Abstract
Advances in genetic research have led to an increased understanding of genotype-phenotype relationships. Excessive eating and weight gain characteristic of Prader-Willi syndrome (PWS) have been the understandable focus of much of the research. The intense preoccupation with food, lack of satiation, and incessant food seeking are among the most striking features of PWS. It has become increasingly clear that the behavioral phenotype of PWS also includes symptoms similar to obsessive compulsive disorder, which in all probability interact with the incessant hunger and lack of satiation to engender the intense preoccupation and food seeking behavior that is characteristic of this disorder. Several lines of evidence suggest that genetic material on chromosome 15 may alter synthesis, release, metabolism, binding, intrinsic activity, or reuptake of specific neurotransmitters, or alter the receptor numbers and/or distribution involved in modulating feeding. Among the likely candidates are GABAnergic, serotonergic, and neuropeptidergic mechanisms. This review summarizes what is known about the appetitive behavior and compulsivity in PWS and discusses the possible mechanisms underlying these behaviors.
Keywords: Prader-Willi syndrome, chromosome 15q deletion, maternal uniparental disomy, appetitive behavior, eating disorder, compulsive behavior, OCD, GABA, serotonin, neuropeptides, neurochemical mechanisms
In Mental Affections of Children and Youth [1887] J. Langdon Down described a 25-year-old woman with intellectual disability who had short stature, short fingers, and small feet, who had a voracious appetite and was very obese, whom he labeled as having polysarcia. Down distinguished this young woman and other similar individuals from other people with intellectual disabilities, also of short stature with short hands who, he said, had Mongolism, now recognized as usually being trisomy 21. He noted the two groups differed in the former’s exceptional appetite and universal obesity. Prader, Labhart, and Willi [1956] described several cases of such individuals and proposed that they constituted a distinct syndrome. Since 1981, it has been known that Prader-Willi syndrome (PWS) is associated with an abnormality of chromosome 15, usually (60% of the time) a paternally derived deletion of the 15q11-q13 region, yet nearly 40% of individuals with features of PWS appeared to have normal appearing chromosomes. In 1989, Nicholls et al., using more recently developed techniques, found that many individuals who did not have a deletion, but appeared to have classic phenotypic features of PWS, had received both copies of chromosome 15 from their mothers. These cases of maternal uniparental disomy constitute 30–35% of all cases of PWS. It would be some time until it was discovered the remaining 5% of cases, usually less severely affected, had microdeletions, translocations, or imprinting mutations of portions of the 15q11-q13 region.
Genomic imprinting refers to the differential expression of genetic traits depending on whether the trait has been inherited from a mother or a father. Another way to think of genomic imprinting is as “parent-of-origin differences” in the expression of inherited traits. In imprinted regions of a chromosome, only one copy of the genes is transcribed, the other remaining genetically silent (at least in somatic cells). The two maternally-derived 15th chromosomes may be the same (both chromosome 15s derived from one 15, called isodisomy), different (the result of the child’s inheritance of two different maternal 15s, called heterodisomy), or, most commonly, a combination of the two maternal chromosomes due to recombination. In all cases, the chromosomes themselves are structurally normal. This type of PWS is referred to as uniparental disomy (UPD).
The paternal contribution is believed to be necessary because the homologous maternally–derived genes are inactivated by methylation. Methylation involves a structural modification of DNA that occurs mostly in inactive genes. This modification involves the addition of a methyl group (–CH3) to cytosine bases in the DNA chain. It typically inactivates genes that are not required for expression in a particular type of cell. An important feature of the methylation of cytosine lies in its ability to be copied, so that methyl groups in a dividing cell’s DNA will result in methyl groups in the same positions in the DNA of both daughter cells.
Angelman syndrome also involves imprinting of the same chromosome region as PWS, except that the maternal contribution ofthe critical region is missing instead of the paternal contribution. The terminology used to describe the role of imprinting in these two disorders is somewhat confusing but goes as follows below.
Individuals with PWS lacking the 15q11–13 region (i.e., deletions) are missing at least two genes believed to play a critical role in features of the syndrome, SNRPN [Ozcelik et al., 1992] and NECDIN [Sutcliffe et al., 1997]. Other contiguous genes also may be implicated in features of the syndrome as well, such as UBE3A [Matsuura et al., 1997]. People with two maternal copies are missing the paternal copies of SNRPN, NECDIN, and other genes that are dependent on parent of origin. The severity of features in Prader-Willi syndrome appears to depend on how many copies of each parent’s genes in this region are missing.
The intellectual and cognitive characteristics of people with PWS have been reviewed recently [Dykens, 1999; Thompson, et al. 1999] and will be summarized here. Decreased intelligence and related adaptive behavior deficits are commonly reported characteristics of persons with PWS; however not all people with PWS have mental retardation. People with PWS typically score in the low-average or mild to moderate range of mental retardation. There is evidence that cognitive profiles of individuals with PWS vary with genetic subtype. In 1999, Dykens et al. reported that subjects with UPD genetic subtype had higher full-scale IQ scores than subjects with a diagnosis of deletion subtype. However, because some subjects were tested using the Kaufman Brief Intelligence Test (K-BIT) [Kaufman and Kaufman, 1990], while IQ scores from other subjects were obtained via parental report of scores from different tests, these findings should be interpreted with caution.
Recently, Roof et al. [2000] provided additional evidence of an uneven profile of intelligence among the PWS genetic subtypes. They found subjects with UPD subtype had significantly higher verbal IQ scores than those with deletion subtype, although there were no significant differences between the two groups in performance IQ or full-scale IQ scores. Verbal IQ differences were driven by significantly higher scores for subjects with UPD subtype on the following subtests of the Wechsler scales (either the WAIS-R or the WISC-III) [Wechsler, 1981; Wechsler, 1991]: Vocabulary, Information, Comprehension and Similarities. Interestingly, subjects with UPD subtype did not exhibit higher performance on academic achievement measures (e.g., reading achievement) as might be expected given their higher verbal IQ score. Roof et al. [2000] found that another factor might be mediating academic performance. Data indicated that subjects with UPD subtype performed significantly more poorly on a measure of visual organization (e.g., Object Assembly) than those with deletion status. Fox et al. [1999] also found that the performance of the same UPD PWS subtype group was substantially inferior to the deletion group on a task of random element stereo form discrimination. Roof and colleagues [2000] comment that this specific visual perceptual deficit may relate directly to individuals with UPD subtype performing lower than expected on specific learning tasks.
APPETITIVE BEHAVIOR IN PRADER-WILLI SYNDROME
The intense preoccupation with food, lack of satiation, and incessant food seeking among people with PWS are the most striking features of the syndrome. People with PWS often report being hungry, seem never satiated, and rarely vomit. The increased food intake, along with the decreased physical activity and low metabolic rate ultimately result in rapid weight gain and obesity. As newborns, infants often exhibit a poor suck reflex and thus require gavage feeding for several months before the onset of the eating disorder occurs [Butler, 1990; Greenswag and Alexander, 1995], usually between the age of 18 months and 5 years [Zellweger and Schnieder, 1968; Hall and Smith, 1972; Cassidy, 1984; Clarke, 1993; Greenswag and Alexander, 1995]. Recently, Dimitropoulos et al. [1999] investigated the onset of eating disorder and the emergence of behavior problems; they found that the majority of children with PWS exhibited the eating disorder at approximately two years of age.
Anecdotal reports from parents and caregivers of people with PWS suggest that, in addition to the compulsive eating, people with PWS often display atypical eating behaviors. Research to date has focused on identifying food preferences and excessive appetite characteristics in this population but has yet to address these often-reported atypical behaviors [Caldwell et al., 1983; Taylor and Caldwell, 1985; Glover et al., 1996; Rankin and Mattes, 1996]. Holm and Pipes [1976] reported clinical findings of peculiar food-related behavior among those with PWS. These included sneaking food, gorging food, and consuming products that are commonly considered as unappealing or unappetizing (e.g., dog food, garbage, sticks of butter, and decayed apples). In 1996, Sarimski used the Children’s Eating Behavior Inventory (CEBI) [Archer et al., 1991] to assess food preferences, feeding skills, behavior compliance, and mealtime interactions between family members in 28 subjects with PWS and 32 subjects with Williams syndrome, ranging from 2 to 25 years of age. Parents of people with PWS reported their children enjoy eating, ask for food between meals, eat foods that they should not eat, and hide food. To identify the appetite patterns of children with PWS, Zipf and Berntson [1987] assessed appetite in 10 children with PWS and 9 obese matched control children. The appetite test consisted of continuously consuming available sandwich quarters for one hour’s duration. Although the rate of eating did not differ between groups, children with PWS ate for a significantly longer period of time indicating the presence of a satiation deficit.
Researchers are now trying to link the odd eating characteristics commonly seen in PWS to other aspects of their behavior. For example, Dimitropoulos et al. [2000] observed 26 people with PWS during mealtime in order to determine whether people with PWS engage in eating patterns that are ritualistic in nature and whether these behaviors relate to compulsive characteristics often seen in this population. Other mealtime characteristics such as meal duration, bite-rate, and the effect of social situation on rate of eating also were targeted. Subjects with PWS (and 13 obese and mental-age matched control subjects) between the ages of 10 and 49 years were given a standard meal (approx. 700 kcal). Scores were coded for duration of meal, eating patterns, abnormal meal-time behavior, and bite-rate within various social conditions (e.g., TV on/off or other people present during meal). Subjects also were assessed for compulsive behavior using the Yale-Brown Obsessive Compulsive Scale (Y-BOCS) [Goodman et al., 1989] and the Compulsive Behavior Checklist (CBC) for Clients with Mental Retardation [Gedye, 1992]. Results indicated that groups differed in frequency of switching from one food type to another food type on the plate. People with PWS switched from food to food less often than matched controls. In addition, the ‘ritualized eating behavior’ item from the Y-BOCS symptom checklist was more frequently endorsed in PWS than matched controls. Among subjects with PWS, bite rate was negatively correlated with ordering nonfood objects into patterns and arranging nonfood objects to fit into a particular location [Dimitropoulos et al., 2000].
COMPULSIVE BEHAVIOR IN PRADER-WILLI SYNDROME
Adults
People with PWS exhibit behaviors that are repetitive and ritualistic in nature. Often these behaviors are related to preoccupation with food or food seeking, but non-food-related behaviors also are frequently observed. Older children and adults with PWS exhibit statistically significant elevations on several compulsivity subscales of the Y-BOCS. Stein et al. [1994] found that compulsive behaviors such as hoarding, arranging, and counting, as well as other repetitive actions, were common in patients with PWS. In 1996, Dykens and colleagues found that a high proportion of people with PWS exhibited moderate to severe symptom severity ratings on the Y-BOCS. Approximately 80% showed adaptive impairment associated with the compulsive behavior. The study also found that people with obsessive compulsive disorder (OCD) showed similar levels of symptom severity and numbers of compulsions when compared to people with PWS. There appeared to be more similarities than differences when comparing the two groups. An increased risk of OCD in people with PWS was indicated. Hoarding and the need to tell, ask, or know were compulsions seen in over 50% of the subject sample. Other commonly endorsed behaviors included ordering and arranging items and repeating rituals.
Recent subtype differences with regard to compulsive behavior were identified by Dykens et al. [1999]. They found that the subgroup with a chromo-some 15 deletion had a higher proportion of clinically elevated scores on the Child Behavior Checklist (CBCL) [Achenbach and Edelbrock, 1983] as well as significantly more symptom related distress on the Y-BOCS than did the subgroup with UPD. In addition, they noted a reduction of symptom severity on the same measures among the 23 age-and gender-matched UPD subjects. These behaviors, which affect a large portion ofthe PWS population, are considered to be compulsions, yet OCD has not been readily diagnosed in PWS. It appears that while people with PWS have a compulsive disorder that overlaps with that seen in other conditions, it may involve different mechanisms.
Although studies have identified compulsive behavior as a salient characteristic of PWS, the research regarding obsessive behavior in PWS is less clear. Researchers report that detection of obsessive symptoms in people with mental delay has been challenging because of the difficulty in assessing the internalizing characteristics of obsessive symptoms [Gedye, 1992]. Because of this difficulty, the relationship between the behavior exhibited by people with PWS and those diagnosed with obsessive-compulsive disorder remains to be seen.
Self-injurious behavior in the form of self-inflicted skin picking (to the point of drawing blood) also is associated with PWS and often is reported as a compulsion along with the other repetitive and ritualistic behaviors [Clarke et al., 1989; Dykens et al., 1992; Stein et al., 1994]. Whitman and Accardo [1987] found skin picking to be a problematic behavior for 69% of a sample of adolescents with PWS. Symons et al. [1999] found 82% of people surveyed with PWS engaged in self-injury, with skin picking being the predominant form of injury. They also reported that individuals with the deletion-based subtype skin pick significantly more body sites than subjects with UPD genetic status. In 1998, Feurer et al. reported a detailed analysis of the factor structure of the Compulsive Behavior Checklist (CBC) [Gedye, 1992]. The CBC is a 25-item checklist specifically designed to obtain information about compulsive behavior in people with mental retardation. The recommended scoring system for the CBC, which includes tallying endorsed behaviors and clustering behaviors into theoretically defined groups, has not been validated in people with PWS. Feurer et al. [1998] found that 24 of 25 CBC items comprised a general factor (item loadings ≥ 0.46) and should be tallied as a single score in PWS. Skin picking, the only CBC item that specifically describes a self-injurious behavior, did not load on the general factor (loading = 0.06) and therefore should be scored as a unique item. The factor structure of compulsive behaviors in people with PWS does not typify what would be expected for psychiatric patients diagnosed with OCD [Feurer et al., 1998]. Furthermore, the mechanisms that drive self-injurious behavior are not well understood, although several neurotransmitters are thought to be involved and will be discussed further.
Early Childhood
Before advances in early diagnosis, young children with PWS received little attention, particularly with regard to their behavioral and emotional characteristics. For this reason, behavior problems have been reported mainly in older people with PWS, while identification of early-onset phenotypically expressed behavior has not been undertaken until recently. For example, compulsive behavior has not been studied systematically in younger children with the syndrome even though some suggest that compulsive-like symptomology develops before adulthood [Dykens et al., 1996; Stein et al., 1994]. Recently, several studies have included children. Dykens and Kasari [1997] compared children with PWS between the ages of 4 and 19 years with children with Down syndrome and nonspecific mental retardation using the CBCL. Results indicated that compulsive behavior was prevalent in the PWS group. Sixty-eight percent of children with PWS exhibited compulsions whereas only 32% of Downs and 37% of children with nonspecific mental retardation displayed compulsive behavior. State et al. [1999] compared obsessive-compulsive symptoms in eight subjects with PWS-deletion subtype and eight subjects who did not present the PWS genotype but who had similar phenotypic expression (age range: 4 to 20 years). The groups did not differ with regard to obesity, IQ, food related problems, and overall maladaptive behavior, but subjects with PWS deletion-based status did exhibit a significantly greater number of obsessive-compulsive symptoms and greater symptom severity than the non- PWS group. State et al. [1999] theorized that obsessive-compulsive symptoms are part of the behavioral phenotype that is related to a deletion on chromosome 15.
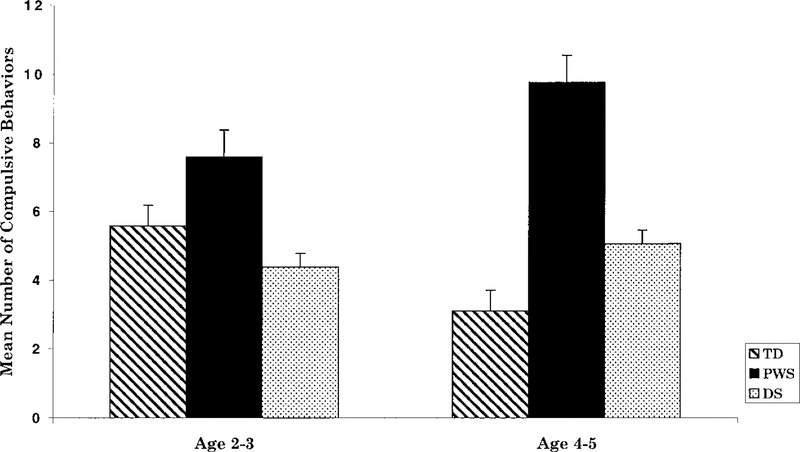
Recently, Dimitropoulos et al. [1999] investigated the development of behavior problems in a sample of children with PWS and identified the approximate age at which various types of behavior problems developed, including compulsive behavior. The sample included 84 children with PWS between 2 and 5 years of age compared with 56 children with Down syndrome and 76 typically developing peers. Measures obtained from children with PWS as young as two years of age were compared with those from a sample of older preschoolers with PWS between the ages of four and five. Children with PWS displayed more types of compulsive behavior than either children with Down syndrome or typically developing peers (Fig. 1). In addition, while older typically developing children exhibited fewer compulsive behaviors than the younger group, there was a trend towards an increase in compulsive behaviors among the children with PWS. Skin picking was also prevalent among children with PWS, while not prevalent in the Down syndrome or typically developing groups. Approximately one-third of the sample of children with PWS engaged in skin picking [Dimitropoulos et al., 1999].
Fig. 1.
Mean (±SE) number of compulsive behaviors by age group among children with typical development, PWS, and Down syndrome. Analysis of variance tests indicated a significant interaction effect (P < 0.05) between age group and subject group. From “Emergence of compulsive behavior and tantrums in children with Prader-Willi Syndrome,” by Dimitropoulos et al. [1999], with permission.
POSSIBLE MECHANISMS UNDERLYING EXCESSIVE EATING AND BEHAVIOR PROBLEMS
Several lines of evidence suggest genetic material on chromosome 15 may alter synthesis, release, metabolism, binding, intrinsic activity, or reuptake of specific neurotransmitters, or alter the receptor numbers and/or distribution, involved in modulating feeding. Among the likely candidates are GABAnergic, serotonergic, and neu-ropeptidergic mechanisms.
Possible Serotonergic Mechanisms in Food Intake and Compulsive Behavior
That serotonin influences eating is well known. Laboratory animal and human clinical evidence indicates drugs that increase serotonin reduce food intake and lead to weight loss. Luo and Li [1990, 1991] found fluoxetine, a serotonin reuptake inhibitor, selectively reduced consumption of a high carbohydrate-low protein diet. Kanarek et al. [1991] also found weight loss and food intake reduction by rats treated with fluoxetine, but the effects on a high fat diet were greater than on a high carbohydrate diet.
Dech and Budow [1991] treated a 17-year-old youth with mild mental retardation and PWS with fluoxetine, and reported a marked improvement in weight control and some improvement in other compulsive behavior. Hellings and Warnock et al. [1992] saw marked improvement in skin picking in two patients with PWS, which they hypothesized was a variant in the spectrum of obsessive compulsive disorder. Benjamin et al. [1993] found that skin picking, as well as food foraging and temper outbursts, was successfully managed by a combination of naltrexone and fluoxetine. Stein et al. [1994] used survey reports of 384 persons with PWS to determine if caregivers felt that selective serotonin reuptake inhibitors helped with certain impulsive, aggressive, and compulsive behaviors. Forty-seven percent of the PWS patients surveyed displayed at least one classical OCD behavior, but only 18% were receiving pharmacological agents to decrease this behavior. They found that SS-Rl’s were helpful in treating temper outbursts and compulsions, antipsychotics in treating skin picking and compulsions, and stimulants in treating overeating. Selikowitz et al. [1990] treated 15 people with PWS, having an average age of 27 years, for 6 weeks with another serotonin agonist, fenfluramine, obtaining significant weight loss and food-related emotional behavior, but no change in skin picking. There have been few systematic studies looking at how SSRI’s and other medications alleviate compulsive and impulsive/aggressive behaviors. Recently, Akefeldt and coworkers [1998] reported increased concentration of the serotonin metabolite 5-HIAA in CSF of individuals with PWS, which supports the hypothesis of an increased serotonin turnover in PWS and possibly decreased synaptic serotonin transmission.
Possible GABAnergic Mechanisms in Feeding
Dahir and Butler [1991] postulated that the GABA-A beta-3 receptor gene (GABRB3) may play a role in the abnormal eating behavior in PWS subjects. Wagstaff et al. [1991] reported that the gene for the beta-3 subunit of the GABA-A receptor is located on the proximal long arm of chromosome 15, which is deleted in most people with PWS. Since that time two additional GABA receptor genes (i.e., GABA alpha 5 and gamma 3) have been located in the same chromosome region. If binding of GABA to the GABA-A receptor or GABA-A mediated function is decreased due to a reduction in the presence of the beta-3, alpha-5 or gamma-3 subunits of the GABA-A receptor, there may be a compensatory, feedback-induced increase in GABA release. Recently GABRB3, GABRA5, and GABRG3 genes were found to be differentially expressed using micro-cell mediated chromosome transfer implying that these GABA-A receptor subunit genes may be candidates for features seen in PWS [Me- guro et al., 1997].
Ebert and colleagues [1997] found elevated blood GABA levels in people with PWS as compared to age- and weight-matched controls. High densities of GABA-A receptors are found in the anterior and medial hypothalamus and more modest levels throughout the entire hypothalamic region [Wagstaff et al., 1991]. Since the ventromedial hypothalamus is a satiety center and the lateral hypothalamus is a feeding center, it may be reasonable to suspect alterations of the GABA-A receptor distributions in these regions may result in elevated GABA levels in the presence of normal GABA-B receptors. That such alterations could reduce satiety or increase feeding is a reasonable hypothesis. Another indirect mechanism by which inadequate GA-BAnergic inhibition could influence food intake is via exacerbation of repetitive compulsive eating mediated by andrenergic neurons in the head of the caudate and the frontal cortex.
Evidence of Other Neuropeptide Involvement in PWS
Many appetite regulating factors are involved in hunger and satiation, including cholecystokinin, bombesin, gastrin releasing peptide, insulin, pancreatic glucagon, glucagon-like peptide 1, calcitonin, neuropeptide Y, satietin, ceruletide, leptin (Ob protein), pancreatic polypeptide, orexins, and several separate protein receptors in the brain. Until recently, some of these factors have been understudied in PWS and in human obesity in general. Holland et al. [1993] studied behavioral, cognitive, and metabolic responses to 1-hour food intake sessions in 13 adults with PWS and 10 matched controls. Findings indicated that increases in plasma glucose levels were inversely correlated with changes in hunger ratings among people with PWS but not among matched controls. In addition, there was a significantly greater increase in the levels of serum cholecystokinin during the meal in the PWS group than in the controls, indicating that in PWS, failure of peripheral CCK release in response to food was not the explanation for the impaired satiety response [Holland et al., 1993].
Cells in the paraventricular nucleus ofthe hypothalamus are related to satiety in animal laboratory studies [Leibowitz et al., 1981; De Vries and Buijs, 1983]. Paraventricular peptides may be involved specifically in eating abnormalities in PWS, as well. Swaab et al. [1995] found 42% to 54% decreases in parvocellular oxytocin neurons in the hypothalamic paraventricular nuclei in 5 individuals with PWS compared with 27 matched controls. Martin et al. [1998] found that CSF oxytocin was elevated in PWS as compared with normal control subjects, especially for female subjects. Gabreels et al. [1994] studied the supraoptic and paraventricular nuclei of five subjects with PWS using antibodies against the 7B2 polypeptide. This peptide is of specific interest because the 7B2 gene is located close to the q11–13 region on chromosome 15, which is typically deleted in PWS. However, Butler and Mbikay (personal communication, 1989) found no deletion of the 7B2 gene in 15 PWS subjects using quantitative Southern hybridization methods. Three of five PWS subjects reported by Gabreels et al. [1994] showed no reaction to the 7B2 antibodies, meaning the 7B2 gene products were missing from the paraventricular nuclei of over half of the PWS individuals.
COMPULSIVE BEHAVIOR AND GABA
Wagstaff et al. [1991] showed that the gene encoding the beta-3 subunit of the GABA-A receptor (GABRB3) maps to the region of 15q involved in Angelman syndrome and Prader-Willi syndrome. The gene was found to be deleted in individuals of both types with interstitial cytogenetic deletions. The gene was also deleted in an Angelman syndrome patient with an unbalanced 13;15 translocation but not in a PWS patient with an unbalanced 9;15 translocation. Wagstaff et al. [1991] suggested that this receptor gene may be involved in the pathogenesis of one or both of these syndromes. Since we have previously reported [Ebert et al., 1997] elevated levels of GABA in plasma of individuals with PWS and Angelman syndrome (presumably reflecting up-regulation of GABA due to improper binding at GABA receptors), it might be hypothesized that lack of GABAnergic inhibition of dopaminergic and serotonergic neurons in orbitofrontal and pre-frontal cortex and the head of the caudate nucleus may be implicated in the compulsive symptoms seen in PWS. This hypothesis is consistent with recent neuroimaging findings (see recent review by Brody et al. [1998]) that suggest failure of GABAnergic inhibition of glutamaner-gic, dopaminergic, and serotonergic pathways between frontal cortex and basal ganglia are involved in obsessive compulsive disorder in psychiatric patients.
CONCLUSION
Excessive eating and weight gain characteristic of Prader-Willi syndrome have been the understandable focus of much of the research. Researchers also have become increasingly interested in understanding the compulsivity and other maladaptive behavior that exist in this population. The fact that compulsive behaviors emerge as early as two years of age and continue into adulthood strongly suggests that biological mechanisms play a significant role. It has become increasingly clear that the behavioral phenotype of PWS includes compulsive symptoms similar to obsessive compulsive disorder, which in all probability interact with the incessant hunger and lack of satiation to engender the intense preoccupation and food seeking characteristic of Prader-Willi syndrome. This raises the possibility of dysregulation of GABAnergic inhibition of adrenergic structures known to be involved in obsessive compulsive disorder, since genes regulating subunits ofthe GABA-A receptor are located in the usually deleted region. Additional research regarding the link between the eating disorder and compulsive behavior in PWS and the neurochemical mechanisms that are thought to be involved is warranted in this population.
The intense preoccupation with food, lack of satiation, and incessant food seeking among people with PWS are the most striking features of the syndrome. People with PWS often report being hungry, seem never satiated, and rarely vomit.
Acknowledgments
Grant sponsor: NICHD; Grant numbers: P01HD30329, P30HD15052, and T32HD07226. Grant sponsor: Prader-Willi Syndrome Association of America.
REFERENCES
- Achenbach TM, Edelbrock C. 1983. Manual for the child behavior checklist and revised behavior profile. Burlington: University of Vermont Department of Psychiatry. [Google Scholar]
- Akefeldt A, Ekman R, Gillberg C, et al. 1998. Cerebral fluid monoamines in Prader-Willi syndrome. Biol Psychiatry 44:1321–1328. [DOI] [PubMed] [Google Scholar]
- Archer L, Rosenbaum P, Streiner D. 1991. The Children’s Eating Behavior Inventory: reliability and validity results. J Pediatr Psychol 16:629–642. [DOI] [PubMed] [Google Scholar]
- Benjamin E, Buot-Smith T. 1993. Naltrexone and fluoxetine in Prader-Willi syndrome. J Am Acad Child Adolesc Psychiatry 32: 870–873. [DOI] [PubMed] [Google Scholar]
- Brody SS, Schwartz AL, Baxter LR. 1998. Neuroimaging and frontal-cortical circuitry in obsessive-compulsive disorder. Br J Psychiatry 35:26–37. [PubMed] [Google Scholar]
- Butler MG. 1990. Prader-Willi syndrome: Current understanding of cause and diagnosis. Am J Med Genet 35:319–332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butler MG, Mbikay M. 1989. Personal communication to Travis Thompson. [Google Scholar]
- Caldwell ML, Taylor RL. 1983. A clinical note on food preference of individuals with Prader-Willi syndrome: The need for empirical research. J Ment Defic Res 27:45–49. [DOI] [PubMed] [Google Scholar]
- Clarke DJ, Waters J, Corbett JA. 1989. Adults with Prader-Willi syndrome: Abnormalities of sleep and behavior. J R Soc Med 82:21–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cassidy SB. 1984. Prader-Willi syndrome. Curr Probl Pediatr 14:1–55. [DOI] [PubMed] [Google Scholar]
- Dahir GA, Butler MG. 1991. Is GABA-A receptor B-3 subunit abnormality responsible for obesity in persons with Prader-Willi syndrome? Dysmorph Clin Genet 5:112–113. [Google Scholar]
- Dech B, Budow L. 1991. The use of fluoxetine in an adolescent with Prader-Willi syndrome. J Am Acad Child Adolesc Psychiatry 30:298–302. [DOI] [PubMed] [Google Scholar]
- De Vries GJ, Buijs RM. 1983. The origin of the vasopressinergic and oxytocinergic innervation of the rat brain with special reference to the lateral septum. Brain Res 273:307–317. [DOI] [PubMed] [Google Scholar]
- Dimitropoulos A, Feurer I, Butler M, et al. 1999. Emergence of compulsive behavior and tantrums in children with Prader-Willi syndrome. Under review. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dimitropoulos A, Tapp J, Feurer I, et al. 2000. Ritualistic eating behavior and mealtime characteristics in Prader-Willi syndrome. Poster presented at the 33rd Annual Gatlin- burg Conference on Research and Theory in Mental Retardation and Developmental Disabilities, San Diego, CA. [Google Scholar]
- Down JL. 1887. Mental affectations of children and youth. London: J. and A. Churchill. [Google Scholar]
- Dykens EM. 1999. Prader-Willi syndrome: Toward a behavioral phenotype In Tager-Flus-berg H, editor. Neurodevelopmental disorders. Cambridge: The MIT Press; p 137–154. [Google Scholar]
- Dykens EM, Cassidy SB, King BH. 1999. Maladaptive behavior differences in Prader-Willi syndrome due to paternal deletion versus maternal uniparental disomy. Am J Ment Retard 104:67–77. [DOI] [PubMed] [Google Scholar]
- Dykens EM, Hodapp RM, Walsh K, et al. 1992. Adaptive and maladaptive behavior in Prader-Willi syndrome. J Am Acad Child Adolesc Psychiatry 31:1131–1136. [DOI] [PubMed] [Google Scholar]
- Dykens EM, Leckman JF, Cassidy SB. 1996. Obsessions and compulsions in Prader-Willi syndrome. J Child Psychol Psychiatry 37:995–1002. [DOI] [PubMed] [Google Scholar]
- Ebert MH, Schmidt DE, Thompson T, et al. 1997. Elevated plasma Gamma-Aminobutyric Acid (GABA) levels in individuals with either Prader-Willi syndrome or Angelman syndrome. J Neuropsychiatry Clin Neurosci 9:75–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feurer ID, Dimitropoulos A, Stone WL, et al. The latent variable structure of the Compulsive Behaviour Checklist in people with Prader-Willi syndrome. J Intellect Dis-abil Res 42:472–480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox R, Sinatra R, Mooney M, et al. 1999. Visual capacity in PWS. J Pediatric Opthamol Strabismus 36:331–336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gabreels BA, Swaab DF, Seidah NG, et al. 1994. Differential expression of the neuroendocrine polypeptide 7B2 in hypothalami of Prader-(Labhart)-Willi syndrome patients. Brain Res 657:281–293. [DOI] [PubMed] [Google Scholar]
- Gedye A. 1992. Compulsive behavior checklist for clients with mental retardation. The Habilitative Mental Healthcare Newsletter 11:73–77. [Google Scholar]
- Glover D, Maltzman I, Williams C. 1996. Food preferences among individuals with and without Prader-Willi syndrome. Am J Ment Retard 101:195–205. [PubMed] [Google Scholar]
- Goodman WK, Price LH, Woods SW, et al. 1989. The Yale-Brown Obsessive Compulsive Scale: Part I: Development, use and reliability. Arch Gen Psychiatry 46:1006–1011. [DOI] [PubMed] [Google Scholar]
- Greenswag LR, Alexander RC. 1995. Management of Prader-Willi syndrome. New York: Springer-Verlag. [Google Scholar]
- Hall BD, Smith DW. 1972. Prader-Willi syndrome. J Pediatr 81:286–293. [DOI] [PubMed] [Google Scholar]
- Hellings JS, Warnock JK. 1994. Self-injurious behavior and serotonin in Prader-Willi syndrome. Psychopharmacol Bull 30:245–250. [PubMed] [Google Scholar]
- Holland AJ, Treasure J, Coskeran J, et al. 1993. Measurement of excessive appetite and metabolic changes in Prader-Willi syndrome. Int J Obes 17:527–532. [PubMed] [Google Scholar]
- Holm VA, Pipes PL. 1976. Food and children with Prader-Willi syndrome. Am J Dis Child 130: 1063–1067. [DOI] [PubMed] [Google Scholar]
- Kanarek RB, Glick AL, Marks-Kaufman R. 1991. Dietary influences on the acute effects of anorectic drugs. Physiol Behav 49:149–152. [DOI] [PubMed] [Google Scholar]
- Kaufman AS, Kaufman NL. 1990. Kaufman Brief Intelligence Test. Circle Pines, MN: American Guidance Service. [Google Scholar]
- Leibowitz SF, Hammer NJ, Chang K. 1981. Hypothalamic paraventricular nucleus lesions produce overeating and obesity in the rat. Physiol Behav 27:1031–1040. [DOI] [PubMed] [Google Scholar]
- Luo S, Li ETS. 1990. Food intake and selection pattern of rats treated with dexfenfluramine, fluoxetine and RU 24969. Brain Res Bull 24:729–33. [DOI] [PubMed] [Google Scholar]
- Luo S, Li ETS. 1991. Effects of repeated administration of serotonergic agonists on diet selection and body weight in rats. Pharmacol Bio-chem Behav 3:495–500. [DOI] [PubMed] [Google Scholar]
- Martin A, State M, Anderson GM, et al. 1998. Cerebrospinal fluid levels of oxytocin in Prader-Willi syndrome: A preliminary report. Biol Psychiatry 44:1349–1352. [DOI] [PubMed] [Google Scholar]
- Matsuura T, Sutcliffe JS, Fang P, et al. 1997. De novo truncating mutations in E6-AP ubiquitin-protein ligase gene (UBE3A) in Angelman syndrome. Nat Genet 15:74–77. [DOI] [PubMed] [Google Scholar]
- Meguro M, Mitsuya K, Sui H, et al. 1997. Evidence for uniparental, paternal expression of the human GABAA receptor subunit genes, using microcell-mediated chromosome transfer. Hum Mol Genet 6:2127–2133. [DOI] [PubMed] [Google Scholar]
- Nicholls RD, Knoll JHM, Butler MG, et al. 1989. Genetic imprinting suggested by maternal heterodisomy in non-deletion Prader-Willi syndrome. Nature 342:281–285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozcelik T, Leff S, Robinson W, et al. (1992). Small nuclear ribonucleoprotein polypeptide N (SNRPN), an expressed gene in the Prader-Willi syndrome critical region. Nat Genet 2:265–269. [DOI] [PubMed] [Google Scholar]
- Prader A, Labhart A, Willi H. 1956. Ein Syndrome von Adipositas, Kleinwuchs, Kryptochismus und Oligophrenie nach myatonieartigem Zustand in Neugeborenenalter. Schweizerische Medizinische Wochenschrift 86:1260–1261. [Google Scholar]
- Rankin KM, Mattes RD. 1996. Role of food familiarity and taste quality in food preferences of individuals with Prader-Willi syndrome. Int J Obes Relat Metab Disord 20: 759–762. [PubMed] [Google Scholar]
- Roof E, Stone W, MacLean W, et al. 2000. Intellectual characteristics of Prader-Willi syndrome: comparison of genetic subtypes. J Intellect Disabil Res 44:25–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarimski K. 1996. Specific eating and sleeping problems in Prader-Willi and Williams-Beuren syndrome. Child Care Health Dev 22:143–150. [PubMed] [Google Scholar]
- Selikowitz M, Sunman J, Pendergast A, et al. 1990. Fenfluramine in Prader-Willi syndrome: A double-blind, placebo controlled trial. Arch Dis Child 65:112–114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- State MW, Dykens EM, Rosner B, et al. 1999. Obsessive-compulsive symptoms in Prader-Willi and “Prader-Willi-Like” patients. J Am Acad Child Adolesc Psychiatry 38: 329–334. [DOI] [PubMed] [Google Scholar]
- Stein DJ, Keating J, Zar HJ, et al. 1994. A survey of the phenomenology and pharmacotherapy of compulsive and impulsive-aggressive symptoms in Prader-Willi syndrome. J Neuropsychiatry Clin Neurosci 6:23–29. [DOI] [PubMed] [Google Scholar]
- Sutcliffe JS, Han M, Christian SI, et al. 1997. Neuronally expressed necdin gene is an imprinted candidate gene in Prader-Willi syndrome. Lancet 350:1520. [DOI] [PubMed] [Google Scholar]
- Swaab DF, Purba JS, Hofman MA. 1995. Alterations in the hypothalamic paraventricular nucleus and its oxytocin neurons (putative satiety cells) in Prader-Willi syndrome: A study of five cases. J Clin Endocrinol Metab 80:573–579. [DOI] [PubMed] [Google Scholar]
- Symons FJ, Butler MG, Sanders MD, et al. 1999. Self-injurious behavior and Prader-Willi syndrome: Behavioral forms and body locations. Am J Ment Retard 104:260–269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor RL, Caldwell ML. 1985. Type and strength of food preferences of individuals with Prader-Willi syndrome. J Ment Defic Res 29:109–112. [DOI] [PubMed] [Google Scholar]
- Thompson T, Butler MG, MacLean WE Jr, et al. 1999. Cognition, behavior, neurochemistry, and genetics in Prader-Willi syndrome In Tager-Flusberg H, editor. Neurodevelopmental disorders. Cambridge: The MIT Press; p 155–178. [Google Scholar]
- Wagstaff J, Knoll JHM, Fleming J, et al. 1991. Localization of gene encoding the GABA-A receptor beta-3 subunit to the Angelman/ Prader-Willi region of human chromosome 15. Am J Hum Genet 49:330–337. [PMC free article] [PubMed] [Google Scholar]
- Wechsler D. 1981. Manual for the Wechsler Intelligence Scale-Revised (WEIS-R). San Antonio: The Psychological Corporation. [Google Scholar]
- Wechsler D. 1991. Manual for the Wechsler Intelligence Scale for Children, 3rd edition (WEIS-III). San Antonio: The Psychological Corporation. [Google Scholar]
- Whitman B, Accardo P. 1987. Emotional symptoms in Prader-Willi syndrome adolescents. Am J Med Genet 28:897–905. [DOI] [PubMed] [Google Scholar]
- Zellweger H. Schneider HJ. 1968. Syndrome of hypotonia-hypomentia-hypogonadism-obesity (HHHO) or Prader-Willi syndrome. Am J Disabled Child 115:588–598. [DOI] [PubMed] [Google Scholar]
- Zipf WB, Berntson GG. 1987. Characteristics of abnormal food-intake patterns in children with Prader-Willi syndrome and study of effects of naloxone. Am J Clin Nutrition 46: 277–281. [DOI] [PubMed] [Google Scholar]