild cognitive impairments: a resting-state fMRI study. Front Aging Neurosci 2016; 8: 247.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang C, Mattis P, Tang C, Perrine K, Carbon M, Eidelberg D.. Metabolic brain networks associated with cognitive function in Parkinson's disease. Neuroimage 2007; 34: 714–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes AJ, Daniel SE, Kilford L, Lees AJ.. Accuracy of clinical diagnosis of idiopathic Parkinson's disease: a clinico-pathological study of 100 cases. J Neurol Neurosurg Psychiatry 1992; 55: 181–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hurtig HI, Trojanowski JQ, Galvin J, Ewbank D, Schmidt ML, Lee VM, et al. Alpha-synuclein cortical Lewy bodies correlate with dementia in Parkinson's disease. Neurology 2000; 54: 1916–21. [DOI] [PubMed] [Google Scholar]
- Hwang KS, Beyer MK, Green AE, et al. Mapping cortical atrophy in Parkinson's disease patients with dementia. J Parkinsons Dis 2013; 3: 69–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irwin DJ, Grossman M, Weintraub D, Hurtig HI, Duda JE, Xie SX, et al. Neuropathological and genetic correlates of survival and dementia onset in synucleinopathies: a retrospective analysis. Lancet Neurol 2017; 16: 55–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irwin DJ, White MT, Toledo JB, Xie SX, Robinson JL, Van Deerlin V, et al. Neuropathologic substrates of Parkinson disease dementia. Ann Neurol 2012; 72: 587–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janvin CC, Larsen JP, Aarsland D, Hugdahl K.. Subtypes of mild cognitive impairment in Parkinson's disease: progression to dementia. Mov Disord 2006; 21: 1343–9. [DOI] [PubMed] [Google Scholar]
- Janvin CC, Larsen JP, Salmon DP, Galasko D, Hugdahl K, Aarsland D.. Cognitive profiles of individual patients with Parkinson's disease and dementia: comparison with dementia with lewy bodies and Alzheimer's disease. Mov Disord 2006; 21: 337–42. [DOI] [PubMed] [Google Scholar]
- Jones EG. Synchrony in the interconnected circuitry of the thalamus and cerebral cortex. Ann N Y Acad Sci 2009; 1157: 10–23. [DOI] [PubMed] [Google Scholar]
- Joutsa J, Horn A, Hsu J, Fox MD.. Localizing parkinsonism based on focal brain lesions. Brain 2018; 141: 2445.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joutsa J, Shih LC, Horn A, Reich MM, Wu O, Rost NS, et al. Identifying therapeutic targets from spontaneous beneficial brain lesions. Ann Neurol 2018; 84: 153–7. [DOI] [PubMed] [Google Scholar]
- Kalbe E, Rehberg SP, Heber I, Kronenbuerger M, Schulz JB, Storch A, et al. Subtypes of mild cognitive impairment in patients with Parkinson's disease: evidence from the LANDSCAPE study. J Neurol Neurosurg Psychiatry 2016; 87: 1099–105. [DOI] [PubMed] [Google Scholar]
- Kandiah N, Zainal NH, Narasimhalu K, Chander RJ, Ng A, Mak E, et al. Hippocampal volume and white matter disease in the prediction of dementia in Parkinson's disease. Parkinsonism Relat Disord 2014; 20: 1203–8. [DOI] [PubMed] [Google Scholar]
- Karunanayaka PR, Lee E-Y, Lewis MM, Sen S, Eslinger PJ, Yang QX, et al. Default mode network differences between rigidity- and tremor-predominant Parkinson's disease. Cortex 2016; 81: 239–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kehagia AA, Barker RA, Robbins TW.. Cognitive impairment in Parkinson's disease: the dual syndrome hypothesis. Neurodegener Dis 2013; 11: 79–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiesmann M, Chanson J-B, Godet J, Vogel T, Schweiger L, Chayer S, et al. The Movement Disorders Society criteria for the diagnosis of Parkinson's disease dementia: their usefulness and limitations in elderly patients. J Neurol 2013; 260: 2569–79. [DOI] [PubMed] [Google Scholar]
- Klein JC, Eggers C, Kalbe E, Weisenbach S, Hohmann C, Vollmar S, et al. Neurotransmitter changes in dementia with Lewy bodies and Parkinson disease dementia in vivo. Neurology 2010; 74: 885–92. [DOI] [PubMed] [Google Scholar]
- Lanskey JH, McColgan P, Schrag AE, Acosta-Cabronero J, Rees G, Morris HR, et al. Can neuroimaging predict dementia in Parkinson's disease? Brain 2018; 141: 2545–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larsen JP, Dupont E, Tandberg E.. Clinical diagnosis of Parkinson's disease. Proposal of diagnostic subgroups classified at different levels of confidence. Acta Neurol Scand 1994; 89: 242–51. [DOI] [PubMed] [Google Scholar]
- Lee WW, Yoon EJ, Lee JY, Park SW, Kim YK.. Visual hallucination and pattern of brain degeneration in Parkinson's disease. Neurodegener Dis 2017; 17: 63–72. [DOI] [PubMed] [Google Scholar]
- Leroi I, McDonald K, Pantula H, Harbishettar V.. Cognitive impairment in Parkinson disease: impact on quality of life, disability, and caregiver burden. J Geriatr Psychiatry Neurol 2012; 25: 208–14. [DOI] [PubMed] [Google Scholar]
- Litvan I, Goldman JG, Tröster AI, Schmand BA, Weintraub D, Petersen RC, et al. Diagnostic criteria for mild cognitive impairment in Parkinson's disease: Movement Disorder Society Task Force guidelines. Mov Disord 2012; 27: 349–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Llinas RR, Steriade M.. Bursting of thalamic neurons and states of vigilance. J Neurophysiol 2006; 95: 3297–308. [DOI] [PubMed] [Google Scholar]
- Mak E, Su L, Williams GB, Firbank MJ, Lawson RA, Yarnall AJ, et al. Baseline and longitudinal grey matter changes in newly diagnosed Parkinson's disease: ICICLE-PD study. Brain 2015; 138: 2974–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mak E, Zhou J, Tan LC, Au WL, Sitoh YY, Kandiah N.. Cognitive deficits in mild Parkinson's disease are associated with distinct areas of grey matter atrophy. J Neurol Neurosurg Psychiatry 2014; 85: 576–80. [DOI] [PubMed] [Google Scholar]
- Mattis PJ, Tang CC, Ma Y, Dhawan V, Eidelberg D.. Network correlates of the cognitive response to levodopa in Parkinson disease. Neurology 2011; 77: 858–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melzer TR, Watts R, MacAskill MR, Pitcher TL, Livingston L, Keenan RJ, et al. Grey matter atrophy in cognitively impaired Parkinson's disease. J Neurol Neurosurg Psychiatry 2012; 83: 188–94. [DOI] [PubMed] [Google Scholar]
- Mihaescu AS, Masellis M, Graff-Guerrero A, et al. Brain degeneration in Parkinson's disease patients with cognitive decline: a coordinate-based meta-analysis. Brain Imaging Behav 2018. [DOI] [PubMed] [Google Scholar]
- Minkova L, Habich A, Peter J, Kaller CP, Eickhoff SB, Kloppel S.. Gray matter asymmetries in aging and neurodegeneration: a review and meta-analysis. Hum Brain Mapp 2017; 38: 5890–904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morales DA, Vives-Gilabert Y, Gómez-Ansón B, Bengoetxea E, Larrañaga P, Bielza C, et al. Predicting dementia development in Parkinson's disease using Bayesian network classifiers. Psychiatry Res 2013; 213: 92–8. [DOI] [PubMed] [Google Scholar]
- Muslimovic D, Schmand B, Speelman JD, de Haan RJ.. Course of cognitive decline in Parkinson's disease: a meta-analysis. J Int Neuropsychol Soc 2007; 13: 920–32. [DOI] [PubMed] [Google Scholar]
- Noe E, Marder K, Bell KL, Jacobs DM, Manly JJ, Stern Y.. Comparison of dementia with Lewy bodies to Alzheimer's disease and Parkinson's disease with dementia. Mov Disord 2004; 19: 60–7. [DOI] [PubMed] [Google Scholar]
- Onofrj M, Carrozzino D, D'Amico A, Di Giacomo R, Delli Pizzi S, Thomas A, et al. Psychosis in parkinsonism: an unorthodox approach. Neuropsychiatr Dis Treat 2017; 13: 1313–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pagonabarraga J, Corcuera-Solano I, Vives-Gilabert Y, Llebaria G, García-Sánchez C, Pascual-Sedano B, et al. Pattern of regional cortical thinning associated with cognitive deterioration in Parkinson's disease. PLoS One 2013; 8: e54980.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pagonabarraga J, Martinez-Horta S, Fernández de Bobadilla R, Pérez J, Ribosa-Nogué R, Marín J, et al. Minor hallucinations occur in drug-naive Parkinson's disease patients, even from the premotor phase. Mov Disord 2016; 31: 45–52. [DOI] [PubMed] [Google Scholar]
- Pan PL, Shi HC, Zhong JG, Xiao PR, Shen Y, Wu LJ, et al. Gray matter atrophy in Parkinson's disease with dementia: evidence from meta-analysis of voxel-based morphometry studies. Neurol Sci 2013; 34: 613–9. [DOI] [PubMed] [Google Scholar]
- Pereira JB, Svenningsson P, Weintraub D, Brønnick K, Lebedev A, Westman E, et al. Initial cognitive decline is associated with cortical thinning in early Parkinson disease. Neurology 2014; 82: 2017–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petersen RC, Doody R, Kurz A, Mohs RC, Morris JC, Rabins PV, et al. Current concepts in mild cognitive impairment. Arch Neurol 2001; 58: 1985–92. [DOI] [PubMed] [Google Scholar]
- Poldrack RA, Baker CI, Durnez J, Gorgolewski KJ, Matthews PM, Munafò MR, et al. Scanning the horizon: towards transparent and reproducible neuroimaging research. Nat Rev Neurosci 2017; 18: 115–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramirez-Ruiz B, Marti MJ, Tolosa E, et al. Cerebral atrophy in Parkinson's disease patients with visual hallucinations. Eur J Neurol 2007; 14: 750–6. [DOI] [PubMed] [Google Scholar]
- Ravina B, Marder K, Fernandez HH, Friedman JH, McDonald W, Murphy D, et al. Diagnostic criteria for psychosis in Parkinson's disease: report of an NINDS, NIMH work group. Mov Disord 2007; 22: 1061–8. [DOI] [PubMed] [Google Scholar]
- Reid WG, Hely MA, Morris JG, Loy C, Halliday GM.. Dementia in Parkinson's disease: a 20-year neuropsychological study (Sydney Multicentre Study). J Neurol Neurosurg Psychiatry 2011; 82: 1033–7. [DOI] [PubMed] [Google Scholar]
- Rektorova I, Biundo R, Marecek R, Weis L, Aarsland D, Antonini A.. Grey matter changes in cognitively impaired Parkinson's disease patients. PLoS One 2014; 9: e85595.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ricciardi L, Piano C, Rita BA, Fasano A.. Pedunculopontine nucleus stimulation in Parkinson's disease dementia. Biol Psychiatry 2015; 77: e35–e40. [DOI] [PubMed] [Google Scholar]
- Schneider CB, Donix M, Linse K, Werner A, Fauser M, Klingelhoefer L, et al. Accelerated age-dependent hippocampal volume loss in Parkinson disease with mild cognitive impairment. Am J Alzheimers Dis Other Demen 2017; 32: 313–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seeley WW, Crawford RK, Zhou J, Miller BL, Greicius MD.. Neurodegenerative diseases target large-scale human brain networks. Neuron 2009; 62: 42–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shine JM, Muller AJ, O'Callaghan C, Hornberger M, Halliday GM, Lewis SJ.. Abnormal connectivity between the default mode and the visual system underlies the manifestation of visual hallucinations in Parkinson's disease: a task-based fMRI study. NPJ Parkinsons Dis 2015; 1: 15003.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shine JM, O'Callaghan C, Halliday GM, Lewis SJ.. Tricks of the mind: visual hallucinations as disorders of attention. Prog Neurobiol 2014; 116: 58–65. [DOI] [PubMed] [Google Scholar]
- Song SK, Lee JE, Park HJ, Sohn YH, Lee JD, Lee PH.. The pattern of cortical atrophy in patients with Parkinson's disease according to cognitive status. Mov Disord 2011; 26: 289–96. [DOI] [PubMed] [Google Scholar]
- Spottke AE, Reuter M, Machat O, Bornschein B, von Campenhausen S, Berger K, et al. Cost of illness and its predictors for Parkinson's disease in Germany. Pharmacoeconomics 2005; 23: 817–36. [DOI] [PubMed] [Google Scholar]
- Tessitore A, Esposito F, Vitale C, Santangelo G, Amboni M, Russo A, et al. Default-mode network connectivity in cognitively unimpaired patients with Parkinson disease. Neurology 2012; 79: 2226–32. [DOI] [PubMed] [Google Scholar]
- Toledo JB, Gopal P, Raible K, Irwin DJ, Brettschneider J, Sedor S, et al. Pathological alpha-synuclein distribution in subjects with coincident Alzheimer's and Lewy body pathology. Acta Neuropathol 2016; 131: 393–409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y-Q, Tang B-S, Yan X-X, Chen Z-h, Xu Q, Liu Z-h, et al. A neurophysiological profile in Parkinson's disease with mild cognitive impairment and dementia in China. J Clin Neurosci 2015; 22: 981–5. [DOI] [PubMed] [Google Scholar]
- Weigand A, Horn A, Caballero R, Cooke D, Stern AP, Taylor SF, et al. Prospective validation that subgenual connectivity predicts antidepressant efficacy of transcranial magnetic stimulation sites. Biol Psychiatry 2018; 84: 28–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weil RS, Pappa K, Schade RN, et al. The cats-and-dogs test: a tool to identify visuoperceptual deficits in Parkinson's disease. Mov Disord 2017; 32: 1789–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weintraub D, Dietz N, Duda JE, Wolk DA, Doshi J, Xie SX, et al. Alzheimer's disease pattern of brain atrophy predicts cognitive decline in Parkinson's disease. Brain 2012; 135: 170–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weintraub D, Doshi J, Koka D, et al. Neurodegeneration across stages of cognitive decline in Parkinson disease. Arch Neurol 2011; 68: 1562–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weir S, Samnaliev M, Kuo T-C, Tierney TS, Walleser Autiero S, Taylor RS, et al. Short- and long-term cost and utilization of health care resources in Parkinson's disease in the UK. Mov Disord 2018; 33: 974–81. [DOI] [PubMed] [Google Scholar]
- Whittington CJ, Podd J, Kan MM.. Recognition memory impairment in Parkinson's disease: power and meta-analyses. Neuropsychology 2000; 14: 233–46. [DOI] [PubMed] [Google Scholar]
- Williams-Gray CH, Mason SL, Evans JR, Foltynie T, Brayne C, Robbins TW, et al. The CamPaIGN study of Parkinson's disease: 10-year outlook in an incident population-based cohort. J Neurol Neurosurg Psychiatry 2013; 84: 1258–64. [DOI] [PubMed] [Google Scholar]
- Winblad B, Palmer K, Kivipelto M, Jelic V, Fratiglioni L, Wahlund L-O, et al. Mild cognitive impairment–beyond controversies, towards a consensus: report of the International Working Group on Mild Cognitive Impairment. J Intern Med 2004; 256: 240–6. [DOI] [PubMed] [Google Scholar]
- Xuereb JH, Perry RH, Candy JM, Perry EK, Marshall E, Bonham JR.. Nerve cell loss in the thalamus in Alzheimer's disease and Parkinson's disease. Brain 1991; 114 (Pt 3): 1363–79. [PubMed] [Google Scholar]
- Yao N, Shek-Kwan Chang R, Cheung C, Pang S, Lau KK, Suckling J, et al. The default mode network is disrupted in Parkinson's disease with visual hallucinations. Hum Brain Mapp 2014; 35: 5658–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yarkoni T, Poldrack RA, Nichols TE, Van Essen DC, Wager TD.. Large-scale automated synthesis of human functional neuroimaging data. Nat Methods 2011; 8: 665–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeo BT, Krienen FM, Sepulcre J, et al. The organization of the human cerebral cortex estimated by intrinsic functional connectivity. J Neurophysiol 2011; 106: 1125–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zarei M, Ibarretxe-Bilbao N, Compta Y, Hough M, Junque C, Bargallo N, et al. Cortical thinning is associated with disease stages and dementia in Parkinson's disease. J Neurol Neurosurg Psychiatry 2013; 84: 875–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou J, Gennatas ED, Kramer JH, Miller BL, Seeley WW.. Predicting regional neurodegeneration from the healthy brain functional connectome. Neuron 2012; 73: 1216–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data on which this study is based were all obtained from published and publically available reports (see Tables 1–3 for details).