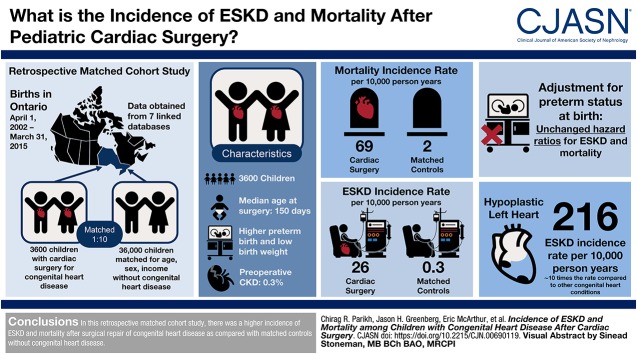
Visual Abstract
Keywords: cardiovascular, chronic kidney disease, clinical epidemiology, ESRD, pediatric nephrology, pediatrics, renal function, renal function decline, child, children, humans, hypoplastic left heart syndrome, incidence, Ontario, follow-up studies, congenital heart defects, cardiac surgical procedures, risk, death, chronic kidney failure, low birth weight infant
Abstract
Background and objectives
Survival after surgical repair for congenital heart disease has markedly improved; however, there are limited data on long-term ESKD and mortality during childhood.
Design, setting, participants, & measurements
We conducted an observational, population-based cohort study of children who had their first surgery for congenital heart disease within 10 years of birth. The study was conducted in Ontario, Canada, where residents have universal access to health care services. Each child who underwent surgical repair was matched to ten children from the general population who were similar in age, sex, index date, rurality, and neighborhood income. Primary outcomes of all-cause mortality and ESKD were reported until March 2015.
Results
We followed 3600 children with congenital heart disease for a median of 5.9 (interquartile range, 2.9–9.0) years after their surgical repair. Median age at first surgery was 150 (interquartile range, 40–252) days and 22% were low birth weight (<2500 g). During follow-up, 140 (4%) children who had surgery for congenital heart disease died and 52 (1%) reached ESKD. The cumulative incidence of death and ESKD at 1, 5, and 10 years was higher in children with surgical repair of congenital heart disease (death: 3%, 4%, and 5%, respectively; ESKD: 1%, 2%, and 2%, respectively) compared with the matched control population without any congenital heart disease (death: 0.06%, 0.10%, and 0.13%, respectively; ESKD: 0.00%, 0.02%, and 0.02%, respectively). The risk of ESKD and death increased with severity of congenital heart disease, with the highest risk in children with hypoplastic left heart syndrome and increased in children who had surgical repair of congenital heart disease compared with those without surgical repair.
Conclusions
The risk of mortality and ESKD is high in children who undergo surgical repair for congenital heart disease compared to the general population.
Introduction
Congenital heart disease is the most common type of birth defect, affecting approximately 1% of live births. One in four children with congenital heart disease requires cardiac surgery (1). With advances in surgical and medical care today more children survive this surgery, with the emphasis now shifting to long-term health, including maintaining normal kidney function.
There is emerging evidence suggesting that pathologic changes occur after surgical repair of congenital heart disease not only in the cardiovascular system but also in the kidneys (2). An excess risk of CKD, ESKD, and death has been previously reported in adults with congenital heart disease (3,4). We recently demonstrated in a multicenter, pediatric cohort that CKD prevalence was four-fold higher than the age-matched general population, just 5 years after cardiac surgery (5). The adverse cardiovascular outcomes associated with CKD and ESKD are especially concerning in these children who are already at higher risk of atrial fibrillation, stroke, and heart failure (4). Dimopoulos et al. (3) showed that mortality was three-fold higher in children and adults with congenital heart disease with GFR<60 ml/min per 1.73 m2. The actual risk of mortality and ESKD may be higher as most of the research to date is restricted to single center studies with variable follow-up and suboptimal controls (6–11).
Understanding the natural history of ESKD and death after childhood cardiac surgery informs the need to prevent and treat kidney disease in an at-risk population. The goal of the present study was to determine the incidence of ESKD and death after surgical repair of congenital heart disease in a large Canadian province.
Materials and Methods
Study Design
We conducted a retrospective matched cohort study in Ontario, Canada using linked administrative health care databases. In Ontario, residents have universal access to healthcare services. These databases were linked using unique, encoded identifiers and analyzed ICES (https://www.ices.on.ca). The reporting of the study follows guidelines for observational studies conducted using routinely collected health data (Supplemental Table 1). The use of data in this project was authorized under section 45 of Ontario’s Personal Health Information Protection Act, which does not require review by a Research Ethics Board.
Data Sources
We obtained data from seven linked databases. The MOMBABY database includes all inpatient maternal-newborn admissions and was used to identify and characterize delivering mothers and their newborns. The Registered Persons Database has demographics and vital statistics on all individuals in Ontario with a valid health card. The Canadian Institute for Health Information (CIHI) Discharge Abstract Database has diagnostic and procedural information for all hospitalizations in the province, and was used to identify relevant exclusions, baseline characteristics, and outcomes. Similarly, the CIHI Same-Day Surgery database has diagnostic and procedural information for all same-day surgeries, with baseline characteristics and outcomes also accrued using this database. Provider characteristics, including specialty, were obtained from the ICES-derived Physician Database. The Ontario Health Insurance Plan database captures physician claims data, which was used in this study to assess exclusion, baseline, and outcome variables. The Canadian Organ Replacement Register is a Canada-wide database that captures all transplant and maintenance dialysis activity in the country. These databases have been used previously in epidemiologic research, including studies of congenital heart disease, newborn outcomes, and kidney diseases (12,13).
Congenital heart disease-related surgeries were identified using Ontario Health Insurance Plan surgical billing codes. The International Classification of Diseases Tenth Revision was the coding system used to identify diagnoses and the Canadian Classification of Health Interventions was used to ascertain procedures (Supplemental Table 2). Hospital coders use structured guidelines established by CIHI to assign relevant diagnostic codes to a patient chart. They rely only upon diagnoses listed in the patient’s chart and do not interpret reports, diagnostic imaging, or laboratory data. To comply with privacy regulations for minimizing the chance of identification of a study patient, numbers of patients are suppressed in the case of five or fewer patients (reported as ≤5).
Population
We identified all newborns in Ontario born between April 1, 2002 and March 31, 2015. Congenital heart disease was identified as evidence of a congenital heart disease–related surgery within 10 years of birth. The surgery date was the date of cohort entry (also referred to as the index date) for each specific patient. To ensure these surgeries were truly related to congenital heart disease, individuals without a congenital heart disease diagnosis before or on their index date were excluded. Similarly, individuals with a patent ductus arteriosus ligation and no other surgical code during their index hospitalization were excluded as this was not considered a congenital heart disease surgery. Children with ESKD before the index date were excluded to facilitate the study of incident kidney outcomes. For individuals with multiple eligible congenital heart disease–related surgical hospitalizations, the first surgery after birth was chosen as the index date.
Severity of congenital heart disease was defined using a previously published hierarchy of congenital heart disease diagnosis codes (14). Atrioventricular septal defect, Tetralogy of Fallot, univentricular heart, transposition complex, truncus arteriosus, and hypoplastic left heart syndrome were classified as severe. The complexity of cardiac surgery was categorized on the basis of the Risk Adjustment for Congenital Heart Surgery-1 (RACHS-1) score (15).
To provide context to congenital heart disease outcomes, we assembled a comparison cohort of children without congenital heart disease. Newborns with no evidence of congenital heart disease diagnosis were considered as potential unexposed controls. As these individuals did not undergo surgery, a random index date was assigned on the basis of the distribution of times from birth to the index date of surgery in the group that received surgery for congenital heart disease. Similar exclusions were applied to this unexposed group, on the basis of the assigned index date. We then matched each patient with surgical repair for congenital heart disease to ten controls using a greedy algorithm without replacement on index date (±365 days), age at index date (±90 days), sex, neighborhood income quintile, and rural residence (municipality with a population <10,000) (16). As a secondary analysis, we identified a cohort of children with a diagnosis of congenital heart disease in the first 10 years of life, but with no evidence of a related surgery and compared these individuals to those with a congenital heart disease surgery. These children without congenital heart disease surgery were matched 1:1 with those with a congenital heart disease surgery using the same exclusion criteria and matching algorithm as in our primary analysis. For children without surgery the index date was defined as the date of congenital heart disease diagnosis. Loss to follow-up was expected to be minimal, as annual emigration from Ontario is estimated at 0.1% (17).
Study Outcomes
Children were followed until death, ESKD, or the end of data availability (March 31, 2015). The primary outcomes were all-cause mortality and ESKD, each examined separately. These outcomes have been shown to be reliably coded in Ontario with a sensitivity of 94% and positive predictive value of 100% for all-cause mortality, and a sensitivity exceeding 96% for ESKD (18–20). Diagnostic codes are detailed in Supplemental Table 3, along with information on validation of the codes.
Statistical Analyses
Baseline characteristics are presented as proportions for categorical variables and medians (interquartile range [IQR]) for continuous variables. We compared baseline characteristics between individuals undergoing surgery for congenital heart disease and matched controls using standardized differences, with a standardized difference >10% considered meaningful (21). This statistic describes differences in group means relative to the pooled SD and is not influenced by large sample sizes to the same degree as traditional hypothesis testing. All the variables in the analysis were complete except for neighborhood income quintile and rural residence, which were missing for <0.5% of the cohort. Missing values were imputed as “3 (income quintile)” and “no (rural residence).”
We used Cox proportional hazards regression, stratified on matched sets, to obtain hazard ratios (HRs) and tested the proportionality assumption by adding a time-dependent exposure covariate to the model (22). Death was treated as a censoring event for nonmortality outcomes. To assess the degree to which preterm birth may have influenced the association between congenital heart disease and outcomes, we modeled a multivariable Cox proportional hazards model, adjusting for preterm (<37 weeks) birth. Low birth weight had high collinearity with preterm birth (r>0.6) and was thus not included in multivariable models. We also performed four subgroup analyses to examine the outcomes of individuals stratified by sex, specific congenital heart disease diagnoses, with versus without chromosomal anomalies, and neonate status (age <28 days at the time of surgery). We assessed potential effect measure modification by adding an interaction term to the Cox model. To assess the potential contribution of receipt of dialysis for AKI (AKI-D) on ESKD and death, we modeled AKI-D during the index surgery hospitalization as a covariate in a Cox model restricted to just those with congenital heart disease. We performed an additional analysis to assess the association of multiple surgeries with ESKD and death. Follow-up time for this analysis began on day 366 and was restricted to those who survived the first year after discharge from their index hospitalization to avoid immortal time bias. To estimate survival functions, we used the Kaplan–Meier product-limit estimator. These survival functions were graphically presented as curves (23). A two-sided P value <0.05 was considered statistically significant and analyses were performed using SAS version 9.4 (SAS Institute).
Results
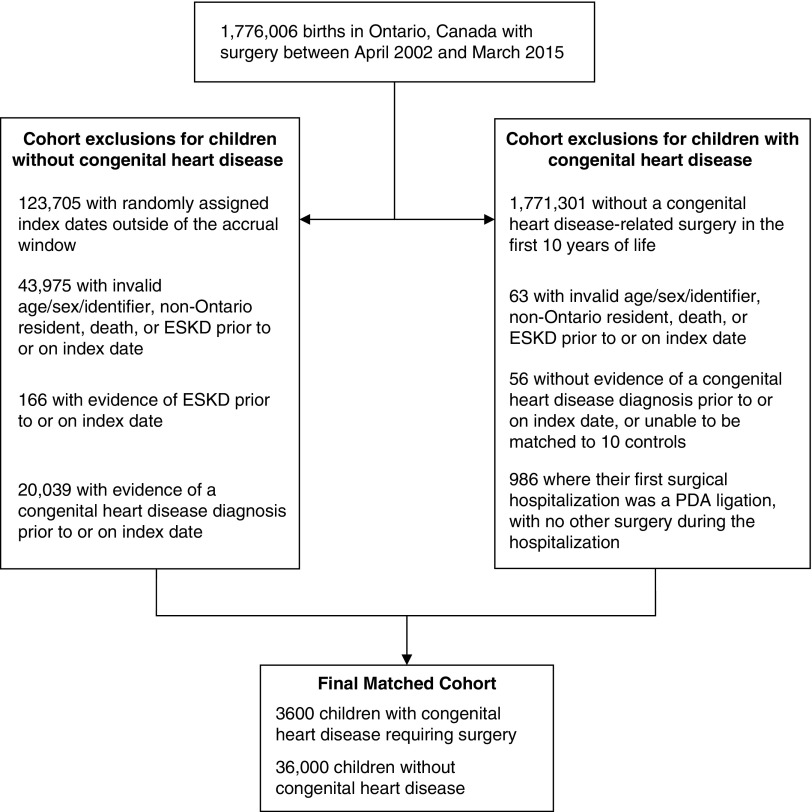
After exclusions and matching, we had 3600 children with surgical repair for congenital heart disease and 36,000 matched controls (Figure 1). The median age at first surgery was 150 days (IQR, 40–252) and 44% of the cohort was female. Maternal age was higher in the cardiac surgery group compared with the controls. Preterm birth and low birth weight were also higher in children with surgical repair for congenital heart disease than in controls (Table 1). The occurrence of concurrent chromosomal abnormalities, noncardiac malformations, and malformations of the urinary system were higher in the cardiac surgery group. Hypertension and CKD was present in 2% and 0.3% of cases before surgery compared with 0.2% and <0.1%, respectively in controls.
Figure 1.
Patient selection for those with and without congenital heart disease.
Table 1.
Characteristics of patients in the study
| Variable | Statistic | Congenital Heart Disease Requiring Surgery | Controls | Standardized Difference |
|---|---|---|---|---|
| n=3600 | n=36,000 | |||
| Demographics | ||||
| Age, yr, median (IQR) | 0.41 (0.11–0.69) | 0.41 (0.12–0.68) | ||
| Female | 1595 (44%) | 15,950 (44%) | 0% | |
| Rural residence | 386 (11%) | 3860 (11%) | 0% | |
| Income quintile | 1 (lowest) | 790 (22%) | 7900 (22%) | 0% |
| 2 | 721 (20%) | 7210 (20%) | 0% | |
| 3 | 751 (21%) | 7510 (21%) | 0% | |
| 4 | 765 (21%) | 7650 (21%) | 0% | |
| 5 (highest) | 573 (16%) | 5730 (16%) | 0% | |
| Year of surgery (reference date for normal) | 2002–2004 | 566 (16%) | 5652 (16%) | 0% |
| 2005–2007 | 781 (22%) | 7827 (22%) | 0% | |
| 2008–2010 | 958 (27%) | 9579 (27%) | 0% | |
| 2011–2013 | 931 (26%) | 9310 (26%) | 0% | |
| 2014–2015 | 364 (10%) | 3632 (10%) | 0% | |
| Maternal age, yr, median (IQR) | 32 (28–36) | 31 (27–35) | ||
| Gestational age, wk | Mean (SD) | 38 (2.42) | 38.9 (2.07) | 94% |
| Median (IQR) | 38(37–40) | 39 (38–40) | ||
| Preterm (<37 wk) | 652 (18%) | 2694 (8%) | 76% | |
| Very preterm (<32 wk) | 98 (3%) | 263 (1%) | 36% | |
| Extremely preterm (<28 wk) | 29 (1%) | 67 (0.2%) | 21% | |
| Birth weight, g | Mean (SD) | 3066 (711) | 3377 (556) | 115% |
| Median (IQR) | 3140 (2670–3532) | 3400 (3053–3730) | ||
| Low birth weight (<2500 g) | 681 (19%) | 2042 (6%) | 97% | |
| Very low birth weight (<1500 g) | 104 (3%) | 171 (1%) | 44% | |
| Hypertension | 72 (2%) | 80 (0.2%) | 17% | |
| CKD | 10 (0.3%) | 16 (0%) | 8% | |
| Chromosomal anomaly | 470 (13%) | 22 (0.1%) | 54% | |
| Multibirth | Yes | 190 (5%) | 1074 (3%) | 12% |
| Surgery hospitalization characteristics | ||||
| Any congenital heart disease diagnosis | 3600 (100%) | |||
| Severe congenital heart disease diagnosis | 1866 (52%) | |||
| Risk Adjustment for Congenital Heart Surgery-1 (RACHS-1) score | 1 | 424 (12%) | ||
| 2 | 1598 (44%) | |||
| 3 | 1051 (29%) | |||
| ≥4 | 519 (14%) | |||
| Length of stay in hospital, d | Mean (SD) | 24.7 (66.8) | ||
| Median (IQR) | 10 (5–23) | |||
| Length of stay in hospital after surgery, d (surgery date to discharge date) | Mean (SD) | 19.7 (63.3) | ||
| Median (IQR) | 8 (5–17) | |||
| Length of stay in ICU, d | Mean (SD) | 11.7 (25.0) | ||
| Median (IQR) | 5 (2–11) | |||
| Time on ventilation, d | Mean (SD) | 9.3 (20.7) | ||
| Median (IQR) | 4 (2–9) | |||
| Dialysis during index hospitalization | 126 (4%) | |||
IQR, interquartile range; ICU, intensive care unit.
Using diagnosis codes, 1866 (52%) cases were classified as severe congenital heart disease. The complexity of surgery by RACHS-1 classification was 12% (category 1), 44% (category 2), 29% (category 3), and 14% (≥ category 4) (Table 1). The most common diagnoses were ventricular septal defect (16%), Tetralogy of Fallot (13%), atrioventricular septal defect (12%), and coarctation of the aorta (11%) (Supplemental Table 4). The most common procedures during the surgical hospitalization were closure of secundum atrial septal defect (37%) and closure of ventricular septal defect (35%). The median length of stay in the hospital postsurgery was 10 days (IQR, 5–23), with 126 (4%) of cardiac surgery patients receiving dialysis during the hospitalization.
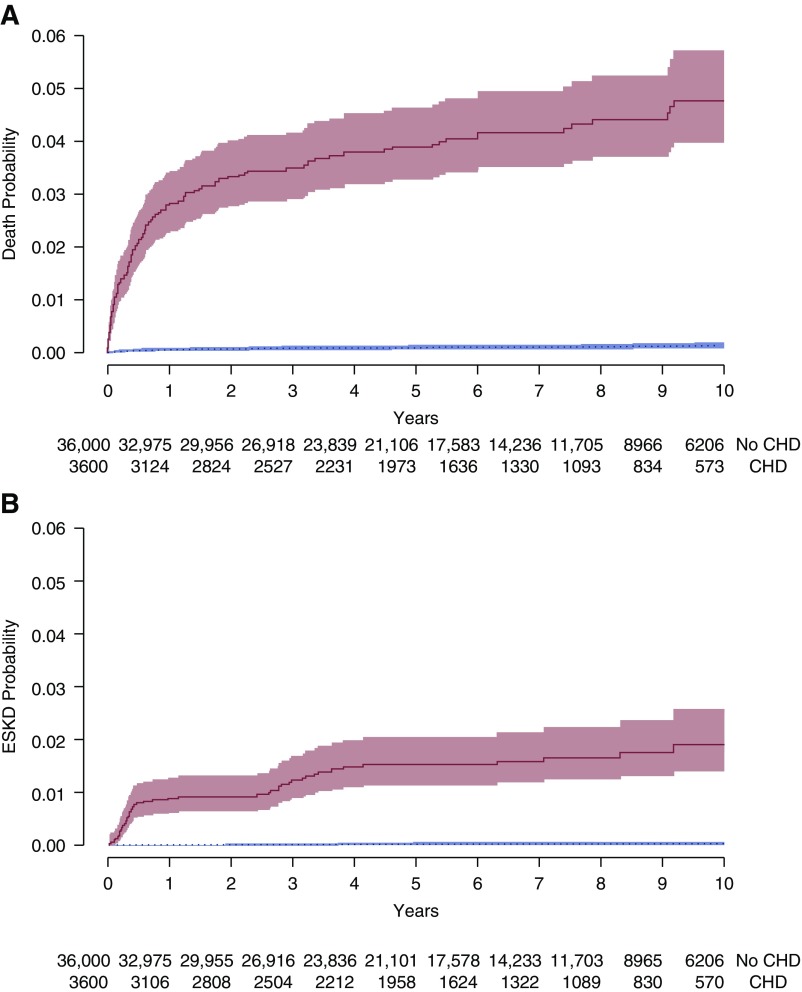
We studied the cohort for a total of 236,224 person-years (20,279 person-years in the cardiac surgery group), with a median follow-up of 5.9 years (IQR, 2.9–9.0) and a maximum of 13.0 years. Hospitalizations and visits with pediatricians and specialists were more frequent in the cardiac surgery cohort compared with the controls (Supplemental Table 5). The 10-year cumulative incidence of death was 5% in the cardiac surgery group and 0.1% in controls (Table 2). Over half of deaths occurred in the first year of follow-up. The mortality incidence rate was 69 per 10,000 person-years in the cardiac surgery group compared with two per 10,000 person-years in matched controls (HR, 42.3; 95% confidence interval [95% CI], 28.2 to 61.4) (Figure 2). Fifty-two (1%) patients with surgical repair of congenital heart disease reached ESKD in follow-up, with a 10-year cumulative incidence of 1.9% in the cardiac surgery group and 0.2% in controls. The ESKD incidence rate was 25.8 per 10,000 person-years compared with matched controls, who experienced ESKD at a rate of 0.28 per 10,000 person-years (HR, 86.5; 95% CI, 37.5 to 201.6). As seen with mortality, 50% of all ESKD events also occurred within the first year after surgery. Children who received dialysis for AKI-D during their index cardiac surgery admission were at a five-fold higher risk of ESKD (crude HR, 5.0; 95% CI, 2.0 to 12.6) compared with those who did not receive dialysis during their cardiac surgery admission. The crude HR for death was 1.5 (95% CI, 0.6 to 3.7) for those who received versus did not receive dialysis during their cardiac surgery admission.
Table 2.
Cumulative incidences of death and ESKD in children with congenital heart disease (CHD) requiring surgical repair
| Outcome | Status | N | No. of Events | % | Incidence Rate per 10,000 Person-Years | Cumulative Incidence | |||
|---|---|---|---|---|---|---|---|---|---|
| 1-yr (%) | 5-yr (%) | 10-yr (%) | 13-yr (%) | ||||||
| Death | No CHD | 36,000 | 35 | 0.10 | 1.62 | 0.06 | 0.10 | 0.13 | 0.13 |
| CHD | 3600 | 140 | 3.89 | 69.04 | 2.79 | 3.90 | 4.77 | 5.37 | |
| ESKD | No CHD | 36,000 | 6 | 0.02 | 0.28 | 0.00 | 0.02 | 0.02 | 0.02 |
| CHD | 3600 | 52 | 1.44 | 25.80 | 0.89 | 1.53 | 1.89 | 2.92 | |
Figure 2.
Time-to-event analysis of death and ESKD. The cumulative probability of incident death (A) and ESKD (B) are shown for the congenital heart disease requiring surgery (solid line) and no congenital heart disease group (dotted line). Shaded areas indicate the 95% CI. Number of individuals at risk at each timepoint is presented below the x axis. CHD, congenital heart disease.
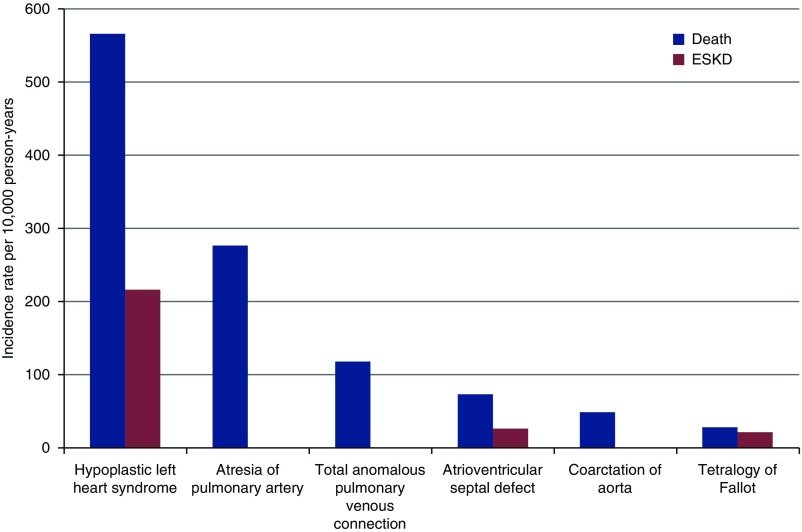
According to the type of congenital heart disease diagnosis, we found the highest cumulative incidence for ESKD and death in children with hypoplastic left heart syndrome (Figure 3, Supplemental Table 6). When stratifying the cohort by those with versus without chromosomal anomalies there was no change in the association with ESKD and mortality (Supplemental Table 7). When comparing outcomes between individuals with index dates as neonates versus those with index dates as infants or children, there was no significant interaction with long-term ESKD or mortality (Supplemental Table 8). There was no clinically significant interaction by gender for outcomes. The HRs of ESKD and mortality were largely unchanged after adjustment for preterm status at birth (Supplemental Table 9).
Figure 3.
Long-term risk of death and ESKD after cardiac surgery by type of congenital heart disease.
Among children who survived the first year, those who received more than one cardiac surgery were at a nine-fold higher risk of death (HR, 9.1; 95% CI, 5.1 to 16.4) and seven-fold higher risk of ESKD (HR, 7.4; 95% CI, 3.2 to 17.3) compared with those who received only one cardiac surgery. According to RACHS-1 severity classification, the children with the most severe, complex surgeries (RACHS-1, category 4–6) had the highest risk of death and kidney outcomes (death rate of 141.3 per 10,000 person-years compared with 48.0 per 10,000 person-years in RACHS-1, category 1) (Supplemental Figure 1). The same trend occurred when classifying congenital heart disease severity on the basis of diagnosis codes (death rate of 88.3 per 10,000 person-years for severe congenital heart disease compared with 47.3 per 10,000 person-years in nonsevere congenital heart disease). We also examined the long-term outcomes of children with congenital heart disease who did not receive surgery. After exclusions and matching, we had 3078 children with congenital heart disease who did not receive surgery and 3078 matched children with congenital heart disease who received surgery (Supplemental Table 10). Compared with those children who did not receive surgery for congenital heart disease, those who received surgery were at an increased risk of ESKD (HR, 5.1; 95% CI, 2.6 to 10.1) and death (HR, 1.3; 95% CI, 1.0 to 1.7) (Supplemental Table 11).
Discussion
We demonstrated a higher cumulative incidence of ESKD and mortality after surgical repair of congenital heart disease compared with matched controls without congenital heart disease. The risk of these complications was highest in the first year after surgery. We also demonstrated that children with the most severe heart defects, particularly hypoplastic left heart syndrome, were at the greatest risk of ESKD and mortality. However, children with non-severe congenital heart disease diagnosis codes were also at an increased risk of these adverse outcomes, relative to matched controls.
Our findings expand on prior studies of long-term kidney health after cardiac surgery. Population-based studies have also identified an excess burden of kidney dysfunction and mortality in adults with congenital heart disease (3,4). In a case-control study, adults with congenital heart disease had a 3.4-fold higher risk of CKD (4). Our research suggests that the excess kidney disease previously identified in adults with congenital heart disease, may start during childhood. Our study is one of the largest and most comprehensive studies of children who underwent surgical repair of congenital heart disease with complete follow-up of long-term ESKD and mortality. A considerable proportion of children undergoing surgeries were born preterm, had low birth weight, and underwent a cardiac surgery before 5 months of age. The 10-year survival probability of over 95% in this cohort is a testament to the remarkable medical and surgical advances over the past few decades. However, as survival improves focus shifts to minimizing long-term comorbidities.
After cardiac surgery, children have several risk factors that may increase their risk of developing CKD and ESKD. CKD and ESKD may be caused by impaired cardiac function, chronic kidney hypoperfusion, high venous pressure, nephrotoxin exposure, multiple surgeries with cardiopulmonary bypass, and recurrent AKI (6–11). Additionally, in children with hypoplastic left heart syndrome, a cyanotic heart disease with the highest risk of long-term outcomes in our study, risk factors also include polycythemia and chronic hypoxia (2). Chronic hypoxia is known to play a role in the initiation and progression of CKD by inducing fibrosis in the glomerular and tubulointerstitial space (10). Single ventricle repair results in elevated central venous pressure as a result of the bidirectional Glenn shunt and completion of the total cavopulmonary anastomosis (2). This results in a nonfavorable preglomerular to postglomerular pressure gradient, linked to altered eGFR. A surgical valvotomy for aortic stenosis can result in significant decreased kidney perfusion, and with aortic insufficiency there can be a nonoptimal reversal of kidney blood flow in diastole. Additionally, prematurity and low birth weight, both known risk factors for CKD, were more common in children with surgical repair of congenital heart disease (24,25). However, adjusting for these characteristics in our study did not change the risk of ESKD or mortality. Malformations of the urinary tract were found to occur in 3% of children with surgical repair of congenital heart disease versus 0.4% of matched controls, which confers additional risk for kidney disease. We also observed that children with more than one cardiac surgery and those who received dialysis for AKI during the index cardiac surgery admission were at an increased risk for ESKD.
The strengths of our study include reliable ascertainment of cardiac surgery cases, careful selection of children without congenital heart disease, and minimal loss to follow-up (<0.2%) (17). Our study population had access to a system of universal health care benefits, in which all health care encounters were recorded, and both groups had high levels of health surveillance.
Our study has certain limitations. First, data with respect to BP, kidney function, and medication use were not available. Second, accurate information on race and ethnicity was not available (26). We do not suspect any bias in the reporting of mortality or ESKD.
Advances in surgical and medical care have increased lifespans for individuals after surgical repair of congenital heart disease (14). As this group is significantly growing in numbers and age, it has become evident that we need an improved understanding of the long-term risk of comorbidities (14). We observed that surgical repair of congenital heart disease confers an excess burden of ESKD and mortality for children with both severe and nonsevere congenital heart disease. Furthermore, children with cyanotic heart disease, specifically hypoplastic left heart syndrome, may be at the highest risk for ESKD and mortality. Kidney disease, and its associated cardiovascular morbidity, is especially concerning after cardiac surgery as these children are already at a higher risk of cardiovascular events (4). As such, children after cardiac surgery should be recognized as a population at risk for ESKD.
Our findings highlight the importance of monitoring for long-term kidney dysfunction after cardiac surgery. Kidney follow-up after congenital heart disease surgery should include monitoring kidney function, avoiding nephrotoxins, and treating hypertension and proteinuria to limit the progression of kidney disease. The risk of ESKD and mortality was highest in the first year after surgery, which highlights the importance of closer outpatient follow-up after surgery and new risk stratification approaches to identify those children at highest risk. Routine monitoring can identify kidney disease earlier and limit progression by aggressively treating BP and proteinuria. We also need to ensure that the many children after cardiac surgery, who are often lost to cardiology follow-up, have continuity of care including a seamless transition to an adult specialist (27). Future research should study novel biomarkers or multivariable prediction models that identify children at high risk for ESKD after cardiac surgery and test interventions aimed at limiting incident disease. Our findings provide data to start creating consensus guidelines for kidney follow-up after cardiac surgery in children.
Disclosures
Dr. Parikh reports personal fees for consulting from Akebia Therapeutics, Inc. and Genfit Biopharmaceutical Company and other fees from RenaltixAI. Dr. Chanchlani, Dr. Everett, Dr. Garg, Dr. Greenberg, Mr. McArthur, Dr. Thiessen-Philbrook, Dr. Wald, and Dr. Zappitelli have nothing to disclose.
Funding
Dr. Garg is supported by a Clinician Investigator Award from the Canadian Institutes of Health Research and the Dr. Adam Linton Chair in Kidney Health Analytics. Dr. Greenberg is supported by the National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Disorders (NIDDK) career development grant K08DK110536. Dr. Parikh is supported by a grant from the National Heart, Lung, and Blood Institute (R01HL085757), which funds the TRIBE-AKI Consortium to study novel biomarkers of AKI in cardiac surgery. Dr. Parikh is also supported by grants from NIDDK (K24DK090203) and O'Brien Center (P30 DK079310-07). Dr. Wald is supported by a grant from Baxter.
Supplementary Material
Acknowledgments
Dr. Parikh is a member of the National Institutes of Health (NIH)-sponsored Assess, Serial Evaluation, and Subsequent Sequelae in AKI Consortium (U01DK082185). Dr. Parikh and Mr. McArthur had full access to all the data in the study and takes responsibility for its integrity and the data analysis. Ontario’s health administrative data are legally restricted under the Personal Health Information Privacy Act. Data used in this study are maintained by ICES. A request for the data used in preparation of the results can be made through ICES’ Data Analytical Service (www.ices.on.ca/Data-Services).
Parts of this material are based on data and/or information compiled and provided by the Canadian Institute for Health Information (CIHI). However, the analyses, conclusions, opinions, and statements expressed in the material are those of the author(s), and not necessarily those of CIHI. This study was supported by ICES, which is funded by an annual grant from the Ontario Ministry of Health and Long-Term Care (MOHLTC). The opinions, results, and conclusions reported in this paper are those of the authors and are independent from the funding sources. No endorsement by ICES or the Ontario MOHLTC is intended or should be inferred.
Footnotes
Published online ahead of print. Publication date available at www.cjasn.org.
Supplemental Material
This article contains the following supplemental material online at http://cjasn.asnjournals.org/lookup/suppl/doi:10.2215/CJN.00690119/-/DCSupplemental.
Supplemental Table 1. STROBE and RECORD checklists.
Supplemental Table 2. Cohort build and baseline characteristic codes.
Supplemental Table 3. Outcome codes.
Supplemental Table 4. International Classification of Diseases Tenth Revision (ICD-10) congenital heart disease (CHD) diagnosis and surgical billing codes.
Supplemental Table 5. Health care utilization among the cardiac surgery (CS) and matched controls.
Supplemental Table 6. Long-term death and ESKD by type of congenital heart disease.
Supplemental Table 7. Long-term outcomes stratified by chromosomal anomaly status amongst cohort of children undergoing surgical repair for congenital heart disease.
Supplemental Table 8. Cumulative incidences of the outcomes in cardiac surgery (CS) group by neonatal status.
Supplemental Table 9. Long-term outcomes after surgical repair of congenital heart disease (S-CHD)
Supplemental Table 10. Characteristics of children with congenital heart disease by whether they did or did not receive surgery.
Supplemental Table 11. Long-term outcomes in children with congenital heart disease by whether they did or did not receive surgery.
Supplemental Figure 1. Hazard ratios for death and ESKD stratified by RACHS-1 category. ESKD collapsed into only two RACHS-1 categories because of a low number of events. Error bars represent the 95% confidence interval.
References
- 1.Hoffman JI, Kaplan S: The incidence of congenital heart disease. J Am Coll Cardiol 39: 1890–1900, 2002 [DOI] [PubMed] [Google Scholar]
- 2.Morgan C, Al-Aklabi M, Garcia Guerra G: Chronic kidney disease in congenital heart disease patients: A narrative review of evidence. Can J Kidney Health Dis 2: 27, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dimopoulos K, Diller GP, Koltsida E, Pijuan-Domenech A, Papadopoulou SA, Babu-Narayan SV, Salukhe TV, Piepoli MF, Poole-Wilson PA, Best N, Francis DP, Gatzoulis MA: Prevalence, predictors, and prognostic value of renal dysfunction in adults with congenital heart disease. Circulation 117: 2320–2328, 2008 [DOI] [PubMed] [Google Scholar]
- 4.Billett J, Cowie MR, Gatzoulis MA, Vonder Muhll IF, Majeed A: Comorbidity, healthcare utilisation and process of care measures in patients with congenital heart disease in the UK: Cross-sectional, population-based study with case-control analysis. Heart 94: 1194–1199, 2008 [DOI] [PubMed] [Google Scholar]
- 5.Greenberg JH, Zappitelli M, Devarajan P, Thiessen-Philbrook HR, Krawczeski C, Li S, Garg AX, Coca S, Parikh CR; TRIBE-AKI Consortium : Kidney outcomes 5 years after pediatric cardiac surgery: The TRIBE-AKI study. JAMA Pediatr 170: 1071–1078, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Magri P, Rao MA, Cangianiello S, Bellizzi V, Russo R, Mele AF, Andreucci M, Memoli B, De Nicola L, Volpe M: Early impairment of renal hemodynamic reserve in patients with asymptomatic heart failure is restored by angiotensin II antagonism. Circulation 98: 2849–2854, 1998 [DOI] [PubMed] [Google Scholar]
- 7.Ishikawa S, Miyauchi T, Sakai S, Ushinohama H, Sagawa K, Fusazaki N, Kado H, Sunagawa H, Honda S, Ueno H, Yamaguchi I, Sugishita Y, Goto K: Elevated levels of plasma endothelin-1 in young patients with pulmonary hypertension caused by congenital heart disease are decreased after successful surgical repair. J Thorac Cardiovasc Surg 110: 271–273, 1995 [DOI] [PubMed] [Google Scholar]
- 8.Lang RE, Unger T, Ganten D, Weil J, Bidlingmaier F, Dohlemann D: Alpha atrial natriuretic peptide concentrations in plasma of children with congenital heart and pulmonary diseases. Br Med J (Clin Res Ed) 291: 1241, 1985 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Schrier RW, Abraham WT: Hormones and hemodynamics in heart failure. N Engl J Med 341: 577–585, 1999 [DOI] [PubMed] [Google Scholar]
- 10.Madsen NL, Goldstein SL, Frøslev T, Christiansen CF, Olsen M: Cardiac surgery in patients with congenital heart disease is associated with acute kidney injury and the risk of chronic kidney disease. Kidney Int 92: 751–756, 2017 [DOI] [PubMed] [Google Scholar]
- 11.Tulevski II, Groenink M, van Der Wall EE, van Veldhuisen DJ, Boomsma F, Stoker J, Hirsch A, Lemkes JS, Mulder BJ: Increased brain and atrial natriuretic peptides in patients with chronic right ventricular pressure overload: Correlation between plasma neurohormones and right ventricular dysfunction. Heart 86: 27–30, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Agha MM, Glazier RH, Moineddin R, Moore AM, Guttmann A: Socioeconomic status and prevalence of congenital heart defects: Does universal access to health care system eliminate the gap? Birth Defects Res A Clin Mol Teratol 91: 1011–1018, 2011 [DOI] [PubMed] [Google Scholar]
- 13.Garg AX, Nevis IF, McArthur E, Sontrop JM, Koval JJ, Lam NN, Hildebrand AM, Reese PP, Storsley L, Gill JS, Segev DL, Habbous S, Bugeja A, Knoll GA, Dipchand C, Monroy-Cuadros M, Lentine KL; DONOR Network : Gestational hypertension and preeclampsia in living kidney donors. N Engl J Med 372: 124–133, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Marelli AJ, Mackie AS, Ionescu-Ittu R, Rahme E, Pilote L: Congenital heart disease in the general population: Changing prevalence and age distribution. Circulation 115: 163–172, 2007 [DOI] [PubMed] [Google Scholar]
- 15.Jenkins KJ, Gauvreau K, Newburger JW, Spray TL, Moller JH, Iezzoni LI: Consensus-based method for risk adjustment for surgery for congenital heart disease. J Thorac Cardiovasc Surg 123: 110–118, 2002 [DOI] [PubMed] [Google Scholar]
- 16.Rosenbaum PR: Optimal matching for observational studies. J Am Stat Assoc 84: 1024–1032, 1989 [Google Scholar]
- 17.Ontario Population Projections Update : Spring 2017, Based on the 2011 Census, Oshawa, Canada, Ontario Ministry of Finance, 2017 [Google Scholar]
- 18.Lam NN, McArthur E, Kim SJ, Knoll GA: Validation of kidney transplantation using administrative data. Can J Kidney Health Dis 2: 20, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Moist LM, Richards HA, Miskulin D, Lok CE, Yeates K, Garg AX, Trpeski L, Chapman A, Amuah J, Hemmelgarn BR: A validation study of the Canadian organ replacement register. Clin J Am Soc Nephrol 6: 813–818, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jha P, Deboer D, Sykora K, Naylor CD: Characteristics and mortality outcomes of thrombolysis trial participants and nonparticipants: A population-based comparison. J Am Coll Cardiol 27: 1335–1342, 1996 [DOI] [PubMed] [Google Scholar]
- 21.Austin PC: Using the standardized difference to compare the prevalence of a binary variable between two groups in observational research. Commun Stat Simul Comput 38: 1228–1234, 2009 [Google Scholar]
- 22.Cox DR: Regression models and life-tables (with discussion). J R Stat Soc Series B 34: 187–220, 1972 [Google Scholar]
- 23.Kaplan EL, Meier P: Nonparametric estimation from incomplete observations. J Am Stat Assoc 53: 457–481, 1958 [Google Scholar]
- 24.Carmody JB, Charlton JR: Short-term gestation, long-term risk: Prematurity and chronic kidney disease. Pediatrics 131: 1168–1179, 2013 [DOI] [PubMed] [Google Scholar]
- 25.White SL, Perkovic V, Cass A, Chang CL, Poulter NR, Spector T, Haysom L, Craig JC, Salmi IA, Chadban SJ, Huxley RR: Is low birth weight an antecedent of CKD in later life? A systematic review of observational studies. Am J Kidney Dis 54: 248–261, 2009 [DOI] [PubMed] [Google Scholar]
- 26.Visible minority population, by province and territory (2006 Census) (Quebec, Ontario, Manitoba, Saskatchewan), Ottawa, Canada, Statistics Canada, 2007 [Google Scholar]
- 27.Mackie AS, Ionescu-Ittu R, Therrien J, Pilote L, Abrahamowicz M, Marelli AJ: Children and adults with congenital heart disease lost to follow-up: Who and when? Circulation 120: 302–309, 2009 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.