Visual Abstract
Keywords: IgA nephropathy, glycation, kidney development, IGA glomerulonephritis, immunoglobulin A, galactose, proportional hazards models, follow-up studies, risk factors, universities, proteinuria, chronic renal insufficiency, chronic kidney failure, complement c3, biopsy, complement activation, hypertension, adrenal cortex hormones, demography
Abstract
Background and objectives
Increased circulating galactose-deficient IgA1 and subsequently complement activation both play important roles in the pathophysiology of IgA nephropathy. However, their relationship to disease severity and progression remains unclear.
Design, setting, participants, & measurements
We assessed 1210 participants in a cohort study of biopsy-proven IgA nephropathy at Peking University First Hospital. Plasma concentrations of galactose-deficient IgA1 and complement component C3 were measured at the time of biopsy. We tested associations of galactose-deficient IgA1 and galactose-deficient IgA1/C3 ratio with CKD progression event, defined as ESKD or 50% decline in eGFR, using Cox proportional hazards models and restricted cubic splines.
Results
After a median follow-up of 43 months (interquartile range, 24–76 months), 172 (14%) participants reached the CKD progression event. The association of galactose-deficient IgA1 levels and CKD progression event showed a nonlinear relationship. The risk of CKD progression events was greater with higher plasma galactose-deficient IgA1 levels but reached a plateau when galactose-deficient IgA1>325 U/ml, whereas the risk of CKD progression events monotonically increased with higher galactose-deficient IgA1/C3 ratio. After adjustment for traditional risk factors (demographics, eGFR, proteinuria, hypertension, Oxford pathologic score, and corticosteroids/immunosuppressive therapy), higher levels of galactose-deficient IgA1/C3 ratio were independently associated with CKD progression event (per natural log-transformed [galactose-deficient IgA1/C3], hazard ratio, 2.03; 95% confidence interval [95% CI], 1.25 to 3.29; P=0.004). In reference to the first quartile of the galactose-deficient IgA1/C3 ratio, hazard ratios were 1.71 (95% CI, 1.01 to 2.89) for the second quartile, 1.55 (95% CI, 0.91 to 2.63) for the third quartile, and 2.17 (95% CI, 1.33 to 3.56) for the fourth quartile.
Conclusions
In IgA nephropathy, plasma galactose-deficient IgA1/C3 ratio was associated with CKD progression event independent of clinical and biopsy characteristics.
Introduction
IgA nephropathy is one of the most common primary glomerular diseases worldwide, especially in young Asian adults (1–3). IgA nephropathy is characterized by a highly variable clinical course ranging from a totally benign incidental condition to rapidly progressive kidney failure. Although most affected individuals develop chronic, slowly progressive kidney injury, many participants will develop ESKD (4). The strongest clinical or pathologic risk factors include hypertension, proteinuria, eGFR, and Oxford pathologic score (5–8). In addition, various plasma (soluble CD89-IgA complexes, advanced oxidation protein products, ceptin, and mannose-binding lectin) and urine (soluble transferrin receptor, α1- and β2-microglobulin, kidney injury molecule-1) biomarkers are found to be associated with disease prognosis but have not been confirmed in other populations (9–15).
Aberrant glycosylation of O-linked glycans in the hinge region of IgA1 plays a key role in the pathogenesis of IgA nephropathy (4,16–19). Most participants with IgA nephropathy have elevated levels of circulating IgA1 molecules with O-linked glycans that are deficient in galactose and exposed N-acetylgalactosamine residues (20–23). Several studies have evaluated plasma galactose-deficient IgA1 levels as a diagnostic marker for IgA nephropathy (22,24). Galactose-deficient IgA1 levels were also associated with pathologic lesions or kidney progression in small cohort studies (25). Thus, galactose-deficient IgA1 is a potential marker for predicting the prognosis, monitoring disease activity, and evaluating the response to treatment of IgA nephropathy (26,27). However, published data on plasma galactose-deficient IgA1 levels as a clinical prognostic marker are limited by small sample sizes and inconsistent findings (5,9,28). On the other hand, complement activation by IgA1-containing immune complexes via the alternative or lectin complement pathway has been confirmed to involve the development and progression in IgA nephropathy (29,30). Prior studies showed that serum IgA1/C3 ratio was associated with the pathologic lesions or kidney progression events in IgA nephropathy (31,32). It is still unknown if the galactose-deficient IgA1/C3 ratio is a more reliable biomarker for kidney progression because the IgA on the kidney is galactose deficient.
In this cohort study, we measured plasma galactose-deficient IgA1 at baseline in 1210 participants with IgA nephropathy using a lectin-based ELISA, and aimed to evaluate the effect of galactose-deficient IgA1 and galactose-deficient IgA1/C3 ratio on CKD progression in IgA nephropathy. We also examined changes in galactose-deficient IgA1 levels in 171 participants with sequential blood samples collected during follow-up. Longitudinal analyses were performed to determine whether galactose-deficient IgA1 levels changed during the course of the disease or responded to specific treatment regimens.
Materials and Methods
Participants and Healthy Controls
From 2003 to September 2017, a total of 1360 participants participated in the IgA nephropathy cohort based at Peking University First Hospital (http://ckd.edc-china.com.cn/). For 60 participants, blood samples collected at the time of kidney biopsy were unavailable. The serum C3 levels of 90 participants were unavailable. Therefore, a total of 1210 participants were enrolled in our study. Oxford scores of 23 participants were unavailable because each of them had fewer than eight glomeruli. In addition, the treatment regimens of eight participants were unavailable. The cases with missing data of Oxford scores or treatment regimens were not included in the multivariable-adjusted analysis. All participants were followed up for at least 12 months (range, 12–177 months). A total of 171 participants had blood samples collected during follow-up to repeat the measurements. Diagnosis of IgA nephropathy was on the basis of the presence of dominant of IgA deposition in the mesangial area by immunofluorescence (IF) microscopy. Participants with secondary IgA nephropathy, such as SLE, rheumatic disease, IgA vasculitis, hepatitis B virus–associated GN, and liver cirrhosis, were excluded. In addition, 208 age-, sex-, and geographically-matched healthy individuals were enrolled as healthy controls. The research was approved by the Peking University First Hospital. All participants provided written informed consent before study inclusion. The study was conducted in accordance with the principles contained in the Declaration of Helsinki.
Clinical and Histologic Manifestations
Complete clinical data, including sex, age, systolic/diastolic pressure, baseline plasma creatinine, and baseline 24-hour urine protein excretion, were collected at the time of kidney biopsy. All kidney biopsy specimens were reviewed and graded by an independent pathologist who was blind to the participants’ clinical data. The Oxford classification (including crescent scores) was used for the evaluation of pathologic lesions (33). The eGFR was calculated using the CKD Epidemiology Collaboration two-level rate equation (34). For the purpose of our study, hypertension was defined as systolic pressure ≥140 mm Hg and/or diastolic pressure ≥90 mm Hg at resting or use of antihypertension medication. The CKD progression event was defined by a 50% decline in eGFR or ESKD that was confirmed by a second evaluation obtained at least 4 weeks later. ESKD was defined as eGFR<15 ml/min per 1.73 m2 or need for kidney replacement therapy (such as hemodialysis, peritoneal dialysis, or kidney transplantation).
Detection of Plasma Galactose-Deficient IgA1 by ELISA
For measurement of galactose-deficient IgA1, F(ab')2 polyclonal fragments of goat anti-human IgA1 (Jackson ImmunoResearch Laboratories, West Grove, PA) in bicarbonate at 2.5 µg/ml were coated onto high-binding MaxiSorp 96-well plates (Nunc MaxiSorp) at 4°C overnight. After blockade with 1% bovine plasma albumin (Sigma-Aldrich, St. Louis, MO) in 1X PBS with 0.1% Tween 20 at room temperature for 2 hours, two-fold sample dilution (1:4000) was added at 4°C overnight. The purified monomeric IgA1 fractions from a patient with IgA nephropathy were digested with neuraminidase from Clostridium perfringens (Sigma, St. Louis, MO) and β-galactosidase from bovine testis (Sigma) to obtain desialylated/degalactosylated IgA1 molecules. Then, the standard was prepared by mixing desialylated/degalactosylated IgA1 molecules with commercial IgA1 (GenWay Biotech, Inc.) in equal proportions. For accurate detection of the galactose-deficient glycans on plasma IgA1, 1 mU/well sialidase A (Prozyme, Inc.) in phosphate buffer (pH 6) was added to the plates and incubated for 3 hours at 37°C to remove the terminal sialic acid from O-linked N-acetylgalactosamine on the IgA1 molecule of the samples and the standard. Then, each well was incubated with 50 µl Helix pomatia (1:500 dilution; Sigma) for 3 hours at 37°C. After the addition of 50 µl horseradish peroxidase-ExtrAvidin (1:2000 dilution; Sigma) to each well, the plates were incubated for 1 hour at 37°C. The color was then developed with TMB Substrate (Kangbeiyuan, Beijing, China) for 35 minutes at 37°C in a dark place. The reaction was stopped with 50 ml/well 1 M H2SO4. The absorbance was read at 450/570 nm using an ELISA reader (Bio-Rad, Tokyo, Japan). The plasma galactose-deficient IgA1 levels were expressed as units per milliliters. One unit of galactose-deficient IgA1 was defined as 1 µg of the standard. Helix pomatia-based ELISA for galactose-deficient IgA1 was highly reproducible. The intraassay coefficient of variation was 6.6%. C3 was measured by rate turbidimetry and rate nephelometry in Beckman Image 800 nephelometer (Beckman Coulter Inc., CA).
Statistical Analyses
Quantitative variables are expressed as the mean and SD for normally distributed variables or the median and interquartile range (IQR) for non-normally distributed variables. Categorical data are summarized as percentages and absolute ratios. Kaplan–Meier analysis was used to derive cumulative kidney survival curves, and differences between curves were analyzed using a log-rank test. Unadjusted and multivariable-adjusted Cox proportional hazards models were adopted to evaluate the relationship between galactose-deficient IgA1 levels and risk of end point. Demographic characteristics (age, sex), prognostic factors of IgA nephropathy (eGFR, proteinuria, BP, Oxford classification scores), and use of immunosuppression were adjusted in multivariable-adjusted Cox proportional hazards models. The Schoenfeld residuals test was used to check the proportional hazards assumption. P values for trend in the Cox proportional hazards models were calculated using the quartile median values. The results are expressed as hazard ratios with 95% confidence intervals (95% CIs). For determination of the association of galactose-deficient IgA1 levels with the kidney outcome, restricted cubic splines were used to model the level of galactose-deficient IgA1 after multivariable adjustment. Knots for the cubic splines were placed at the quartiles. We also performed sensitivity analyses on the basis of outcomes of 50% decline in eGFR or ESKD. The significance of trends across quintiles of galactose-deficient IgA1 ratio and kidney outcomes was tested by Cochran–Armitage trend test. The statistical software SPSS version 23.0 (SPSS, Chicago, IL) and Stata Edition 14.0 (StataCorp., College Station, TX) were used. P values <0.05 (two-sided) were considered statistically significant.
Results
Study Cohort
The characteristics of the study population and measurements of galactose-deficient IgA1, IgA1, and C3 at the time of kidney biopsy are described in Table 1. This cohort included 615 men (52%) with a mean age of 35±12 years. At the time of diagnosis, the average eGFR was 83±32 ml/min per 1.73 m2 (range, 6–169 ml/min per 1.73 m2) and the median proteinuria level was 1.30 g/24 h (IQR, 0.68–2.51 g/24 h). A total of 514 participants (43%) were hypertensive at biopsy. Overall, 550 participants (46%) received steroids or other immunosuppressive agents. After a median follow-up of 43 months (IQR, 24–76 months), 172 (14%) participants reached the CKD progression event, including 114 with ESKD events and 165 with 50% eGFR decline events.
Table 1.
Characteristics of participants in the Peking University First Hospital IgA nephropathy cohort at the time of kidney biopsy
| Characteristics | Total Cohort | Quartiles of Galactose-Deficient IgA1/C3 (U/mg, range) | |||
|---|---|---|---|---|---|
| 1st Quartile | 2nd Quartile | 3rd Quartile | 4th Quartile | ||
| 123.2–268.4 | 268.7–322.3 | 322.4–386.9 | 387.2–982.3 | ||
| N | 1210 | 303 | 302 | 302 | 303 |
| Sex (men, %) | 615 (52) | 142 (47) | 167 (55) | 174 (57) | 132 (43) |
| Age, yr, mean±s.d. | 35±12 | 36±13 | 34±13 | 35±12 | 35±10 |
| Hypertension (%) | 514 (43) | 141 (47) | 127 (42) | 132(44) | 114 (38) |
| Initial proteinuria, g/24 h, median, IQR | 1.30 (0.68 to 2.51) | 1.50 (0.87 to 2.86) | 1.32 (0.69 to 2.63) | 1.26 (0.62 to 2.28) | 1.20 (0.61 to 2.4) |
| eGFR, ml/min per 1.73 m2, mean±s.d. | 83±32 | 85±20 | 86±33 | 83±33 | 78±31 |
| Oxford classification (%)a | |||||
| M1 | 481 (40) | 97 (32) | 113 (38) | 121 (41) | 150 (50) |
| E1 | 391 (33) | 112 (37) | 90 (31) | 96 (33) | 93 (31) |
| S1 | 746(63) | 186 (62) | 175 (60) | 187 (63) | 198 (66) |
| T1 | 300 (25) | 67 (22) | 74 (25) | 75 (25) | 84 (28) |
| T2 | 124 (11) | 19 (6) | 20 (7) | 33 (11) | 52 (17) |
| C1 | 562 (47) | 133 (44) | 136 (46) | 145 (49) | 148 (49) |
| C2 | 144 (12) | 35(12) | 36 (12) | 36 (12) | 37 (12) |
| Plasma galactose-deficient IgA1, U/ml, mean±s.d. | 325±51 | 293±39 | 312±37 | 331±39 | 364±59 |
| Plasma IgA1, mg/ml, median, IQR | 2.64 (1.82 to 3.36) | 2.43 (1.69 to 3.19) | 2.54 (1.73 to 3.23) | 2.59 (1.9 to 3.23) | 2.76 (2.04 to 3.69) |
| Plasma C3, mg/dl, mean±s.d. | 102±23 | 129±21 | 105±13 | 95±12 | 79±13 |
| Treated with immunosuppressive agents or prednisone (%)b | 550 (46) | 128 (42) | 155 (51) | 129 (43) | 138 (46) |
IQR, interquartile range; M, mesangial hypercellularity; E, the presence of endocapillary proliferation; S, segmental glomerulosclerosis/adhesion; T, severity of tubular atrophy/interstitial fibrosis; C, presence of crescent.
The Oxford classification was developed by the Working Group of the International IgA Nephropathy Network and the Renal Pathology Society. Oxford scores of 23 participants were unavailable because each of them had fewer than eight glomeruli.
The treatment regimens of eight participants were unavailable.
Association of Plasma Levels of Galactose-Deficient IgA1 and Galactose-Deficient IgA1/C3 Ratio and Clinicopathologic Characteristics
The mean galactose-deficient IgA1 level in participants with IgA nephropathy at biopsy was 325±51 U/ml, significantly higher than that of healthy controls (270±45 U/ml; P<0.001) (Supplemental Figure 1). The characteristics according to ascending quartiles of plasma galactose-deficient IgA1 were presented in Supplemental Table 1. The mean C3 level in participants with IgA nephropathy at biopsy was 102±23 mg/dl. The characteristics according to ascending quartiles of serum C3 were presented in Supplemental Table 2. The galactose-deficient IgA1 levels showed a negative correlation with C3 levels (r=−0.06; P=0.04). Median galactose-deficient IgA1/C3 ratio in participants with IgA nephropathy was 322.3 U/mg (IQR, 268.4–387.2 U/mg). The participants were next divided into four equal groups according to the quartiles of the galactose-deficient IgA1/C3 ratio distribution. Characteristics of the participants with IgA nephropathy were presented for the overall population, as well as for participants stratified according to quartiles of galactose-deficient IgA1/C3 ratio, in Table 1. The follow-up characteristics of four groups defined by quartiles of plasma galactose-deficient IgA1/C3 levels were shown in Table 2. The incidence rate of composite kidney progression event was higher in participants with high levels of galactose-deficient IgA1/C3 ratio (P for trend <0.001). Similar results were obtained when analyzed ESKD (P for trend <0.001) and 50% eGFR decline (P for trend <0.001) separately (Table 2).
Table 2.
Associations of plasma galactose-deficient IgA1/C3 ratio with CKD progression
| Galactose-Deficient IgA1/C3 Levels (U/mg, range) | No. at Risk | Events (Incidence Rate)a | Hazard Ratio (95% Confidence Interval) | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Composite End Pointb | ESKD | 50% eGFR Decline | Unadjusted | Model 1c | Model 2d | Model 3e | |||
| CKD progression event per ln (galactose-deficient IgA1/C3) | 123.2–982.3 | 1210 | 172 (3.1) | 114 (2.0) | 165 (3.0) | 2.90 (1.80 to 4.67) | 2.92 (1.8 to 4.72) | 2.31 (1.47 to 3.64) | 2.03 (1.25 to 3.29) |
| Galactose-deficient IgA1/C3 quartiles | |||||||||
| 1 | 123.2–268.4 | 303 | 24 (1.7) | 18 (1.3) | 22 (1.6) | 1 (Reference) | 1 (Reference) | 1 (Reference) | 1 (Reference) |
| 2 | 268.7–322.3 | 302 | 41 (3.0) | 22 (1.5) | 39 (2.8) | 1.60 (0.97 to 2.66) | 1.52 (0.91 to 2.53) | 1.59 (0.95 to 2.66) | 1.71 (1.01 to 2.89) |
| 3 | 322.4–386.9 | 302 | 45 (3.2) | 28 (2.0) | 43 (3.1) | 1.83 (1.12 to 3.01) | 1.72 (1.04 to 2.84) | 1.89 (1.14 to 3.12) | 1.55 (0.91 to 2.63) |
| 4 | 387.2–982.3 | 303 | 62 (4.4) | 46 (3.1) | 61 (4.3) | 2.55 (1.59 to 4.1) | 2.51 (1.57 to 4.03) | 2.28 (1.41 to 3.66) | 2.17 (1.33 to 3.56) |
| P for trendf | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | 0.001 | 0.003 | ||
Annual incidence rate of events per 100 participants.
CKD progression event was defined as a 50% decline in eGFR or ESKD. The events are not mutually exclusive.
Model 1 was adjusted for sex and age. Sex was analyzed as dichotomous data.
Model 2 was adjusted for covariates in model 1 plus eGFR, proteinuria, and hypertension (yes or no).
Model 3 was adjusted for covariates in model 2 plus Oxford M (mesangial hypercellularity), E (the presence of endocapillary proliferation), S (segmental glomerulosclerosis/adhesion), T (severity of tubular atrophy/interstitial fibrosis), and C (presence of crescent) scores and steroids or other immunosuppressive agents (yes or no).
Test for trend of composite end point, ESKD or 50% eGFR decline across quartiles of galactose-deficient IgA1/C3 levels according to the Cochran–Armitage trend test. Test for trend in Cox regression models according to the variable containing the median value for each quartile.
Association of Galactose-Deficient IgA1 and Galactose-Deficient IgA1/C3 Ratio with Kidney Disease Progression
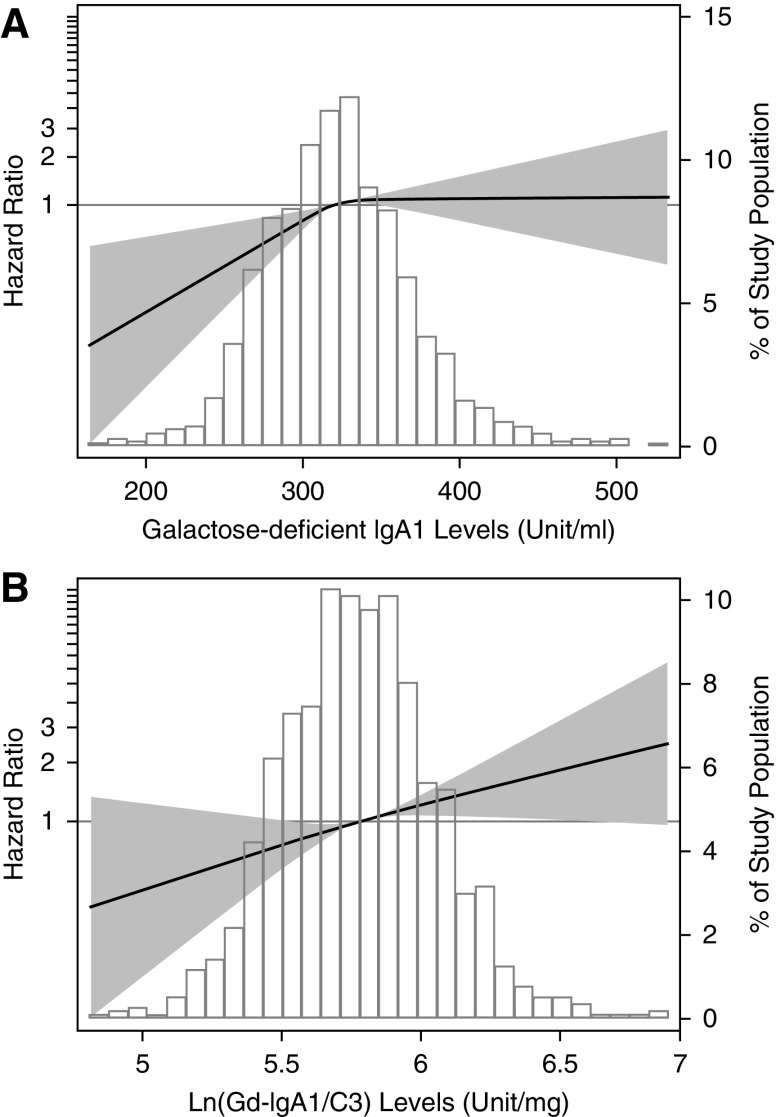
To demonstrate the relationship between galactose-deficient IgA1 levels and kidney outcomes in IgA nephropathy, we modeled galactose-deficient IgA1 level as a continuous variable using restricted cubic splines. We found a nonlinear association between galactose-deficient IgA1 and risk of the CKD progression events (Figure 1A). The risk of CKD progression events was greater with higher plasma galactose-deficient IgA1 levels but reached a plateau when galactose-deficient IgA1 was >325 U/ml. C3 levels also showed a nonlinear relationship with the risk of CKD progression events. Supplemental Figure 2 shows the restricted cubic splines displaying the association between C3 levels and the CKD progression event. Galactose-deficient IgA1 levels and C3 levels were separately tested for association with the CKD progression event using Cox proportional hazards model. We found that higher levels of galactose-deficient IgA1 and lower levels of C3 were associated with a greater risk of CKD progression. However, the significances were lost after adjustment for traditional risk factors (Supplemental Tables 3 and 4).
Figure 1.
Associations of plasma galactose-deficient IgA1 concentration and plasma galactose-deficient IgA1/C3 ratio with CKD progression. Models were performed using restricted cubic splines with knots at the 25th, 50th, and 75th percentiles. The reference value was set at the medians. The solid line represents the estimated hazard ratio, the shaded area represents the 95% confidence bands, and the histogram represents the distribution of galactose-deficient IgA1/C3 ratio in participants with IgA nephropathy. Models were adjusted for age; sex; eGFR; proteinuria; hypertension; Oxford M (mesangial hypercellularity score), E (the presence of endocapillary proliferation), S (segmental glomerulosclerosis/adhesion), T (severity of tubular atrophy/interstitial fibrosis), and C (presence of crescent) scores; and corticosteroids/immunosuppressive therapy. Gd-IgA1, galactose-deficient IgA1; ln, natural log transformation.
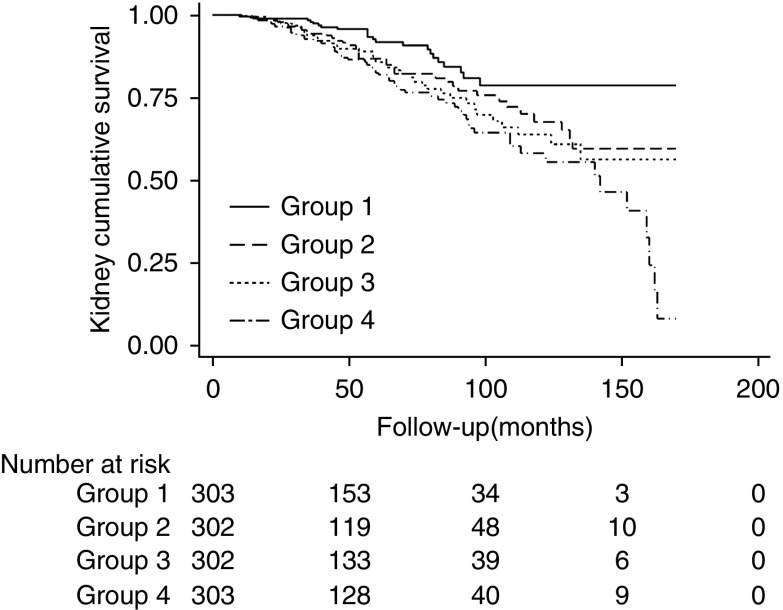
Restricted cubic spline models were built to explore the association between galactose-deficient IgA1/C3 ratio and the CKD progression event. The restricted cubic splines displayed a linear relationship between plasma galactose-deficient IgA1/C3 ratio and risk of CKD progression events (Figure 1B). We further used Cox proportional hazards model to assess the association between galactose-deficient IgA1/C3 ratio and CKD progression events. In unadjusted analysis, sex, lower baseline eGFR, heavier proteinuria, hypertension, higher Oxford scores, use of immunosuppression, and higher levels of galactose-deficient IgA1/C3 ratio were all significantly associated with a progression risk (Supplemental Table 5). After adjusted for traditional risk factors, higher levels of galactose-deficient IgA1/C3 ratio was independently associated with a greater risk of CKD progression events with a hazard ratio of 2.03 (95% CI, 1.25 to 3.29; P=0.004) per natural log-transformed (galactose-deficient IgA1/C3). In reference to the first quartile of galactose-deficient IgA1/C3 ratio, the risk of CKD progression events was higher, that the hazard ratio was 1.71 (95% CI, 1.01 to 2.89) for the second quartile, 1.55 (95% CI, 0.91 to 2.63) for the third quartile, and 2.17 (95% CI, 1.33 to 3.56) for the fourth quartile of the galactose-deficient IgA1/C3 ratio (Table 2). Similar results were obtained in sensitivity analyses that including ESKD alone (Supplemental Figure 3, Supplemental Table 6). We also did a sensitivity analysis in participants with or without immunosuppressive therapy, and the results were consistent (Supplemental Table 7). Kaplan–Meier curves showed similar results with a significant association between quartiles of galactose-deficient IgA1/C3 and the CKD progression event (log-rank test, P=0.001) (Figure 2).
Figure 2.
Kaplan–Meier kidney survival curves of participants with IgA nephropathy according to plasma galactose-deficient IgA1/C3 ratio. The time zero was kidney biopsy. The division between the four groups of participants was on the basis of quartiles of galactose-deficient IgA1/C3.
Longitudinal Change in Galactose-Deficient IgA1
We performed repeated measurements of plasma galactose-deficient IgA1 with blood samples collected during follow-up from 171 participants. Mean levels of galactose-deficient IgA1 did not change over a median of 36 months of follow-up (313±47 versus 319±41 U/ml; P=0.13). Plasma galactose-deficient IgA1 levels in participants who received steroids/immunosuppressive therapy also did not change over time (317±49 versus 320±45 U/ml; P=0.58). The longitudinal change in galactose-deficient IgA1 is shown in Supplemental Figure 4.
Discussion
Numerous studies have now firmly established that altered O-glycosylation of serum IgA1 has a central role in the development of IgA nephropathy, but few cohort studies ever evaluate its role as a biomarker in the disease progression. In this large study with 1210 participants and 172 CKD progression events, we demonstrated the association between galactose-deficient IgA1 and CKD progression event in IgA nephropathy. Although there was a trend that high levels of circulating galactose-deficient IgA1 were associated with greater risk of CKD progression, we firstly found that this association was not in a linear relationship. The risk of CKD progression events increased before the galactose-deficient IgA1 <325 U/ml, and then the risk reached a plateau (Figure 1A). However, the galactose-deficient IgA1/C3 ratio showed a much stronger association with CKD progression events. The risk continuously increased with the levels of galactose-deficient IgA1/C3 supporting this ratio as biomarkers for CKD progression event in IgA nephropathy. These findings also support the hypothesis that complement activated by IgA1-containing immune complexes play an important role in the development and progression of IgA nephropathy.
Participants with IgA nephropathy have abnormalities in the O-glycosylation of the IgA1 hinge region, leading to increased levels of circulating galactose-deficient IgA1. It has been proposed as a key biomarker for disease diagnosis and disease progression. There has been conflicting evidence regarding whether galactose-deficient IgA1 levels also were associated with disease progression. Circulating galactose-deficient IgA1 levels were evaluated in 107 Chinese participants and were associated with pathologic lesions (25). In another cross-sectional study with 41 Japanese participants, higher galactose-deficient IgA1 was not associated with severity of lesions in kidney biopsy. In a recent United Kingdom cohort with 379 participants over 5 years follow-up, plasma galactose-deficient IgA1 levels were significantly increased in participants with progressive IgA nephropathy, compared with nonprogressive IgA nephropathy (35). Berthoux et al. (36) also reported that participants with a high risk for progression to dialysis/death had increased plasma galactose-deficient IgA1 levels in a French cohort with 97 participants. In our prior study with 275 Chinese participants, we found that plasma galactose-deficient IgA1 levels independently affected disease progression (28). These findings were limited by the small sample size or lack adjustments in a multivariable model. In this large study with 1210 participants, we found lower levels of galactose-deficient IgA1 were associated decreased risk of kidney function decline. Compared with the previous studies, this large cohort study showed the correlation of galactose-deficient IgA1 and risk of kidney progression was not in a linear association (Figure 1A). The risk of CKD progression events did not increase after galactose-deficient IgA1 reached 325 U/ml, which limited its prognostic utility in IgA nephropathy.
Recent studies had suggested complement activation by the pathogenic immune complexes consisting of galactose-deficient IgA1 play important roles in the development or progression of IgA nephropathy. Colocalization of glomerular complement C3c with IgA is present in 90% of participants. Serum levels of activated C3 and mesangial C3 deposition correlate with loss of kidney function. In previous studies circulating IgA/C3 ratio has been shown associated with kidney progression in Japanese and Chinese population (37,38). In this study we combined galactose-deficient IgA1 and C3 levels to predict disease progression in IgA nephropathy and found high galactose-deficient IgA1/C3 ratio was also independently associated with CKD progression events. Thus, our data suggest that high levels of galactose-deficient IgA1/C3 ratio showed much better prognostic utility than galactose-deficient IgA1 itself in IgA nephropathy.
These findings have implications for clinical practice. Firstly, lower levels of galactose-deficient IgA1 were associated with decreased risk of CKD progression events when galactose-deficient IgA1 <325 U/ml. Several studies evaluated galactose-deficient IgA1 changes over time. In a United Kingdom longitudinal cohort of participants with IgA nephropathy and healthy controls, the galactose-deficient IgA1 level in individuals did not change significantly over 5 years (35). In this study, we found that therapies including renin-angiotensin-aldosterone system inhibitors or immunosuppressive therapy was not associated with decline of galactose-deficient IgA1 levels in the follow-up. In IgA nephropathy, mucosal B lymphocytes located in Peyer patches were believed to be primed to produce galactose-deficient IgA1 (39). The recent targeted-release budesonide trial (NEFIGAN) was designed to reduce galactose-deficient IgA1 producing and succeeded in reducing proteinuria by 24% during the 9-month therapy (40). Future studies should evaluate if the galactose-deficient IgA1 reduction improve kidney outcomes. Secondly, current studies mainly focus on the levels of galactose-deficient IgA1. This study showed that increased levels of galactose-deficient IgA1 as a diagnostic biomarker for IgA nephropathy with the sensitivity and specificity values were 54% and 90%, respectively, which was comparable with that in Japanese participants but much lower than the white population. Galactose-deficient IgA1 levels were also not stably associated with risk of CKD progression events when they were >325 U/ml. So levels of galactose-deficient IgA1 only have limited clinical utility in IgA nephropathy. The galactose-deficient IgA1/C3 ratio was linearly associated with the risk of CKD progression events that suggest the pathogenicity of galactose-deficient IgA1 depended on not only their levels, but also their ability for complement activation. The IgA1 hinge region has nine potential sites for O-glycan attachment. Although lectin binding provided an overall assessment of hinge galactose content, it did not provide specific information on the positioning of hinge O-glycans. Additionally, polymeric IgA activated the lectin complement pathway through the N-linked glycans of IgA1. Future studies warranted to evaluate the structures of IgA1 O-glycans and N-glycans in the development or progression in IgA nephropathy.
The strengths of this study are the large sample size and the reliable ELISA methods used to detect galactose-deficient IgA1. In addition, we use the galactose-deficient IgA1/C3 ratio to assess the pathogenic activity of galactose-deficient IgA1. To the best of our knowledge, this study is the largest study to explore the association of galactose-deficient IgA1 and kidney progression. We first demonstrated that the association had a nonlinear relationship, whereas galactose-deficient IgA1/C3 ratio was linearly associated with CKD progression events. However, our study has several limitations. First, sequential galactose-deficient IgA1 measurements were not prospectively collected. Second, this was a single-center study and the findings need to be confirmed in other populations.
In conclusion, in this study we use a large cohort study demonstrating the association between plasma galactose-deficient IgA1 level and CKD progression. The risk of CKD progression events was greater with higher galactose-deficient IgA1 levels and the risk reached a plateau when galactose-deficient IgA1 level was >325 U/ml. Further, galactose-deficient IgA1/C3 ratio was linearly associated with CKD progression events independently of clinical and biopsy characteristics.
Disclosures
The authors have nothing to disclose.
Funding
This study was supported by grants from the National Natural Science Foundation of China (81670649). Dr. Lv reports grants from National Natural Science Foundation of China during the conduct of the study.
Supplementary Material
Footnotes
Published online ahead of print. Publication date available at www.cjasn.org.
See related editorial, “Biomarkers to Predict Progression in IgA Nephropathy,” on pages 1421–1423.
Supplemental Material
This article contains the following supplemental material online at http://cjasn.asnjournals.org/lookup/suppl/doi:10.2215/CJN.13711118/-/DCSupplemental.
Supplemental Table 1. Demographic and clinical characteristics of four groups defined by quartiles of plasma galactose-deficient IgA1 levels.
Supplemental Table 2. Demographic and clinical characteristics of four groups defined by quartiles of serum C3 levels.
Supplemental Table 3. Associations of plasma galactose-deficient IgA1 with CKD progression.
Supplemental Table 4. Associations of serum C3 with CKD progression.
Supplemental Table 5. Unadjusted and multivariable-adjusted Cox regression models testing associations of plasma galactose-IgA1/C3 ratio with CKD progression.
Supplemental Table 6. Associations of plasma galactose-deficient IgA1/C3 ratio with ESKD.
Supplemental Table 7. Associations of plasma galactose-deficient IgA1/C3 ratio with ESKD stratified by use of immunosuppression.
Supplemental Figure 1. Galactose-deficient IgA1 levels were higher in 1210 patients with IgA nephropathy than 208 healthy controls (P<0.001).
Supplemental Figure 2. Associations of serum C3 concentration with CKD progression.
Supplemental Figure 3. Associations of plasma galactose-deficient IgA1/C3 ratio with ESKD.
Supplemental Figure 4. Spaghetti plots of serial plasma galactose-deficient IgA1 levels.
References
- 1.D’Amico G: The commonest glomerulonephritis in the world: IgA nephropathy. Q J Med 64: 709–727, 1987 [PubMed] [Google Scholar]
- 2.Woo KT, Chan CM, Chin YM, Choong HL, Tan HK, Foo M, Anantharaman V, Lee GS, Chiang GS, Tan PH, Lim CH, Tan CC, Lee E, Tan HB, Fook-Chong S, Lau YK, Wong KS: Global evolutionary trend of the prevalence of primary glomerulonephritis over the past three decades. Nephron Clin Pract 116: c337–c346, 2010 [DOI] [PubMed] [Google Scholar]
- 3.McGrogan A, Franssen CF, de Vries CS: The incidence of primary glomerulonephritis worldwide: A systematic review of the literature. Nephrol Dial Transplant 26: 414–430, 2011 [DOI] [PubMed] [Google Scholar]
- 4.Wyatt RJ, Julian BA: IgA nephropathy. N Engl J Med 368: 2402–2414, 2013 [DOI] [PubMed] [Google Scholar]
- 5.Berthoux F, Mohey H, Laurent B, Mariat C, Afiani A, Thibaudin L: Predicting the risk for dialysis or death in IgA nephropathy. J Am Soc Nephrol 22: 752–761, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Reich HN, Troyanov S, Scholey JW, Cattran DC; Toronto Glomerulonephritis Registry : Remission of proteinuria improves prognosis in IgA nephropathy. J Am Soc Nephrol 18: 3177–3183, 2007 [DOI] [PubMed] [Google Scholar]
- 7.Barbour SJ, Reich HN: Risk stratification of patients with IgA nephropathy. Am J Kidney Dis 59: 865–873, 2012 [DOI] [PubMed] [Google Scholar]
- 8.Goto M, Wakai K, Kawamura T, Ando M, Endoh M, Tomino Y: A scoring system to predict renal outcome in IgA nephropathy: A nationwide 10-year prospective cohort study. Nephrol Dial Transplant 24: 3068–3074, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Camilla R, Suzuki H, Daprà V, Loiacono E, Peruzzi L, Amore A, Ghiggeri GM, Mazzucco G, Scolari F, Gharavi AG, Appel GB, Troyanov S, Novak J, Julian BA, Coppo R: Oxidative stress and galactose-deficient IgA1 as markers of progression in IgA nephropathy. Clin J Am Soc Nephrol 6: 1903–1911, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Delanghe SE, Speeckaert MM, Segers H, Desmet K, Vande Walle J, Laecke SV, Vanholder R, Delanghe JR: Soluble transferrin receptor in urine, a new biomarker for IgA nephropathy and Henoch-Schönlein purpura nephritis. Clin Biochem 46: 591–597, 2013 [DOI] [PubMed] [Google Scholar]
- 11.Guo WY, Zhu L, Meng SJ, Shi SF, Liu LJ, Lv JC, Zhang H: Mannose-binding lectin levels could predict prognosis in IgA nephropathy. J Am Soc Nephrol 28: 3175–3181, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Peters HP, van den Brand JA, Wetzels JF: Urinary excretion of low-molecular-weight proteins as prognostic markers in IgA nephropathy. Neth J Med 67: 54.–, 2009 [PubMed] [Google Scholar]
- 13.Peters HP, Waanders F, Meijer E, van den Brand J, Steenbergen EJ, van Goor H, Wetzels JF: High urinary excretion of kidney injury molecule-1 is an independent predictor of end-stage renal disease in patients with IgA nephropathy. Nephrol Dial Transplant 26: 3581–3588, 2011 [DOI] [PubMed] [Google Scholar]
- 14.Vuong MT, Hahn-Zoric M, Lundberg S, Gunnarsson I, van Kooten C, Wramner L, Seddighzadeh M, Fernström A, Hanson LA, Do LT, Jacobson SH, Padyukov L: Association of soluble CD89 levels with disease progression but not susceptibility in IgA nephropathy. Kidney Int 78: 1281–1287, 2010 [DOI] [PubMed] [Google Scholar]
- 15.Zittema D, van den Brand JA, Bakker SJ, Wetzels JF, Gansevoort RT: Copeptin, a surrogate marker for arginine vasopressin, is associated with disease severity and progression in IgA nephropathy patients. Nephrol Dial Transplant 32[suppl_1]: i146–i153, 2017 [DOI] [PubMed] [Google Scholar]
- 16.Coppo R, Fonsato V, Balegno S, Ricotti E, Loiacono E, Camilla R, Peruzzi L, Amore A, Bussolati B, Camussi G: Aberrantly glycosylated IgA1 induces mesangial cells to produce platelet-activating factor that mediates nephrin loss in cultured podocytes. Kidney Int 77: 417–427, 2010 [DOI] [PubMed] [Google Scholar]
- 17.Hiki Y: O-linked oligosaccharides of the IgA1 hinge region: Roles of its aberrant structure in the occurrence and/or progression of IgA nephropathy. Clin Exp Nephrol 13: 415–423, 2009 [DOI] [PubMed] [Google Scholar]
- 18.Suzuki H, Kiryluk K, Novak J, Moldoveanu Z, Herr AB, Renfrow MB, Wyatt RJ, Scolari F, Mestecky J, Gharavi AG, Julian BA: The pathophysiology of IgA nephropathy. J Am Soc Nephrol 22: 1795–1803, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Suzuki H, Moldoveanu Z, Hall S, Brown R, Vu HL, Novak L, Julian BA, Tomana M, Wyatt RJ, Edberg JC, Alarcón GS, Kimberly RP, Tomino Y, Mestecky J, Novak J: IgA1-secreting cell lines from patients with IgA nephropathy produce aberrantly glycosylated IgA1. J Clin Invest 118: 629–639, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hastings MC, Moldoveanu Z, Julian BA, Novak J, Sanders JT, McGlothan KR, Gharavi AG, Wyatt RJ: Galactose-deficient IgA1 in African Americans with IgA nephropathy: Serum levels and heritability. Clin J Am Soc Nephrol 5: 2069–2074, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lin X, Ding J, Zhu L, Shi S, Jiang L, Zhao M, Zhang H: Aberrant galactosylation of IgA1 is involved in the genetic susceptibility of Chinese patients with IgA nephropathy. Nephrol Dial Transplant 24: 3372–3375, 2009 [DOI] [PubMed] [Google Scholar]
- 22.Moldoveanu Z, Wyatt RJ, Lee JY, Tomana M, Julian BA, Mestecky J, Huang WQ, Anreddy SR, Hall S, Hastings MC, Lau KK, Cook WJ, Novak J: Patients with IgA nephropathy have increased serum galactose-deficient IgA1 levels. Kidney Int 71: 1148–1154, 2007 [DOI] [PubMed] [Google Scholar]
- 23.Shimozato S, Hiki Y, Odani H, Takahashi K, Yamamoto K, Sugiyama S: Serum under-galactosylated IgA1 is increased in Japanese patients with IgA nephropathy. Nephrol Dial Transplant 23: 1931–1939, 2008 [DOI] [PubMed] [Google Scholar]
- 24.Yanagawa H, Suzuki H, Suzuki Y, Kiryluk K, Gharavi AG, Matsuoka K, Makita Y, Julian BA, Novak J, Tomino Y: A panel of serum biomarkers differentiates IgA nephropathy from other renal diseases. PLoS One 9: e98081, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Xu LX, Zhao MH: Aberrantly glycosylated serum IgA1 are closely associated with pathologic phenotypes of IgA nephropathy. Kidney Int 68: 167–172, 2005 [DOI] [PubMed] [Google Scholar]
- 26.Canetta PA, Kiryluk K, Appel GB: Glomerular diseases: Emerging tests and therapies for IgA nephropathy. Clin J Am Soc Nephrol 9: 617–625, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Coppo R: Biomarkers and targeted new therapies for IgA nephropathy. Pediatr Nephrol 32: 725–731, 2017 [DOI] [PubMed] [Google Scholar]
- 28.Zhao N, Hou P, Lv J, Moldoveanu Z, Li Y, Kiryluk K, Gharavi AG, Novak J, Zhang H: The level of galactose-deficient IgA1 in the sera of patients with IgA nephropathy is associated with disease progression. Kidney Int 82: 790–796, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Maillard N, Wyatt RJ, Julian BA, Kiryluk K, Gharavi A, Fremeaux-Bacchi V, Novak J: Current understanding of the role of complement in IgA nephropathy. J Am Soc Nephrol 26: 1503–1512, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zhu L, Zhai YL, Wang FM, Hou P, Lv JC, Xu DM, Shi SF, Liu LJ, Yu F, Zhao MH, Novak J, Gharavi AG, Zhang H: Variants in complement factor H and complement factor H-related protein genes, CFHR3 and CFHR1, affect complement activation in IgA nephropathy. J Am Soc Nephrol 26: 1195–1204, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kawasaki Y, Maeda R, Ohara S, Suyama K, Hosoya M: Serum IgA/C3 and glomerular C3 staining predict severity of IgA nephropathy. Pediatr Int 60: 162–167, 2018 [DOI] [PubMed] [Google Scholar]
- 32.Mizerska-Wasiak M, Małdyk J, Rybi-Szumińska A, Wasilewska A, Miklaszewska M, Pietrzyk J, Firszt-Adamczyk A, Stankiewicz R, Bieniaś B, Zajączkowska M, Gadomska-Prokop K, Grenda R, Pukajło-Marczyk A, Zwolińska D, Szczepańska M, Turczyn A, Roszkowska-Blaim M: Relationship between serum IgA/C3 ratio and severity of histological lesions using the Oxford classification in children with IgA nephropathy. Pediatr Nephrol 30: 1113–1120, 2015 [DOI] [PubMed] [Google Scholar]
- 33.Trimarchi H, Barratt J, Cattran DC, Cook HT, Coppo R, Haas M, Liu Z-H, Roberts IS, Yuzawa Y, Zhang H, Feehally J; IgAN Classification Working Group of the International IgA Nephropathy Network and the Renal Pathology Society; Conference Participants : Oxford classification of IgA nephropathy 2016: An update from the IgA nephropathy classification working group. Kidney Int 91: 1014–1021, 2017 [DOI] [PubMed] [Google Scholar]
- 34.Kong X, Ma Y, Chen J, Luo Q, Yu X, Li Y, Xu J, Huang S, Wang L, Huang W, Wang M, Xu G, Zhang L, Zuo L, Wang H; Chinese eGFR Investigation Collaboration : Evaluation of the Chronic Kidney Disease Epidemiology Collaboration equation for estimating glomerular filtration rate in the Chinese population. Nephrol Dial Transplant 28: 641–651, 2013 [DOI] [PubMed] [Google Scholar]
- 35.Gale DP, Molyneux K, Wimbury D, Higgins P, Levine AP, Caplin B, Ferlin A, Yin P, Nelson CP, Stanescu H, Samani NJ, Kleta R, Yu X, Barratt J: Galactosylation of IgA1 is associated with common variation in C1GALT1. J Am Soc Nephrol 28: 2158–2166, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Berthoux F, Suzuki H, Thibaudin L, Yanagawa H, Maillard N, Mariat C, Tomino Y, Julian BA, Novak J: Autoantibodies targeting galactose-deficient IgA1 associate with progression of IgA nephropathy. J Am Soc Nephrol 23: 1579–1587, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Komatsu H, Fujimoto S, Hara S, Sato Y, Yamada K, Eto T: Relationship between serum IgA/C3 ratio and progression of IgA nephropathy. Intern Med 43: 1023–1028, 2004 [DOI] [PubMed] [Google Scholar]
- 38.Zhang J, Wang C, Tang Y, Peng H, Ye ZC, Li CC, Lou TQ: Serum immunoglobulin A/C3 ratio predicts progression of immunoglobulin A nephropathy. Nephrology (Carlton) 18: 125–131, 2013 [DOI] [PubMed] [Google Scholar]
- 39.Lafayette RA, Canetta PA, Rovin BH, Appel GB, Novak J, Nath KA, Sethi S, Tumlin JA, Mehta K, Hogan M, Erickson S, Julian BA, Leung N, Enders FT, Brown R, Knoppova B, Hall S, Fervenza FC: A randomized, controlled trial of rituximab in IgA nephropathy with proteinuria and renal dysfunction. J Am Soc Nephrol 28: 1306–1313, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Fellström BC, Barratt J, Cook H, Coppo R, Feehally J, de Fijter JW, Floege J, Hetzel G, Jardine AG, Locatelli F, Maes BD, Mercer A, Ortiz F, Praga M, Sørensen SS, Tesar V, Del Vecchio L; NEFIGAN Trial Investigators : Targeted-release budesonide versus placebo in patients with IgA nephropathy (NEFIGAN): A double-blind, randomised, placebo-controlled phase 2b trial. Lancet 389: 2117–2127, 2017 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.