Abstract
Behavioral states such as arousal and attention have profound effects on sensory processing, determining how – even whether – a stimulus is perceived. This state-dependence is believed to arise, at least in part, in response to inputs from subcortical structures that release neuromodulators such as acetylcholine, often non-synaptically. The mechanisms that underlie the interaction between these non-synaptic signals and the more point-to-point synaptic cortical circuitry are not well understood. This review highlights the state of the field, with a focus on cholinergic action in early visual processing. Key anatomical and physiological features of both the cholinergic and the visual systems are discussed. Furthermore, presenting evidence of cholinergic modulation in visual thalamus and primary visual cortex, we explore potential functional roles of acetylcholine and its effects on the processing of visual input over the sleep-wake cycle, sensory gain control during wakefulness, and consider evidence for cholinergic support of visual attention.
Keywords: LGN, V1, visual cortex, neuromodulation, primate, macaque
Graphical Abstract

The cholinergic and visual systems intersect at the level of the thalamus and the cortex, but the source of cholinergic modulation to these structures differs. In reviewing the literature regarding the structure and function of cholinergic modulation in early vision, interesting questions emerge regarding this dual-source modulation.
The processing state of cortical circuits is dynamically controlled by brain areas whose activity reflects cognitive and behavioral variables. In vision, these non-visual inputs can determine whether and how visual information is processed. This state-dependence of visual processing arises, at least in part, from the influence upon cortex of inputs from subcortical neuromodulatory systems, including the cholinergic system. In the visual system, nearly all information about the visual world passes through the lateral geniculate nucleus of the thalamus (LGN) and the primary visual cortex (V1). Modifying activity in this early, and largely obligatory, circuit is thus a powerful means for altering the outcome of all subsequent processing of visual information. The cholinergic system interacts with the visual system at this crucial point in the pathway; that interaction is the focus of our review.
We begin by describing the known anatomical features of both the cholinergic and visual systems and their intersection at the levels of the LGN and V1. One notable contrast between these two brain regions is that they receive cholinergic input from different sources: the LGN being principally innervated by brainstem cholinergic structures and V1 by the basal forebrain. The functional consequences of this arrangement remain to be determined, but we will explore ideas towards the end of the review. Along the way, we highlight seminal physiological and behavioral studies looking at cholinergic modulation of information processing in early vision. We further consider the different roles acetylcholine (ACh) plays in LGN versus V1 and how they might affect thalamocortical interactions and visual processing more broadly, taking the examples of sleep-wake state control and sensory gating as models.
Key anatomical and pharmacological features of the cholinergic system
The cholinergic system is a major neuromodulatory network in the brain implicated in a variety of brain functions. It has widespread projection patterns and features two classes of receptors. Muscarinic acetylcholine receptors (mAChRs) are G protein-coupled (metabotropic) and can interact directly with ion channels (Bernheim et al., 1991) or act via second messenger cascades. The five genetic subtypes of mAChR - categorized by the G protein to which they are coupled - are subtypes m2 and m4, which are Gi/o-coupled, and subtypes m1, m3, and m5, which are Gq-coupled. mAChRs are commonly classified by their affinity for certain exogenous ligands; these pharmacological classes are traditionally denoted by a capital ‘M’ (e.g. M1, M2), while the genetic types are referred to with the lower case ‘m’. We will observe this convention throughout our review. The M2 pharmacological class comprises the m2 and m4 genetic types and is characterized by insensitivity to the antagonist pirenzepine. Receptors in the M1 pharmacological class (m1, m3, and m5) can all be blocked by pirenzepine, but vary in their affinity for that ligand.
Nicotinic receptors (nAChRs), on the other hand, are ligand-gated cation channels (ionotropic) usually classified by their affinity for the exogenous ligand nicotine. In the central nervous system of mammals, there are two classes of nAChRs: homopentameric receptors comprising five α7 subunits with low affinity for nicotine and heteropentameric receptors of diverse subunit composition with high affinity for nicotine. In general, low-affinity nAChRs desensitize more rapidly than do high-affinity nAChRs (McGehee and Role, 1995; Dani and Bertrand, 2007). Depending on species and brain area, both mAChRs and nAChRs can be found on both excitatory and inhibitory neurons (see below).
Cholinergic neurons synthesize ACh from choline, brought across the membrane by the choline transporter and then catalyzed by the enzyme choline acetyltransferase (ChAT). ChAT is the primary anatomical marker for cholinergic neurons as it is strongly expressed throughout the cytoplasm. Degradation of ACh is accomplished by acetylcholinesterase, which is expressed by both cholinergic and non-cholinergic neurons (Mesulam et al., 1984). In most circuits in the central nervous system, ACh is disseminated, at least in part, by volume transmission; a signaling mode in which release sites are not apposed to receptive membrane specializations, and/or the signaling molecules routinely escape the confines of a classical synapse [for discussions of volume transmission see (Fuxe and Agnati, 1991; Aoki and Kabak, 1992; Umbriaco et al., 1994; Mrzljak et al., 1995), for synaptic transmission see (Turrini et al., 2001)].
Traditionally, the cholinergic system has been described as providing a ‘diffuse and loosely topographic’ innervation of the cortex and, as a result, many conceptualizations of cholinergic modulatory effects assume that the release of ACh is likely to be uniform over relatively large swaths of cortex. It has, however, been shown that the cholinergic innervation of cortex has a higher degree of topographic specificity than previously appreciated (Pearson et al., 1983; Price and Stern, 1983; Zaborszky et al., 2015). Furthermore, the existence of modulatory compartments in cortex has been proposed, again challenging the conceptualization of cholinergic signaling as necessarily non-specific (Coppola et al., 2016). This latter idea is anchored in the claim that - regardless of projection topography - factors such as receptor expression, local differences in the capacity for ACh synthesis and degradation, and the structure and composition of the extracellular space yield local circuit motifs within which the cholinergic system can accomplish exquisite signaling precision.
Cholinergic pathways have been studied both structurally and functionally since the early 1950s. First identified in rodents (Shute and Lewis, 1963) and cats (Krnjevic and Silver, 1965), substantial progress has been made in characterizing the two major ascending brainstem cholinergic pathways, dorsal and ventral, innervating the thalamus and the basal forebrain respectively (Dale et al., 1967; Jones, 1993; Wainer et al., 1993). Cholinergic inputs to both the sensory thalamus and basal forebrain arise in the brainstem (Figure 1a), specifically in the pontomesencephalic tegmental complex, which includes the laterodorsal tegmental nucleus and pedunculopontine nucleus [rodents (Woolf and Butcher, 1986; Hallanger et al., 1987; Jones and Cuello, 1989) and cats/primates (Jones and Webster, 1988; Pare et al., 1988; Smith et al., 1988; Steriade et al., 1988)]. Various basal forebrain nuclei then provide cholinergic input to other forebrain structures; of interest in this review is the nucleus basalis, which provides cholinergic input to cortex [rodents (Lehmann et al., 1980; Bigl et al., 1982) and primates/humans (Kievit and Kuypers, 1975; Mesulam and Van Hoesen, 1976; Mesulam et al., 1983)] including visual cortex (Figure 1a). Interesting here is a profound species difference in the cellular composition of the basal forebrain. In rodents, only a small percentage of basal forebrain neurons are cholinergic [~5%; (Gritti et al., 1997; Gritti et al., 2006)]. This contrasts greatly with human and non-human primates in which, for example, at least 90% of neurons in the nucleus basalis are cholinergic (Mesulam et al., 1983; Raghanti et al., 2008; reviewed by Coppola and Disney, 2018).
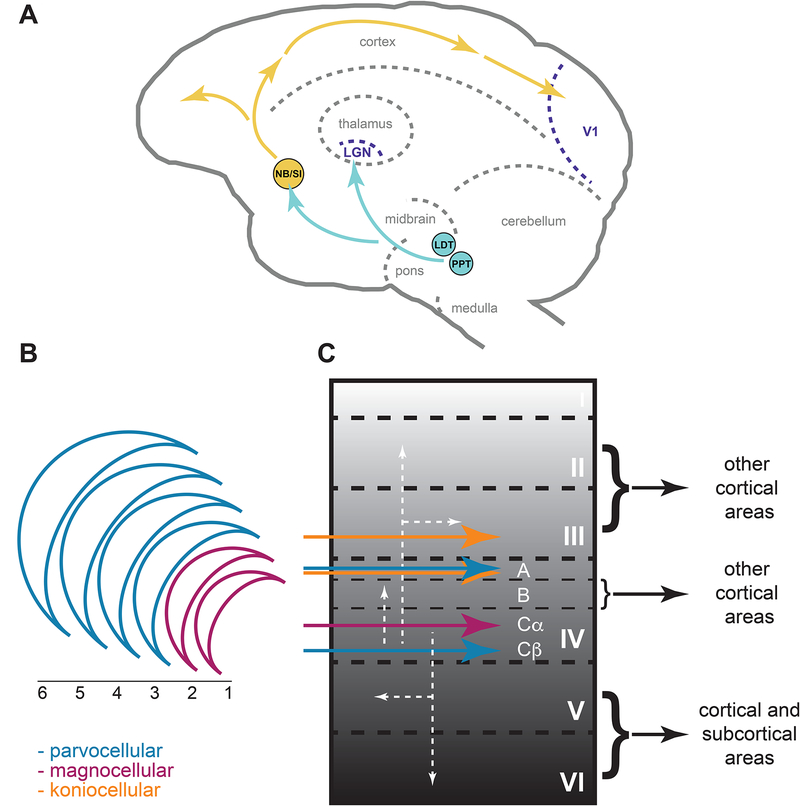
Figure 1:
(A) Schematic depiction of cholinergic innervation of primate visual thalamus and visual cortex. The lateral geniculate nucleus (LGN) receives cholinergic projections from the pontomesencephalic tegmental complex, including the laterodorsal tegmental nucleus (LTD) and the pedunculopontine nucleus (PPT), while the primary visual cortex (V1) receives cholinergic projections from the nucleus basalis (NB) and substantia innominata (SI) of the basal forebrain. (B) LGN of old world monkeys and apes is made up of six layers. Layers 1 and 2 are part of the magnocellular (M) pathway (magenta), while layers 3 through 6 belong to the parvocellular (P) pathway (blue). Koniocellular layers (orange, not shown) are situated in between M and P layers. (C) The laminar and sublaminar structure of V1 and the mapping of LGN projections (colored arrows) are shown. Projections within V1 are denoted by the white arrows and projections from V1 are denoted by the black arrows.
Key anatomical features of the early visual system in primates
Most visual information travels from the retina, along the optic nerve, to the LGN (and other subcortical structures such as the superior colliculus), and then to V1 (also known as the striate cortex), before being distributed through various ‘extrastriate’ visual cortical areas [V2, V3, V3A, V4, etc; for reviews of the visual pathway see for example (Hendry and Reid, 2000; Boyd et al., 2009; Jeffries et al., 2014; Usrey and Alitto, 2015; Weyand, 2016)]. Much of what we know about this circuitry arises from the work of Vivien Casagrande [for example see (Lachica et al., 1992; Casagrande and Kaas, 1994; Xu et al., 2004)].
In old world monkeys and apes (including humans), the LGN is made up of six layers that receive different feedforward inputs from the retina (Figure 1b) and also receive feedback projections from V1. LGN layers 2, 3, and 5 receive signals from the ipsilateral eye, and layers 1, 4, and 6 receive signals from the contralateral eye. Driven by distinct classes of retinal ganglion cells, LGN layers subserve the magnocellular (M; layers 1 and 2), parvocellular (P; layers 3 through 6), or koniocellular (K) visual processing pathways. The M pathway is thought to be responsible for supporting low contrast vision and motion processing, while the P pathway is involved in color processing and high acuity vision. The K layers are situated in between M and P layers and represent a third visual pathway, extensively studied by Casagrande and colleagues [for reviews see (Casagrande, 1994; Casagrande and Xu, 2004)] and hypothesized to contribute to color vision.
V1, like other neocortical areas, broadly comprises six layers although it has a complex sub-laminar structure that is unique (Figure 1c). The M layers of the LGN preferentially terminate in the upper portion of layer IVc (IVcα), while the P layers preferentially innervate the lower portion of layer IVc (IVcβ) and layer IVa. The K layers make terminal fields in the cytochrome oxidase (CO)-rich “blobs” of layer III, in layer IVa (Figure 1b and 1c), and in layer I. Notable here is that, in primates, the inputs to layer IVc are still segregated by eye (as they are in the LGN), giving rise to ocular dominance columns, which constitute a key anatomical (and functional) feature of V1. From layer IVc, information flows on to layer IVb, the supra-, and then the infra-granular layers. It is within this circuitry that signals from the eyes are integrated, yielding binocular receptive fields. From layers II, III, and IVb arise projections to other cortical areas, while layers V and VI project both cortico-cortically and back to subcortical structures, including the LGN (from layer VI).
The CO-rich blobs in layers II and III are a prominent anatomical characteristic of V1. Neurons in these blobs receive inputs from the K layers of the LGN and from layer IVc of V1, make lateral connections within the supragranular layers, and also make selective projections to functional subdivisions known as ‘thin stripes’ (also defined by CO histochemistry) of the second visual cortical area, extrastriate area V2 (Lund, 1988; Callaway, 2004; Sincich and Horton, 2005; Boyd et al., 2009). Neurons in the ‘interblob’ regions in V1 connect with ‘pale CO stripes’ in V2 (Callaway, 1998), while layer IVb of V1 projects to the ‘thick CO stripes’ (Van Essen and Deyoe, 1995; Callaway, 1998).
The intersection of the cholinergic and early visual systems
Both LGN and V1 receive cholinergic projections. Indeed, it has long been known that these structures are strongly influenced by the differences in cholinergic tone that are observed across the sleep-wake cycle (Evarts et al., 1962; Amzica and Steriade, 1996; Jimenez-Capdeville and Dykes, 1996). Interestingly, the source of the afferents providing this cholinergic innervation differs; while LGN receives cholinergic input from brainstem nuclei, V1 is innervated by the basal forebrain.
The LGN shows a significant degree of state-dependence in its processing of visual information, and the cholinergic system appears to play a crucial role in dynamically determining LGN functional states (see below). In cats and primates, cholinergic axons from the brainstem densely innervate the LGN [and other thalamic nuclei; (Pare et al., 1988; Smith et al., 1988; Zou et al., 2009)]. Electron microscopic data from cats reveal that these cholinergic fibers form synapses with both the excitatory LGN neurons that project to V1 (also known as relay cells) and local circuit LGN inhibitory interneurons (Dolabela de Lima et al., 1985; Sherman and Koch, 1986). Dense ChAT staining has also been observed in LGN of human and non-human primates (Hirai and Jones, 1989; McDonald et al., 1993; Bickford et al., 2000).
Studies of cholinergic receptor distribution in the LGN are scarce and mostly undertaken in rodent or cat models. We will focus on the admittedly sparse data from primates, as species differences have been identified in this domain. For data on receptor expression in the LGN of rodents and cats see (Shaw and Cynader, 1986; Plummer et al., 1999). In primates, autoradiographic binding studies (which do not allow for precise receptor localization or subtype differentiation) indicate a broad distribution of mAChRs across all six LGN laminae (Figure 2a top panel), with a tendency for greater ligand binding in the lateral portion of the nucleus (Shaw and Cynader, 1986). Studies utilizing immunolabeling of specific mAChR subtypes are needed to delineate the anatomical substrates of cholinergic action on primate LGN neurons.
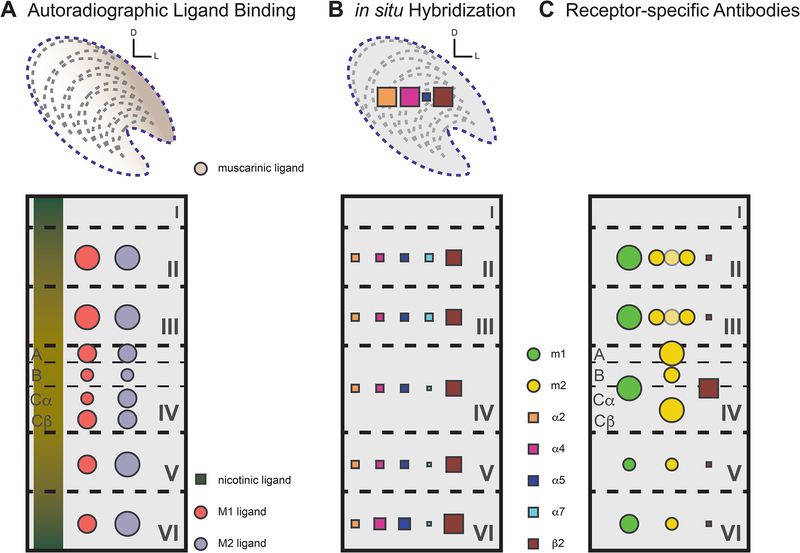
Figure 2:
Cholinergic receptor distribution in lateral geniculate nucleus (LGN) and primary visual cortex (V1) in nonhuman primates (unless noted otherwise) separated by methodology. Size of symbol indicates relative density of the receptor. (A) Top panel: Muscarinic binding sites are present throughout LGN with higher binding in the lateral portion. Bottom panel: Nicotinic binding sites are denser in supra- and infragranular layers in V1 while muscarinic binding sites are fairly uniform across cortex with slight variations in the subdivisions of layer IV (cortex data from humans). (B) Top panel: mRNA copies of α2, α4, α5, and β2 are present in LGN. Localization by layers is unknown. Bottom panel: Distribution of mRNA for different muscarinic and nicotinic receptor subtypes in V1. β2 mRNA is remarkably dense in layer IV, while all other types tend to be most strongly expressed in the deeper layers, specifically layer VI. Note: data is derived from a study looking at the entire cortex, not just specifically V1. (C) Laminar patterns of mRNA copy detection and receptor protein expression are not perfectly matched, but receptor expression across laminae mimics in situ data from (B), particularly in layer IV (m2 was densest in layer IVa and IVc). m2 labeling also showed patches of intermittent light and dark staining in layers II and III overlapping with the CO-rich blobs (data not shown). Data adapted from Shaw & Cynader 1986 (A, top panel); Eickhoff et al 2007 (A, bottom panel); Han et al 2000 (B); and Tigges et al 1997, Disney et al 2006, 2007, and Disney & Aoki 2008 (C).
Investigating nAChRs has been complicated by the lack of antibodies directed against nAChR subunits that are both sensitive and selective. However, in situ hybridization assays have been conducted and reveal differences between primate species. A study in squirrel monkeys found moderate expression of α4 and α7 subunit mRNA, low levels for β2, and very high levels of β4 (Quik et al., 2000), while a study in macaques (Figure 2b top panel) detected very strong α2, α4, and β2 levels and weak levels of α5 mRNAs in the LGN (Han et al., 2000).
In humans (as in non-human primates) mAChRs are abundantly expressed throughout the brain, while nAChRs are comparatively scarce (Paterson and Nordberg, 2000). However, ligand-binding studies have shown that the human thalamus boasts high levels of nAChR expression in comparison to the rest of the brain, and the LGN specifically is one of the thalamic nuclei with the highest nAChR density (Adem et al., 1988; Spurden et al., 1997).
In contrast to the state of affairs with respect to the LGN, cortical acetylcholine receptor (AChR) expression has been more thoroughly studied in primates than in other species, although knowledge gaps still exist. At the level of cortical layers and visual pathways, the two most strongly-expressed mAChR subtypes (m1 and m2) have distinct patterns of expression. Tigges and colleagues (1997) reported that m1 AChRs were found most often in somata or proximal dendrites, and were most densely expressed in layers II and III followed by a small dip in layer VI and weaker expression in layer V. m2 AChRs, on the other hand, were densest in layer IV specifically the parvocellular subdivisions IVa and IVcβ, followed by alternating strong and weak expression in layers II and III (Figure 2c) complementing the CO-rich blobs. Examination at higher magnification revealed this m2-immunoreactivity to be punctate, labeling fine granular structures in the neuropil and short strands of processes. Fascinatingly, expression of the m2 AChR is specifically associated with the parvocellular visual pathway in V1 (Mrzljak et al., 1996; Disney et al., 2007) and pharmacological manipulation of cholinergic signaling has a profound impact on the McCollough effect [an orientation-dependent color after-effect; (Byth et al., 2000)].
At the level of cellular populations, the majority of mAChRs are found on inhibitory neurons. Fewer than 10% of excitatory neurons in V1 express m1 or m2 mAChRs, while 61% of γ-aminobutyric acid-containing (GABAergic) interneurons express m1 AChRs and 28% express m2 AChRs (Disney et al., 2006). Furthermore, specific subclasses of interneurons show differential patterns, for example: m1 AChRs are expressed by 87% of parvalbumin-expressing interneurons, 60% of calbindin-expressing interneurons, and 40% of calretinin-expressing interneurons in macaque V1 (Disney and Aoki, 2008). Yet again, we find differences in these patterns when we compare across species; while the vast majority of parvalbumin-expressing neurons in both macaques and humans express m1 AChRs, fewer than 25% of these neurons in rats express the same receptor (Disney and Reynolds, 2014). At the electron microscopic level, m1 and m2 AChRs have been reported as a small population of both excitatory and inhibitory synapses in primates (Mrzljak et al., 1993; Disney et al., 2007; 2012).
Few immunohistochemical studies of receptor localization have been undertaken in humans, more commonly autoradiographic labeling is used and shows that the M1 pharmacological class of mAChRs is relatively densely expressed in layers II – IVa of V1, expression of the M2 class is fairly uniformly high with a small dip in density in layer IVb [Figure 2a bottom panel; (Eickhoff et al., 2007)].
nAChRs are differentially expressed across laminae in V1 and (as was the case in studies of LGN) in situ hybridization studies are the main source of evidence regarding nAChR expression patterns. Distributions of the various α and β subunits overlap across the brain in a manner that gives rise to multiple possible nAChR combinations. The only study to date looking at laminar distribution of nAChR mRNA in the cortex, but not specifically V1, of macaques (Figure 2b bottom panel) reported that there were no differences between cortical areas and that β2 nAChR subunit mRNA is densely expressed in all layers of cortex, with layer VI showing the highest expression (Han et al., 2000). Interestingly, these data are not reflected at the protein and functional levels, at least in V1; by antibody-based detection, the β2 subunit is only expressed by a small population of GABAergic interneurons across the cortical layers, and is strongly expressed by the axons of LGN relay cells arriving in layer IVc. Furthermore, the physiological effects of delivering nicotine in V1 are also strongest in layer IVc (Disney et al., 2007). By in situ hybridization, layer VI was also reported by Han and colleagues (2000) to have the highest level of expression for the α4 and α5 nAChR subunits, while it was layers II and III that were densest in their expression of the α7 subunit [levels actually being barely detectable in other layers; (Han et al., 2000)].
Autoradiographic data shows that in humans, while nAChR expression is weak outside the thalamus, it is present. There is a low-level, broad distribution of nicotine binding sites across all layers of V1, with slightly higher binding in the supra- and infragranular layers [Figure 2a bottom panel; (Eickhoff et al., 2007)]. Again, this conflicts with protein-level data from non-human primates. Whether this is a genuine species difference or is due to different detection methods is not known. Altogether, while much anatomical research remains to be done, it is clear that LGN and V1 are rich with cholinergic innervation and have unique distributions of cholinergic receptors on both inhibitory and excitatory neurons, allowing for diverse cholinergic action during visual processing.
Cholinergic modulation of visual processing in thalamocortical circuits
Cholinergic effects during the sleep-wake cycle are evident in thalamus and cortex
The cholinergic system is involved in the sleep-wake cycle (Pepeu and Mantegazzini, 1964; Phillis and Chong, 1965; Shouse and Siegel, 1992). Cholinergic tone appears to be stereotyped during different states of arousal such that cholinergic neurons in both the brainstem and basal forebrain discharge strongly during periods of waking, decrease their activity during slow-wave (SWS or non-rapid-eye-movement (REM) sleep, and increase their firing again during REM sleep (Jones, 2005). Reflecting these activity levels in the innervating modulatory structures, both thalamus and cortex have characteristic activity patterns that correlate with the organism’s state of arousal. During waking, fast rhythms and sustained depolarization dominate oscillatory brain activity, as assessed globally by electroencephalogram (EEG). Non-REM sleep, on the other hand, is characterized by low-frequency EEG oscillations and sustained hyperpolarization. Interestingly, REM sleep appears to constitute an intermediate state with periods of high-frequency oscillations (similar to waking), interleaved with periods of SWS-like slow oscillations (Steriade, 1993; 2004).
Neuronal activity in the thalamus shows two modes of discharge across the sleep-wake cycle: a bursting mode during SWS, and tonic firing during REM sleep and wakefulness (McCormick and Bal, 1997). The discharge mode of LGN relay cells is dependent on voltage-gated T-type calcium (Ca2+) channels (Sherman, 2001; 2005). During tonic mode, an LGN neuron is relatively depolarized and action potential firing reflects the intensity of the input arriving from the retina. Burst mode, on the other hand, is associated with a hyperpolarized state and the release of a short volley of action potentials in response to visual input (Lu et al., 1992; Sherman, 2001). The tonic mode thus yields more linear (and burst mode nonlinear) signal transmission. With these distinct firing patterns comes a different responsiveness to sensory signals such that the tonic mode promotes a more faithful representation of sensory input while neurons in burst mode are less reliably responsive to external visual stimulation (McCormick, 1992). Interestingly, while unreliable, LGN spikes during burst mode lead to much stronger cortical activation than in tonic mode (Swadlow and Gusev, 2001).
Sleep-wake cycle effects in visual cortex may be reflective of changes in basal forebrain activity as well as changes in the activity of the LGN (or a combination thereof). Brainstem cholinergic control of V1 thus is likely exerted through at least two pathways: brainstem-forebrain-cortex and/or brainstem-LGN-cortex (Steriade, 2004). A third pathway, via the reticular thalamic nucleus (TRN), is likely of enormous functional importance, given the capacity of the TRN to inhibit relay neurons of the LGN and thus to serve a gating function on visual input. There is, sadly, very little data on the modulatory control of the TRN, and so it is not considered further in this review.
Cortical neurons show a similar state-dependence of activity patterns as do their thalamic relay partners; a depolarization leads to increased excitability when transitioning from sleep to waking, which also appears to depend on local levels of ACh (Steriade et al., 1991). This cholinergic modulation must be relayed from the brainstem to V1 via the basal forebrain since in adult primates there are no local cholinergic interneurons in cortex (Mesulam et al., 1983; Hendry et al., 1987; Raghanti et al., 2008). These state changes appear to be governed by mAChR activation as scopolamine (a muscarinic antagonist), but not mecamylamine (a nicotinic antagonist), promotes cortical slow oscillations during brainstem stimulation (Steriade et al., 1993). As in the case of burst mode operation of the LGN, in cortex, non-REM sleep is associated with poor fidelity of representation of visual input and high response thresholds.
Sensory gain control by ACh during wakefulness
Beyond the sleep-wake cycle, the cholinergic ascending reticular activating system of the brainstem also plays a crucial role in managing arousal during waking. The LGN receives inputs from these nuclei and cholinergic modulation is evident in the within-waking activity patterns of neurons (McCormick and Bal, 1994). Indeed, increased ACh release, which is associated with alertness while awake, leads to an increase in the magnitude of visually-evoked responses in LGN (Uhlrich et al., 1995). This has been argued to enhance the visual resolution of LGN neurons (Hartveit et al., 1993) and increase sensitivity to spatial features of a visual stimulus (McCormick and Bal, 1994). As discussed above, brainstem cholinergic axons form synapses with both relay cells and interneurons and, accordingly, both direct excitation (McCormick and Prince, 1987; Sherman, 1996) and inhibition of interneurons (i.e. disinhibition) has been associated with this arousal-related response increase (McCormick and Pape, 1988; McCormick, 1989; Funke and Eysel, 1993).
The nucleus basalis of the basal forebrain sends projections to the entire neocortex, allowing ACh to modulate any cortical neuron expressing an AChR (Mesulam and Van Hoesen, 1976; Mesulam et al., 1983; Rasmusson, 1993). Data from basal forebrain stimulation studies show that ACh release into somatosensory, auditory, and visual cortex is modality-specific with greater release, for example, in visual regions during visual stimulation (Rasmusson, 1993). This release directly impacts visual processing. Studies in cat V1 have shown that ACh enhances responses to visual stimuli over an unmodulated baseline, without a loss of receptive field selectivity (Sillito and Kemp, 1983; Sato et al., 1987; Murphy and Sillito, 1991).
Changing the strength (or gain) of input at thalamocortical synapses is one way to control the flow of incoming sensory information in cortex. At the most basic level, sensory gain control can implement a ‘yes-no’ switch in which information either propagates into cortex, or does not (for example during sleep). Clearly, reducing the extent to which visual information has access to visual cortex (i.e. ‘closing’ the thalamocortical gate) will have a profound impact on downstream processing. During waking or alertness, sensory gain control takes on a subtler form; the ‘gate’ to cortex is generally open, but information from some sources (for example behaviorally-relevant signals) is emphasized over others. Gating and gain control in vision almost certainly both occur in both the LGN and V1.
A role for ACh in gain control, the more subtle within-waking control of the gate to cortex, was originally proposed by Hasselmo and Bower (Hasselmo and Bower, 1992) in a study of olfactory cortex. Since that time, it has been shown that ACh controls the gain of the ascending input to cortex across species and sensory systems, including in V1 (Gil et al., 1997; Kimura et al., 1999; Hsieh et al., 2000; Disney et al., 2007).
In the case of macaque V1, a two-stage gain control mechanism is suggested by data that show that the commonly-observed input gain control by ACh, mediated by activation of nAChRs in layer IVc (expressed on the terminals of thalamic relay cells), is supported at later stages of processing by an m1 AChR-mediated increase in inhibition strength (Disney et al., 2012). Interestingly, there may be a similar dual action of ACh in visual cortex of cats (Müller and Singer, 1989; Sillito, 1993) but likely not in rodents (Kawaguchi, 1997; Gulledge et al., 2007; Alitto and Dan, 2012; Pinto et al., 2013; Disney and Reynolds, 2014)
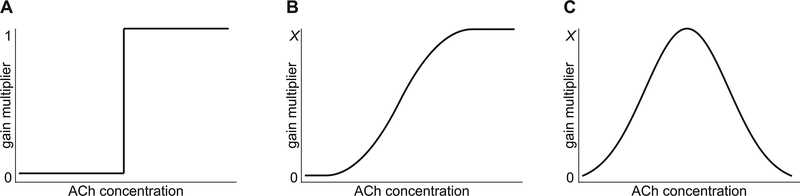
While it is common to think of the within-waking (gain) and sleep-wake (gate) functions of ACh as fundamentally different, on careful consideration it can be seen that the cholinergic system in both modes simply applies a multiplier to whatever visual input is currently running through the circuit. In the case of gating this is a binary operation in which the system multiplies by 0 (gate closed) or 1 (gate open; Figure 3a). In the case of gain control, it has been shown that in layer IVc of V1 the cholinergic system applies a multiplicative response gain on the input from the LGN [Figure 3b; (Disney et al., 2007)]. It has been noted previously that the effect of this gain operation is similar to that observed during states of attention or vigilance [(Disney et al., 2007); discussed further below]. Note that gating can be seen as equivalent to gain control with a very steep slope (compare Figures 3a and 3b), and gain need not be monotonically increasing. U-shaped relationships between gain and concentration (Figure 3c) are commonly observed for neuromodulatory molecules [for example dopamine (Zahrt et al., 1997; Stewart and Plenz, 2006; Gjedde et al., 2010; Cools and D’Esposito, 2011) or serotonin (Hulsken et al., 2013)].
Figure 3:
Multiplicative control of visual signaling mediated by acetylcholine concentration. (A) Acetylcholine may act by means of a binary gate during sleep-wake cycle where visual input gets either multiplied by 0 (gate closed) or by 1 (gate open). (B) Acetylcholine may act on gain control during waking in a way that visual input gets multiplied by a value of 0 to × resulting in gradual increases of visual signaling. (C) Gain need not increase with concentration; in fact non-monotonic gain control such as the inverted U-shaped gain function has been observed for other neuromodulatory systems.
Is there a role for ACh in (visual) attention?
It has been hypothesized that the cholinergic system is involved in attention and goal-oriented behaviors during sensory processing (Muir et al., 1993; Everitt and Robbins, 1997; Robbins, 1997; Smythies, 1997; Kobayashi and Isa, 2002; Roberts et al., 2007; Herrero et al., 2008; Deco and Thiele, 2011; Klinkenberg et al., 2011). A thorough coverage and critique of this literature is well beyond the scope of this review, but we will touch on key issues. First of all, it is important to note that attention-like effects of ACh could be an extension of cholinergic gain control discussed above (Sarter et al., 2005; Disney et al., 2007; Hasselmo and Sarter, 2011).
A challenge in this field is the lack of a universal operational definition for attention. The tasks that are commonly used in rodents to assay cholinergic effects on “attention” would generally be considered vigilance tasks by the community of researchers who study selective attention in the visual system of human and non-human primates. When added to the profound species differences between the cholinergic systems of rodents and primates (discussed briefly above and reviewed by Coppola and Disney, 2018), it is not at all clear that it is possible, or even appropriate, to attempt to merge the vast literature on cholinergic modulation of vigilance and task-demand with the similarly vast literature on the mechanisms of visual attention in primates (Desimone and Duncan, 1995; Egeth and Yantis, 1997; Treue, 2001; Reynolds and Chelazzi, 2004; Moore, 2006; Herrero et al., 2008; Moore and Zirnsak, 2017). We will briefly address the evidence for ACh playing a role in attention, or attentive-like behaviors in primates.
Studies in the 1990s showed that directing attention toward a neuron’s spatial receptive field (the region of retinotopically-mapped visual space to which the neuron is responsive) alters visually-evoked responses in primate V1 (Motter, 1993; Roelfsema et al., 1998; Ito and Gilbert, 1999). It quickly became evident that the mechanisms underlying such effects are likely to be as varied as are the differences between various types of attention [for a review of definitions of attention see (Oken et al., 2006)]. Visual selective attention, for example, plays a crucial role when multiple, potentially competing, visual stimuli are present within one visual scene or within a single neuron’s receptive field (Posner and Gilbert, 1999; Reynolds et al., 1999; Reynolds and Desimone, 2003; Reynolds and Chelazzi, 2004; Moore and Zirnsak, 2017), allowing for a filtering of sensory information in favor of that immediately relevant to behavior (Lubar, 1997; Treue, 2001). These effects are commonly attributed to the impact of glutamatergic feedback from higher cortical areas, which themselves are under tight modulatory control (Noudoost and Moore, 2011). However, a study in primates revealed a local modulation of V1 by ACh in the context of a visuospatial selective attention task (Herrero et al., 2008). It has also been shown that ACh application mimics effects observed when the monkeys were prompted to direct attention toward a specific spatial location (Roberts et al., 2005; Disney et al., 2007; Roberts et al., 2007; Herrero et al., 2008).
Interestingly, modulatory effects during visuospatial attention tasks are also observed in the LGN, where attentional modulation is associated with response increases in neurons of both the M and P pathways (McAlonan et al., 2008). It is unclear whether effects of attention observed in the LGN might be mediated by ACh, but it is imperative to recall here that the cholinergic innervation of the LGN arises from the brainstem, not the basal forebrain. The existence of attention effects in LGN is not addressed in current formulations of the glutamatergic feedback or the basal forebrain cholinergic hypotheses of attention.
Concluding remarks
In this review, we have emphasized the intersection between the cholinergic early visual systems in primates and potential cholinergic roles in the wake-sleep cycle, sensory gating, and visual attention. It is important to acknowledge here that ACh modulatory action has also been studied in the context of plasticity [see for example (Bear and Singer, 1986; Keuroghlian and Knudsen, 2007; Origlia et al., 2008; Bruel-Jungerman et al., 2011; Lin et al., 2015)], reward [see for example (Sarter and Bruno, 1997; Sarter et al., 2009; Chubykin et al., 2013; Hangya et al., 2015; Liu et al., 2015; Sarter et al., 2016)], and development [see for example (Filogamo and Marchisio, 1971; Court et al., 1995; Role and Berg, 1996; Rice and Barone, 2000)]. Whether ACh subserves many functions or whether all the above-mentioned roles have one (or a few) unifying processes, which is (are) mediated through ACh remains to be determined. It is also crucial to recall that ACh overlaps structurally and functionally with other neuromodulatory networks such as the serotonergic, dopaminergic, and noradrenergic systems, which will simultaneously influence visual processing in a higher dimensional modulatory state space.
A potentially interesting question for future research would focus on the functional implications of the dual-source nature of cholinergic modulation of the input to primary cortex from the thalamus. As noted above, the somata of thalamic relay cells are subject to cholinergic modulation arising from the brainstem, which carries information about the arousal state of the animal (Pepeu and Mantegazzini, 1964; Phillis and Chong, 1965; Shouse and Siegel, 1992) and probably other variables, including reward (Hangya et al., 2015). Thus, the computation being performed by these cell bodies integrates visual information (from the retina) with arousal information from the brainstem (and of course information from other sources, including other modulatory systems, but importantly in this context, NOT information directly from the basal forebrain). The axonal output from these cells (i.e. the thalamocortical terminals in V1) reflect this computation but are then additionally subject to modulation by ACh released from basal forebrain neurons (Disney et al., 2007), as are some of the cortical cells receiving the feedforward visual input (Disney et al., 2006; Disney and Aoki, 2008; Disney et al., 2012). This means that the computation being performed in cortex adds information carried by basal forebrain afferents that was absent at the immediately preceding processing stage. To re-state in another way; the input of thalamic relay cells is under the cholinergic control of the brainstem, while the output is under the cholinergic control of the basal forebrain.
This brings into focus the question of what the computation being performed by the basal forebrain might be; i.e. what purpose is served by running brainstem cholinergic arousal signals through the basal forebrain before delivering them to cortex? What is the information being added in that additional basal forebrain step? And what is the relevance (or value) of adding it to the processing stream at the level of V1, and not earlier? It is important to point out that this is a question that applies to the auditory and somatosensory pathways as well since they too see basal forebrain input arriving first in the primary sensory cortices for hearing (A1) and touch (S1), while the brainstem innervates the auditory and somatosensory thalamic nuclei.
One distinguishing feature of V1 (and A1 and S1) is the sheer number of neurons dedicated to representing the external world, and their organization into maps (e.g. the orientation and spatial frequency tuning columns of V1) within functional streams (e.g. the magnocellular pathway). Directing a modulatory signal to this stage of processing would avail the system of high resolution (i.e. the ability to direct a signal to an exquisitely small region of sensory space) and of emerging feature addressability (i.e. the ability to direct a signal to neurons carrying information about red things, or vertical lines). Whether the cholinergic input from the basal forebrain can capitalize on these features of V1 functional architecture depends critically on the extent to which the modulatory signal is global versus local, a question which is currently not resolved. The failure to conclusively answer this question to date arises, in part, because it is enormously challenging to determine the degree of anatomical overlap that exists between axons providing the cholinergic innervation of the cortex. And even when one has that answer, without further functional characterization of the basal forebrain (for example the number of co-active basal forebrain neurons), it remains unknown to what extent the available innervation specificity is actually used. One promising way forward is the concept of the (cortical) cholinergic compartment, briefly discussed earlier in this review (Coppola et al., 2016). Compartments allow for differential modulatory function depending on the architecture of the receiving circuit. This model implies that, in fact, local circuits in cortex manage their own ACh signaling in a dynamic fashion that could allow for very fine control of cortical processing by multiple interacting modulatory systems.
Acknowledgments
The authors would like to acknowledge Vivien Casagrande’s contributions to the field of neuroscience, and her friendship and mentorship to us personally. We would also like to thank Jennifer Coppola for her critical eye and helpful comments on drafts of this review. This work was supported by NIH grant R00 MH-93567 (AAD) and NIH grant R01 EY0254022 (JK).
References
- Adem A, Jossan SS, D’Argy R, Brandt I, Winblad B, Nordberg A. 1988. Distribution of nicotinic receptors in human thalamus as visualized by 3h-nicotine and 3h-acetylcholine receptor autoradiography. Journal of Neural Transmission 73:77–83. https://www.ncbi.nlm.nih.gov/pubmed/3404147 [DOI] [PubMed] [Google Scholar]
- Alitto HJ, Dan Y. 2012. Cell-type-specific modulation of neocortical activity by basal forebrain input. Front Syst Neurosci 6:79 10.3389/fnsys.2012.00079 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amzica F, Steriade M. 1996. Progressive cortical synchronization of ponto-geniculo-occipital potentials during rapid eye movement sleep. Vol 72 p 309–314. 10.1016/0306-4522(96)00012-7 [DOI] [PubMed] [Google Scholar]
- Aoki C, Kabak S. 1992. Cholinergic terminals in the cat visual cortex: Ultrastructural basis for interaction with glutamate-immunoreactive neurons and other cells. Vis Neurosci 8(3):177–191. https://www.ncbi.nlm.nih.gov/pubmed/1347700 [DOI] [PubMed] [Google Scholar]
- Bear MF, Singer W. 1986. Modulation of visual cortical plasticity by acetylcholine and noradrenaline. Nature 320(6058):172–176. 10.1038/320172a0 [DOI] [PubMed] [Google Scholar]
- Bernheim L, Beech DJ, Hille B. 1991. A diffusible second messenger mediates one of the pathways coupling receptors to calcium channels in rat sympathetic neurons. Neuron 6(6):859–867. 10.1016/0896-6273(91)90226-P [DOI] [PubMed] [Google Scholar]
- Bickford ME, Ramcharan E, Godwin DW, Erisir A, Gnadt J, Sherman SM. 2000. Neurotransmitters contained in the subcortical extraretinal inputs to the monkey lateral geniculate nucleus. J Comp Neurol 424(4):701–717. 10.1002/1096-9861(20000904)424:4 [DOI] [PubMed] [Google Scholar]
- Bigl V, Woolf NJ, Butcher LL. 1982. Cholinergic projections from the basal forebrain to frontal, parietal, temporal, occipital, and cingulate cortices: A combined fluorescent tracer and acetylcholinesterase analysis. Brain Res Bull 8(6):727–749. 10.1016/0361-9230(82)90101-0 [DOI] [PubMed] [Google Scholar]
- Boyd JD, Khaytin I, Casagrande VA. 2009. Evolution of the visual system in mammals – comparative evolutionary aspects across orders. p 1448–1459. [Google Scholar]
- Bruel-Jungerman E, Lucassen PJ, Francis F. 2011. Cholinergic influences on cortical development and adult neurogenesis. Behav Brain Res 221(2):379–388. 10.1016/j.bbr.2011.01.021 [DOI] [PubMed] [Google Scholar]
- Byth W, McMahon D, King DJ. 2000. Cholinergic agents and the mccollough effect. Perception 29(4):461–480. 10.1068/p2892 [DOI] [PubMed] [Google Scholar]
- Callaway EM. 1998. Local circuits in primary visual cortex of the macaque monkey. Annu Rev Neurosci 21(1):47–74. 10.1146/annurev.neuro.21.1.47 [DOI] [PubMed] [Google Scholar]
- Callaway EM. 2004. Feedforward, feedback and inhibitory connections in primate visual cortex. Vol 17 p 625–632. 10.1016/j.neunet.2004.04.004 [DOI] [PubMed] [Google Scholar]
- Casagrande VA. 1994. A third parallel visual pathway to primate area v1. Vol 17: American Physiological Society. p 305–310. 10.1016/0166-2236(94)90065-5 [DOI] [PubMed] [Google Scholar]
- Casagrande VA, Kaas JH. 1994. The afferent, intrinsic, and efferent connections of primary visual cortex in primates. Cerebral Cortex 10:201–259. 10.1007/978-1-4757-9628-5_5 [DOI] [Google Scholar]
- Casagrande VA, Xu X. 2004. Parallel visual pathways: A comparative perspective. p 494–506. [Google Scholar]
- Chubykin AA, Roach EB, Bear MF, Shuler MG. 2013. A cholinergic mechanism for reward timing within primary visual cortex. Neuron 77(4):723–735. 10.1016/j.neuron.2012.12.039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cools R, D’Esposito M. 2011. Inverted-u-shaped dopamine actions on human working memory and cognitive control. Biol Psychiatry 69(12):e113–125. 10.1016/j.biopsych.2011.03.028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coppola JJ, Disney AA. 2018. Is there a canonical cortical circuit for the cholinergic system? Anatomical differences across common model systems. Front Neural Circuits 12:8 10.3389/fncir.2018.00008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coppola JJ, Ward NJ, Jadi MP, Disney AA. 2016. Modulatory compartments in cortex and local regulation of cholinergic tone. J Physiol Paris 110(1–2):3–9. 10.1016/j.jphysparis.2016.08.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Court JA, Perry EK, Spurden D, Griffiths M, Kerwin JM, Morris CM, Johnson M, Oakley AE, Birdsall NJ, Clementi F, et al. 1995. The role of the cholinergic system in the development of the human cerebellum. Brain Res Dev Brain Res 90(1–2):159–167. 10.1016/0165-3806(96)83496-1 [DOI] [PubMed] [Google Scholar]
- Dale H, William S, Stedmans DT, Mann P, Feldberg W, Hebb C, Burn J. 1967. Cholinergic cells and pathways.
- Dani JA, Bertrand D. 2007. Nicotinic acetylcholine receptors and nicotinic cholinergic mechanisms of the central nervous system. Annu Rev Pharmacol Toxicol 47(1):699–729. 10.1146/annurev.pharmtox.47.120505.105214 [DOI] [PubMed] [Google Scholar]
- Deco G, Thiele A. 2011. Cholinergic control of cortical network interactions enables feedback-mediated attentional modulation. Eur J Neurosci 34(1):146–157. 10.1111/j.1460-9568.2011.07749.x [DOI] [PubMed] [Google Scholar]
- Desimone R, Duncan J. 1995. Neural mechanisms of selective visual attention. Annu Rev Neurosci 18:193–222. 10.1146/annurev.ne.18.030195.001205 [DOI] [PubMed] [Google Scholar]
- Disney AA, Aoki C. 2008. Muscarinic acetylcholine receptors in macaque v1 are most frequently expressed by parvalbumin-immunoreactive neurons. J Comp Neurol 507(5):1748–1762. 10.1002/cne.21616 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Disney AA, Aoki C, Hawken MJ. 2007. Gain modulation by nicotine in macaque v1. Neuron 56(4):701–713. 10.1016/j.neuron.2007.09.034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Disney AA, Aoki C, Hawken MJ. 2012. Cholinergic suppression of visual responses in primate v1 is mediated by gabaergic inhibition. J Neurophysiol 108(7):1907–1923. 10.1152/jn.00188.2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Disney AA, Domakonda KV, Aoki C. 2006. Differential expression of muscarinic acetylcholine receptors across excitatory and inhibitory cells in visual cortical areas v1 and v2 of the macaque monkey. J Comp Neurol 499(1):49–63. 10.1002/cne.21096 [DOI] [PubMed] [Google Scholar]
- Disney AA, Reynolds JH. 2014. Expression of m1-type muscarinic acetylcholine receptors by parvalbumin-immunoreactive neurons in the primary visual cortex: A comparative study of rat, guinea pig, ferret, macaque, and human. J Comp Neurol 522(5):986–1003. 10.1002/cne.23456 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dolabela de Lima A, Montero VM, Singer W. 1985. The cholinergic innervation of the visual thalamus: An em immunocytochemical study. Experimental Brain Research 59(1):206–212. 10.1007/bf00237681 [DOI] [PubMed] [Google Scholar]
- Egeth HE, Yantis S. 1997. Visual attention: Control, representation, and time course. Annu Rev Psychol 48:269–297. 10.1146/annurev.psych.48.1.269 [DOI] [PubMed] [Google Scholar]
- Eickhoff SB, Rottschy C, Zilles K. 2007. Laminar distribution and co-distribution of neurotransmitter receptors in early human visual cortex. Brain Struct Funct 212(3–4):255–267. 10.1007/s00429-007-0156-y [DOI] [PubMed] [Google Scholar]
- Evarts EV, Bental E, Bihari B, Huttenlocher PR. 1962. Spontaneous discharge of single neurons during sleep and waking. Science 135(3505):726–728. https://www.ncbi.nlm.nih.gov/pubmed/13891034 [DOI] [PubMed] [Google Scholar]
- Everitt BJ, Robbins TW. 1997. Central cholinergic systems and cognition. Annu Rev Psychol 48(1):649–684. 10.1146/annurev.psych.48.1.649 [DOI] [PubMed] [Google Scholar]
- Filogamo G, Marchisio PC. 1971. Acetylcholine system and neural development. Elsevier; p 29–64. 10.1016/B978-0-12-512504-8.50008-1 [DOI] [PubMed] [Google Scholar]
- Funke K, Eysel U. 1993. Modulatory effects of acetylcholine, serotonin and noradrenaline on the activity of cat perigeniculate neurons. Experimental Brain Research 95(3):409–420. 10.1007/bf00227133 [DOI] [PubMed] [Google Scholar]
- Fuxe K, Agnati LF. 1991. Volume transmission in the brain: Novel mechanisms for neural transmission. 10.1016/0166-2236(92)90174-7 [DOI] [Google Scholar]
- Gil Z, Connors BW, Amitai Y. 1997. Differential regulation of neocortical synapses by neuromodulators and activity. Neuron 19(3):679–686. 10.1016/S0896-6273(00)80380-3 [DOI] [PubMed] [Google Scholar]
- Gjedde A, Kumakura Y, Cumming P, Linnet J, Moller A. 2010. Inverted-u-shaped correlation between dopamine receptor availability in striatum and sensation seeking. Proc Natl Acad Sci U S A 107(8):3870–3875. 10.1073/pnas.0912319107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gritti I, Henny P, Galloni F, Mainville L, Mariotti M, Jones BE. 2006. Stereological estimates of the basal forebrain cell population in the rat, including neurons containing choline acetyltransferase, glutamic acid decarboxylase or phosphate-activated glutaminase and colocalizing vesicular glutamate transporters. Neuroscience 143(4):1051–1064. 10.1016/j.neuroscience.2006.09.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gritti I, Mainville L, Mancia M, Jones BE. 1997. Gabaergic and other noncholinergic basal forebrain neurons, together with cholinergic neurons, project to the mesocortex and isocortex in the rat. J Comp Neurol 383(2):163–177. 10.1002/(SICI)1096-9861(19970630)383:2 [DOI] [PubMed] [Google Scholar]
- Gulledge AT, Park SB, Kawaguchi Y, Stuart GJ. 2007. Heterogeneity of phasic cholinergic signalling in neocortical neurons. J Neurophysiol 97(3):2215–2229. 10.1152/jn.00493.2006 [DOI] [PubMed] [Google Scholar]
- Hallanger AE, Levey AI, Lee HJ, Rye DB, Wainer BH. 1987. The origins of cholinergic and other subcortical afferents to the thalamus in the rat. J Comp Neurol 262(1):105–124. 10.1002/cne.902620109 [DOI] [PubMed] [Google Scholar]
- Han ZY, Le Novere N, Zoli M, Hill JA Jr., Champtiaux N, Changeux JP. 2000. Localization of nachr subunit mrnas in the brain of macaca mulatta. Eur J Neurosci 12(10):3664–3674. 10.1046/j.1460-9568.2000.00262.x [DOI] [PubMed] [Google Scholar]
- Hangya B, Ranade SP, Lorenc M, Kepecs A. 2015. Central cholinergic neurons are rapidly recruited by reinforcement feedback. Cell 162(5):1155–1168. 10.1016/j.cell.2015.07.057 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartveit E, Ramberg SI, Heggelund P. 1993. Brain stem modulation of spatial receptive field properties of single cells in the dorsal lateral geniculate nucleus of the cat. J Neurophysiol 70(4):1644–1655. 10.1152/jn.1993.70.4.1644 [DOI] [PubMed] [Google Scholar]
- Hasselmo ME, Bower JM. 1992. Cholinergic suppression specific to intrinsic not afferent fiber synapses in rat piriform (olfactory) cortex. Journal of neurophysiology 67(5):1222–1229. 10.1152/jn.1992.67.5.1222 [DOI] [PubMed] [Google Scholar]
- Hasselmo ME, Sarter M. 2011. Modes and models of forebrain cholinergic neuromodulation of cognition. Vol 36: Nature Publishing Group; p 52–73. 10.1038/npp.2010.104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hendry SH, Jones EG, Killackey HP, Chalupa LM. 1987. Choline acetyltransferase-immunoreactive neurons in fetal monkey cerebral cortex. Brain Res 465(1–2):313–317. 10.1016/0165-3806(87)90252-5 [DOI] [PubMed] [Google Scholar]
- Hendry SH, Reid RC. 2000. The koniocellular pathway in primate vision. Annu Rev Neurosci 23(1):127–153. 10.1146/annurev.neuro.23.1.127 [DOI] [PubMed] [Google Scholar]
- Herrero JL, Roberts MJ, Delicato LS, Gieselmann MA, Dayan P, Thiele A. 2008. Acetylcholine contributes through muscarinic receptors to attentional modulation in v1. Nature 454(7208):1110–1114. 10.1038/nature07141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirai T, Jones EG. 1989. A new parcellation of the human thalamus on the basis of histochemical staining. Vol 14 p 1–34. 10.1016/0165-0173(89)90007-6 [DOI] [PubMed] [Google Scholar]
- Hsieh CY, Cruikshank SJ, Metherate R. 2000. Differential modulation of auditory thalamocortical and intracortical synaptic transmission by cholinergic agonist. Vol 880 p 51–64. 10.1016/S0006-8993(00)02766-9 [DOI] [PubMed] [Google Scholar]
- Hulsken S, Martin A, Mohajeri MH, Homberg JR. 2013. Food-derived serotonergic modulators: Effects on mood and cognition. Nutr Res Rev 26(2):223–234. 10.1017/S0954422413000164 [DOI] [PubMed] [Google Scholar]
- Ito M, Gilbert CD. 1999. Attention modulates contextual influences in the primary visual cortex of alert monkeys. Neuron 22(3):593–604. 10.1016/S0896-6273(00)80713-8 [DOI] [PubMed] [Google Scholar]
- Jeffries AM, Killian NJ, Pezaris JS. 2014. Mapping the primate lateral geniculate nucleus: A review of experiments and methods. Vol 108: Elsevier; p 3–10. 10.1016/j.jphysparis.2013.10.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jimenez-Capdeville ME, Dykes RW. 1996. Changes in cortical acetylcholine release in the rat during day and night differences between motor and sensory areas. Neuroscience 71(2):567–579. 10.1016/0306-4522(95)00439-4 [DOI] [PubMed] [Google Scholar]
- Jones BE. 1993. The organization of central cholinergic systems and their functional importance in sleep-waking states. Prog Brain Res 98(August):61–71. 10.1016/S0079-6123(08)62381-X [DOI] [PubMed] [Google Scholar]
- Jones BE. 2005. From waking to sleeping: Neuronal and chemical substrates. Vol 26 p 578–586. 10.1016/j.tips.2005.09.009 [DOI] [PubMed] [Google Scholar]
- Jones BE, Cuello AC. 1989. Afferents to the basal forebrain cholinergic cell area from pontomesencephalic—catecholamine, serotonin, and acetylcholine—neurons. Neuroscience 31(1):37–61. 10.1016/0306-4522(89)90029-8 [DOI] [PubMed] [Google Scholar]
- Jones BE, Webster HH. 1988. Neurotoxic lesions of the dorsolateral pontomesencephalic tegmentum-cholinergic cell area in the cat. I. Effects upon the cholinergic innervation of the brain. Brain Research 451(1–2):13–32. 10.1016/0006-8993(88)90745-7 [DOI] [PubMed] [Google Scholar]
- Kawaguchi Y 1997. Selective cholinergic modulation of cortical gabaergic cell subtypes. J Neurophysiol 78(3):1743–1747. 10.1152/jn.1997.78.3.1743 [DOI] [PubMed] [Google Scholar]
- Keuroghlian AS, Knudsen EI. 2007. Adaptive auditory plasticity in developing and adult animals. Vol 82 p 109–121. 10.1016/j.pneurobio.2007.03.005 [DOI] [PubMed] [Google Scholar]
- Kievit J, Kuypers HGJM. 1975. Basal forebrain and hypothalamic connections to frontal and parietal cortex in the rhesus monkey. Science 187(21):660–662. 10.1126/science.1114317 [DOI] [PubMed] [Google Scholar]
- Kimura F, Fukuda M, Tsumoto T. 1999. Acetylcholine suppresses the spread of excitation in the visual cortex revealed by optical recording: Possible differential effect depending on the source of input. Eur J Neurosci 11(10):3597–3609. 10.1046/j.1460-9568.1999.00779.x [DOI] [PubMed] [Google Scholar]
- Klinkenberg I, Sambeth A, Blokland A. 2011. Acetylcholine and attention. Behav Brain Res 221(2):430–442. 10.1016/j.bbr.2010.11.033 [DOI] [PubMed] [Google Scholar]
- Kobayashi Y, Isa T. 2002. Sensory-motor gating and cognitive control by the brainstem cholinergic system. Vol 15 p 731–741. 10.1016/S0893-6080(02)00059-X [DOI] [PubMed] [Google Scholar]
- Krnjevic K, Silver A. 1965. A histochemical study of cholinergic fibres in the cerebral cortex. J Anat 99(Pt 4):711–759. https://www.ncbi.nlm.nih.gov/pubmed/4160130 [PMC free article] [PubMed] [Google Scholar]
- Lachica EA, Beck PD, Casagrande VA. 1992. Parallel pathways in macaque monkey striate cortex: Anatomically defined columns in layer iii. Proc Natl Acad Sci U S A 89(8):3566–3570. 10.1073/pnas.89.8.3566 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehmann J, Nagy JI, Atmadia S, Fibiger HC. 1980. The nucleus basalis magnocellularis: The origin of a cholinergic projection to the neocortex of the rat. Neuroscience 5(7):1161–1174. 10.1016/0306-4522(80)90195-5 [DOI] [PubMed] [Google Scholar]
- Lin SC, Brown RE, Hussain Shuler MG, Petersen CC, Kepecs A. 2015. Optogenetic dissection of the basal forebrain neuromodulatory control of cortical activation, plasticity, and cognition. J Neurosci 35(41):13896–13903. 10.1523/JNEUROSCI.2590-15.2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu CH, Coleman JE, Davoudi H, Zhang K, Hussain Shuler MG. 2015. Selective activation of a putative reinforcement signal conditions cued interval timing in primary visual cortex. Curr Biol 25(12):1551–1561. 10.1016/j.cub.2015.04.028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu SM, Guido W, Sherman SM. 1992. Effects of membrane voltage on receptive field properties of lateral geniculate neurons in the cat: Contributions of the low-threshold ca2+ conductance. J Neurophysiol 68(6):2185–2198. 10.1152/jn.1992.68.6.2185 [DOI] [PubMed] [Google Scholar]
- Lubar JF. 1997. Neocortical dynamics: Implications for understanding the role of neurofeedback and related techniques for the enhancement of attention. Appl Psychophysiol Biofeedback 22(2):111–126. 10.1023/A:1026276228832 [DOI] [PubMed] [Google Scholar]
- Lund JS. 1988. Anatomical organization of macaque monkey striate visual cortex. Annu Rev Neurosci 11(1):253–288. 10.1146/annurev.ne.11.030188.001345 [DOI] [PubMed] [Google Scholar]
- McAlonan K, Cavanaugh J, Wurtz RH. 2008. Guarding the gateway to cortex with attention in visual thalamus. Nature 456(7220):391–394. 10.1038/nature07382 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCormick DA. 1989. Cholinergic and noradrenergic modulation of thalamocortical processing. Trends Neurosci 12(6):215–221. 10.1016/0166-2236(89)90125-2 [DOI] [PubMed] [Google Scholar]
- McCormick DA. 1992. Cellular mechanisms underlying cholinergic and noradrenergic modulation of neuronal firing mode in the cat and guinea pig dorsal lateral geniculate nucleus. J Neurosci 12(1):278–289. 10.1523/JNEUROSCI.12-01-00278.1992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCormick DA, Bal T. 1994. Sensory gating mechanisms of the thalamus. Vol 4 p 550–556. 10.1016/0959-4388(94)90056-6 [DOI] [PubMed] [Google Scholar]
- McCormick DA, Bal T. 1997. Sleep and arousal: Thalamocortical mechanisms. Annu Rev Neurosci 20(1):185–215. 10.1146/annurev.neuro.20.1.185 [DOI] [PubMed] [Google Scholar]
- McCormick DA, Pape HC. 1988. Acetylcholine inhibits identified interneurons in the cat lateral geniculate nucleus. Nature 334(6179):246–248. 10.1038/334246a0 [DOI] [PubMed] [Google Scholar]
- McCormick DA, Prince DA. 1987. Actions of acetylcholine in the guinea-pig and cat medial and lateral geniculate nuclei, in vitro. J Physiol 392(1):147–165. 10.1113/jphysiol.1987.sp016774 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDonald CT, McGuinness ER, Allman JM. 1993. Laminar organization of acetylcholinesterase and cytochrome oxidase in the lateral geniculate nucleus of prosimians. Neuroscience 54(4):1091–1101. 10.1016/0306-4522(93)90598-A [DOI] [PubMed] [Google Scholar]
- McGehee DS, Role LW. 1995. Physiological diversity of nicotinic acetylcholine receptors expressed by vertebrate neurons. Annu Rev Physiol 57(1):521–546. 10.1146/annurev.ph.57.030195.002513 [DOI] [PubMed] [Google Scholar]
- Mesulam MM, Mufson EJ, Levey AI, Wainer BH. 1983. Cholinergic innervation of cortex by the basal forebrain: Cytochemistry and cortical connections of the septal area, diagonal band nuclei, nucleus basalis (substantia innominata), and hypothalamus in the rhesus monkey. J Comp Neurol 214(2):170–197. 10.1002/cne.902140206 [DOI] [PubMed] [Google Scholar]
- Mesulam MM, Mufson EJ, Levey AI, Wainer BH. 1984. Atlas of cholinergic neurons in the forebrain and upper brainstem of the macaque based on monoclonal choline acetyltransferase immunohistochemistry and acetylcholinesterase histochemistry. Neuroscience 12(3):669–686. 10.1016/0306-4522(84)90163-5 [DOI] [PubMed] [Google Scholar]
- Mesulam MM, Van Hoesen GW. 1976. Acetylcholinesterase-rich projections from the basal forebrain of the rhesus monkey to neocortex. Brain Res 109(1):152–157. 10.1016/0006-8993(76)90385-1 [DOI] [PubMed] [Google Scholar]
- Moore T 2006. The neurobiology of visual attention: Finding sources. Vol 16 p 159–165. 10.1016/j.conb.2006.03.009 [DOI] [PubMed] [Google Scholar]
- Moore T, Zirnsak M. 2017. Neural mechanisms of selective visual attention. Annu Rev Psychol 68(1):47–72. 10.1146/annurev-psych-122414-033400 [DOI] [PubMed] [Google Scholar]
- Motter BC. 1993. Focal attention produces spatially selective processing in visual cortical areas v1, v2, and v4 in the presence of competing stimuli. J Neurophysiol 70(3):909–919. 10.1152/jn.1993.70.3.909 [DOI] [PubMed] [Google Scholar]
- Mrzljak L, Levey AI, Goldman-Rakic PS. 1993. Association of m1 and m2 muscarinic receptor proteins with asymmetric synapses in the primate cerebral cortex: Morphological evidence for cholinergic modulation of excitatory neurotransmission. Proc Natl Acad Sci U S A 90(11):5194–5198. 10.1073/pnas.90.11.5194 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mrzljak L, Levey AI, Rakic P. 1996. Selective expression of m2 muscarinic receptor in the parvocellular channel of the primate visual cortex. Proc Natl Acad Sci U S A 93(14):7337–7340. https://www.ncbi.nlm.nih.gov/pubmed/8692994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mrzljak L, Pappy M, Leranth C, Goldman-Rakic PS. 1995. Cholinergic synaptic circuitry in the macaque prefrontal cortex. J Comp Neurol 357(4):603–617. 10.1002/cne.903570409 [DOI] [PubMed] [Google Scholar]
- Muir JL, Page KJ, Sirinathsinghji DJ, Robbins TW, Everitt BJ. 1993. Excitotoxic lesions of basal forebrain cholinergic neurons: Effects on learning, memory and attention. Behav Brain Res 57(2):123–131. 10.1016/0166-4328(93)90128-D [DOI] [PubMed] [Google Scholar]
- Müller CM, Singer W. 1989. Acetylcholine-induced inhibition in the cat visual cortex is mediated by a gabaergic mechanism. Brain Research 487(2):335–342. 10.1016/0006-8993(89)90837-8 [DOI] [PubMed] [Google Scholar]
- Murphy PC, Sillito AM. 1991. Cholinergic enhancement of direction selectivity in the visual cortex of the cat. Neuroscience 40(1):13–20. 10.1016/0306-4522(91)90170-S [DOI] [PubMed] [Google Scholar]
- Noudoost B, Moore T. 2011. The role of neuromodulators in selective attention. Vol 15: NIH Public Access; p 585–591. 10.1016/j.tics.2011.10.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oken BS, Salinsky MC, Elsas SM. 2006. Vigilance, alertness, or sustained attention: Physiological basis and measurement. Vol 117: NIH Public Access; p 1885–1901. 10.1016/j.clinph.2006.01.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Origlia N, Kuczewski N, Pesavento E, Aztiria E, Domenici L. 2008. The role of cholinergic system in neuronal plasticity: Focus on visual cortex and muscarinic receptors. Vol 146 p 165–188. 10.4449/AIB.V146I3.767 [DOI] [PubMed] [Google Scholar]
- Pare D, Smith Y, Parent A, Steriade M. 1988. Projections of brainstem core cholinergic and non-cholinergic neurons of cat to intralaminar and reticular thalamic nuclei. Neuroscience 25(1):69–86. 10.1016/0306-4522(88)90007-3 [DOI] [PubMed] [Google Scholar]
- Paterson D, Nordberg A. 2000. Neuronal nicotinic receptors in the human brain. Vol 61 p 75–111. 10.1016/S0301-0082(99)00045-3 [DOI] [PubMed] [Google Scholar]
- Pearson RC, Sofroniew MV, Cuello AC, Powell TP, Eckenstein F, Esiri MM, Wilcock GK. 1983. Persistence of cholinergic neurons in the basal nucleus in a brain with senile dementia of the alzheimer’s type demonstrated by immunohistochemical staining for choline acetyltransferase. Brain Res 289(1–2):375–379. 10.1016/0006-8993(83)90046-X [DOI] [PubMed] [Google Scholar]
- Pepeu G, Mantegazzini P. 1964. Midbrain hemisection: Effect on cortical acetylcholine in the cat. Science 145(3636):1069–1070. 10.1126/science.145.3636.1069 [DOI] [PubMed] [Google Scholar]
- Phillis JW, Chong GC. 1965. Acetylcholine release from the cerebral and cerebellar cortices: Its role in cortical arousal. Nature 207(5003):1253–1255. 10.1038/2071253a0 [DOI] [PubMed] [Google Scholar]
- Pinto L, Goard MJ, Estandian D, Xu M, Kwan AC, Lee SH, Harrison TC, Feng G, Dan Y. 2013. Fast modulation of visual perception by basal forebrain cholinergic neurons. Nat Neurosci 16(12):1857–1863. 10.1038/nn.3552 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plummer KL, Manning KA, Levey AI, Rees HD, Uhlrich DJ. 1999. Muscarinic receptor subtypes in the lateral geniculate nucleus: A light and electron microscopic analysis. J Comp Neurol 404(3):408–425. [DOI] [PubMed] [Google Scholar]
- Posner MI, Gilbert CD. 1999. Attention and primary visual cortex. Proc Natl Acad Sci U S A 96(6):2585–2587. 10.1073/pnas.96.6.2585 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price JL, Stern R. 1983. Individual cells in the nucleus basalis--diagonal band complex have restricted axonal projections to the cerebral cortex in the rat. Brain Res 269(2):352–356. 10.1016/0006-8993(83)90145-2 [DOI] [PubMed] [Google Scholar]
- Quik M, Polonskaya Y, Gillespie A, Jakowec M, Lloyd GK, Langston JW. 2000. Localization of nicotinic receptor subunit mrnas in monkey brain by in situ hybridization. J Comp Neurol 425(1):58–69. [pii] [DOI] [PubMed] [Google Scholar]
- Raghanti MA, Stimpson CD, Marcinkiewicz JL, Erwin JM, Hof PR, Sherwood CC. 2008. Cholinergic innervation of the frontal cortex: Differences among humans, chimpanzees, and macaque monkeys. J Comp Neurol 506(3):409–424. 10.1002/cne.21546 [DOI] [PubMed] [Google Scholar]
- Rasmusson DD. 1993. Cholinergic modulation of sensory information. Prog Brain Res 98:357–364. 10.1016/S0079-6123(08)62419-X [DOI] [PubMed] [Google Scholar]
- Reynolds JH, Chelazzi L. 2004. Attentional modulation of visual processing. Annu Rev Neurosci 27(1):611–647. 10.1146/annurev.neuro.26.041002.131039 [DOI] [PubMed] [Google Scholar]
- Reynolds JH, Chelazzi L, Desimone R. 1999. Competitive mechanisms subserve attention in macaque areas v2 and v4. J Neurosci 19(5):1736–1753. 10.1523/JNEUROSCI.19-05-01736.1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reynolds JH, Desimone R. 2003. Interacting roles of attention and visual salience in v4. Neuron 37(5):853–863. 10.1016/S0896-6273(03)00097-7 [DOI] [PubMed] [Google Scholar]
- Rice D, Barone S Jr. 2000. Critical periods of vulnerability for the developing nervous system: Evidence from humans and animal models. Environ Health Perspect 108 Suppl 3(SUPPL. 3):511–533. 10.1289/ehp.00108s3511 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robbins TW. 1997. Arousal systems and attentional processes. Biol Psychol 45(1–3):57–71. 10.1016/S0301-0511(96)05222-2 [DOI] [PubMed] [Google Scholar]
- Roberts M, Delicato LS, Herrero J, Gieselmann MA, Thiele A. 2007. Attention alters spatial integration in macaque v1 in an eccentricity-dependent manner. Nat Neurosci 10(11):1483–1491. 10.1038/nn1967 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts MJ, Zinke W, Guo K, Robertson R, McDonald JS, Thiele A. 2005. Acetylcholine dynamically controls spatial integration in marmoset primary visual cortex. J Neurophysiol 93(4):2062–2072. 10.1152/jn.00911.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roelfsema PR, Lamme VA, Spekreijse H. 1998. Object-based attention in the primary visual cortex of the macaque monkey. Nature 395(6700):376–381. 10.1038/26475 [DOI] [PubMed] [Google Scholar]
- Role LW, Berg DK. 1996. Nicotinic receptors in the development and modulation of cns synapses. Vol 16 p 1077–1085. 10.1016/S0896-6273(00)80134-8 [DOI] [PubMed] [Google Scholar]
- Sarter M, Bruno JP. 1997. Cognitive functions of cortical acetylcholine: Toward a unifying hypothesis. Vol 23 p 28–46. 10.1016/S0165-0173(96)00009-4 [DOI] [PubMed] [Google Scholar]
- Sarter M, Hasselmo ME, Bruno JP, Givens B. 2005. Unraveling the attentional functions of cortical cholinergic inputs: Interactions between signal-driven and cognitive modulation of signal detection. Vol 48 p 98–111. 10.1016/j.brainresrev.2004.08.006 [DOI] [PubMed] [Google Scholar]
- Sarter M, Lustig C, Berry AS, Gritton H, Howe WM, Parikh V. 2016. What do phasic cholinergic signals do? Neurobiol Learn Mem 130:135–141. 10.1016/j.nlm.2016.02.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarter M, Parikh V, Howe WM. 2009. Phasic acetylcholine release and the volume transmission hypothesis: Time to move on. Nat Rev Neurosci 10(5):383–390. 10.1038/nrn2635 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato H, Hata Y, Hagihara K, Tsumoto T. 1987. Effects of cholinergic depletion on neuron activities in the cat visual cortex. J Neurophysiol 58(4):781–794. 10.1152/jn.1987.58.4.781 [DOI] [PubMed] [Google Scholar]
- Shaw C, Cynader M. 1986. Laminar distribution of receptors in monkey (macaca fascicularis) geniculostriate system. J Comp Neurol 248(3):301–312. 10.1002/cne.902480302 [DOI] [PubMed] [Google Scholar]
- Sherman SM. 1996. Dual response modes in lateral geniculate neurons: Mechanisms and functions. Vis Neurosci 13(2):205–213. 10.1017/S0952523800007446 [DOI] [PubMed] [Google Scholar]
- Sherman SM. 2001. Tonic and burst firing: Dual modes of thalamocortical relay. Trends Neurosci 24(2):122–126. 10.1016/S0166-2236(00)01714-8 [DOI] [PubMed] [Google Scholar]
- Sherman SM. Thalamic relays and cortical functioning; 2005. 2005 p 107–126. 10.1016/S0079-6123(05)49009-3 [DOI] [PubMed] [Google Scholar]
- Sherman SM, Koch C. 1986. The control of retinogeniculate transmission in the mammalian lateral geniculate nucleus. Exp Brain Res 63(1):1–20. 10.1007/BF00235642 [DOI] [PubMed] [Google Scholar]
- Shouse MN, Siegel JM. 1992. Pontine regulation of rem sleep components in cats: Integrity of the pedunculopontine tegmentum (ppt) is important for phasic events but unnecessary for atonia during rem sleep. Brain Research 571(1):50–63. 10.1016/0006-8993(92)90508-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shute CC, Lewis PR. 1963. Cholinesterase-containing systems of the brain of the rat. Nature 199(4899):1160–1164. 10.1038/1991160a0 [DOI] [PubMed] [Google Scholar]
- Sillito AM. 1993. The cholinergic neuromodulatory system: An evaluation of its functional roles. Prog Brain Res 98:371–378. https://www.ncbi.nlm.nih.gov/pubmed/8248525 [DOI] [PubMed] [Google Scholar]
- Sillito AM, Kemp JA. 1983. Cholinergic modulation of the functional organization of the cat visual cortex. Brain Res 289(1–2):143–155. 10.1016/0006-8993(83)90015-X [DOI] [PubMed] [Google Scholar]
- Sincich LC, Horton JC. 2005. The circuitry of v1 and v2: Integration of color, form, and motion. Annu Rev Neurosci 28(1):303–326. 10.1146/annurev.neuro.28.061604.135731 [DOI] [PubMed] [Google Scholar]
- Smith Y, Pare D, Deschenes M, Parent A, Steriade M. 1988. Cholinergic and non-cholinergic projections from the upper brainstem core to the visual thalamus in the cat. Exp Brain Res 70(1):166–180. 10.1007/BF00271858 [DOI] [PubMed] [Google Scholar]
- Smythies J 1997. The functional neuroanatomy of awareness: With a focus on the role of various anatomical systems in the control of intermodal attention. Conscious Cogn 6(4):455–481. 10.1006/ccog.1997.0315 [DOI] [PubMed] [Google Scholar]
- Spurden DP, Court JA, Lloyd S, Oakley A, Perry R, Pearson C, Pullen RG, Perry EK. 1997. Nicotinic receptor distribution in the human thalamus: Autoradiographical localization of [3h]nicotine and [125i] alpha-bungarotoxin binding. J Chem Neuroanat 13(2):105–113. 10.1016/S0891-0618(97)00038-0 [DOI] [PubMed] [Google Scholar]
- Steriade M 1993. Cholinergic blockage of network- and intrinsically generated slow oscillations promotes waking and rem sleep activity patterns in thalamic and cortical neurons. Prog Brain Res 98:345–355. 10.1016/S0079-6123(08)62418-8 [DOI] [PubMed] [Google Scholar]
- Steriade M Acetylcholine systems and rhythmic activities during the waking-sleep cycle; 2004. 2004 p 179–196. 10.1016/S0079-6123(03)45013-9 [DOI] [PubMed] [Google Scholar]
- Steriade M, Amzica F, Nunez A. 1993. Cholinergic and noradrenergic modulation of the slow (approximately 0.3 hz) oscillation in neocortical cells. J Neurophysiol 70(4):1385–1400. 10.1152/jn.1993.70.4.1385 [DOI] [PubMed] [Google Scholar]
- Steriade M, Dossi RC, Pare D, Oakson G. 1991. Fast oscillations (20–40 hz) in thalamocortical systems and their potentiation by mesopontine cholinergic nuclei in the cat. Proceedings of the National Academy of Sciences 88(10):4396–4400. 10.1073/pnas.88.10.4396 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steriade M, Pare D, Parent A, Smith Y. 1988. Projections of cholinergic and non-cholinergic neurons of the brainstem core to relay and associational thalamic nuclei in the cat and macaque monkey. Neuroscience 25(1):47–67. 10.1016/0306-4522(88)90006-1 [DOI] [PubMed] [Google Scholar]
- Stewart CV, Plenz D. 2006. Inverted-u profile of dopamine-nmda-mediated spontaneous avalanche recurrence in superficial layers of rat prefrontal cortex. J Neurosci 26(31):8148–8159. 10.1523/JNEUROSCI.0723-06.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swadlow HA, Gusev AG. 2001. The impact of ‘bursting’ thalamic impulses at a neocortical synapse. Nat Neurosci 4(4):402–408. 10.1038/86054 [DOI] [PubMed] [Google Scholar]
- Tigges M, Tigges J, Rees H, Rye D, Levey AI. 1997. Distribution of muscarinic cholinergic receptor proteins m1 to m4 in area 17 of normal and monocularly deprived rhesus monkeys. J Comp Neurol 388(1):130–145. [DOI] [PubMed] [Google Scholar]
- Treue S 2001. Neural correlates of attention in primate visual cortex. Vol 24 p 295–300. 10.1016/S0166-2236(00)01814-2 [DOI] [PubMed] [Google Scholar]
- Turrini P, Casu MA, Wong TP, De Koninck Y, Ribeiro-da-Silva A, Cuello AC. 2001. Cholinergic nerve terminals establish classical synapses in the rat cerebral cortex: Synaptic pattern and age-related atrophy. Neuroscience 105(2):277–285. 10.1016/S0306-4522(01)00172-5 [DOI] [PubMed] [Google Scholar]
- Uhlrich DJ, Tamamaki N, Murphy PC, Sherman SM. 1995. Effects of brain stem parabrachial activation on receptive field properties of cells in the cat’s lateral geniculate nucleus. J Neurophysiol 73(6):2428–2447. 10.1152/jn.1995.73.6.2428 [DOI] [PubMed] [Google Scholar]
- Umbriaco D, Watkins KC, Descarries L, Cozzari C, Hartman BK. 1994. Ultrastructural and morphometric features of the acetylcholine innervation in adult rat parietal cortex: An electron microscopic study in serial sections. J Comp Neurol 348(3):351–373. 10.1002/cne.903480304 [DOI] [PubMed] [Google Scholar]
- Usrey WM, Alitto HJ. 2015. Visual functions of the thalamus. Annu Rev Vis Sci 1(1):351–371. 10.1146/annurev-vision-082114-035920 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Essen D, Deyoe Ea. 1995. Concurrent processing in the primate visual cortex. p 383–400. 10.1016/0166-2236(88)90130-0 [DOI] [Google Scholar]
- Wainer BH, Steininger TL, Roback JD, Burke-Watson MA, Mufson EJ, Kordower J. 1993. Chapter 2: Ascending cholinergic pathways: Functional organization and implications for disease models Cholinergic function and dysfunction. Vol Volume 98 Progress in brain research; p 9–30. 10.1016/s0079-6123(08)62378-x [DOI] [PubMed] [Google Scholar]
- Weyand TG. 2016. The multifunctional lateral geniculate nucleus. Rev Neurosci 27(2):135–157. 10.1515/revneuro-2015-0018 [DOI] [PubMed] [Google Scholar]
- Woolf NJ, Butcher LL. 1986. Cholinergic systems in the rat brain: Iii. Projections from the pontomesencephalic tegmentum to the thalamus, tectum, basal ganglia, and basal forebrain. Brain Research Bulletin 16(5):603–637. 10.1016/0361-9230(86)90134-6 [DOI] [PubMed] [Google Scholar]
- Xu X, Bosking W, Sary G, Stefansic J, Shima D, Casagrande V. 2004. Functional organization of visual cortex in the owl monkey. J Neurosci 24(28):6237–6247. 10.1523/JNEUROSCI.1144-04.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaborszky L, Csordas A, Mosca K, Kim J, Gielow MR, Vadasz C, Nadasdy Z. 2015. Neurons in the basal forebrain project to the cortex in a complex topographic organization that reflects corticocortical connectivity patterns: An experimental study based on retrograde tracing and 3d reconstruction. Cereb Cortex 25(1):118–137. 10.1093/cercor/bht210 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zahrt J, Taylor JR, Mathew RG, Arnsten AF. 1997. Supranormal stimulation of d1 dopamine receptors in the rodent prefrontal cortex impairs spatial working memory performance. J Neurosci 17(21):8528–8535. 10.1523/JNEUROSCI.17-21-08528.1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zou Q, Long X, Zuo X, Yan C, Zhu C, Yang Y, Liu D, He Y, Zang Y. 2009. Functional connectivity between the thalamus and visual cortex under eyes closed and eyes open conditions: A resting-state fmri study. Hum Brain Mapp 30(9):3066–3078. 10.1002/hbm.20728 [DOI] [PMC free article] [PubMed] [Google Scholar]