Abstract
Drug delivery to the brain remains challenging mainly due to the blood-brain barrier (BBB) that regulates the entrance of substances to the brain. Advances in nanotechnology have enabled the engineering of nanomedicines for biomedical applications including enhanced drug delivery into the brain. In this review, we describe strategies of nanomedicines engineered to traverse the BBB and deliver therapeutic molecules to target brain sites. We highlight the representative applications with materials including polymers, lipids, and inorganic elements for brain drug delivery. We finalize this review with the current challenges and future perspective of nanotherapeutics for advanced drug delivery to the brain.
Keywords: blood-brain barrier, engineered nanoparticles, nanomedicine, drug delivery, brain disease
Graphical Abstract
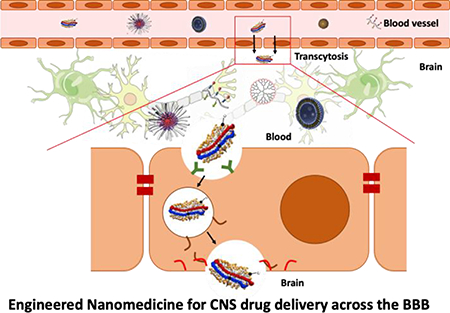
Introduction
According to American Brain Foundation, brain diseases affect the lives of 1 in 6 people, and treatment of the disease costs over a trillion dollars annually. Moreover, the number of patients suffering from brain disease is expected to be growing due to the prolonged aging; therefore, there is a growing need for development of effective therapeutics for brain diseases [1]. Nevertheless, there has been minimal successful development of therapeutic molecules for brain disease despite remarkable achievements in scientific and medical research for treatment of other diseases [2]. This challenge is largely because of the blood-brain barrier (BBB) that controls the accumulation and excretion of small molecules, protein, and even cells in the brain to protect it from the unexpected passage of substances from blood [3]. The barrier function of the BBB mainly comes from tightly connected vascular endothelial cells (ECs) that are surrounded by and communicate with pericytes and astrocytes. Highly expressed tight junctions of the ECs do not allow large molecules (approximately greater than 400 Da) to cross the barrier in a paracellular way while pericytes and astrocytes help ECs to maintain the barrier homeostasis by stabilizing the basement membrane and communicating with neural cells [4]. The challenge in brain drug delivery lies in transporting a sufficient amount of therapeutic agents across this BBB without disrupting the native barrier function required for the brain homeostasis [5]. Other approaches to the artificial opening of the BBB for drug delivery (e.g., using external stimulation such as ultrasound) won’t be included in this review.
Nanotechnology has revealed the distinct properties of diverse nanomaterials and paved the path for the applications for therapeutics, which is known as nanomedicine for promising next generation therapeutics with the following advantages for advanced drug delivery [6, 7]. First, various therapeutic molecules including biomolecules, genes, and small molecular agents can be loaded into diverse types of nanoparticles stable in circulation for the sustained delivery to target regions [8]. Second, the nanomedicine surface engineered with desirable properties by chemical modification enhances the stability and targeting ability [9]. Third, the intrinsic unique optical or electrical properties of nanomaterials can be exploited for additional functionality of nanoparticles for various biomedical applications [10]. Taking advantages of these features, nanoparticles have been designed to cross the BBB for the treatment of brain diseases.
This review begins with the introduction of the BBB physiology and describes the possible mechanisms with which drugs or drug-loaded nanoparticles can cross the barrier. We then describe the general use of nanoparticles for non-invasive delivery of therapeutic molecules and highlight various engineered nanomaterials including polymer, lipid, and inorganic nanoparticles for the delivery of therapeutic molecules across the BBB upon systemic administration. We will discuss the representative case studies to enhance the delivery efficiency for the treatment of brain diseases without altering brain microenvironment.
The BBB: Definition and Physiology
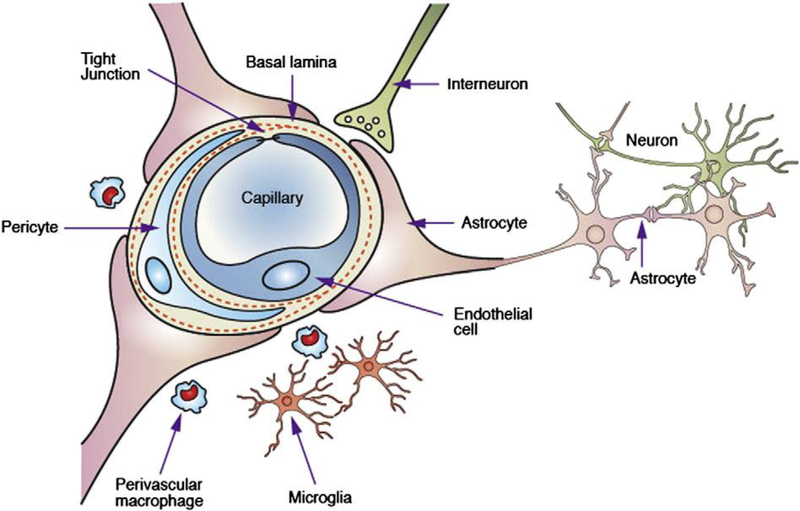
The BBB is a brain-specific, selective barrier that regulates the transport of substances between the circulation and the brain. The BBB mainly consists of endothelial cells, pericytes, and astrocytes, while other cellular components like microglia and neurons actively communicate with BBB cells, leading to the structural integrity of the BBB (Figure 1). Therefore, it is critical to understand the physiological and structural feature of the BBB for the administration of therapeutics into the brain.
Figure 1.
Schematic illustration of the blood-brain barrier (BBB) and other components of a neurovascular unit. Reproduced with permission [3]. Copyright 2006, Springer Nature.
Compared to other vascular endothelial cells, brain vascular endothelial cells, the key components of the barrier function of the BBB, have highly expressed tight junction proteins, such as zonula occludens-1 (ZO-1) and claudins, primarily inhibiting random diffusion of substances between the blood and the brain [11] and strictly regulating the paracellular transport of molecules reportedly with a size up to approximately 4 nm [12]. Pericytes reside in the basement membrane or luminal surface of the endothelium. The number of pericytes in the brain vasculature is much higher than that of other vasculature, even similar to the number of endothelial cells [13]. This high number of pericytes contributes to the regulation of blood flow, angiogenesis, and immune cell infiltration in response to several neural activities [14]. Astrocytes are the most common and diverse neuroglial cells and provide a cellular link between the neurons and blood vessels, contributing to the formation, function, and structural integrity of the BBB through intricate signaling pathways [5, 15]. Microglia are immune cells that regulate neuronal development, several innate and adaptive immune responses, and wound healing in the BBB [16, 17]. These complex activities and interactions of the BBB cells enable the maintenance of the physiological activity and flux of biomolecules across the barrier. The BBB integrity is often disrupted in pathological disease conditions including stroke, multiple sclerosis, brain injuries, Parkinson’s disease, and Alzheimer disease. The BBB breakdown is largely due to the remodeling of the protein complex located in the endothelial cell junctions [18].
From blood to brain: Mechanisms
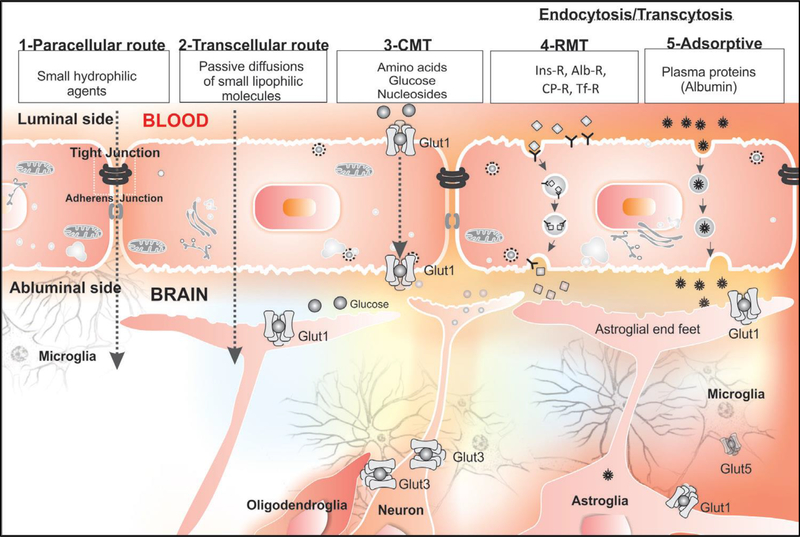
Despite the strict regulation of the material transport across the BBB, essential materials such as glucose can selectively penetrate into the brain for the maintenance of brain metabolism. The penetrance of molecules in circulation into the brain parenchyma generally occurs in a paracellular way (between endothelial cells), through passive diffusion across the endothelial cells, or via solute carriers and vesicular transport (Figure 2). The following section presents brief mechanistic features by which molecules can get into the brain through several routes.
Figure 2.
Transport of substances from blood to brain by several routes; paracellular route, transcellular route, carrier-mediated transporter (CMT), receptor-mediated transcytosis (RMT), and adsorptive-mediated transcytosis. Reproduced with permission [32] from Tabriz University of Medical Sciences licensed under a Creative Commons.
Paracellular transportation
Paracellular transportation represents the movement of substances through the gaps between two adjacent endothelial cells. Intercellular junctions between endothelial cells do not allow large molecules to pass them; however, molecules smaller than the gap can pass it through. At the BBB, tight junctions of the endothelium block the passage of molecules larger than ~ 4 nm, whereas small and water solutes can penetrate through paracellular transportation [19]. Studies have attempted to disrupt intercellular junctions to enhance the paracellular delivery of molecules using internal or external stimuli [20, 21]. This review does not include these BBB-disrupting or ‘invasive’ approaches.
Transcellular passive diffusion
Transcellular passive diffusion is a main pathway of small and hydrophobic molecules to interact with the endothelial plasma membrane of the BBB upon the concentration gradient across the barrier [22]. Modification of the chemical structure of small and hydrophobic molecules can further improve the kinetics of transcellular passive diffusion [23]. However, not all small and hydrophobic molecules can similarly cross the endothelial layer through transcellular passive diffusion, the principle of which remains to be studied for the discovery of small molecular therapeutics for brain diseases [24].
Transporters
Brain endothelial cells express various solute carriers to transport essential substances that could otherwise not pass through the BBB. Vital and small components such as specific ion, hexoses, amino acids, and fatty acids for brain metabolic activities can penetrate into the brain using the specific transporters [3]. For example, glucose is selectively delivered by the glucose transporter1 (GLUT-1) for energy production in brain, and amino acids cross the BBB using the specific transporters, such as L-type amino acid transporter (LAT)1 and 2, and cationic/anionic transporters, depending on the charge and size [25]. In addition, fatty acids and nucleic acids can also cross the barrier by their own specific transporters [26].
Transcytosis
Other substances unable to cross the BBB in a paracellular way, via transcellular diffusion, or by specific transporters may get into the brain through transcytosis [24, 27]. Transcytosis is one mechanism of a material transport through a cell through endocytosis and exocytosis, which is commonly observed in polarized cells [28, 29]. Transcytosis allows relatively large substances such as proteins to pass through a cell whereas other transport mechanisms involve with small molecules. The process of transcytosis is divided into three steps: endocytosis of a cargo on the surface of a plasma membrane, intracellular trafficking in the vesicle without lysosomal degradation, and the exocytosis on the opposite cellular orientation. This transport occurs in an adsorption-mediated way or receptor-mediated way. Adsorption-mediated transcytosis happens to cationic and large substances on the surface of endothelial cells largely followed by caveolin or clathrin, while receptor-mediated transcytosis is one common pathway to deliver specific ligands such as transferrin, insulin, deltrophin, and lipoproteins [24, 30, 31]. Both adsorption-mediated transcytosis and receptor-mediated transcytosis are widely used for nanomaterial transport into the brain [27–29]. Adsorption-mediated transcytosis is a main transport pathway for nanomaterials made of cationic components or positively charged surface, while receptor-mediated transcytosis is a strategy for nanoparticles functionalized to interact with cellular receptors.
Nanoparticles for Therapeutic Molecule Delivery (Nanomedicines)
Development of biotechnology has extended the concept of therapeutic molecules from conventional small molecular drugs to several biopharmaceutical agents such as recombinant proteins, functional peptides, monoclonal antibodies, and genetic molecules [33–35]. In a well-defined and controlled laboratory protocol, such therapeutic molecules have exhibited an enormous potential; however, the systemic effect in animal models has hardly reproducible largely due to the unexpected structural instability and non-specific distribution throughout the animal organs [36, 37]. This challenge in bench-to-bed translation of drugs underscores the needs for highly reproducible and effective drug delivery system [38]. In order to improve therapeutic efficacy of drugs, many delivery carriers have been developed to maintain the structural stability of therapeutic molecules and minimize undesired leakage of drugs in off-target regions. To date, various formulations such as micro/nanoparticles, patches, scaffolds, and hydrogels used for drug delivery have exhibited better therapeutic efficacy with the improved pharmacokinetics [39].
There are several administration routes for the delivery of therapeutic molecules into target sites, including transdermal, pulmonary, oral, and systemic delivery. Administrated formulations exhibit different pharmacokinetic profiles per route [40, 41]. Parenteral systemic delivery directly through blood vessels is commonly performed in drug delivery due to the fast effect and low loss of therapeutic molecules via the simple and direct injection of the therapeutic molecules into blood [42]. For effective drug delivery upon systemic administration into blood, the carriers are required to maintain the stability in circulation, to accumulate in a target tissue with a minimal off-target effect, and to internalize into a cellular compartment and release the cargo on a desirable site for better efficacy [43]. In this case, nanoscale formulations including polymeric, lipid, and inorganic nanoparticles are commonly exploited due to the unique physicochemical characteristics. Furthermore, by engineering the surface of nanoparticles with shielding materials, blood circulation time of those nanoparticles becomes longer for increased accumulation in target tissues. Conjugation of targeting ligands further enhances cell-type specific internalization after accumulation of nanoparticles in diseased tissues, finally exhibiting therapeutic effect at a cellular level.
In that regard, various nanoparticles have been utilized to deliver of therapeutic molecules across the BBB for the treatment of brain diseases [44, 45]. Surface modification of nanoparticles with specific ligands capable of binding to target receptors in brain endothelial cells may lead to receptor-mediated transcytosis of the engineered nanoparticles loaded with drug cargos, thereby delivering therapeutic molecules into the brain [46, 47]. In addition, the efflux system mainly operated by p-glycoproteins can inhibit the penetration of drug molecules across the BBB [48]. In that regard, delivery of therapeutic molecules by nanoparticles via receptor-mediated transcytosis is advantageous because the internalization process can exploit vesicular trafficking that reduces the efflux of external molecules. Several in vitro and in vivo studies have demonstrated the advantages of nanoparticle-based drug delivery to the brain (Figure 3). In the following section, we highlight representative studies that leverage various nanoparticles to effectively deliver therapeutic molecules across the BBB in a non-invasive manner.
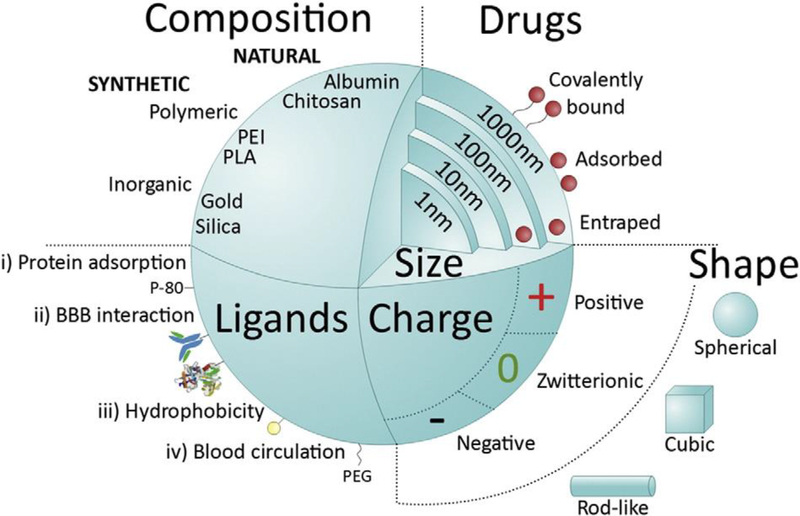
Figure 3.
Main features of nanomedicine influencing on systemic delivery and the BBB passage: size, shape, charge, and composition. Reproduced with permission [46]. Copyright 2016, Elsevier.
Nanoparticles Engineered to Deliver Therapeutic Molecules across the BBB
Polymer nanoparticles
Synthetic or natural polymers are highly promising materials for drug delivery thanks to the high versatility and biocompatibility depending on their backbone structure. Controlling the degradation/bond cleavage of polymers can also regulate the release kinetics in the body and facilitate the in vivo clearance of delivery carriers. Here we review three types of polymer nanoparticles for drug delivery; dendrimers, entangled solid polymer nanoparticles, and self-assembled micelles.
Dendrimers are highly symmetric, monodisperse, and spherical three-dimensional polymer networks [49]. In general, drug molecules are attached on the surface of dendrimers in a covalent manner. The size of dendrimers is determined by its generation of polymerization. A recent study demonstrated that the use of hydroxyl polyamidoamine (PAMAM) exhibited site-specific delivery of small molecular drugs across the BBB and blood-CSF barriers, specifically targeting the injured brain [50]. More recently, the same group further engineered PAMAM dendrimers with different generations and monitored the accumulation in a brain injury mouse model. Interestingly, the result showed a higher generation (6th generation) of the PAMAM dendrimer in a size of 6.7 nm that has a longer blood circulation time and more brain accumulation than those of a lower generation (4th generation) in a size of 4.3 nm [51]. Another study demonstrated that a PAMAM with cationic surface property also can cross the BBB followed by localization in neurons and glial cells when administered to the carotid artery [52]. Despite several studies showing the dendrimer-based BBB penetrance, the mechanism by which dendrimers can cross the BBB remains unclear.
Entangled polymer nanoparticles form a solid nanoparticle by intermolecular interactions between several polymers via multiple interactions such as Van der Waals or electrostatic interactions. For example, poly(D,L-lactide-co-glycolide) (PLGA), which is a copolymer of lactic acid and glycolic acid, is commonly used to deliver various therapeutic agents incorporated in the core of PLGA. Moreover, the chemical structure of PLGA enables slow hydrolysis and subsequent passive drug release in cellular environment, resulting in a sustained drug effect. In order to deliver nanoparticles across the BBB, a 65-nm PLGA nanoparticle has been developed to cross the BBB for the delivery to glioblastoma cells in the brain parenchyma [53] Furthermore, a poly(ethylene glycol) (PEG)-coated PLGA nanoparticle enhanced the circulation time by escaping from the elimination by the reticuloendothlial system (RES), thus enabling higher internalization of polymer nanoparticles in the brain (Figure 4) [54]. In addition to PLGA, several biodegradable or biocompatible polymers including poly(D,L-lactide) (PLA), polyglycolide (PGA), heparin, polycaprolactone (PCL), and polystyrene (PS) have been applied for the preparation of polymer nanoparticles. Size-dependent brain penetrance has been demonstrated using PS-PEG nanoparticles (20, 40, 100, and 500 nm) [55]. However, all models used in these studies are either brain injury or brain tumor models, where the BBB structure is often disrupted. On the other hand, targeting ligands are attached on the surface of nanoparticles in order to deliver those across the healthy or intact BBB, including cRGD [56, 57], PS-80 [58], transferrin [59], IgG [60], and Pep-1 [61]. Electrostatic interaction-based entangled polymer nanoparticles designed to deliver therapeutic molecules across the BBB used a nanocomplex that has a cationic polymer network, polyethyleneimine (PEI) and poly-L-lysine (PLL) with plasmid DNA [62]. The nanocomplex was further modified with Angiopep-2 to facilitate the transcytosis through LDLR-related protein (LRP)-receptor in brain endothelial cells, showing effective treatment of glioma at both cellular and animal levels.
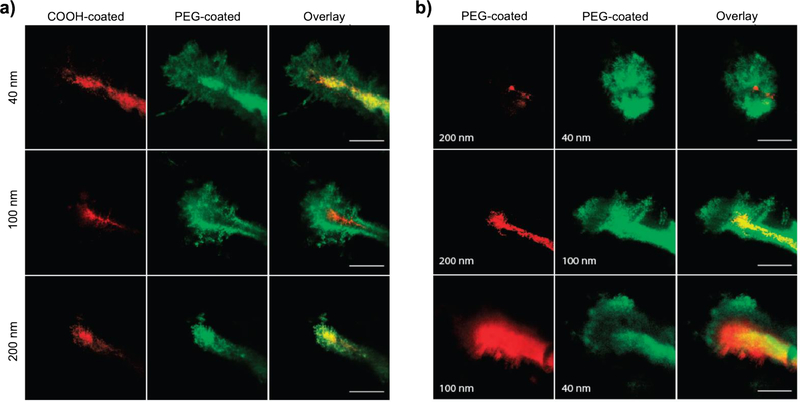
Figure 4.
Penetration of PLGA and PLGA-PEG nanoparticles into the brain in living mice. (a) Direct comparison of the distribution of fluorophore-loaded nanoparticles of similar sizes with different surface coatings 60 min after injection into the mice. (b) Direct comparison of the distribution of PEG-coated nanoparticles of various sizes 60 min after injection into the mice. Scale bar is 50 mm. Reproduced with permission [54]. Copyright 2012, AAAS.
Self-assembled micelles are composed of hydrophilic and hydrophobic block copolymers. The hydrophobic blocks are assembled in the middle of a nanoparticle to the core for the loading of hydrophobic drugs, while hydrophilic blocks spread out to the nanoparticle corona for maintaining the structure in an aqueous solution. Different from the additional coating of surfactants or stealth polymers on entangled polymer nanoparticles needed for anti-adsorption and anti-phagocytosis in circulation, self-assembled micelles form a stable and stealth nanoparticle itself due to the amphiphilic nature. Furthermore, by modifying the surface of hydrophilic corona with selective ligands, drug-loaded micelles can be internalized into the brain via transcytosis, followed by the release of therapeutic molecules upon micelle disruption in intracellular environment. Several types of micelles composed of various block copolymer formulations such as PAA-PEG [63], PLA-PEG [64, 65], DGL-PEG [66], PTMC-PEG [67], and PDSGM-PEG [68] have been reported for the delivery of therapeutic molecules across the BBB. Paclitaxel (PTX)-loaded PLA-PEG micelles modified the PEG block with t-Lyp1 ligand exhibited higher brain accumulation and internalization into the glioma cells, inhibiting the progression of glioma in an animal model [69]. A recent interesting study designed advanced wormlike polymer micelles made of PEG-grafted poly(2-diisopropyl methacrylate) (PDPA) block copolymers (mPEG-b-PDPA) to be degraded in response to brain tumor microenvironment so that drugs could be released into the target brain tumor (Figure 5) [70]. With further modification of the PEG with a RGD peptide, the micelle even enabled highly specific delivery of drugs into the specific brain tumor while the wormlike shape facilitated deeper penetration into the three-dimensional tumor tissue.
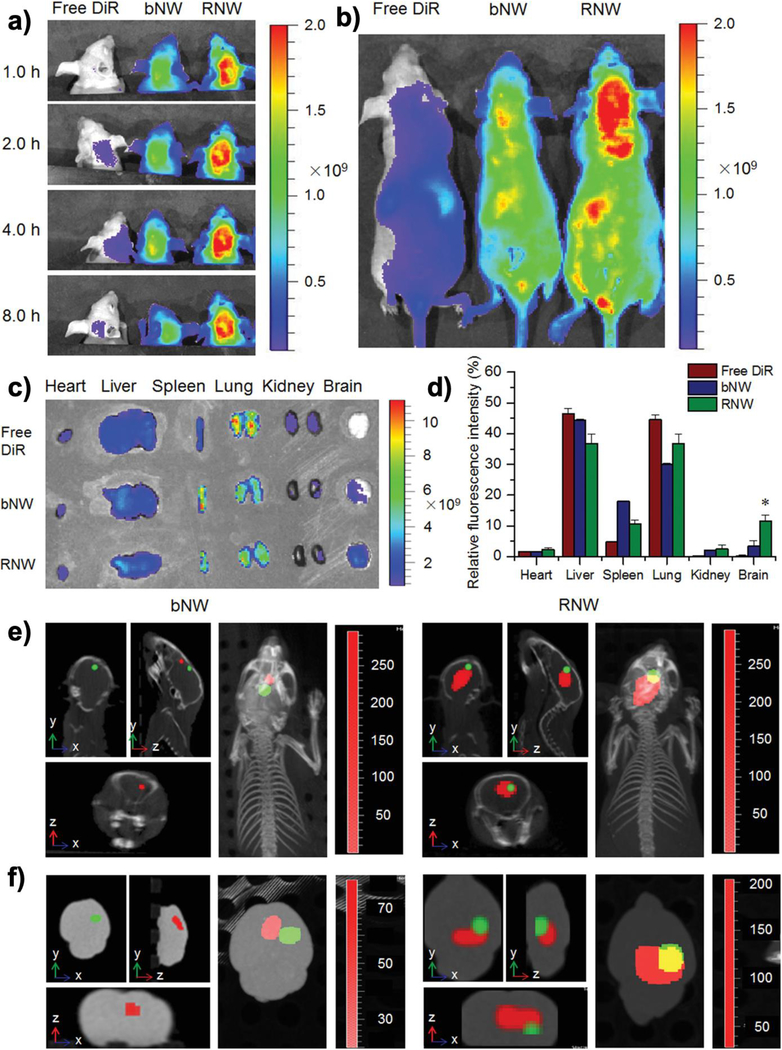
Figure 5.
In vivo distribution profiles of RGD-DM1 loaded mPEG-b-PDPA micelles (RNW) in a U87-Luc induced orthotopic brain tumor model and its specific targeting to the tumor tissues. a) The in vivo fluorescence images of RNW and blank mPEG-b-PDPA micelles (bNW) at different time points after intravenous injection. b) the typical fluorescence images of the entire animal at 4 h after injection; c) the ex vivo fluorescence images of the major organs at 4 h after injection; d) the calculated relative fluorescence intensity of each major organ at 4 h after injection, * p < 0.05; e) the in vivo specific targeting of bNW and RNW to the sites of brain tumors. The in vivo imaging was detected under the in vivo multimodal imaging system of Spectrum/CT at 4 h after injection, and the 3D images were reconstructed by the accompanied software. f) the ex vivo multimodal images of brain tissues under the Spectrum/CT system. Reproduced with permission [70]. Copyright 2016, Wiley-VCH.
Lipid nanoparticles
Lipid-based nanoparticles such as liposomes has relatively low toxicity compared to other nanoparticle formulations with high in vivo stability. We highlight representative lipid-based nanoparticles, including liposomes, solid lipid nanoparticles, high-and low-density lipoprotein (HDL and LDL) nanoparticles.
Liposomes have a spherical vesicle structure that consists of an amphiphilic phospholipid bilayer such as sphingomyelin and phosphatidylcholine. Both the surface and core of a liposome are hydrophilic because hydrophilic heads of phospholipids are oriented to the inner and outer side of a liposome while the shell compartment of a liposome has hydrophobic tails, enabling the loading of both hydrophilic and hydrophobic cargos into a single nanoparticle. Furthermore, modification of the liposome surface with grafting or conjugating specific ligands capable of binding to brain endothelial cells has facilitated the transport of therapeutic molecules loaded in the liposome across the BBB [71, 72]. The first delivery of small molecules was reported in an animal model in 1996, using a liposome surface engineered with PEG and OX26 monoclonal antibody to create an antibody-directed immunoliposome [73]. The immunoliposome was internalized into the brain via transferrin receptor-mediated transcytosis, therefore exhibiting successful delivery of a drug molecule, doxorubicin, 24 hours after intravenous injection. In order to induce additional function after brain penetration, a dual targeting liposome was developed by modifying the surface with both folate and transferrin [74]. Transferrin modified liposomes enabled the effective transcytosis, and subsequent delivery of doxorubicin-loaded liposomes to glioma cells specifically via folate-mediated endocytosis. Therapeutic efficacy of this liposome demonstrated minimal systemic toxicity and high glioma regression upon non-invasive systemic administration in a glioma-bearing mice model (Figure 6). Up to date, a number of liposome-based delivery systems have been introduced, including paclitaxel [75–77], doxorubicin [78, 79], siRNA [80–82], cisplatin [83], and imaging agents [84–86], which suggests the high potential of engineered liposomes for the delivery of therapeutic molecules across the BBB.
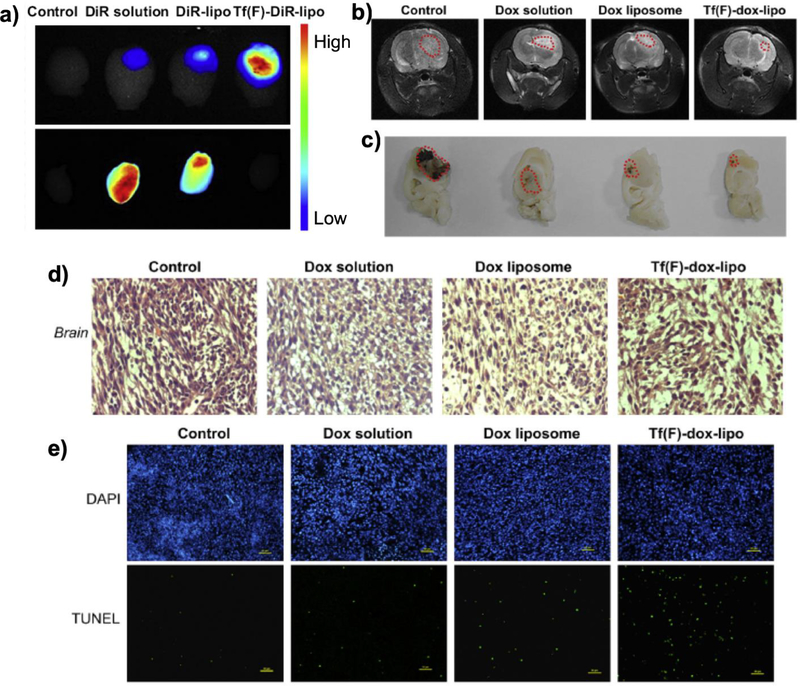
Figure 6.
Systemic evaluation of folate and transferrin modified liposome (Tf(F)-dox-lipo). (a) Ex vivo fluorescence images of dissected brains of rats bearing C6 glioma sacrificed at 24 h after intravenous injection. (b-d) BBB penetration of Tf(F)-dox-lipo monitored by (b) MRI, (c) brain column, and d) H&E staining of 10 μm sections of glioma tumors after each treatment excised on day 23 post-inoculation. (e) TUNEL assay of brain tumors. Blue represents nuclei and green represents apoptotic cells. Reproduced with permission [74]. Copyright 2013, Elsevier.
Solid lipid nanoparticles are micelle-like spheres that consist of a solid hydrophobic lipid core surrounded by triglycerides and fatty acids with several surfactants including polysorbate and phospholipid on the particle surface. The loading efficiency of hydrophobic drugs in solid lipid nanoparticles is higher than that of liposomes due to the large volume of a hydrophobic core for drug entrapment. Studies have reported up to date that solid lipid nanoparticles penetrate into the brain parenchyma via adsorption-mediated transcytosis. This is because the polysorbate on solid lipid nanoparticles are easily adsorbed on the plasma membrane of brain endothelial cells, thereby contributing to the particle internalization and subsequent excretion from brain endothelial cells to the brain parenchyma. Recently, a solid lipid nanoparticle used for the delivery of resveratrol, a polyphenolic anticancer drug, showed the ability to penetrate into the brain tumor [87]. In this study, the particle formulation with triglyceride trimyristin and lipids was optimized with the drug in a 1:10 drug-to-lipid ratio, and the particle surface was further modified with polysorbate 80 (PS 80) and poly(vinyl alcohol) (PVA). Owing to the high volume of the particle core, approximately 30% of resveratrol was loaded into the nanoparticle, exhibiting high efficacy with minimal systemic toxicity.
HDL and LDL nanoparticles are natural particles that are composed of lipid precursors and apolipoproteins. HDL transports cholesterols from peripheral tissues to the liver while LDL delivers cholesterols from the liver to peripheral tissues, contributing to cholesterol homeostasis in the body. In addition, small molecules or proteins are naturally assembled with these nanoparticles, either loaded into the particles or bound on the surface of the particles, for natural delivery to the brain parenchyma in vivo. A recent study showed that HDL can also deliver endogenous miRNA [88]. Researchers have reconstituted HDL or LDL nanoparticles using apolipoprotein mimetics. Delivery of curcumin into the brain was shown to treat Alzheimer’s disease using a LDL-mimetic nanocarrier [89]. Due to the limited availability of the primary apolipoproteins of LDL, apolipoprotein B100 (apoB100), researchers used positively charged lactoferrin instead of apoB100 to mimic LDL, demonstrating the transcytosis of LDL-mimetic nanoparticles through lactoferrin receptors on brain endothelial cells. High serum stability of the LDL-mimetic nanoparticles enhanced the pharmacokinetic properties, resulting in higher accumulation in the brain of a mouse model. Natural HDL nanoparticles are highly stable in circulation with a long half-life (6 ~ 24 hours) [90]. This high stability, biocompatibility, and long circulation time allow reconstituted HDL-mimetic nanoparticles to have attracted great attention for the potential to deliver therapeutic molecules. Furthermore, the major apolipoprotein of HDL, apolipoprotein A1 (apoA1), can cross the BBB via transcytosis mediated by several receptors on brain endothelial cells such as scavenger receptor class B-I (SR-B1), inducing effective delivery of HDL-mimetic nanoparticles with no additional modifications [91]. Different from other nanoparticle platforms, reconstituted HDL-mimetic nanoparticles can serve a nanomedicine without further loading of drug molecules because of its intrinsic biological functions. For example, HDL-mimetic nanoparticles with apoA1 themselves reduced the level of brain amyloid beta (Aβ) after 24 hours of intravenous administration in symptomatic APP/PS1 mice [92]. Similarly, HDL-mimetic nanoparticles with apoE3 were also internalized into the cortex and hippocampus upon systemic injection, exhibiting accelerated Aβ clearance and relieving memory impairment (Figure 7) [93, 94].
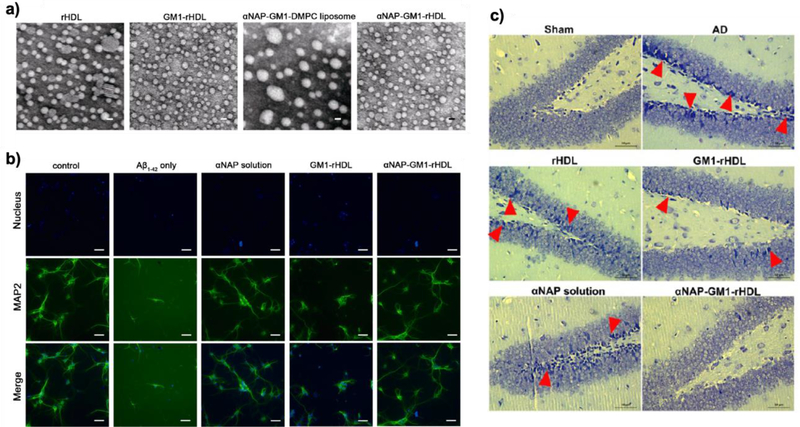
Figure 7.
HDL-mimetic nanoparticles with apoE3 for Aβ clearance. (a) TEM images of reconstituted HDL with apoE3 (rHDL), GM1 modified rHDL (GM1-rHDL), neuroprotective peptide-modified liposomes without apoE3 (αNAP-GM1-DMPC liposome), and neuroprotective peptide-modified rHDL (reconstituted HDL) (αNAP-GM1-rHDL). Scale bar is 20 nm. (b) Morphology of the primary neurons after treatment of the samples. (c) Histological assay of neurons in the dentate gyrus region after treatment of the samples. Reproduced with permission [93]. Copyright 2015, ACS.
Inorganic nanoparticles
Inorganic nanoparticles consisting of heavy metals or semi-conductive elements have the unique physical, optical, chemical, and electrical properties. Inorganic materials-based nanomedicine can support the nanostructure more firmly than that of other organic materials-based ones. However, the reactivity of inorganic nanoparticles requires surface modification with biocompatible materials with targeting ligands for the use as non-invasive nanomedicine.
Gold nanoparticles are the most commonly used inorganic nanomedicine for biomedical applications because of the easy synthesis and surface modification with high biocompatibility. Transferrin modified gold nanoparticles incorporated with PEG linkers were internalized into the brain parenchyma [95]. Size-dependent internalization of the engineered gold nanoparticles, as well as the density of transferrin modification on the gold surface were monitored. Interestingly, 20 nm gold nanoparticle showed the lowest accumulation in the brain due to the lower amount of transferrin on the surface, while other 45 and 80 nm gold nanoparticles showed increased association with brain endothelial cells through enhanced particle-cell interaction via transferrin receptors. More recent studies showed that transferrin-gold nanoparticles leverage the cleavable bond in the endosome to facilitate the transcytosis into the brain while inhibiting the re-efflux to the blood [96]. Gold nanoparticles with highly packed oligonucleotide on the surface called spherical nucleic acid (SNA) could get into the brain through scavenger receptor class A [97], and this approach also showed delivery of siRNA for the treatment of glioblastoma [98]. The successful BBB-crossing ability of SNA was evaluated by in vitro and in vivo models, and this targeted glioblastoma therapy showed a remarkable effect with minimal systemic toxicity upon intravenous injection (Figure 8).
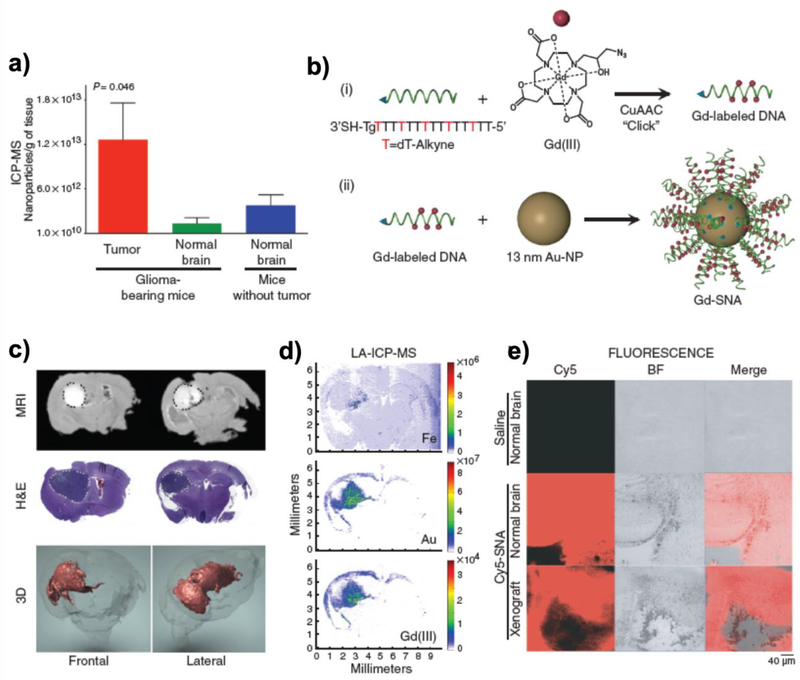
Figure 8.
Delivery of siRNA into glioblastoma using SNA. (a) Accumulation of gold nanoparticles in the brain and glioblastoma region. (b) Schematic illustration of Gd(III)-modified SNA for MR imaging. (c) Accumulation of SNA by crossing the BBB monitored by MRI section, H&E staining, and 3D reconstituted MRI. (d) LA-ICP-MS mapping of Gd(III)-modified SNA in the brain. (e) Confocal microscopic image of coronal brain sections derived from normal and glioblastoma-bearing mice upon injection of Cy-5 modified SNA. Reproduced with permission [98]. Copyright 2013, AAAS.
Magnetic nanoparticles have also been utilized for biomedical applications including brain nanomedicine. The paramagnetism of magnetic nanoparticles enables additional function of magnetic resonance image (MRI)-guided delivery of therapeutic molecules into the brain [99]. Furthermore, internalization of magnetic nanoparticles into the brain was enhanced by an external magnetic force which attract the nanoparticles to the magnet direction [100]. The external magnetic force induced more interaction with endothelial cells for receptor- or adsorptive-mediated transcytosis, delivering more nanoparticles into the brain without disrupting brain microenvironment.
Even though gold nanoparticles and magnetic nanoparticles are the most commonly applied inorganic material-based nanomedicines, other nanoparticles with different inorganic cores have been utilized as a carrier for BBB-crossing nanomedicine. Quantum dots have excellent optical properties for fluorescent diagnostic probes [101]. Coating of the outer shell of quantum dots with bioactive molecules such as targeting ligands and stealth materials enabled targeted delivery of therapeutic molecules to the brain [102]. Silica nanoparticles known to be relatively more biocompatible than other heavy metal-based inorganic nanoparticles have been widely used for various nanomedicine applications [103, 104]. In particular, mesoporous silica nanoparticles can efficiently load therapeutic or diagnostic molecules in the pore and the surface is engineered to exhibit great potential to cross the BBB [105, 106]. Carbon nanoparticles, such as fullerene, carbon nanotube, graphene (oxide), and carbon nanodot, have also been applied for brain nanomedicine upon their surface modification [107–110]. Although advantages of inorganic nanoparticles such as additional functionality with the solid structural backbone, long-term toxicity of inorganic nanomedicine should be addressed for further clinical translations.
Conclusion and Future Prospects
There is considerable progress in the field of engineered nanomedicine for central nervous system (CNS) drug delivery past several years. Several nanoscale formulations have demonstrated the enhanced efficacy by successful delivery of therapeutic molecules across the BBB at the cellular and animal levels. Nevertheless, few clinically approved therapeutics across the BBB is reported yet, although many nanomedicines for the treatment of other diseases like cancers or cardiovascular diseases are already commercialized or in clinical trials. That is mainly because of the inability of drugs or drug-loaded nanoparticles to cross the BBB and also due to the lack of cost-effective and predictive power of assessing the efficacy of drugs across the BBB. Recent innovative models called organs-on-chips in which tissues can be cultured in an environment that is engineered for better replicating the in vivo microenvironment may provide better chance to prescreen drugs and test the BBB-crossing ability of drug candidates [111]. Different from animal models that arise the concern of cross-species differences, human organ-on-chips can provide a better opportunity for the understanding of human organs including human cell-based brain neurovascular unit in well-defined and controlled system [112]. Besides, further engineering of various nanoparticles for clinical translations are required for the clinical translation of nanotherapeutics for the CNS drug delivery across the BBB. The priority now is to standardize the production of nanotherapeutics to reduce the heterogeneity of nanomedicine synthesis and modification at a bulk scale. Along with a better prescreening platform for the testing of the BBB-crossing ability, as well as standardized bulk synthesis of nanomedicines, advanced nanotherapeutics engineered to cross the BBB for CNS disease would be realized in the near future.
Acknowledgement
This work was supported by the National Institutes of Health (NIH) Director’s New Innovator Award 1DP2HL142050 and the NIH National Institute on Aging (1R21AG056781).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].Lang AE, Nat Med, 16 (2010) 1223–1226. [DOI] [PubMed] [Google Scholar]
- [2].Pantoni L, Lancet Neurol, 9 (2010) 689–701. [DOI] [PubMed] [Google Scholar]
- [3].Abbott NJ, Ronnback L, Hansson E, Nat Rev Neurosci, 7 (2006) 41–53. [DOI] [PubMed] [Google Scholar]
- [4].Zlokovic BV, Nat Rev Neurosci, 12 (2011) 723–738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Daneman R, Zhou L, Kebede AA, Barres BA, Nature, 468 (2010) 562–566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Farokhzad OC, Langer R, Adv Drug Deliv Rev, 58 (2006) 1456–1459. [DOI] [PubMed] [Google Scholar]
- [7].Moghimi SM, Hunter AC, Murray JC, FASEB J, 19 (2005) 311–330. [DOI] [PubMed] [Google Scholar]
- [8].Wagner V, Dullaart A, Bock AK, Zweck A, Nat Biotechnol, 24 (2006) 1211–1217. [DOI] [PubMed] [Google Scholar]
- [9].Mout R, Moyano DF, Rana S, Rotello VM, Chem Soc Rev, 41 (2012) 2539–2544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Kim J, Kim J, Jeong C, Kim WJ, Adv Drug Deliv Rev, 98 (2016) 99–112. [DOI] [PubMed] [Google Scholar]
- [11].Coomber BL, Stewart PA, Microvasc Res, 30 (1985) 99–115. [DOI] [PubMed] [Google Scholar]
- [12].Van Itallie CM, Holmes J, Bridges A, Gookin JL, Coccaro MR, Proctor W, Colegio OR, Anderson JM, J Cell Sci, 121 (2008) 298–305. [DOI] [PubMed] [Google Scholar]
- [13].Shepro D, Morel NML, Faseb Journal, 7 (1993) 1031–1038. [DOI] [PubMed] [Google Scholar]
- [14].Armulik A, Genove G, Betsholtz C, Dev Cell, 21 (2011) 193–215. [DOI] [PubMed] [Google Scholar]
- [15].Janzer RC, Raff MC, Nature, 325 (1987) 253–257. [DOI] [PubMed] [Google Scholar]
- [16].Streit WJ, Conde JR, Fendrick SE, Flanary BE, Mariani CL, Neurol Res, 27 (2005) 685–691. [DOI] [PubMed] [Google Scholar]
- [17].Ajami B, Bennett JL, Krieger C, Tetzlaff W, Rossi FM, Nat Neurosci, 10 (2007) 1538–1543. [DOI] [PubMed] [Google Scholar]
- [18].Komarova YA, Kruse K, Mehta D, Malik AB, Circulation Research, 120 (2017) 179–206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Sarin H, J Angiogenes Res, 2 (2010) 14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Smith NM, Gachulincova I, Ho D, Bailey C, Bartlett CA, Norret M, Murphy J, Buckley A, Rigby PJ, House MJ, St Pierre T, Fitzgerald M, Iyer KS, Dunlop SA, Sci Rep, 6 (2016) 22595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Jain KK, Nanomedicine (Lond), 7 (2012) 1225–1233. [DOI] [PubMed] [Google Scholar]
- [22].Pardridge WM, Mol Interv, 3 (2003) 90–105, [DOI] [PubMed] [Google Scholar]
- [23].Pajouhesh H, Lenz GR, NeuroRx, 2 (2005) 541–553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Kreuter J, Adv Drug Deliv Rev, 47 (2001) 65–81. [DOI] [PubMed] [Google Scholar]
- [25].Uchida Y, Ohtsuki S, Katsukura Y, Ikeda C, Suzuki T, Kamiie J, Terasaki T, J Neurochem, 117 (2011) 333–345. [DOI] [PubMed] [Google Scholar]
- [26].Witt KA, Gillespie TJ, Huber JD, Egleton RD, Davis TP, Peptides, 22 (2001) 2329–2343. [DOI] [PubMed] [Google Scholar]
- [27].Sahay G, Alakhova DY, Kabanov AV, J Control Release, 145 (2010) 182–195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Muro S, Koval M, Muzykantov V, Curr Vasc Pharmacol, 2 (2004) 281–299. [DOI] [PubMed] [Google Scholar]
- [29].Bareford LM, Swaan PW, Adv Drug Deliv Rev, 59 (2007) 748–758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Pardridge WM, Adv Drug Deliv Rev, 36 (1999) 299–321. [DOI] [PubMed] [Google Scholar]
- [31].Mooberry LK, Sabnis NA, Panchoo M, Nagarajan B, Lacko AG, Front Pharmacol, 7 (2016) 466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Barar J, Rafi MA, Pourseif MM, Omidi Y, Bioimpacts, 6 (2016) 225–248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Rathore AS, Winkle H, Nat Biotechnol, 27 (2009) 26–34. [DOI] [PubMed] [Google Scholar]
- [34].Schellekens H, Nat Rev Drug Discov, 1 (2002) 457–462. [DOI] [PubMed] [Google Scholar]
- [35].Walsh G, Trends Biotechnol, 23 (2005) 553–558. [DOI] [PubMed] [Google Scholar]
- [36].Daniel RM, Cowan DA, Cell Mol Life Sci, 57 (2000) 250–264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Jiskoot W, Randolph TW, Volkin DB, Middaugh CR, Schoneich C, Winter G, Friess W, Crommelin DJ, Carpenter JF, J Pharm Sci, 101 (2012) 946–954. [DOI] [PubMed] [Google Scholar]
- [38].Biondi M, Ungaro F, Quaglia F, Netti PA, Adv Drug Deliv Rev, 60 (2008) 229–242. [DOI] [PubMed] [Google Scholar]
- [39].Lienemann PS, Lutolf MP, Ehrbar M, Adv Drug Deliv Rev, 64 (2012) 1078–1089. [DOI] [PubMed] [Google Scholar]
- [40].Harivardhan Reddy L, Sharma RK, Chuttani K, Mishra AK, Murthy RS, J Control Release, 105 (2005) 185–198. [DOI] [PubMed] [Google Scholar]
- [41].Ray PC, Chem Rev, 110 (2010) 5332–5365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Torchilin V, Adv Drug Deliv Rev, 63 (2011) 131–135. [DOI] [PubMed] [Google Scholar]
- [43].Blanco E, Shen H, Ferrari M, Nat Biotechnol, 33 (2015) 941–951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Tsou YH, Zhang XQ, Zhu H, Syed S, Xu X, Small, 13 (2017) 1701921. [DOI] [PubMed] [Google Scholar]
- [45].Furtado D, Bjornmalm M, Ayton S, Bush AI, Kempe K, Caruso F, Adv Mater, (2018) e1801362. [DOI] [PubMed] [Google Scholar]
- [46].Saraiva C, Praca C, Ferreira R, Santos T, Ferreira L, Bernardino L, J Control Release, 235 (2016) 34–47. [DOI] [PubMed] [Google Scholar]
- [47].Dong X, Theranostics, 8 (2018) 1481–1493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Banks WA, Nat Rev Drug Discov, 15 (2016) 275–292. [DOI] [PubMed] [Google Scholar]
- [49].Abbasi E, Aval SF, Akbarzadeh A, Milani M, Nasrabadi HT, Joo SW, Hanifehpour Y, Nejati-Koshki K, Pashaei-Asl R, Nanoscale Res Lett, 9 (2014) 247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Mishra MK, Beaty CA, Lesniak WG, Kambhampati SP, Zhang F, Wilson MA, Blue ME, Troncoso JC, Kannan S, Johnston MV, Baumgartner WA, Kannan RM, ACS Nano, 8 (2014) 2134–2147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Zhang F, Trent Magruder J, Lin YA, Crawford TC, Grimm JC, Sciortino CM, Wilson MA, Blue ME, Kannan S, Johnston MV, Baumgartner WA, Kannan RM, J Control Release, 249 (2017) 173–182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Srinageshwar B, Peruzzaro S, Andrews M, Johnson K, Hietpas A, Clark B, McGuire C, Petersen E, Kippe J, Stewart A, Lossia O, Al-Gharaibeh A, Antcliff A, Culver R, Swanson D, Dunbar G, Sharma A, Rossignol J, Int J Mol Sci, 18 (2017) 628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Zhou J, Patel TR, Sirianni RW, Strohbehn G, Zheng MQ, Duong N, Schafbauer T, Huttner AJ, Huang Y, Carson RE, Zhang Y, Sullivan DJ Jr., Piepmeier JM, Saltzman WM, Proc Natl Acad Sci U S A, 110 (2013) 11751–11756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Nance EA, Woodworth GF, Sailor KA, Shih TY, Xu Q, Swaminathan G, Xiang D, Eberhart C, Hanes J, Sci Transl Med, 4 (2012) 149ra119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Bharadwaj VN, Lifshitz J, Adelson PD, Kodibagkar VD, Stabenfeldt SE, Sci Rep, 6 (2016) 29988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Jiang X, Sha X, Xin H, Xu X, Gu J, Xia W, Chen S, Xie Y, Chen L, Chen Y, Fang X, Biomaterials, 34 (2013) 2969–2979. [DOI] [PubMed] [Google Scholar]
- [57].Wang JH, Yang YT, Zhang YH, Huang M, Zhou ZJ, Luo WX, Tang J, Wang JG, Xiao Q, Chen HJ, Cai YQ, Sun XL, Wang Y, Ke YQ, Advanced Functional Materials, 26 (2016) 7873–7885. [Google Scholar]
- [58].Kolter M, Ott M, Hauer C, Reimold I, Fricker G, J Control Release, 197 (2015) 165–179. [DOI] [PubMed] [Google Scholar]
- [59].Li Y, He H, Jia X, Lu WL, Lou J, Wei Y, Biomaterials, 33 (2012) 3899–3908. [DOI] [PubMed] [Google Scholar]
- [60].Kolhar P, Anselmo AC, Gupta V, Pant K, Prabhakarpandian B, Ruoslahti E, Mitragotri S, Proc Natl Acad Sci U S A, 110 (2013) 10753–10758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].Wang B, Lv L, Wang Z, Jiang Y, Lv W, Liu X, Wang Z, Zhao Y, Xin H, Xu Q, Sci Rep, 5 (2015) 16589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [62].Gao S, Tian H, Xing Z, Zhang D, Guo Y, Guo Z, Zhu X, Chen X, J Control Release, 243 (2016) 357–369. [DOI] [PubMed] [Google Scholar]
- [63].Zhang C, Nance EA, Mastorakos P, Chisholm J, Berry S, Eberhart C, Tyler B, Brem H, Suk JS, Hanes J, J Control Release, 263 (2017) 112–119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [64].Zhang B, Sun X, Mei H, Wang Y, Liao Z, Chen J, Zhang Q, Hu Y, Pang Z, Jiang X, Biomaterials, 34 (2013) 9171–9182. [DOI] [PubMed] [Google Scholar]
- [65].Zhang C, Zheng X, Wan X, Shao X, Liu Q, Zhang Z, Zhang Q, J Control Release, 192 (2014) 317–324. [DOI] [PubMed] [Google Scholar]
- [66].Yao H, Wang K, Wang Y, Wang S, Li J, Lou J, Ye L, Yan X, Lu W, Huang R, Biomaterials, 37 (2015) 345–352. [DOI] [PubMed] [Google Scholar]
- [67].Jiang X, Xin H, Ren Q, Gu J, Zhu L, Du F, Feng C, Xie Y, Sha X, Fang X, Biomaterials, 35 (2014) 518–529. [DOI] [PubMed] [Google Scholar]
- [68].Chen YC, Chiang CF, Chen LF, Liang PC, Hsieh WY, Lin WL, Biomaterials, 35 (2014) 4066–4081. [DOI] [PubMed] [Google Scholar]
- [69].Hu Q, Gao X, Gu G, Kang T, Tu Y, Liu Z, Song Q, Yao L, Pang Z, Jiang X, Chen H, Chen J, Biomaterials, 34 (2013) 5640–5650. [DOI] [PubMed] [Google Scholar]
- [70].Zeng LJ, Zou LL, Yu HJ, He XY, Cao HQ, Zhang ZW, Yin Q, Zhang PC, Gu WW, Chen LL, Li YP, Advanced Functional Materials, 26 (2016) 4201–4212. [Google Scholar]
- [71].Sakamoto A, Ido T, Brain Res, 629 (1993) 171–175. [DOI] [PubMed] [Google Scholar]
- [72].Gennuso R, Spigelman MK, Chinol M, Zappulla RA, Nieves J, Vallabhajosula S, Alberto Paciucci P, Goldsmith SJ, Holland JF, Cancer Invest, 11 (1993) 118–128. [DOI] [PubMed] [Google Scholar]
- [73].Huwyler J, Wu D, Pardridge WM, Proc Natl Acad Sci U S A, 93 (1996) 14164–14169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [74].Gao JQ, Lv Q, Li LM, Tang XJ, Li FZ, Hu YL, Han M, Biomaterials, 34 (2013) 5628–5639. [DOI] [PubMed] [Google Scholar]
- [75].Liu Y, Mei L, Xu C, Yu Q, Shi K, Zhang L, Wang Y, Zhang Q, Gao H, Zhang Z, He Q, Theranostics, 6 (2016) 177–191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [76].Shi K, Long Y, Xu C, Wang Y, Qiu Y, Yu Q, Liu Y, Zhang Q, Gao H, Zhang Z, He Q, ACS Appl Mater Interfaces, 7 (2015) 21442–21454. [DOI] [PubMed] [Google Scholar]
- [77].Liu Y, Mei L, Yu Q, Xu C, Qiu Y, Yang Y, Shi K, Zhang Q, Gao H, Zhang Z, He Q, ACS Appl Mater Interfaces, 7 (2015) 16792–16801. [DOI] [PubMed] [Google Scholar]
- [78].Yang Y, Yan Z, Wei D, Zhong J, Liu L, Zhang L, Wang F, Wei X, Xie C, Lu W, He D, Nanotechnology, 24 (2013) 405101. [DOI] [PubMed] [Google Scholar]
- [79].Gong W, Wang Z, Liu N, Lin W, Wang X, Xu D, Liu H, Zeng C, Xie X, Mei X, Lu W, Biol Pharm Bull, 34 (2011) 1058–1064. [DOI] [PubMed] [Google Scholar]
- [80].Zong T, Mei L, Gao H, Shi K, Chen J, Wang Y, Zhang Q, Yang Y, He Q, J Pharm Sci, 103 (2014) 3891–3901. [DOI] [PubMed] [Google Scholar]
- [81].Yue PJ, He L, Qiu SW, Li Y, Liao YJ, Li XP, Xie D, Peng Y, Mol Cancer, 13 (2014) 191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [82].Zhang Y, Boado RJ, Pardridge WM, J Gene Med, 5 (2003) 1039–1045. [DOI] [PubMed] [Google Scholar]
- [83].Charest G, Sanche L, Fortin D, Mathieu D, Paquette B, J Neurooncol, 115 (2013) 365–373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [84].Joshi S, Singh-Moon RP, Ellis JA, Chaudhuri DB, Wang M, Reif R, Bruce JN, Bigio IJ, Straubinger RM, Neurosurgery, 76 (2015) 92–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [85].Qiu LH, Zhang JW, Li SP, Xie C, Yao ZW, Feng XY, J Magn Reson Imaging, 41 (2015) 1056–1064. [DOI] [PubMed] [Google Scholar]
- [86].Chekhonin VP, Baklaushev VP, Yusubalieva GM, Belorusova AE, Gulyaev MV, Tsitrin EB, Grinenko NF, Gurina OI, Pirogov YA, Nanomedicine, 8 (2012) 63–70. [DOI] [PubMed] [Google Scholar]
- [87].Jose S, Anju SS, Cinu TA, Aleykutty NA, Thomas S, Souto EB, Int J Pharm, 474 (2014) 6–13. [DOI] [PubMed] [Google Scholar]
- [88].Tabet F, Vickers KC, Cuesta Torres LF, Wiese CB, Shoucri BM, Lambert G, Catherinet C, Prado-Lourenco L, Levin MG, Thacker S, Sethupathy P, Barter PJ, Remaley AT, Rye KA, Nat Commun, 5 (2014) 3292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [89].Meng F, Asghar S, Gao S, Su Z, Song J, Huo M, Meng W, Ping Q, Xiao Y, Colloids Surf B Biointerfaces, 134 (2015) 88–97. [DOI] [PubMed] [Google Scholar]
- [90].Kuai R, Li D, Chen YE, Moon JJ, Schwendeman A, ACS Nano, 10 (2016) 3015–3041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [91].Fung KY, Wang C, Nyegaard S, Heit B, Fairn GD, Lee WL, Front Physiol, 8 (2017) 841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [92].Robert J, Stukas S, Button E, Cheng WH, Lee M, Fan J, Wilkinson A, Kulic I, Wright SD, Wellington CL, Biochim Biophys Acta, 1862 (2016) 1027–1036. [DOI] [PubMed] [Google Scholar]
- [93].Huang M, Hu M, Song Q, Song H, Huang J, Gu X, Wang X, Chen J, Kang T, Feng X, Jiang D, Zheng G, Chen H, Gao X, ACS Nano, 9 (2015) 10801–10816. [DOI] [PubMed] [Google Scholar]
- [94].Song Q, Song H, Xu J, Huang J, Hu M, Gu X, Chen J, Zheng G, Chen H, Gao X, Mol Pharm, 13 (2016) 3976–3987. [DOI] [PubMed] [Google Scholar]
- [95].Wiley DT, Webster P, Gale A, Davis ME, Proc Natl Acad Sci U S A, 110 (2013) 8662–8667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [96].Clark AJ, Davis ME, Proc Natl Acad Sci U S A, 112 (2015) 12486–12491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [97].Georganopoulou DG, Chang L, Nam JM, Thaxton CS, Mufson EJ, Klein WL, Mirkin CA, Proc Natl Acad Sci U S A, 102 (2005) 2273–2276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [98].Jensen SA, Day ES, Ko CH, Hurley LA, Luciano JP, Kouri FM, Merkel TJ, Luthi AJ, Patel PC, Cutler JI, Daniel WL, Scott AW, Rotz MW, Meade TJ, Giljohann DA, Mirkin CA, Stegh AH, Sci Transl Med, 5 (2013) 209ra152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [99].Zhang R, Li Y, Hu B, Lu Z, Zhang J, Zhang X, Adv Mater, 28 (2016) 6345–6352. [DOI] [PubMed] [Google Scholar]
- [100].Lee C, Hwang HS, Lee S, Kim B, Kim JO, Oh KT, Lee ES, Choi HG, Youn YS, Adv Mater, 29 (2017) 1605563. [DOI] [PubMed] [Google Scholar]
- [101].Petryayeva E, Algar WR, Medintz IL, Appl Spectrosc, 67 (2013) 215–252. [DOI] [PubMed] [Google Scholar]
- [102].Liu L, Guo K, Lu J, Venkatraman SS, Luo D, Ng KC, Ling EA, Moochhala S, Yang YY, Biomaterials, 29 (2008) 1509–1517. [DOI] [PubMed] [Google Scholar]
- [103].Tang L, Cheng J, Nano Today, 8 (2013) 290–312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [104].Kim J, Jo C, Lim WG, Jung S, Lee YM, Lim J, Lee H, Lee J, Kim WJ, Adv Mater, (2018) e1707557. [DOI] [PubMed] [Google Scholar]
- [105].Baghirov H, Karaman D, Viitala T, Duchanoy A, Lou YR, Mamaeva V, Pryazhnikov E, Khiroug L, de Lange Davies C, Sahlgren C, Rosenholm JM, PLoS One, 11 (2016) e0160705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [106].Shi J, Hou S, Huang J, Wang S, Huan W, Huang C, Liu X, Jiang R, Qian W, Lu J, Wang X, Shi W, Huang R, Chen J, Nanoscale, 9 (2017) 8970–8981. [DOI] [PubMed] [Google Scholar]
- [107].Lin CM, Lu TY, Recent Pat Nanotech, 6 (2012) 105–113. [DOI] [PubMed] [Google Scholar]
- [108].Mendonca MC, Soares ES, de Jesus MB, Ceragioli HJ, Ferreira MS, Catharino RR, da Cruz-Hofling MA, J Nanobiotechnology, 13 (2015) 78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [109].Baldrighi M, Trusel M, Tonini R, Giordani S, Front Neurosci, 10 (2016) 250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [110].Kafa H, Wang JT, Rubio N, Venner K, Anderson G, Pach E, Ballesteros B, Preston JE, Abbott NJ, Al-Jamal KT, Biomaterials, 53 (2015) 437–452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [111].Sei Y, Justus K, LeDuc P, Kim Y, Microfluidics and Nanofluidics, 16 (2014) 907–920. [Google Scholar]
- [112].van der Helm MW, van der Meer AD, Eijkel JC, van den Berg A, Segerink LI, Tissue Barriers, 4 (2016) e1142493. [DOI] [PMC free article] [PubMed] [Google Scholar]