Abstract
South Asians (SAs) are at heightened risk for cardiovascular disease as compared to other ethnic groups, facing premature and more severe coronary artery disease, and decreased insulin sensitivity. This disease burden can only be partially explained by conventional risk factors, suggesting the need for a specific cardiovascular risk profile for SAs. Current research, as explored through a comprehensive literature review, suggests the existence of population specific genetic risk factors such as lipoprotein(a), as well as population specific gene modulating factors. This review catalogues the available research on cardiovascular disease and genetics, anthropometry, and pathophysiology, and cancer genetics among SAs, with a geographical focus on the U.S. A tailored risk profile will hinge upon population customized classification and treatment guidelines, informed by continued research.
Keywords: South Asian, Immigrant, Genetics, Epigenetics, Cardiovascular
Background
South Asians (SAs) worldwide are disproportionately affected by several cardiovascular disease (CVD) risk factors, including diabetes, metabolic syndrome (MetS), and obesity [1–7]. Studies confirm that underlying genetic and physiological factors, including body composition, biological predisposition and environmental change due to migration, play a significant role in the pathophysiology of CVD, diabetes, MetS, and abdominal obesity in the SA population [8, 9]. This review catalogues the available research on cardiovascular disease and genetics, anthropometry, and pathophysiology, and cancer genetics among SAs, with a geographical focus on the U.S. Relevant research on SAs in their countries of origin and diaspora (e.g. the United Kingdom and South Africa) is also assessed for topics that lack literature from the U.S. Catalogued literature on the intersection of environment, genetics, biomarkers, and pathophysiology was then used to identify research and health disparities and develop actionable steps to address these gaps in the U.S.
Methods
A search of NCBI PubMed and Scopus databases using the following key terms was conducted: diabetes or genetics or insulin resistance or body mass index or metabolic syndrome or hyperlipidemia or hypertension or genetic risk or cardiac or physiology. All studies that examined the significance, prevalence, and/or mechanism(s) of the association between epigenetics, genetics, epidemiology, pathophysiology, cardiovascular disease, and/or cancer (all types) among SA immigrants in the U.S. or other common host countries were included. Additional articles were added based on Steering Committee suggestions. Articles were excluded from the review if they were not relevant to the genetic, epigenetic, or pathophysiological mechanisms of cardiovascular disease; the cancer risk among the SA Diaspora; or the epidemiological context of this risk.
Results
Epidemiology
Metabolic Syndrome, Diabetes, and Cardiovascular Disease
South Asian immigrants are at a demonstrably heightened risk for CVD, diabetes, MetS, and abdominal obesity as compared to Caucasians and Africans [7, 10–21], to immigrants from other Asian groups [22–24], and even to native SA populations [25–27]. Within the SA immigrant population, similar rates of MetS have been found between Bangladeshi immigrants and other SA immigrant groups [28–30]. Younger age, increased weight, lower level of physical activity, and positive family history of heart disease were significant risk factors for metabolic syndrome. Even after adjusting for age, BMI and other diabetes risk factors, diabetes rates among Asian Indians are high. In an analysis of National Health Interview Survey data, Asian Americans were 30–50% more likely to have diabetes than their white counterparts, a trend seen in other studies among South Asians.
The Diabetes among Indian Americans (DIA) study was the first population-based study of SAs in the U.S. to assess the prevalence of risk factors for type II diabetes (T2DM), MetS and CVD [31]. The sample was comprised of 1038 randomly selected SA immigrants (18 and over) from seven U.S. cities [31]. Overall, 17.4% of respondents had diabetes, while 33% had prediabetes—a higher rate than reported in prior small scale studies. Notable findings included a significantly higher prevalence of diabetes among individuals who had a first-degree relative with the condition than among individuals without a family history of diabetes (11 vs. 5%), and significant gender differences in the presence of criteria for MetS and its risk factors [31]. CVD risk factors present in the sample included high levels of triglycerides, total cholesterol, LDL cholesterol, homocysteine, and C-reactive protein, and low levels of HDL cholesterol, though elevated lipoprotein(a) was not observed [31]. The age-adjusted prevalence of MetS was more than a quarter of the sample (26.9%) by the original National Cholesterol Education Program/Adult Treatment Panel III (NCEP/ATP III) criteria, approximately a third (32.7%) by the modified NCEP/ATP III criteria, and 38.2% by the International Diabetes Federation (IDF) criteria [31]. The MetS rates for women, but not for men, increased with age under all three criteria. As individuals progressed from normal to impaired fasting glucose (IFG) to diabetes, there was a progressive worsening of all metabolic parameters, including glucose and lipid levels [31]. The results warrant further population-based studies assessing MetS among SA immigrants.
Coronary Artery Disease
South Asians have significantly higher rates of incidence, prevalence, morbidity and mortality from coronary artery disease (CAD) and myocardial infarction (MI) [32–36] when compared to the general U.S. population, among whom age-standardized CAD mortality decreased by 69% in the past 30–35 years despite increases in diabetes and obesity rates [37].
Two cardinal features of CAD in SAs are prematurity and heightened severity [32–36, 38]. In large scale studies, including Coronary Artery Disease Among Indians (CADI), the mean age of diagnosis of CAD in SAs was lower as compared to the U.S. general population [35, 39]. In India, an estimated 30% of all CAD deaths occurs in people < 40 years of age [40] as compared to only 1 and 4% of all CAD deaths in U.S. whites and blacks respectively < 45 years of age [41].
CAD has been shown not only to develop at a younger age among SAs compared to other populations, but also to be more severe and malignant, resulting in death at younger ages [42]. Compared to whites in the UK, the relative risk of CAD mortality among SAs in the U.K. is 3.13 between the ages of 20 and 29, as opposed to 1.36 at all ages [43]. In Singapore, the relative risk of CAD mortality among SAs compared to Chinese is 12.5 in men aged 30–39 years and 3.0 in men aged 60–69 [44]. Other studies have shown a fourfold higher rate of hospitalization for CAD in SAs [45].
A European study demonstrated that the relative rate of infarction was higher in Asians than in the white population, and the age at infarction was lower in Asians. Risk factors and atherogenesis arise earlier in Asians, contributing to premature first myocardial infarctions. The authors speculated that increased incidence of diabetes in Asians may not in itself be relevant in the greater incidence of coronary atheroma in Asians [46].
A study of CAD comparing non-Bangladeshis to Bangladeshis in New York City found that Bangladeshis were more likely than non-Bangladeshis to evidence premature onset, clinically aggressive, and angiographically extensive CAD. Specifically, Bangladeshi ethnicity correlated with carrying > 3x the risk of 3-vessel CAD at angiography (p = .011), with Bangladeshis having twice the rate of 3-vessel CAD compared to non-Bangladeshis (53 vs. 26%). Bangladeshis were less likely to be current or recent smokers, younger, and had a lower body-mass index than non-Bangladeshis, highlighting the risk for prematurity and severity in this population even in the absence of several conventional risk factors. Conventional risk factors could not explain the prematurity and severity of CAD in SAs: there were no statistically significant differences between the two groups (Bangladeshis and non-Bangladeshis) in the prevalence of diabetes mellitus, hypertension, dyslipidemia, or family history of CAD [47].
Risk Factors
CAD
All traditional risk factors for CAD are associated with CAD in SAs, but the prevalence is similar or lower than other populations with the exception of diabetes (South Asian paradox) [35, 36, 38]. Consanguinity appears to be an important risk factor in at least some communities [48]. In Pakistan, studies have shown CVD and its risk factors to be high, with one in 4 Pakistanis > 40 years of age having CVD [49, 50].
Several studies have shown marked differences in the predicted and actual standardized mortality rates of SAs in the UK [51, 52], but have failed to explain the excess burden of CAD among SAs and Asian Indians by conventional risk factors and diabetes [53, 54]. A 21-year follow-up of a large prospective study from the UK has shown that baseline diabetes prevalence was 3 times more common among SAs (n = 1517) and African Caribbeans (n = 630) compared to whites (n = 2049). However, the incidence of CAD was 70% higher among SAs but 35% lower among Africans and Caribbeans [1]. Ethnic differences in measured metabolic risk factors such as insulin resistance, dyslipidemia and central obesity and even the 9 modifiable INTERHEART risk factors did not explain differences in coronary heart disease incidence among whites, blacks and SAs. Only one-third of the excess risk of CAD in SAs could be explained by the measured metabolic risk factors [1].
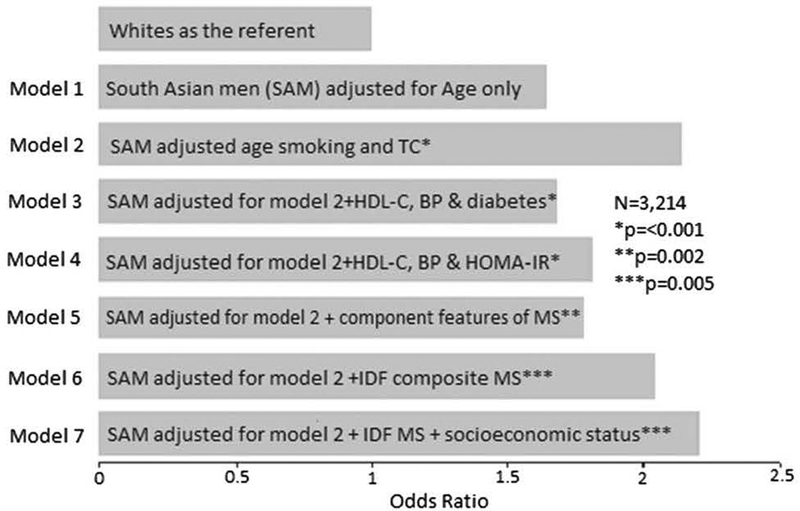
Another prospective study from the UK demonstrated a greater than twofold risk of CHD mortality among SAs. A greater proportion of SA CHD deaths were among individuals with diabetes (nearly half of SAs vs. 13% among Europeans). In multivariable models adjusting for conventional risk factors (diabetes and/or impaired glucose regulation, features of insulin resistance or metabolic syndrome), CHD mortality remained significantly higher in SA men compared to their European counterparts. Including co-morbidity and socio-economic status as covariates in an adjusted model did not significantly affect these findings (Fig. 1). These data confirm that SA men have significantly higher CHD mortality than European men and demonstrate that excess risk in this population cannot be explained by conventional risk factors (e.g. smoking, hypertension, and total cholesterol), insulin resistance parameters, nor metabolic syndrome. Future studies should examine the impact of unmeasured factors, such as genetically elevated lipoprotein(a) levels, on elevated vascular risk in SAs [56].
Fig. 1.
Odds ratio for CAD death in White and South Asian Men in UK. BP blood pressure, IDF International Diabetic Federation, IR insulin resistance, MS metabolic syndrome (Reproduced with permission from Enas et al. [55])
Lipoprotein(a)
Lipoprotein(a) (Lp(a)), is involved in thrombogenesis and atherogenesis. Elevated Lp(a) levels is a major determinant of CAD, MI, and stroke, especially at younger ages [56]. The impact of elevated Lp(a) level on CAD risk is heterogeneous, with greater risk imposed on Asian Indians—a population with the highest rate of premature and malignant CAD [32, 42, 56, 57].
Mechanistic, epidemiological, and recent genetic findings suggest Lp(a) is a causal risk factor for premature atherosclerotic cardiovascular disease (CVD) demonstrating atherogenic and thrombogenic properties, including enhanced uptake by macrophages, interference with fibrinolysis, and increased expression of adhesion molecules [58–60]. Lp(a) powerfully inhibits the fibrinolysis system, leading to atherothrombosis and MI [61].
Elevated Lp(a) levels may be the key factor underlying the excess burden of premature and malignant CAD in SAs around the globe [26, 36, 42, 56, 62–69]. Racial differences in plasma Lp(a) levels are present and expressed at birth; in a Singaporean study comparing newborns of Chinese, Malay, and Indian ethnicity, Indian newborns had the highest plasma levels of Lp(a) and Chinese newborns the lowest, a statistically significant difference [70]. The ranking of Lp(a) levels at birth mirrored adult Singaporean coronary mortality rates by the three ethnic groups [70]. Lipoprotein(a) levels are also increased in healthy young subjects with a family history of premature MI [71–74]. Such observations have also been made among SAs [75]. Elevated Lp(a) level is considered the single best biological marker of family history of premature CAD [56, 72].
Extreme elevation of Lp(a) levels predict a three- to fourfold increase in risk of MI in the general population with an absolute 10-year risk of 20% in women and 35% in men [76, 77]. High Apo B/Apo AI and TC/HDL ratios are powerful risk factors for CAD and MI [78, 79] and the risk may be synergistically increased when Lp(a) levels are also elevated [55]. The combination of elevated Lp(a) and high ApoB/ApoA1 ratio is also typical of Asian Indian dyslipidemia [55, 56, 79].
Lp(a) levels vary more than 1000-fold (from 0 to > 300 mg/dl) in individuals and fivefold in populations, unlike most other lipoproteins [80]. The majority of whites have Lp(a) levels < 10 mg/dl with a median concentration of 6 mg/dl [56, 80]. Lp(a) levels in Asian Indians are intermediate between whites and blacks, with 35–40% of Asian Indians having high levels with a median level of 16 mg/dl [56, 81, 82].
In Europeans, short KIV-2 repeats (a type of gene polymorphism) are associated with very high Lp(a) levels while intermediate and high copy number alleles are associated with low or very low concentrations. This inverse correlation between Lp(a) levels and KIV-2 repeats, while present, is weaker in Asian Indians. Studies of CHD risk in Asian Indians and other populations corroborate the hypothesis that Lp(a) level and not KIV-2 number is the cause of increased CHD risk [80].
High Lp(a) levels are strongly correlated with the severity of CAD [76, 83–89] as well as long-term adverse outcome, irrespective of the presence of traditional risk factors [90–97]. Lp(a) is a strong independent predictor of coronary artery calcification (CAC) and CAD in patients with diabetes [98–101]. The severity of the CAD is markedly increased when both diabetes and elevated Lp(a) levels are present. This combination is particularly common among SAs and possibly explains the greater severity of CAD in this population [42, 55, 98–104].
High levels of Lp(a) is a powerful risk factor for CAD among SAs with or without diabetes [63, 105–108]. It is also related to severity of CAD in this population [109–113]. The risk is further increased in the presence of Asian Indian dyslipidemia [55]. High Lp(a) levels are correlated with vascular complications in diabetes [114].
Several case control studies involving MI or stroke have shown higher levels of Lp(a) in cases than in controls, particularly among young Indians (40–45 years or younger) [72, 111, 115–117]. Lp(a) levels were also a better predictor of premature CAD or stroke than other risk factors in most of these studies [4, 72, 112, 117].
Given the higher prevalence [70] and higher risk of premature CAD [57], the population-attributable risk for elevated Lp(a) level is likely to be much higher among SAs (2–4 times) than in whites [55, 118]. The failure of several prospective studies to show any relationship between elevated Lp(a) and CVD has now been attributed to the use of inaccurate methods used for measuring Lp(a) levels [56, 97]. The Women’s Health Study and all other studies using Lp(a) assays that are not affected by LP(a) isoform size have shown strong relationships between Lp(a) and CAD [119].
Insulin Sensitivity
Studies in the U.S. have shown decreased insulin sensitivity among SAs compared to Caucasians, regardless of total body fat levels, suggesting that the SA propensity for the disorder is associated with significantly lower glucose disposal even among non-diabetics [9, 57, 120, 121]. A study in Europe found insulin levels to be higher in SAs compared to Europeans, regardless of diabetes history, while another reported similar differences even though the SA participants were younger [122, 123].
Compared with other ethnic groups, SAs may also face early declines in beta-cell function, which affects diabetes pathogenesis, though the complete mechanistic pathway of pancreatic beta-cell decline leading to T2DM is not entirely understood. A prospective study of Indians in South Africa with impaired glucose tolerance (IGT) found that SA participants with IGT showed delayed insulin responses with similar plasma glucose levels compared to controls [124]. This finding suggests early beta-cell dysfunction as an underlying pathophysiological abnormality of IGT in this population, a mechanism that may explain T2DM development in SAs, along with their higher degree of insulin resistance than other groups [124]. Moreover, a study of East Asians, SAs, Blacks, and Caucasians found insulin resistance in SA men to be three- to fourfold greater than men of the other ethnic groups, after controlling for lifestyle factors and BMI [125]. Further assessment of beta-cell function in a sub-group comparison of SA and Caucasian men found SAs to have a 30% increase in basal beta-cell responsiveness that did not sufficiently compensate for the degree of insulin resistance in SA men, as shown by a 60% reduction in insulin sensitivity disposition index [125]. Ethnic differences in sensitivity to T2DM development may be explained by variances in developing insulin resistance, the compensatory ability of beta-cells, or influences by genetics, or the environment [5, 126–128].
South Asian Population‑Specific Classification and Treatment Guidelines
There has been significant debate over the past decade on appropriateness of generic metabolic syndrome (MetS) diagnostic criteria for SAs. The WHO and researchers at multiple Indian conferences have recommended optimum BMI to be < 23, BMI 23–24.9 to be overweight, and BMI > 25 to be obese for SAs [129–132]. Use of the IDF criteria for diagnosing metabolic syndrome can underestimate its risk by 25–50% [104].
In 2009 the IDF, the American Heart Association, the National Heart, Lung, and Blood Institute, and several other organizations published a harmonized definition of metabolic syndrome without an obligatory component. A person would qualify for the metabolic syndrome if they met three abnormal findings out of any 5. A single set of cut points would be used for all components except waist circumference, for which further studies are required to determine more reliable cut points for different ethnic groups. In the interim, it was recommended that national or regional cut points for waist circumference be used [133]. Using > 90 cm as the cut point for men and > 80 cm for women, 70% of Indian men and women had abdominal obesity [134].
The effects of risk factors for diabetes are greater for SAs compared to other ethnicities. A 2006 study of immigrant SAs in Canada revealed optimal BMI cutoff points of 22.5 kg/m2 for lipid metabolism and 21 kg/m2 for glucose metabolism. 1176 subjects from 4 ethnic groups (289 SAs, 281 Chinese, 207 Aboriginals, and 301 Europeans) were randomly sampled from 4 regions in Canada and ethnic-specific BMI cut points were derived for three cardiometabolic factors [135]. For a given BMI, elevated levels of glucoseand lipid-related factors were more likely to be present in SAs, Chinese, and Aboriginals compared with Europeans; therefore, revised cut-off points would greatly increase the estimated burden of obesity-related metabolic disorders among non-European populations [135]. These findings are consistent with other studies demonstrating elevated risk of type 2 diabetes, hypertension, and dyslipidemia in SAs with BMIs under 25.0 kg/m2 [23, 136–141]. These and other studies prompted the release of revised BMI guidelines for Asian Indians based on consensus developed through a Prevention and Management of Obesity and Metabolic Syndrome group, defining overweight as a BMI > 23.0 kg/m2 and obesity as a BMI > 25.0 kg/m2 [130]. Studies in the UK have shown that CAD mortality rates among SAs were 50–80% higher than those predicted from using both Framingham Risk Score (FRS) and European SCORE prediction models [51, 52, 142]. The American and European guidelines for the management of blood pressure and lipids for the prevention and control of CAD are based on the levels of various risk factors as well as the risk conferred by them in their particular populations. These guidelines acknowledge the need to consider the heightened risk of premature CAD among SAs when treatment decisions are made [143, 144], and specific recommended thresholds for intervention and treatment can be seen in Table 1.
Table 1.
Indo U.S. health summit: recommended thresholds of intervention & treatment goals for indians [132]
| Parameters | Desirable levels for Asian Indians |
|---|---|
| Waist circumference | < 80 cm for women; < 90 cm for men |
| Body mass index | <23 kg/m2 men and women |
| Total cholesterol | < 160 mg/dl (high-risk Indians) |
| LDL-cholesterol | < 100 mg/dl (high-risk Indians)a |
| <70 for people with CAD or diabetesb | |
| Non-HDL-cholesterol | < 130 mg/dl (high-risk Indians)a |
| < 100 for people with CAD or diabetesb | |
| Triglycerides | < 150 mg/dl |
| HDL-cholesterol | >40 mg/dl (men); >50 mg/dl (women) |
| Hemoglobin A1C | <6.5% |
| Lipoprotein(a) | < 20 mg/dl |
Those who have ≥ 2 risk factors or metabolic syndrome are considered high risk individuals
Those who have diabetes, CAD or CVD are considered very high risk
Genetics
Diabetes
Genome-wide association studies (GWAS) have identified approximately 60 genes associated with T2DM risk, but most of these studies have been conducted in European populations, and few studies have attempted to replicate GWAS in SAs [145–147]. Two recent studies in SAs showed that the common variants associated with T2DM in European populations (PPARG, TCF7L2, FTO, and CDKN2A) are likewise associated with diabetes development among SAs [148, 149]. However, the authors stated that factors that mediate genetic effects, allele frequencies, or varying polymorphisms appeared to possibly differ between groups for at least some of those genes [150]. For example, the effect of FTO variants on T2DM is mediated by BMI in Europeans, but in SAs, the association is inconsistent [151–155]. While inconclusive, current data suggest that mechanistic differences in T2DM and FTO variant associations among the different ethnic groups may cause differences in associations between FTO variants, T2DM and obesity [152–154].
Another possibility for differences in T2DM risk is varying genetic polymorphisms. For example, studies have found that SAs have lower levels of circulating plasma adiponectin, a protein involved in glucose modulation, than Caucasians [121, 156]. Though this association is yet to be verified, a study comparing normal glucose tolerant patients with T2DM patients noted that an adiponectin gene polymorphism is associated with T2DM and obesity in SAs, which may explain the difference in circulating plasma adiponectin between the ethnic groups [157]. Moreover, a recent GWAS of 5561 individuals with T2DM (cases) and 14,458 controls drawn from studies in London, Pakistan and Singapore found 20 independent single nucleotide polymorphisms associated with T2DM, and common genetic variants were detected at six new loci associated with T2DM in SAs [146, 150]. Given the limited data currently available on the genetics of T2DM and obesity in SAs, further GWAS would be instrumental in providing information on underlying T2DM risk.
CVD
At present, genetic variation in the LP(a) gene is arguably the strongest single common genetic risk factor identified for premature CAD, MI, and stroke among diverse populations [56, 77, 158, 159]. In young women Lp(a) appears to be a stronger determinant of CAD than diabetes [160]. Lipoprotein(a) levels stabilize by age 2 and remain constant throughout life [56], suggesting the pathological processes associated with elevated Lp(a) also begin 10–20 years earlier than other risk factors such as high blood pressure, cigarette smoking, and diet-related dyslipidemia.
Apo(a) isoform size (i.e. KIV repeats) and Lp(a) concentrations are inversely correlated. Lp(a) levels and CAD share a causal relationship because a low number of KIV copies (11–22 copies) are associated with high Lp(a) levels and high Lp(a) levels are associated with CAD. This also rules out any reverse causality (CAD causing elevated Lp(a) levels) [80]. Mendelian randomization and other genetic studies have demonstrated a causal role of blood levels of Lp(a) in CAD [60, 161]. A candidate gene study of nearly 8000 CAD cases and 8000 controls found several SNPs in the LPA region to be associated with MI. The most strongly MI-correlated SNPs, rs10455872 and rs3798220, are associated with short KIV-2 repeats and high Lp(a) levels and confer a 1.47 and 1.58-fold increased risk of CAD compared with noncarriers, respectively [60]. Carriers of one variant evidence a 1.51 odds ratio (OR) for CAD and carriers of two or more variant alleles an OR of 2.57 for CAD [60]. A recent meta-analysis showed that the minor alleles of rs10455872 and rs3798220 increased CHD risk in carriers by 42 and 57%, respectively [162]. Another meta-analysis of 14 CAD GWA studies demonstrated a 51% higher OR for the rare versus common allele of SNP rs3798220 [163].
GWA analysis of multiple European myocardial infarction study datasets, including the German Myocardial Infarction Family Study, extended the CAD risk to haplotype information instead of single SNPs [164]. A haplotype comprised of four SNPs, two in LPA (rs7767084 and rs10755578) and two in neighboring genes LPAL2 (rs3127599) and SLC22A3 (rs2048327), was significantly associated with CAD risk. The uncommon haplotype CCTC (frequency of approximately 2%) was correlated with an 82% higher risk (OR 1.82, 95% CI 1.57–2.12) while the more common haplotype CTTG (frequency of about 16%) conferred a 20% higher risk (OR 1.20, 95% CI 1.13–1.28) when compared with the most frequent TCTC haplotype [164].
Prodigious genetic evidence has established Lp(a) as an emerging genetic risk factor for CVD. Lp(a) confers risk independent of other traditional risk factors, including lipids. However, the risk of high Lp(a) is further magnified in patients with high levels of traditional and emerging risk factors [56].
Cancer
In 2003, the Indian Genome Variation Database (IGVdb) was built to catalog and map SNPs among Indian subpopulations, the single most common type of human genome variation [165]. Bag et al. recently released a comprehensive literature review of genetic associations with cancer among Indians. The team’s review of 137 case control studies highlighted significant findings as well as pressing research gaps [165].
The most commonly studied genes in Indians are the Cytochrome P450 enzyme family (CYP) and glutathione S-transferases (GSTs) [165]. The most significant study results emphasized that combinations of genotypes, as opposed to single polymorphisms, had a much higher association with cancer risk [165]. One 2008 case control study with 375 participants found a much greater risk for head and neck cancers (4.47; CI 1.62–12.31) for patients that carried a combination of GSTM1 null, GSTT1 null and GSTP1 variations than those with individual variations [166].
Another study by Srivastava et al. found no associations with bladder cancer risk for individual polymorphisms, but did find a significant increased risk (OR 7.29) for patients with combined genotypes (GSTAT1, GSTM1, and GSTP1) [167]. Similar genotype combinations (GSTM2 and GSTT1) were found to be associated with risk of upper digestive tract cancers in another study that assessed patients with a history of smoking, tobacco chewing, and alcohol abuse [168].
A 2005 study by Mittal et al. among North Indians found a significant gene-environment interaction, with a GSTP1 gene polymorphism combined with tobacco use tremendously increasing risk for bladder cancer (OR 24.06) [169]. A 2008 international collaborative study between American and Indian investigators found that three sequence variations on 8q24 independently increased prostate cancer risk among North Indians, with OR values equivalent to 1.60, 1.77, and 1.85 for each respective sequence studied [170]. A very recent study emerging from India was the first to find that a mitochondrial D310 instability in breast cancer patients may be indicative of early progression of breast cancer to metastasis [171].
Biomarkers
Studies have shown biomarkers such as ROS, leptin, and C-Reactive Protein (CRP) to be associated with an increased risk for diabetes and adiponectin with a decreased risk for T2DM [172–176]. SAs demonstrate elevated levels of leptin and CRP, and decreased levels of adiponectin compared to Caucasians, of similar BMI and waist and hip circumferences. This further supports SA immigrant heightened risk for T2DM development [174–177], and highlights the possibility of defects in adipose tissue metabolism in SAs beyond elevated total abdominal fat [150]. An increased number and size of fat cells leads to an overproduction of hormones such as leptin and cytokines like tumor necrosis factor (TNFa), while reducing the synthesis of adiponectin, which enhances insulin sensitivity [178]. A study of SA Indians in the U.S. found that more truncal fat and large dysfunctional subcutaneous fat cells were related to ethnic differences in the manifestation of insulin resistance [14]. Oxidative stress has also been linked to higher levels of visceral fat [179]. As studies assessing levels of biomarkers of oxidative stress among SAs have yet to be conducted, this area warrants further research.
High levels of adiposity and unfavorable adipokine profiles are hypothesized to cause metabolic disturbances in SAs [14, 135, 156, 174, 180, 181]. Metabolic Syndrome and Atherosclerosis in South Asians Living in America (MASALA) found a strong positive association between leptin and body composition after adjusting for demographic and metabolic covariates [182]. The association between adiponectin and body composition was reduced by metabolic variables [182]. Differences between men and women in body composition and adipokines levels were also reported, similar to prior studies [182].
Prior studies have examined the relationship between adipokine profiles and body composition in SAs compared to other ethnic groups [14]. South Asian men, when compared to their Caucasian counterparts, were found to have higher leptin and lower adiponectin levels, with lower adiponectin levels correlating with larger subcutaneous adipocyte cell size [14]. In a study in Mauritius, SA men and women were found to have higher leptin levels when compared to Creoles and Europids, after adjusting for body composition [180]. A recent study found SAs to have the least favorable adiponectin profile as compared to Europeans, Chinese, and Aboriginal people [135].
An inflammatory marker and independent risk factor for CVD, CRP is associated with T2DM development in both Western and SA populations [177, 183, 184]. In several studies in the UK and U.S., SA immigrants were found to have higher CRP levels than their Caucasian counterparts. This finding suggests an underlying pro-inflammatory state in SAs, which could be another important contributing factor toward increased T2DM risk [150].
Other risk factors suggested as contributing to premature CVD pathogenesis include other inflammatory biomarkers such as interleukin 6 (IL-6), other adipokines and thrombotic risk factors such as fibrinogen 101 and plasminogen activator inhibitor-1 (PAI-1) [21, 64, 185–188]. As reviewed by Eapen et al., abnormalities in markers of endothelial dys-function, such as vascular cell adhesion molecule 1 (VCAM-1), elevated homocysteine levels, and impaired endothelium dependent dilatation have also been described in SA populations [185, 189–193]. Studies in the UK and U.S. comparing Caucasians and Indians found an association between fasting plasma homocysteine concentration and CVD.
Many studies also show that lipid profiles consistent with MetS definitions are also being seen in the SA population [128, 194, 195]. Within the overall SA population (native and diasporic), the lowest levels of HDL are seen in urban Indians and migrant Indians, and hypertriglyceridemia is more commonly seen among more affluent Indians and migrant Indians, when compared to rural Indians [128, 194, 195]. Also, higher levels of the more atherogenic small-dense LDL is seen among SAs, although LDL levels in the population are comparable to those seen in Caucasians [196].
Epigenetics
Examination of the role of environmental markers on phenotypes and subsequent inheritance between generations, without DNA sequence mutations, is an emerging field of research [197–201]. Researchers have found three primary mechanisms that facilitate the epimutation of phenotypes that adversely affect cardiovascular disease risk: DNA methylation, modification of core histone proteins, and alteration of microRNA expression. The latter mechanism, microRNAs (miR) expression alterations, is believed to be the most influential of the three [198–201], regulating several CVD-related disease processes, including cardiac hypertrophy, angiogenesis, glucose metabolism, and lipid metabolism [200].
Preliminary research in SAs suggests a link between miR alterations and dyslipidemia, one of the most common CVD risk phenotypes. One study measured an array of 85 microRNAs in SA cases (with dyslipidemia) and controls in California [202, 203]. Of these 85, 16 (19%) miRs displayed significant differences in expression, with three (miR-106b, miR-125b, miR-21) likely targeting transcription of genes that regulate lipid metabolism [202, 203]. Validation of the miR epigenetic mechanism in dyslipidemia in the SA population indicates a promising area of intervention [202, 203].
As miR is found in blood and can be used as a biomarker, it has great potential as a target for therapy [201]. Flowers et al. suggests that miR can serve as a measurement of CVD risk in clinical practice. It could potentially also serve as a target for risk reduction interventions [202, 203].
Results from EpiMigrant, a European Union commissioned case-control study concerned with identifying the epigenetic markers associated with risk of T2DM in SAs, found that methylation markers at genetic 5 loci were associated with future T2D among SAs [204]. DNA methylation’s ability to discriminate risk of T2D was especially pronounced for obese, normoglycemic SAs, enabling identification of a subset of obese individuals with high (> 20%) or low (< 5%) incidence of T2D at follow up [204]. Moreover, observed differences in methylation accounted for a significant amount of previously unexplained increased risk of T2D among SAs relative to Europeans [204]. These findings suggest DNA methylation may be a key epigenetic marker in identifying SAs who may benefit from early lifestyle and/or pharmacologic interventions [204].
Conclusions
The results of this literature review elucidate certain biological factors that may be linked to SA immigrants’ disproportionate rates of cardiovascular disease and its related risk factors, including diabetes, metabolic syndrome (MetS), and obesity. To accurately reflect these risks, providers should develop consensus risk and diagnosis guidelines tailored to the SA population. While many studies worldwide have investigated potential genetic and pathophysiological mechanisms related to abdominal obesity, MetS, T2DM development and related metabolic diseases, CVD risks, and cancer, more conclusive findings on SAs in the U.S. is needed. Research to date has not adequately addressed the specific cardiovascular and cancer risk profile of U.S. SAs in genetic, epigenetic, or pathophysiological terms. For example, SAs in the U.S. experience markedly premature and malignant coronary artery disease, but current research cannot explain their excess CAD burden by conventional risk factors or diabetes. This knowledge gap may be addressed by focusing more studies on the role of lipoprotein(a) [Lp(a)] in CAD among U.S. SAs.
Diabetes, a cardiovascular risk factor, has also been shown to disproportionately affect SAs in the U.S. More specifically, SAs experience higher rates of T2DM compared to other Asian ethnic groups in the U.S. Yet, the mechanism by which T2DM develops among SAs compared to other ethnic groups has not been uncovered [150]. Despite clinical and experimental studies suggesting that oxidative stress factors significantly determine diabetes pathogenesis by excessively forming free radicals (whose subsequent pathway can lead to the development of insulin resistance), no studies have assessed levels of oxidative stress in SAs [150, 205]. The role of early declines in beta-cell function in T2DM development among SAs in the U.S. also shows promise but remains unclear. Future research on T2DM among SAs should analyze these and other potential mechanisms.
SAs in the U.S. have also demonstrated high prevalence rates of metabolic disease (MetS), a risk factor for cardiovascular disease that has not been systematically analyzed or explained.
Researchers who have published papers on metabolic syndrome in SAs could potentially pool their data to analyze individual patients’ data from all the studies. This collaborative research effort would give more precise estimates for metabolic syndrome among SAs and may point to likely genetic, epigenetic, or pathophysiological determinants. Given the considerable heterogeneity as evidenced by different findings among the varied SA populations, root genetic causes of metabolic disorders also need be examined through large scale studies among SAs of varying cultural, geographic, and economic backgrounds [150]. In assessing the relationship between MetS and CVD in the SA population, an exploration of obesity and insulin resistance and their related risk factors is necessary, as T2DM and obesity are primary causes of insulin resistance, which is in turn associated with MetS. Further assessment of biomarkers as emerging risk factors [interleukin 6 (IL-6), CRP, homo-cysteine, Lp(a), adipokines, thrombotic risk factors such as fibrinogen and plasminogen activator inhibitor-1 (PAI-1) etc.] may partially explain ethnic differences in and SA proclivity towards adverse metabolic conditions.
The environmental causes of epigenetic alterations are another important topic lacking research. More research is needed on the potential environmental markers that affect alteration of microRNA and DNA methylation among SAs. Epigenetic research on hypertension among SAs does not yet exist, and very limited data exist on T2DM and dyslipidemia.
According to Bag et al., more research is needed in several areas of cancer genetics in SA populations. Many studies reviewed had small sample sizes that the authors feel may have generated false-positive associations [165]. The authors also highlighted a need for research on alcohol-related cancers and their genetic etiology, including head and neck and liver cancers, as well as a need for association studies for ovarian cancers in SA women [165].
The potential findings of these suggested research priorities would require new approaches to diagnosing and treating SA populations with cardiovascular disease, cancer, and their related risk factors. Reference value studies could inform modifications to diagnostic/value guidelines for metabolic syndrome, BMI, waist circumference, diabetes risk scores, and other CVD markers so that they specifically target SA groups. A multi-center population study for bio-markers (lipids, oxidative stress load) with a large sample size (e.g. 1000 participants) could be conducted to gather value distributions. Should future research reveal genetic, epigenetic, and pathophysiological explanations for cardiovascular and cancer related disparities among U.S. SAs, broader acceptance and dissemination of harmonized diagnostic criteria should be a policy priority.
Acknowledgements
This study was funded by National Institute on Minority Health and Health Disparities (R13 MD007147–01A1), National Cancer Institute (U54CA137788), National Cancer Institute (P30 CA008748). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. SAHI staff would like to thank the Steering Committee members, all working group co-chairs, the Memorial Sloan Kettering Cancer Center Library, Rohini Rau-Murthy and the SAHI interns for their assistance in assembling this document.
Footnotes
Conflict of interest The authors declare that they have no conflicts of interest.
References
- 1.Tillin T, Hughes AD, Mayet J, Whincup P, Sattar N, Forouhi NG, et al. (2013). The relationship between metabolic risk factors and incident cardiovascular disease in Europeans, South Asians, and African Caribbeans: SABRE (Southall and Brent Revisited)—A prospective population-based study. Journal of the American College of Cardiology, 61(17), 1777–1786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Forouhi NG, Sattar N, Tillin T, McKeigue PM, & Chaturvedi N (2006). Do known risk factors explain the higher coronary heart disease mortality in South Asian compared with European men? Prospective follow-up of the Southall and Brent studies, UK. Diabetologia, 49(11), 2580–2588. [DOI] [PubMed] [Google Scholar]
- 3.Reddy KS, Prabhakaran D, Chaturvedi V, Jeemon P, Thankappan KR, Ramakrishnan L, et al. (2006). Methods for establishing a surveillance system for cardiovascular diseases in Indian industrial populations. Bulletin of the World Health Organization, 84(6), 461–469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sobhani I, Amiot A, Le Baleur Y, Levy M, Auriault ML, Van Nhieu JT, et al. (2013). Microbial dysbiosis and colon carcinogenesis: Could colon cancer be considered a bacteria-related disease? Therapeutic Advances in Gastroenterology, 6(3), 215–229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.McKeigue PM, Shah B, & Marmot MG (1991). Relation of central obesity and insulin resistance with high diabetes prevalence and cardiovascular risk in South Asians. The Lancet, 337(8738), 382–386. [DOI] [PubMed] [Google Scholar]
- 6.Ma S, Cutter J, Tan CE, Chew SK, & Tai ES (2003). Associations of diabetes mellitus and ethnicity with mortality in a multiethnic Asian population: Data from the 1992 Singapore National Health Survey. American Journal of Epidemiology, 158(6), 543–552. [DOI] [PubMed] [Google Scholar]
- 7.Palaniappan LP, Wong EC, Shin JJ, Fortmann SP, & Lauderdale DS (2010). Asian Americans have greater prevalence of metabolic syndrome despite lower body mass index. International Journal of Obesity, 35, 393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yates T, Davies MJ, Gray LJ, Webb D, Henson J, Gill JM, et al. (2010). Levels of physical activity and relationship with markers of diabetes and cardiovascular disease risk in 5474 white European and South Asian adults screened for type 2 diabetes. Preventive Medicine, 51(3–4), 290–294. [DOI] [PubMed] [Google Scholar]
- 9.Hayes L, White M, Unwin N, Bhopal R, Fischbacher C, Harland J, et al. (2002). Patterns of physical activity and relationship with risk markers for cardiovascular disease and diabetes in Indian, Pakistani, Bangladeshi and European adults in a UK population. Journal of Public Health Medicine, 24(3), 170–178. [DOI] [PubMed] [Google Scholar]
- 10.Tillin T, Forouhi N, Johnston DG, McKeigue PM, Chaturvedi N, & Godsland IF (2005). Metabolic syndrome and coronary heart disease in South Asians, African-Caribbeans and white Europeans: A UK population-based cross-sectional study. Diabetologia, 48(4), 649–656. [DOI] [PubMed] [Google Scholar]
- 11.Yates T, Davies M, Gorely T, Bull F, & Khunti K (2008). Rationale, design and baseline data from the Pre-diabetes Risk Education and Physical Activity Recommendation and Encouragement (PREPARE) programme study: A randomized controlled trial. Patient Education and Counseling, 73(2), 264–271. [DOI] [PubMed] [Google Scholar]
- 12.Grundy SM, Cleeman JI, Daniels SR, Donato KA, Eckel RH, Franklin BA, et al. (2005). Diagnosis and management of the metabolic syndrome: An American Heart Association/National Heart, Lung, and Blood Institute Scientific Statement. Circulation, 112(17), 2735–2752. [DOI] [PubMed] [Google Scholar]
- 13.Misra A, & Khurana L (2010). Obesity-related non-communicable diseases: South Asians vs White Caucasians. International Journal of Obesity, 35(2), 167–187. [DOI] [PubMed] [Google Scholar]
- 14.Chandalia M, Lin P, Seenivasan T, Livingston EH, Snell PG, Grundy SM, et al. (2007). Insulin resistance and body fat distribution in South Asian men compared to Caucasian men. PLoS ONE, 2(8), e812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zimmet P, Wischusen J, Cohen M, Raper LR, & Crosbie C (1983). Improved control in self-blood glucose monitoring insulin dependent diabetics treated with acarbose. The Tohoku Journal of Experimental Medicine, 141(Suppl), 687–692. [DOI] [PubMed] [Google Scholar]
- 16.Omar MA, Seedat MA, Dyer RB, Motala AA, Knight LT, & Becker PJ (1994). South African Indians show a high prevalence of NIDDM and bimodality in plasma glucose distribution patterns. Diabetes Care, 17(1), 70–73. [DOI] [PubMed] [Google Scholar]
- 17.Seedat YK, Mayet FG, & Gouws E (1994). Risk factors for coronary heart disease in the white community of Durban. South African Medical Journal, 84(5), 257–262. [PubMed] [Google Scholar]
- 18.Jenum AK, Holme I, Graff-Iversen S, & Birkeland KI (2005). Ethnicity and sex are strong determinants of diabetes in an urban Western society: Implications for prevention. Diabetologia, 48(15729578), 435–439. [DOI] [PubMed] [Google Scholar]
- 19.Lee WR (2000). The changing demography of diabetes mellitus in Singapore. Diabetes Research and Clinical Practice, 50(Suppl 2), S35–S39. [DOI] [PubMed] [Google Scholar]
- 20.Anand SS, Tarnopolsky MA, Rashid S, Schulze KM, Desai D, Mente A, et al. (2011). Adipocyte hypertrophy, fatty liver and metabolic risk factors in South Asians: The Molecular Study of Health and Risk in Ethnic Groups (mol-SHARE). PLoS ONE, 6(7), e22112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Anand SS, Yusuf S, Vuksan V, Devanesen S, Teo KK, Montague PA, et al. (2000). Differences in risk factors, atherosclerosis and cardiovascular disease between ethnic groups in Canada: The Study of Health Assessment and Risk in Ethnic groups (SHARE). Indian Heart Journal, 52(7 Suppl), S35–43. [PubMed] [Google Scholar]
- 22.Oza-Frank R, Ali MK, Vaccarino V, & Narayan KM (2009). Asian Americans: Diabetes prevalence across U.S. and World Health Organization weight classifications. Diabetes Care, 32(9), 1644–1646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wang EJ, Wong EC, Dixit AA, Fortmann SP, Linde RB, & Palaniappan LP (2011). Type 2 diabetes: Identifying high risk Asian American subgroups in a clinical population. Diabetes Research and Clinical Practice, 93(2), 248–254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gupta LS, Wu CC, Young S, & Perlman SE (2011). Prevalence of diabetes in New York City, 2002–2008: Comparing foreign-born South Asians and other Asians with U.S.-born whites, blacks, and Hispanics. Diabetes Care, 34(8), 1791–1793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Patel JV, Vyas A, Cruickshank JK, Prabhakaran D, Hughes E, Reddy KS, et al. (2006). Impact of migration on coronary heart disease risk factors: Comparison of Gujaratis in Britain and their contemporaries in villages of origin in India. Atherosclerosis, 185(2), 297–306. [DOI] [PubMed] [Google Scholar]
- 26.Bhatnagar D, Anand IS, Durrington PN, Patel DJ, Wander GS, Mackness MI, et al. (1995). Coronary risk factors in people from the Indian subcontinent living in west London and their siblings in India. The Lancet, 345(8947), 405–409. [DOI] [PubMed] [Google Scholar]
- 27.Misra A, & Vikram NK (2004). Insulin resistance syndrome (metabolic syndrome) and obesity in Asian Indians: Evidence and implications. Nutrition, 20(5), 482–491. [DOI] [PubMed] [Google Scholar]
- 28.Balasubramanyam A, Rao S, Misra R, Sekhar RV, & Ballantyne CM (2008). Prevalence of metabolic syndrome and associated risk factors in Asian Indians. Journal of Immigrant and Minority Health/Center for Minority Public Health, 10(4), 313–323. [DOI] [PubMed] [Google Scholar]
- 29.Misra KB, Endemann SW, & Ayer M (2006). Measures of obesity and metabolic syndrome in Indian Americans in northern California. Ethnicity and Disease, 16(2), 331–337. [PubMed] [Google Scholar]
- 30.Yajnik CS (2002). The lifecycle effects of nutrition and body size on adult adiposity, diabetes and cardiovascular disease. Obesity Reviews, 3(3), 217–224. [DOI] [PubMed] [Google Scholar]
- 31.Misra R, Patel T, Kotha P, Raji A, Ganda O, Banerji M, et al. (2010). Prevalence of diabetes, metabolic syndrome, and cardiovascular risk factors in US Asian Indians: Results from a national study. Journal of Diabetes and Its Complications, 24(3), 145–153. [DOI] [PubMed] [Google Scholar]
- 32.Enas EA, Yusuf S, & Mehta J (1996). Meeting of the international working group on coronary artery disease in South Asians. 24 March 1996, Orlando, Florida, USA. Indian Heart Journal, 48(6), 727–732. [PubMed] [Google Scholar]
- 33.Enas EA, Yusuf S, & Mehta J (1992). Prevalence of coronary artery disease in Asian Indians. American Journal of Cardiology, 70, 945–949. [DOI] [PubMed] [Google Scholar]
- 34.Enas EA (2000). Coronary artery disease epidemic in Indians: A cause for alarm and call for action. Journal of the Indian Medical Association, 98(11), 694–695. 697–702. [PubMed] [Google Scholar]
- 35.Enas EA, Garg A, Davidson MA, Nair VM, Huet BA, & Yusuf S (1996). Coronary heart disease and its risk factors in first-generation immigrant Asian Indians to the United States of America. Indian Heart Journal, 48(4), 343–353. [PubMed] [Google Scholar]
- 36.Jha P, Enas E, & Yusuf S (1993). Coronary artery disease in Asian Indians: Prevalence and risk factors. Asian American and Pacific Islander Journal of Health, 1(2), 163–175. [PubMed] [Google Scholar]
- 37.Ford ES, Ajani UA, Croft JB, Critchley JA, Labarthe DR, Kottke TE, et al. (2007). Explaining the decrease in U.S. deaths from coronary disease, 1980–2000. New England Journal of Medicine, 356(23), 2388–2398. [DOI] [PubMed] [Google Scholar]
- 38.Coronary Artery Disease among Asian Indians. (2013). CADI research. Retrieved July 25, 2013 from http://www.cadiresearch.org/
- 39.Aarabi M, & Jackson PR (2005). Predicting coronary risk in UK South Asians: An adjustment method for Framingham-based tools. European Journal of Cardiovascular Prevention and Rehabilitation, 12(1), 46–51. [PubMed] [Google Scholar]
- 40.Indrayan A (2010). Forecasting vascular disease cases and associated mortality in India, pp 197–215. Retrieved March 5, 2013 from http://www.whoindia.org/LinkFiles/Commision_on_Macroeconomic_and_Health_Bg_P2_Forecasting_vascular_disease_cases_and_associated_mortality_in_India.pdf [Google Scholar]
- 41.Jolly S, Vittinghoff E, Chattopadhyay A, & Bibbins-Domingo K (2010). Higher cardiovascular disease prevalence and mortality among younger blacks compared to whites. American Journal of Medicine, 123(9), 811–818. [DOI] [PubMed] [Google Scholar]
- 42.Enas EA, & Mehta J (1995). Malignant coronary artery disease in young Asian Indians: Thoughts on pathogenesis, prevention, and therapy. Coronary Artery Disease in Asian Indians (CADI) Study. Clinical Cardiology, 18(3), 131–135. [DOI] [PubMed] [Google Scholar]
- 43.Balarajan R (1991). Ethnic differences in mortality from ischaemic heart disease and cerebrovascular disease in England and Wales. BMJ, 302(6776), 560–564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hughes K, Lun KC, & Yeo PP (1990). Cardiovascular diseases in Chinese, Malays, and Indians in Singapore. I. Differences in mortality. Journal of Epidemiology and Community Health, 44(1), 24–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Klatsky AL, Tekawa I, Armstrong MA, & Sidney S (1994). The risk of hospitalization for ischemic heart disease among Asian Americans in northern California. American Journal of Public Health, 84(10), 1672–1675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hughes LO, Raval U, & Raftery E (1989). First myocardial infarctions in Asian and White men. BMJ, 298, 1345–1350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Silbiger JJ, Ashtiani R, Attari M, Spruill TM, Kamran M, Reynolds D, et al. (2009). Atheroscerlotic heart disease in Bangladeshi immigrants: Risk factors and angiographic findings. International Journal of Cardiology, 146, e38–e40. [DOI] [PubMed] [Google Scholar]
- 48.Ismail J, Jafar TH, Jafary FH, White F, Faruqui AM, & Chaturvedi N (2004). Risk factors for non-fatal myocardial infarction in young South Asian adults. Heart, 90(3), 259–263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Jafar TH (2006). Women in Pakistan have a greater burden of clinical cardiovascular risk factors than men. International Journal of Cardiology, 106(3), 348–354. [DOI] [PubMed] [Google Scholar]
- 50.Jafar TH, Jafary FH, Jessani S, & Chaturvedi N (2005). Heart disease epidemic in Pakistan: Women and men at equal risk. American Heart Journal, 150(2), 221–226. [DOI] [PubMed] [Google Scholar]
- 51.Bhopal R, Fischbacher C, Vartiainen E, Unwin N, White M, & Alberti G (2005). Predicted and observed cardiovascular disease in South Asians: Application of FINRISK, Framingham and SCORE models to Newcastle Heart Project data. Journal of Public Health, 27(1), 93–100. [DOI] [PubMed] [Google Scholar]
- 52.Quirke TP, Gill PS, Mant JW, & Allan TF (2003). The applicability of the Framingham coronary heart disease prediction function to black and minority ethnic groups in the UK. Heart, 89(7), 785–786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Miller GJ, Beckles GL, Maude GH, Carson DC, Alexis SD, Price SG, et al. (1989). Ethnicity and other characteristics predictive of coronary heart disease in a developing community: Principal results of the St James Survey, Trinidad. International Journal of Epidemiology, 18(4), 808–817. [DOI] [PubMed] [Google Scholar]
- 54.Beckles GL, Miller GJ, Kirkwood BR, Alexis SD, Carson DC, & Byam NT (1986). High total and cardiovascular disease mortality in adults of Indian descent in Trinidad, unexplained by major coronary risk factors. The Lancet, 1(8493), 1298–1301. [DOI] [PubMed] [Google Scholar]
- 55.Enas EA, Chacko V, Pazhoor SG, Chennikkara H, & Devarapalli HP (2007). Dyslipidemia in South Asian patients. Current Atherosclerosis Reports, 9(5), 367–374. [DOI] [PubMed] [Google Scholar]
- 56.Enas EA, Chacko V, Senthilkumar A, Puthumana N, & Mohan V (2006). Elevated lipoprotein(a)—A genetic risk factor for premature vascular disease in people with and without standard risk factors: A review. Disease-a-Month, 52(1), 5–50. [DOI] [PubMed] [Google Scholar]
- 57.Banerjee D, Wong EC, Shin J, Fortmann SP, & Palaniappan L (2011). Racial and ethnic variation in lipoprotein (a) levels among Asian Indian and Chinese Patients. Journal of Lipids, 2011, 291954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Nordestgaard BG, Chapman MJ, Ray K, Boren J, Andreotti F, Watts GF, et al. (2010). Lipoprotein(a) as a cardiovascular risk factor: Current status. European Heart Journal, 31(23), 2844–2853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Boffa MB, Marcovina SM, & Koschinsky ML (2004). Lipoprotein(a) as a risk factor for atherosclerosis and thrombosis: Mechanistic insights from animal models. Clinical Biochemistry, 37(5), 333–343. [DOI] [PubMed] [Google Scholar]
- 60.Clarke R, Peden JF, Hopewell JC, Kyriakou T, Goel A, Heath SC, et al. (2009). Genetic variants associated with Lp(a) lipoprotein level and coronary disease. New England Journal of Medicine, 361(26), 2518–2528. [DOI] [PubMed] [Google Scholar]
- 61.Matsuda S, Arima M, Ohigawa T, Tanimoto K, Takagi A, Kanoh T, et al. (2004). Relation between serum lipoprotein (a) and residual lesion stenosis of coronary artery after myocardial Infarction without reperfusion therapy. Japanese Heart Journal, 45(3), 397–407. [DOI] [PubMed] [Google Scholar]
- 62.Enas EA, Dhawan J, & Petkar S (1997). Coronary artery disease in Asian Indians: Lessons learnt and the role of lipoprotein(a). Indian Heart Journal, 49(1), 25–34. [PubMed] [Google Scholar]
- 63.Enas EA (2001). Lipoprotein(a) is an important genetic risk factor for coronary artery disease in Asian Indians. American Journal of Cardiology, 88, 201–202. [DOI] [PubMed] [Google Scholar]
- 64.Hoogeveen RC, Gambhir JK, Gambhir DS, Kimball KT, Ghazzaly K, Gaubatz JW, et al. (2001). Evaluation of Lp[a] and other independent risk factors for CHD in Asian Indians and their USA counterparts. Journal of Lipid Research, 42(4), 631–638. [PubMed] [Google Scholar]
- 65.Anand SS, Enas EA, Pogue J, Haffner S, Pearson T, & Yusuf S (1998). Elevated lipoprotein(a) levels in South Asians in North America. Metabolism, 47(2), 182–184. [DOI] [PubMed] [Google Scholar]
- 66.Hughes K, Aw TC, Kuperan P, & Choo M (1997). Central obesity, insulin resistance, syndrome X, lipoprotein(a), and cardiovascular risk in Indians, Malays, and Chinese in Singapore. Journal of Epidemiology and Community Health, 51(4), 394–399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Chuang CZ, Subramaniam PN, LeGardeur BY, & Lopez A (1998). Risk factors for coronary artery disease and levels of lipoprotein(a) and fat-soluble antioxidant vitamins in Asian Indians of USA. Indian Heart Journal, 50(3), 285–291. [PubMed] [Google Scholar]
- 68.Anand SS, Yusuf S, Vuksan V, Devanesen S, Teo KK, Montague PA, et al. (2000). Differences in risk factors, atherosclerosis, and cardiovascular disease between ethnic groups in Canada: The Study of Health Assessment and Risk in Ethnic groups (SHARE). The Lancet, 356(9226), 279–284. [DOI] [PubMed] [Google Scholar]
- 69.Devanapalli B, Lee S, Mahajan D, & Bermingham M (2002). Lipoprotein (a) in an immigrant Indian population sample in Australia. British Journal of Biomedical Science, 59(2), 119–122. [PubMed] [Google Scholar]
- 70.Low PS, Heng CK, Saha N, & Tay JS (1996). Racial variation of cord plasma lipoprotein(a) levels in relation to coronary risk level: A study in three ethnic groups in Singapore. Pediatric Research, 40(5), 718–722. [DOI] [PubMed] [Google Scholar]
- 71.Gaeta G, Cuomo S, Capozzi G, Foglia MC, Barra S, Madrid A, et al. (2008). Lipoprotein(a) levels are increased in healthy young subjects with parental history of premature myocardial infarction. Nutrition, Metabolism & Cardiovascular Diseases, 18(7), 492–496. [DOI] [PubMed] [Google Scholar]
- 72.Isser HS, Puri VK, Narain VS, Saran RK, Dwivedi SK, & Singh S (2001). Lipoprotein (a) and lipid levels in young patients with myocardial infarction and their first-degree relatives. Indian Heart Journal, 53(4), 463–466. [PubMed] [Google Scholar]
- 73.Marquez A, Mendoza S, Carrasco H, Hamer T, & Glueck CJ (1993). High lipoprotein(a) in children from kindreds with parental premature myocardial infarction. Pediatric Research, 34(5), 670–674. [DOI] [PubMed] [Google Scholar]
- 74.Pay S, Ozcan N, & Tokgozoglu SL (1997). Elevated Lp(a) is the most frequent familial lipoprotein disorder leading to premature myocardial infarction in a country with low cholesterol levels. International Journal of Cardiology, 60(3), 301–305. [DOI] [PubMed] [Google Scholar]
- 75.Shah SM, Karira KA, Soomro Salahuddin MS, & Ghafoor S (2001). Serum lipoprotein (a) in offspring of patients with premature myocardial infarction. Journal of the Pakistan Medical Association, 51(5), 180–183. [PubMed] [Google Scholar]
- 76.Kamstrup PR, Benn M, Tybjaerg-Hansen A, & Nordestgaard BG (2008). Extreme lipoprotein(a) levels and risk of myocardial infarction in the general population: The Copenhagen City Heart Study. Circulation, 117(2), 176–184. [DOI] [PubMed] [Google Scholar]
- 77.Kamstrup PR, Tybjaerg-Hansen A, Steffensen R, & Nordestgaard BG (2009). Genetically elevated lipoprotein(a) and increased risk of myocardial infarction. JAMA, 301(22), 2331–2339. [DOI] [PubMed] [Google Scholar]
- 78.Holme I, Aastveit AH, Jungner I, & Walldius G (2008). Relationships between lipoprotein components and risk of myocardial infarction: Age, gender and short versus longer follow-up periods in the Apolipoprotein MOrtality RISk study (AMORIS). Journal of Internal Medicine, 264(1), 30–38. [DOI] [PubMed] [Google Scholar]
- 79.McQueen MJ, Hawken S, Wang X, Ounpuu S, Snider-man A, Probstfield J, et al. (2008). Lipids, lipoproteins, and apolipoproteins as risk markers of myocardial infarction in 52 countries (the INTERHEART study): A case-control study. The Lancet, 372(9634), 224–233. [DOI] [PubMed] [Google Scholar]
- 80.Kronenberg F, & Utermann G (2013). Lipoprotein(a): Resurrected by genetics. Journal of Internal Medicine, 273(1), 6–30. [DOI] [PubMed] [Google Scholar]
- 81.Anand SS, Enas EA, Pogue J, Haffner S, Pearson T, & Yusuf S (1998). Elevated lipoprotein(a) levels in South Asians in North America. Metabolism, 47(2), 182–184. [DOI] [PubMed] [Google Scholar]
- 82.Superko HR, Enas EA, Kotha P, Bhat NK, & Garrett B (2005). High-density lipoprotein subclass distribution in individuals of asian Indian descent: The National Asian Indian Heart Disease Project. Preventive Cardiology, 8(2), 81–86. [DOI] [PubMed] [Google Scholar]
- 83.Erqou S, Kaptoge S, Perry PL, Di Angelantonio E, Thompson A, White IR, et al. (2009). Lipoprotein(a) concentration and the risk of coronary heart disease, stroke, and nonvascular mortality. JAMA, 302(4), 412–423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Kamstrup PR, Tybjaerg-Hansen A, & Nordestgaard BG (2011). Lipoprotein(a) and risk of myocardial infarction- Genetic epidemiologic evidence of causality. Scandinavian Journal of Clinical and Laboratory Investigation, 71, 87–93. [DOI] [PubMed] [Google Scholar]
- 85.Bolibar I, Thompson SG, von Eckardstein A, Sandkamp M, & Assmann G (1995). Dose-response relationships of serum lipid measurements with the extent of coronary stenosis. Strong, independent, and comprehensive. ECAT Angina Pectoris Study Group. Arteriosclerosis, Thrombosis, and Vascular Biology, 15(8), 1035–1042. [DOI] [PubMed] [Google Scholar]
- 86.Budde T, Fechtrup C, Bosenberg E, Vielhauer C, Enbergs A, Schulte H, et al. (1994). Plasma Lp(a) levels correlate with number, severity, and length-extension of coronary lesions in male patients undergoing coronary arteriography for clinically suspected coronary atherosclerosis. Arteriosclerosis and Thrombosis, 14(11), 1730–1736. [DOI] [PubMed] [Google Scholar]
- 87.Hartmann M, von Birgelen C, Mintz GS, Stoel MG, Eggebrecht H, Wieneke H, et al. (2006). Relation between lipoprotein(a) and fibrinogen and serial intravascular ultrasound plaque progression in left main coronary arteries. Journal of the American College of Cardiology, 48(3), 446–452. [DOI] [PubMed] [Google Scholar]
- 88.Watts GF, Mazurkiewicz JC, Tonge K, Nelson V, Warburton FG, & Slavin BM (1995). Lipoprotein(a) as a determinant of the severity of angiographically defined carotid atherosclerosis. QJM, 88(5), 321–326. [PubMed] [Google Scholar]
- 89.Wang XL, Cranney G, & Wilcken DE (2000). Lp(a) and conventional risk profiles predict the severity of coronary stenosis in high-risk hospital-based patients. Australian and New Zealand Journal of Medicine, 30(3), 333–338. [DOI] [PubMed] [Google Scholar]
- 90.Gomez M, Valle V, Aros F, Sanz G, Sala J, Fiol M, et al. (2009). Oxidized LDL, lipoprotein (a) and other emergent risk factors in acute myocardial infarction (FORTIAM study). Revista Espanola de Cardiologia, 62(4), 373–382. [DOI] [PubMed] [Google Scholar]
- 91.Ornek E, Murat S, Duran M, Turfan M, Kurtul A, Demircelik MB, et al. (2011). The relationship between lipoprotein(a) and coronary artery disease, as well as its variable nature following myocardial infarction. Clinical and Investigative Medicine, 34(1), E14–20. [DOI] [PubMed] [Google Scholar]
- 92.Cho JY, Jeong MH, Ahn Y, Hong YJ, Park HW, Yoon NS, et al. (2010). High lipoprotein(a) levels are associated with long-term adverse outcomes in acute myocardial infarction patients in high Killip classes. Korean Circulation Journal, 40(10), 491–498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Igarashi Y, Aizawa Y, Satoh T, Konno T, Ojima K, & Aizawa Y (2003). Predictors of adverse long-term outcome in acute myocardial infarction patients undergoing primary percutaneous transluminal coronary angioplasty: With special reference to the admission concentration of lipoprotein (a). Circulation Journal, 67(7), 605–611. [DOI] [PubMed] [Google Scholar]
- 94.Batalla AC, Julian JRR, Iglesias GC, Hevia SN, Braga SF, Fernandez EB, et al. (2000). Lipoprotein (a) as a predictor of severity of coronary artery stenosis documented by angiography in male coronary patients under 50 years old. Revista Española de Cardiología, 53(8), 1047–1051. [DOI] [PubMed] [Google Scholar]
- 95.Uusimaa P, Kervinen K, Kesaniemi A, & Peukurinen K (2002). Serum lipoprotein(a) level in relation to severity of coronary artery disease and coronary artery patency in acute myocardial infarction. Heart and Vessels, 16(2), 37–41. [DOI] [PubMed] [Google Scholar]
- 96.Zorio E, Falco C, Arnau MA, Espana F, Osa A, Ramon LA, et al. (2006). Lipoprotein (a) in young individuals as a marker of the presence of ischemic heart disease and the severity of coronary lesions. Haematologica, 91(4), 562–565. [PubMed] [Google Scholar]
- 97.Rifai N, Ma J, Sacks FM, Ridker PM, Hernandez WJ, Stampfer MJ, et al. (2004). Apolipoprotein(a) size and lipoprotein(a) concentration and future risk of angina pectoris with evidence of severe coronary atherosclerosis in men: The Physicians’ Health Study. Clinical Chemistry, 50(8), 1364–1371. [DOI] [PubMed] [Google Scholar]
- 98.Qasim AN, Martin SS, Mehta NN, Wolfe ML, Park J, Schwartz S, et al. (2010). Lipoprotein(a) is strongly associated with coronary artery calcification in type-2 diabetic women. International Journal of Cardiology, 150, 17–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Gazzaruso C, Garzaniti A, Falcone C, Geroldi D, Finardi G, & Fratino P (2001). Association of lipoprotein(a) levels and apolipoprotein(a) phenotypes with coronary artery disease in type 2 diabetic patients and in non- diabetic subjects. Diabetic Medicine, 18(7), 589–594. [DOI] [PubMed] [Google Scholar]
- 100.Koschinsky ML, & Marcovina SM (2003). The relationship between lipoprotein(a) and the complications of diabetes mellitus. Acta Diabetologica, 40(2), 65–76. [DOI] [PubMed] [Google Scholar]
- 101.Murase T, Okubo M, Amemiya-Kudo M, Ebara T, & Mori Y (2008). Impact of elevated serum lipoprotein (a) concentrations on the risk of coronary heart disease in patients with type 2 diabetes mellitus. Metabolism, 57(6), 791–795. [DOI] [PubMed] [Google Scholar]
- 102.Gazzaruso C, Bruno R, Pujia A, De Amici E, Fratino P, Solerte SB, et al. (2006). Lipoprotein(a), apolipoprotein(a) polymorphism and coronary atherosclerosis severity in type 2 diabetic patients. International Journal of Cardiology, 108(3), 354–358. [DOI] [PubMed] [Google Scholar]
- 103.Enas EA, Dhawan J, & Petkar S (1997). Coronary artery disease in Asian Indians: Lessons learnt and the role of lipoprotein(a). Indian Heart Journal, 49(1), 25–34. [PubMed] [Google Scholar]
- 104.Enas EA, Mohan V, Deepa M, Farooq S, Pazhoor S, & Chennikkara H (2007). The metabolic syndrome and dyslipidemia among Asian Indians: A population with high rates of diabetes and premature coronary artery disease. Journal of the Cardiometabolic Syndrome, 2(4), 267–275. [DOI] [PubMed] [Google Scholar]
- 105.Gupta R, Kastia S, Rastogi S, Kaul V, Nagar R, & Enas EA (2000). Lipoprotein(a) in coronary heart disease: A case-control study. Indian Heart Journal, 52(4), 407–410. [PubMed] [Google Scholar]
- 106.Mohan A, Srinivasan V, Deepa R, & Mohan V (2001). Lipoprotein (a): Role in diabetes and its vascular complications. Journal of the Association of Physicians of India, 49, 1100–1105. [PubMed] [Google Scholar]
- 107.Mohan V, Deepa R, Haranath SP, Premalatha G, Rema M, Sastry NG, et al. (1998). Lipoprotein(a) is an independent risk factor for coronary artery disease in NIDDM patients in South India. Diabetes Care, 21(11), 1819–1823. [DOI] [PubMed] [Google Scholar]
- 108.Velmurugan K, Deepa R, Ravikumar R, Lawrence JB, Anshoo H, Senthilvelmurugan M, et al. (2003). Relationship of lipoprotein(a) with intimal medial thickness of the carotid artery in type 2 diabetic patients in south India. Diabetic Medicine, 20(6), 455–461. [DOI] [PubMed] [Google Scholar]
- 109.Geethanjali FS, Jose VJ, & Kanagasabapathy AS (2002). Lipoprotein (a) phenotypes in south Indian patients with coronary artery disease. Indian Heart Journal, 54(1), 50–53. [PubMed] [Google Scholar]
- 110.Geethanjali FS, Luthra K, Lingenhel A, Kanagasaba-Pathy AS, Jacob J, Srivastava LM, et al. (2003). Analysis of the apo(a) size polymorphism in Asian Indian populations: Association with Lp(a) concentration and coronary heart disease. Atherosclerosis, 169(1), 121–130. [DOI] [PubMed] [Google Scholar]
- 111.Christopher R, Kailasanatha KM, Nagaraja D, & Tripathi M (1996). Case-control study of serum lipoprotein(a) and apolipoproteins A-I and B in stroke in the young. Acta Neurologica Scandinavica, 94(2), 127–130. [DOI] [PubMed] [Google Scholar]
- 112.Gupta R, Vasisht S, Bahl VK, & Wasir HS (1996). Correlation of lipoprotein(a) to angiographically defined coronary artery disease in Indians. International Journal of Cardiology, 57(3), 265–270. [DOI] [PubMed] [Google Scholar]
- 113.Ashfaq F, Goel PK, Sethi R, Khan MI, Ali W, & Idris MZ (2013). Lipoprotein (a) levels in relation to severity of coronary artery disease in North Indian patients. Heart Views, 14(1), 12–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Deepa R, Mohan A, Rema M, Haranath SP, Saravanan G, & Mohan V (2002). Lipoprotein(a) in South Indian type 2 diabetic subjects in relation to diabetic vascular complications. Journal of the Association of Physicians of India, 50(5), 657–661. [PubMed] [Google Scholar]
- 115.Gambhir JK, Kaur H, Gambhir DS, & Prabhu KM (2000). Lipoprotein(a) as an independent risk factor for coronary artery disease in patients below 40 years of age. Indian Heart Journal, 52(4), 411–415. [PubMed] [Google Scholar]
- 116.Singh S, Dwivedi S, Melkani GC, Rani C, Gaur SP, Mandal SK, et al. (1999). Lipoprotein(a) and coronary heart disease in Indian population. Journal of the Association of Physicians of India, 47(12), 1157–1160. [PubMed] [Google Scholar]
- 117.Angeline T, Aruna R, Ramadevi K, Mohan G, & Jeyaraj N (2003). Serum lipoprotein (a) and lipid profile in young South Indian patients with myocardial infarction. Indian Journal of Clinical Biochemistry, 18(1), 103–106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Bostom AG, Cupples LA, Jenner JL, Ordovas JM, Seman LJ, Wilson PW, et al. (1996). Elevated plasma lipoprotein(a) and coronary heart disease in men aged 55 years and younger. A prospective study. JAMA, 276(7), 544–548. [DOI] [PubMed] [Google Scholar]
- 119.Suk Danik J, Rifai N, Buring JE, & Ridker PM (2006). Lipoprotein(a), measured with an assay independent of apolipoprotein(a) isoform size, and risk of future cardiovascular events among initially healthy women. JAMA, 296(11), 1363–1370. [DOI] [PubMed] [Google Scholar]
- 120.Kousar R, Burns C, & Lewandowski P (2008). A culturally appropriate diet and lifestyle intervention can successfully treat the components of metabolic syndrome in female Pakistani immigrants residing in Melbourne, Australia. Metabolism, 57(11), 1502–1508. [DOI] [PubMed] [Google Scholar]
- 121.Martin M, Palaniappan LP, Kwan AC, Reaven GM, & Reaven PD (2008). Ethnic differences in the relationship between adiponectin and insulin sensitivity in South Asian and Caucasian women. Diabetes Care, 31(4), 798–801. [DOI] [PubMed] [Google Scholar]
- 122.Yajnik CS, Lubree HG, Rege SS, Naik SS, Deshpande JA, Deshpande SS, et al. (2002). Adiposity and hyperinsulinemia in Indians are present at birth. The Journal of Clinical Endocrinology and Metabolism, 87(12), 5575–5580. [DOI] [PubMed] [Google Scholar]
- 123.Moore SC, Gunter MJ, Daniel CR, Reddy KS, George PS, Yurgalevitch S, et al. (2012). Common genetic variants and central adiposity among Asian-Indians. Obesity, 20(9), 1902–1908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Motala AA, & Omar MA (1994). Evidence for impaired pancreatic beta cell function in South African Indians with impaired glucose tolerance. Diabetic Medicine, 11(5), 437–444. [DOI] [PubMed] [Google Scholar]
- 125.Petersen KF, Dufour S, Feng J, Befroy D, Dziura J, Dalla Man C, et al. (2006). Increased prevalence of insulin resistance and nonalcoholic fatty liver disease in Asian-Indian men. Proceedings of the National Academy of Sciences of the United States of America, 103(48), 18273–18277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.McKeigue PM, & Marmot MG (1988). Mortality from coronary heart disease in Asian communities in London. BMJ, 297(6653), 903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Singh N, & Gupta M (2005). Clinical characteristics of South Asian patients hospitalized with heart failure. Ethnicity and Disease, 15(4), 615–619. [PubMed] [Google Scholar]
- 128.Lubree HG, Rege SS, Bhat DS, Raut KN, Panchnadikar A, Joglekar CV, et al. (2002). Body fat and cardiovascular risk factors in Indian men in three geographical locations. Food and Nutrition Bulletin, 23(3 Suppl), 146–149. [PubMed] [Google Scholar]
- 129.Steering Committee. (2000). The Asia-Pacific perspective: Redefining obesity and its treatment. Retrieved August 20, 2013 from www.diabetes.com.au/research/report_obesity.htm
- 130.Misra A, Chowbey P, Makkar BM, Vikram NK, Wasir JS, Chadha D, et al. (2009). Consensus statement for diagnosis of obesity, abdominal obesity and the metabolic syndrome for Asian Indians and recommendations for physical activity, medical and surgical management. Journal of the Association of Physicians of India, 57, 163–170. [PubMed] [Google Scholar]
- 131.Misra A, Vikram NK, Gupta R, Pandey RM, Wasir JS, & Gupta VP (2006). Waist circumference cutoff points and action levels for Asian Indians for identification of abdominal obesity. International Journal of Obesity, 30(1), 106–111. [DOI] [PubMed] [Google Scholar]
- 132.Enas EA, Singh V, Munjal YP, Gupta R, Patel KC, Bhandari S, et al. (2009). Recommendations of the second Indo-U.S. health summit on prevention and control of cardiovascular disease among Asian Indians. Indian Heart Journal, 61(3), 265–274. [PubMed] [Google Scholar]
- 133.Alberti KG, Eckel RH, Grundy SM, Zimmet PZ, Cleeman JI, Donato KA, et al. (2009). Harmonizing the metabolic syndrome: A joint interim statement of the International Diabetes Federation Task Force on Epidemiology and Prevention; National Heart, Lung, and Blood Institute; American Heart Association; World Heart Federation; International Atherosclerosis Society; and International association for the Study of Obesity. Circulation, 120(16), 1640–1645. [DOI] [PubMed] [Google Scholar]
- 134.Huffman MD, Prabhakaran D, Osmond C, Fall CH, Tandon N, Lakshmy R, et al. (2011). Incidence of cardiovascular risk factors in an Indian urban cohort results from the new delhi birth cohort. Journal of the American College of Cardiology, 57(17), 1765–1774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Mente A, Razak F, Blankenberg S, Vuksan V, Davis AD, Miller R, et al. (2010). Ethnic variation in adiponectin and leptin levels and their association with adiposity and insulin resistance. Diabetes Care, 33(7), 1629–1634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Razak F, Anand SS, Shannon H, Vuksan V, Davis B, Jacobs R, et al. (2007). Defining obesity cut points in a multiethnic population. Circulation, 115(16), 2111–2118. [DOI] [PubMed] [Google Scholar]
- 137.Forouhi NG, Jenkinson G, Thomas EL, Mullick S, Mierisova S, Bhonsle U, et al. (1999). Relation of triglyceride stores in skeletal muscle cells to central obesity and insulin sensitivity in European and South Asian men. Diabetologia, 42(8), 932–935. [DOI] [PubMed] [Google Scholar]
- 138.Jafar TH, Chaturvedi N, & Pappas G (2006). Prevalence of overweight and obesity and their association with hypertension and diabetes mellitus in an Indo-Asian population. CMAJ, 175(9), 1071–1077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Lear SA, Toma M, Birmingham CL, & Frohlich JJ (2003). Modification of the relationship between simple anthropometric indices and risk factors by ethnic background. Metabolism: Clinical and Experimental, 52(10), 1295–1301. [DOI] [PubMed] [Google Scholar]
- 140.Lovegrove JA, Brady LM, Lesauvage SV, Lovegrove SS, Minihane AM, & Williams CM (2003). Lack of association between central adiposity and lipaemia in UK Sikh men. International Journal of Obesity and Related Metabolic Disorders, 27(11), 1373–1382. [DOI] [PubMed] [Google Scholar]
- 141.Unwin N, Harland J, White M, Bhopal R, Winocour P, Stephenson P, et al. (1997). Body mass index, waist circumference, waist-hip ratio, and glucose intolerance in Chinese and Europid adults in Newcastle, UK. Journal of Epidemiology and Community Health, 51(2), 160–166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Cappuccio FP, Oakeshott P, Strazzullo P, & Kerry SM (2002). Application of Framingham risk estimates to ethnic minorities in United Kingdom and implications for primary prevention of heart disease in general practice: Cross sectional population based study. BMJ, 325(7375), 1271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Graham I, Atar D, Borch-Johnsen K, Boysen G, & Durrington PN (2007). European guidelines on cardiovascular disease prevention in clinical practice: Executive summary: Fourth Joint Task Force of the European Society of Cardiology and Other Societies on Cardiovascular Disease Prevention in Clinical Practice (Constituted by representatives of nine societies and by invited experts). European Heart Journal, 28(19), 2375–2414. [DOI] [PubMed] [Google Scholar]
- 144.Grundy SM, Pasternak R, Greenland P, Smith S Jr., & Fuster V (1999). Assessment of cardiovascular risk by use of multiple-risk-factor assessment equations: A statement for healthcare professionals from the American Heart Association and the American College of Cardiology. Circulation, 100(13), 1481–1492. [DOI] [PubMed] [Google Scholar]
- 145.Szuszkiewicz-Garcia M, Li R, Grundy SM, Abate N, & Chandalia M (2012). Fat distribution and insulin resistance in young adult nonobese Asian Indian women. Metabolic Syndrome and Related Disorders, 10(5), 326–330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Kooner JS, Saleheen D, Sim X, Sehmi J, Zhang W, Frossard P, et al. (2011). Genome-wide association study in individuals of South Asian ancestry identifies six new type 2 diabetes susceptibility loci. Nature Genetics, 43(10), 984–989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Cho YS, Chen CH, Hu C, Long J, Ong RT, Sim X, et al. (2012). Meta-analysis of genome-wide association studies identifies eight new loci for type 2 diabetes in east Asians. Nature Genetics, 44(1), 67–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Rees SD, Hydrie MZ, Shera AS, Kumar S, O’Hare JP, Barnett AH, et al. (2011). Replication of 13 genome-wide association (GWA)-validated risk variants for type 2 diabetes in Pakistani populations. Diabetologia, 54(6), 1368–1374. [DOI] [PubMed] [Google Scholar]
- 149.Chauhan G, Spurgeon CJ, Tabassum R, Bhaskar S, Kulkarni SR, Mahajan A, et al. (2010). Impact of common variants of PPARG, KCNJ11, TCF7L2, SLC30A8, HHEX, CDKN2A, IGF2BP2, and CDKAL1 on the risk of type 2 diabetes in 5,164 Indians. Diabetes, 59(8), 2068–2074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Gujral UP, Pradeepa R, Weber MB, Narayan KM, & Mohan V (2013). Type 2 diabetes in South Asians: Similarities and differences with white Caucasian and other populations. Annals of the New York Academy of Sciences, 1281(1), 51–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Kahn SE (2003). The relative contributions of insulin resistance and beta-cell dysfunction to the pathophysiology of type 2 diabetes. Diabetologia, 46(1), 3–19. [DOI] [PubMed] [Google Scholar]
- 152.Yajnik CS, Janipalli CS, Bhaskar S, Kulkarni SR, Freathy RM, Prakash S, et al. (2009). FTO gene variants are strongly associated with type 2 diabetes in South Asian Indians. Diabetologia, 52(2), 247–252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Sanghera DK, Ortega L, Han S, Singh J, Ralhan SK, Wander GS, et al. (2008). Impact of nine common type 2 diabetes risk polymorphisms in Asian Indian Sikhs: PPARG2 (Pro12Ala), IGF2BP2, TCF7L2 and FTO variants confer a significant risk. BMC Medical Genetics, 9, 59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Ramya K, Radha V, Ghosh S, Majumder PP, & Mohan V (2011). Genetic variations in the FTO gene are associated with type 2 diabetes and obesity in south Indians (CURES-79). Diabetes Technology & Therapeutics, 13(1), 33–42. [DOI] [PubMed] [Google Scholar]
- 155.Frayling TM, Timpson NJ, Weedon MN, Zeggini E, Freathy RM, Lindgren CM, et al. (2007). A common variant in the FTO gene is associated with body mass index and predisposes to childhood and adult obesity. Science, 316(5826), 889–894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Wasim H, Al-Daghri NM, Chetty R, McTernan PG, Barnett AH, & Kumar S (2006). Relationship of serum adiponectin and resistin to glucose intolerance and fat topography in South-Asians. Cardiovascular Diabetology, 5(1), 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Vimaleswaran KS, Radha V, Ghosh S, Majumder PP, Deepa R, Babu HN, et al. (2005). Peroxisome proliferator-activated receptor-gamma co-activator-1alpha (PGC-1alpha) gene polymorphisms and their relationship to type 2 diabetes in Asian Indians. Diabetic Medicine, 22(11), 1516–1521. [DOI] [PubMed] [Google Scholar]
- 158.Liu X, Sun L, Li Z, Gao Y, & Hui R (2002). Relation of pentanucleotide repeat polymorphism of apolipoprotein (a) gene to plasma lipoprotein (a) level among Chinese patients with myocardial infarction and cerebral infarction. Zhonghua Yi Xue Za Zhi, 82(20), 1396–1400. [PubMed] [Google Scholar]
- 159.Orth-Gomer K, Mittleman MA, Schenck-Gustafsson K, Wamala SP, Eriksson M, Belkic K, et al. (1997). Lipoprotein(a) as a determinant of coronary heart disease in young women. Circulation, 95(2), 329–334. [DOI] [PubMed] [Google Scholar]
- 160.Enas EA (1998). Lipoprotein(a) as a determinant of coronary heart disease in young women: A stronger risk factor than diabetes? Circulation, 97(3), 293–295. [DOI] [PubMed] [Google Scholar]
- 161.Luke MM, Kane JP, Liu DM, Rowland CM, Shiffman D, Cassano J, et al. (2007). A polymorphism in the protease-like domain of apolipoprotein(a) is associated with severe coronary artery disease. Arteriosclerosis, Thrombosis, and Vascular Biology, 27(9), 2030–2036. [DOI] [PubMed] [Google Scholar]
- 162.Li Y, Luke MM, Shiffman D, & Devlin JJ (2011). Genetic variants in the apolipoprotein(a) gene and coronary heart disease. Circulation Cardiovascular Genetics, 4(5), 565–573. [DOI] [PubMed] [Google Scholar]
- 163.Schunkert H, Konig IR, Kathiresan S, Reilly MP, Assimes TL, Holm H, et al. (2011). Large-scale association analysis identifies 13 new susceptibility loci for coronary artery disease. Nature Genetics, 43(4), 333–338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Tregouet DA, Konig IR, Erdmann J, Munteanu A, Braund PS, Hall AS, et al. (2009). Genome-wide haplotype association study identifies the SLC22A3-LPAL2-LPA gene cluster as a risk locus for coronary artery disease. Nature Genetics, 41(3), 283–285. [DOI] [PubMed] [Google Scholar]
- 165.Bag A, Jyala NS, & Bag N (2012). Indian studies on genetic polymorphisms and cancer risk. Indian Journal of Cancer, 49(1), 144–162. [DOI] [PubMed] [Google Scholar]
- 166.Singh M, Shah PP, Singh AP, Ruwali M, Mathur N, Pant MC, et al. (2008). Association of genetic polymorphisms in glutathione S-transferases and susceptibility to head and neck cancer. Mutation Research, 638(1–2), 184–194. [DOI] [PubMed] [Google Scholar]
- 167.Srivastava DS, Mishra DK, Mandhani A, Mittal B, Kumar A, & Mittal RD (2005). Association of genetic polymorphism of glutathione S-transferase M1, T1, P1 and susceptibility to bladder cancer. European Urology, 48(2), 339–344. [DOI] [PubMed] [Google Scholar]
- 168.Soya SS, Vinod T, Reddy KS, Gopalakrishnan S, & Adithan C (2007). Genetic polymorphisms of glutathione-S-transferase genes (GSTM1, GSTT1 and GSTP1) and upper aerodigestive tract cancer risk among smokers, tobacco chewers and alcoholics in an Indian population. European Journal of Cancer, 43(18), 2698–2706. [DOI] [PubMed] [Google Scholar]
- 169.Mittal RD, & Srivastava DS (2005). A M, B M: Genetic polymorphism of drug metabolizing enzymes (CYP2E1, GSTP1) and susceptibility to bladder cancer in North India. Asian Pacific Journal of Cancer Prevention, 6(1), 6–9. [PubMed] [Google Scholar]
- 170.Tan YC, Zeigler-Johnson C, Mittal RD, Mandhani A, Mital B, Rebbeck TR, et al. (2008). Common 8q24 sequence variations are associated with Asian Indian advanced prostate cancer risk. Cancer Epidemiology, Biomarkers and Prevention, 17(9), 2431–2435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Zheng Y, & Lamoureux EL (2012). Chiang P-CP, Anuar AR, Ding J, Wang JJ, Mitchell P, Tai ES, Wong TY: Language barrier and its relationship to diabetes and diabetic retinopathy. BMC Public Health, 12(1), 781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Chauhan G, Tabassum R, Mahajan A, Dwivedi OP, Mahendran Y, Kaur I, et al. (2011). Common variants of FTO and the risk of obesity and type 2 diabetes in Indians. Journal of Human Genetics, 56(10), 720–726. [DOI] [PubMed] [Google Scholar]
- 173.Indulekha K, Anjana RM, Surendar J, & Mohan V (2011). Association of visceral and subcutaneous fat with glucose intolerance, insulin resistance, adipocytokines and inflammatory markers in Asian Indians (CURES-113). Clinical Biochemistry, 44(21219897), 281–287. [DOI] [PubMed] [Google Scholar]
- 174.Mohan V, Deepa R, Velmurugan K, & Premalatha G (2005). Association of C-reactive protein with body fat, diabetes and coronary artery disease in Asian Indians: The Chennai Urban Rural Epidemiology Study (CURES-6). Diabetic Medicine, 22(7), 863–870. [DOI] [PubMed] [Google Scholar]
- 175.Pradhan AD, Manson JE, Rifai N, Buring JE, & Ridker PM (2001). C-reactive protein, interleukin 6, and risk of developing type 2 diabetes mellitus. JAMA, 286(11466099), 327–334. [DOI] [PubMed] [Google Scholar]
- 176.Abate N, Chandalia M, Snell PG, & Grundy SM (2004). Adipose tissue metabolites and insulin resistance in nondiabetic Asian Indian men. Journal of Clinical Endocrinology and Metabolism, 89(6), 2750–2755. [DOI] [PubMed] [Google Scholar]
- 177.Forouhi NG, Sattar N, & McKeigue PM (2001). Relation of C-reactive protein to body fat distribution and features of the metabolic syndrome in Europeans and South Asians. International Journal of Obesity and Related Metabolic Disorders, 25(9), 1327–1331. [DOI] [PubMed] [Google Scholar]
- 178.Ramachandran A, Chamukuttan S, Shetty SA, Arun N, & Susairaj P (2012). Obesity in Asia–is it different from rest of the world. Diabetes/Metabolism Research and Reviews, 28(Suppl 2), 47–51. [DOI] [PubMed] [Google Scholar]
- 179.Pou KM, Massaro JM, Hoffmann U, Vasan RS, Maurovich-Horvat P, Larson MG, et al. (2007). Visceral and sub-cutaneous adipose tissue volumes are cross-sectionally related to markers of inflammation and oxidative stress: The Framingham Heart Study. Circulation, 116(11), 1234–1241. [DOI] [PubMed] [Google Scholar]
- 180.Lilja M, Rolandsson O, Shaw JE, Pauvaday V, Cameron AJ, Tuomilehto J, et al. (2010). Higher leptin levels in Asian Indians than Creoles and Europids: A potential explanation for increased metabolic risk. International Journal of Obesity, 34(5), 878–885. [DOI] [PubMed] [Google Scholar]
- 181.Soderberg S, Zimmet P, Tuomilehto J, Chitson P, Gareeboo H, Alberti KG, et al. (2007). Leptin predicts the development of diabetes in Mauritian men, but not women: A population-based study. International Journal of Obesity, 31(7), 1126–1133. [DOI] [PubMed] [Google Scholar]
- 182.Shah A, Hernandez A, Mathur D, Budoff MJ, & Kanaya AM (2012). Adipokines and body fat composition in South Asians: Results of the Metabolic Syndrome and Atherosclerosis in South Asians Living in America (MASALA) study. International Journal of Obesity, 36(6), 810–816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Chambers JC, Eda S, Bassett P, Karim Y, Thompson SG, Gallimore JR, et al. (2001). C-reactive protein, insulin resistance, central obesity, and coronary heart disease risk in Indian Asians from the United Kingdom compared with European whites. Circulation, 104(2), 145–150. [DOI] [PubMed] [Google Scholar]
- 184.Ramachandran A, Snehalatha C, Vijay V, Satyavani K, Latha E, & Haffner SM (1997). Plasma leptin in non-diabetic Asian Indians: Association with abdominal adiposity. Diabetic Medicine, 14(11), 937–941. [DOI] [PubMed] [Google Scholar]
- 185.Deepa R, Velmurugan K, Arvind K, Sivaram P, Sientay C, Uday S, et al. (2006). Serum levels of interleukin 6, C-reactive protein, vascular cell adhesion molecule 1, and monocyte chemo-tactic protein 1 in relation to insulin resistance and glucose intolerance–the Chennai Urban Rural Epidemiology Study (CURES). Metabolism, 55(9), 1232–1238. [DOI] [PubMed] [Google Scholar]
- 186.Liew CF, Seah ES, Yeo KP, Lee KO, & Wise SD (2003). Lean, nondiabetic Asian Indians have decreased insulin sensitivity and insulin clearance, and raised leptin compared to Caucasians and Chinese subjects. International Journal of Obesity and Related Metabolic Disorders, 27(7), 784–789. [DOI] [PubMed] [Google Scholar]
- 187.Raji A, Gerhard-Herman MD, Warren M, Silverman SG, Raptopoulos V, Mantzoros CS, et al. (2004). Insulin resistance and vascular dysfunction in nondiabetic Asian Indians. The Journal of Clinical Endocrinology and Metabolism, 89(8), 3965–3972. [DOI] [PubMed] [Google Scholar]
- 188.Snehalatha C, Mukesh B, Simon M, Viswanathan V, Haffner SM, & Ramachandran A (2003). Plasma adiponectin is an independent predictor of type 2 diabetes in Asian indians. Diabetes Care, 26(12), 3226–3229. [DOI] [PubMed] [Google Scholar]
- 189.Bhargava SK, Sachdev HS, Fall CH, Osmond C, Lakshmy R, Barker DJ, et al. (2004). Relation of serial changes in childhood body-mass index to impaired glucose tolerance in young adulthood. New England Journal of Medicine, 350(9), 865–875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Chambers JC, McGregor A, Jean-Marie J, & Kooner JS (1999). Abnormalities of vascular endothelial function may contribute to increased coronary heart disease risk in UK Indian Asians. Heart, 81(5), 501–504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Chambers JC, Obeid OA, Refsum H, Ueland P, Hackett D, Hooper J, et al. (2000). Plasma homocysteine concentrations and risk of coronary heart disease in UK Indian Asian and European men. The Lancet, 355(9203), 523–527. [DOI] [PubMed] [Google Scholar]
- 192.Chandalia M, Cabo-Chan AV Jr., Devaraj S, Jialal I, Grundy SM, & Abate N (2003). Elevated plasma high-sensitivity C-reactive protein concentrations in Asian Indians living in the United States. Journal of Clinical Endocrinology and Metabolism, 88(8), 3773–3776. [DOI] [PubMed] [Google Scholar]
- 193.Eapen D, Kalra GL, Merchant N, Arora A, & Khan BV (2009). Metabolic syndrome and cardiovascular disease in South Asians. Vascular Health and Risk Management, 5, 731–743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Gama R, Elfatih AB, & Anderson NR (2002). Ethnic differences in total and HDL cholesterol concentrations: Caucasians compared with predominantly Punjabi Sikh Indo-Asians. Annals of Clinical Biochemistry, 39(Pt 6), 609–611. [DOI] [PubMed] [Google Scholar]
- 195.Misra A, Luthra K, & Vikram NK (2004). Dyslipidemia in Asian Indians: Determinants and significance. Journal of the Association of Physicians of India, 52, 137–142. [PubMed] [Google Scholar]
- 196.Kulkarni KR, Markovitz JH, Nanda NC, & Segrest JP (1999). Increased prevalence of smaller and denser LDL particles in Asian Indians. Arteriosclerosis, Thrombosis, and Vascular Biology, 19(11), 2749–2755. [DOI] [PubMed] [Google Scholar]
- 197.Huang JB, Liang J, Zhao XF, Wu WS, & Zhang F (2013). Epigenetics: Novel mechanism of pulmonary hypertension. Lung, 191(6), 601–610. [DOI] [PubMed] [Google Scholar]
- 198.Flowers E, & Aouizerat BE (2013). MicroRNA Associated with Dyslipidemia and Coronary Disease in Humans. Physiological Genomics, 45, 1199–1205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Flowers E, Froelicher ES, & Aouizerat BE (2013). Measurement of microRNA: A regulator of gene expression. Biological Research For Nursing, 15(2), 167–178. [DOI] [PubMed] [Google Scholar]
- 200.Flowers E, Froelicher ES, & Aouizerat BE (2013). Micro-RNA regulation of lipid metabolism. Metabolism, 62(1), 12–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Papageorgiou N, Tousoulis D, Androulakis E, Siasos G, Briasoulis A, Vogiatzi G, et al. (2012). The role of microRNAs in cardiovascular disease. Current Medicinal Chemistry, 19(16), 2605–2610. [DOI] [PubMed] [Google Scholar]
- 202.Flowers E (2012). Unique cardiovascular risk profiles in US South Asians: From epidemiology to epigenetic biomarker discovery. Ann Arbor: University of California San Francisco. [Google Scholar]
- 203.Flowers E, Singh K, Molina C, Mathur A, Froelicher ES, & Aouizerat BE (2012). Abstract 11404: MicroRNA associated with atherogenic dyslipidemia in South Asian men. Circulation, 126, A11404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.European Commission Community Research and Development Information Service (CORDIS). Final report summary - EPI-MIGRANT (identification of epigenetic markers underlying increased risk of T2D in South Asians). https://cordis.europa.eu/result/rcn/164149_en.html. Accessed 6 June 2018.
- 205.Maritim AC, Sanders RA, & Watkins JB 3rd. (2003). Diabetes, oxidative stress, and antioxidants: A review. Journal of Biochemical and Molecular Toxicology, 17(1), 24–38. [DOI] [PubMed] [Google Scholar]