Introduction
Infections account for 8% of the total mortality in ESKD patients (1). ESKD patients are at increased risk of infectious complications due to their immunosuppressed status. Metabolic complications of uremia cause abnormalities in monocyte and neutrophil function, as well as antigen processing, antibody formation and cell mediated immune responses (2). The effects on vaccine efficacy include lower seroconversion rate, lower peak antibody titers and faster decline in antibody titers. Consequently, the Centers for Disease Control and Prevention (CDC) in the United States has summarized recommendations from the Advisory Committee on Immunization Practices (ACIP), and issued specific guidelines for vaccinations in ESKD patients. Additionally, the CDC provides updated recommendations via the Morbidity and Mortality Weekly Report. Besides age appropriate vaccine recommendations, augmented vaccination regimens have been detailed for hepatitis B, pneumococcus and influenza vaccines, in an effort to boost immune reactivity and prolong the vaccines’ protective effects (3). We explain specific hepatitis B and influenza guidelines and gaps in knowledge around their application to patients with ESKD via the following cases. Box 1 summarizes the recommendations for these immunization protocols (Figure 1).
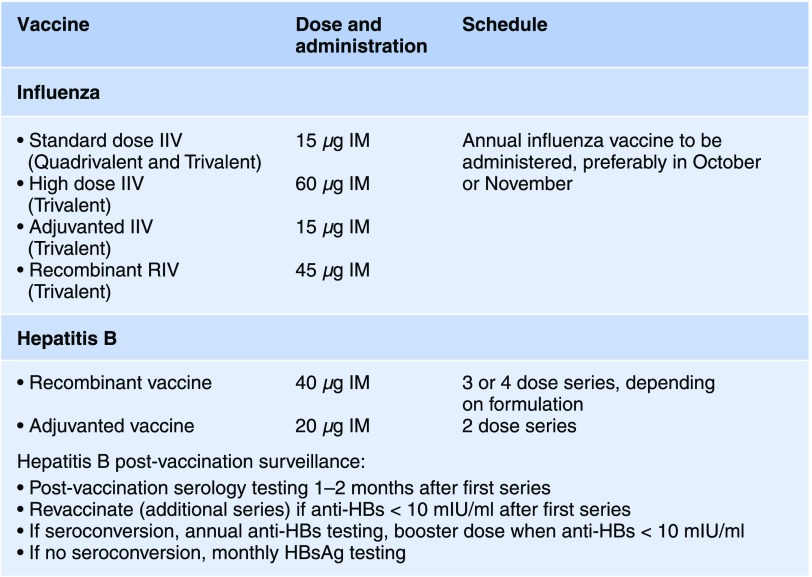
Figure 1.
Recommended ESKD specific influenza and hepatitis B immunization guidelines. IIV, inactivated influenza vaccine; IM, intramuscular; RIV, recombinant influenza vaccine; anti-HBs, hepatitis B surface antibody; HBsAg, hepatitis B surface antigen.
Patient 1
A 58-year-old female with ESKD contacts the dialysis unit to inform the staff she will not be attending treatment today. The patient reports she presented to the emergency department over the weekend with fever, cough and cold symptoms ×36 hours. The patient is diagnosed with influenza A by rapid PCR testing and was prescribed oseltamivir 30 mg postdialysis for 5 days. You review the patient’s immunizations at dialysis and note she received the standard 15 µg trivalent influenza vaccine 2 months prior as part of unit wide vaccination protocol.
Patient 2
A 65-year-old male with ESKD is admitted to the hospital for a diabetic foot ulcer. Routine hospital testing for hepatitis B surface antigen (HBsAg) is positive, and the patient is dialyzed with isolation practices while awaiting results of an acute hepatitis B panel. The patient is irate about faulty vaccinations, stating he was told his recent vaccination would help prevent hepatitis B infection, not cause it. Detailed information from the patient’s dialysis unit revealed administration of hepatitis B booster dose 2 weeks prior to admission.
Overview of Special Recommendations in the ESKD Population
Current guidelines on vaccination practices recommend annual vaccination with seasonal influenza vaccine, unless contraindicated (prior history of Guillain–Barre syndrome within 6 weeks of prior dose of influenza vaccine) (4). Several formulations of the inactivated influenza virus are approved for use: standard dose (15 µg hemagglutinin) quadrivalent (four influenza strains) and trivalent (three influenza strains) inactivated influenza virus, high dose (60 µg hemagglutinin) trivalent inactivated influenza virus and adjuvanted standard dose trivalent inactivated influenza virus. A vaccine containing recombinant hemagglutinin is also available. There are no official recommendations regarding choice of vaccine specific to ESKD patients. Small studies have shown improved seroconversion rates with adjuvanted vaccine use (5). There is currently no data regarding recombinant influenza vaccine in dialysis patients. Similarly, there are no head to head trials of standard dose inactivated influenza virus versus high dose inactivated influenza virus. However, a recent retrospective study suggested high dose trivalent inactivated influenza virus was associated with a modest reduction in hospitalization rates compared with standard dose trivalent and quadrivalent vaccines (6). Unfortunately, studies of inactivated influenza virus are limited by either small samples evaluating only seroconversion or by retrospective designs vulnerable to residual confounding. In addition, seasonal changes in the overall inactivated influenza virus efficacy due to antigenic drift make comparisons of vaccine types across various flu seasons challenging.
Current ACIP guidelines recommend hepatitis B vaccination series for all ESKD patients, preceded by serologic testing and followed by post vaccination confirmation of immunity (7). Serologic testing consists of hepatitis B surface antigen (HBsAg), antibodies to hepatitis B surface antigen (anti-HBs), and antibodies to hepatitis B core antigen (anti-HBc). Currently, one of two single recombinant antigen vaccines are administered intramuscularly in ESKD patients, with a three or four dose series of double antigen dose (40 µg) depending on the formulation. Alternatively, an adjuvanted recombinant hepatitis B vaccine can administered in a two-dose series. Current recommendations are for postvaccination serologic testing at 1–2 months after completion of the primary series. Anti-HBs levels >10 mIU/ml are noted to be protective per CDC guidelines. A second hepatitis B vaccination series should be administered in patients with anti-HBs <10 mIU/ml after the primary vaccine series. Patients without protective levels of anti-HBs are screened for HBsAg monthly. Responders to the vaccine series have anti-HBs levels monitored annually with one booster dose (40 µg recombinant hepatitis B) administered if anti-HBs is <10 mIU/ml (7). There are currently no recommendations regarding monitoring HBsAg in vaccine responders, and there have been reports of false negative HBsAg, as well as reverse seroconversion (8,9).
Our Practice
Since 2015, our program has provided high dose influenza vaccine in all adult patients with ESKD, regardless of age. This policy is informed by the concept of ESKD as an immune-suppressed state and high rates of confirmed influenza within our centers during the 2013–2014 flu season despite 90% of patients receiving standard dose vaccine (national average 71.3% (1)). As with many topics in ESKD, data providing clear direction for medical directors remains elusive. Trials illustrating the relative efficacy of standard dose inactivated influenza virus versus high dose versus adjuvanted type are sorely needed to optimize care for this most vulnerable population. We recommend continued encouragement for patients regarding adherence to vaccination schedules and counseling that vaccine efficacy varies from year to year. For patient 1, in addition to oseltamivir, we require flu-positive patients to wear a droplet mask for 7 to 10 days post diagnosis—the approximate duration of viral shedding, in hopes of reducing intra-facility transmission.
Turning to hepatitis B vaccinations, patient 2 illustrates a familiar scenario in which a patient receives hepatitis B serologic testing in close temporal proximity to vaccination resulting in a positive HBsAg test. Most often this occurs in admitted patients where protocols dictate routine hepatitis B screening, or in outpatient dialysis clinics due to mis-timed lab draws. Transient HBsAg positivity is a known consequence of vaccination and resolves in approximately 20 days (10). In this situation, we obtain a rapid hepatitis B viral load test to confirm the likely false positive result, as was the situation with patient 2. While awaiting testing results, patients are dialyzed on a dedicated machine, which is terminally cleaned following use. Most inpatient laboratories provide hepatitis B viral load testing knowing this is required for discharge placement in a dialysis unit. In outpatient units, there are no guidelines as to whether these patients should be dialyzed in hepatitis B isolation or among the unit population, especially given the high likelihood of a false positive result. Pending a negative viral load test, we dialyze these patients in hepatitis B isolation rooms; however, the room is thoroughly disinfected, the patient has a dedicated machine and no other known hepatitis B confirmed patients are run concurrently. We feel this approach best balances the risk of exposing the general population to a potential true infection while protecting the affected patient from infection from known hepatitis B infected patients.
Disclosures
Dr. Bowman and Dr. Khan have nothing to disclose.
Footnotes
Published online ahead of print. Publication date available at www.cjasn.org.
References
- 1.United States renal data system. 2018. Annual Report. Available at: https://www.usrds.org/2018/view/v2_05.aspx. Accessed February 17, 2019
- 2.Hauser AB, Stinghen AE, Kato S, Bucharles S, Aita C, Yuzawa Y, Pecoits-Filho R: Characteristics and causes of immune dysfunction related to uremia and dialysis. Perit Dial Int 28[Suppl 3]: S183–S187, 2008 [PubMed] [Google Scholar]
- 3.Guidelines for vaccinating kidney dialysis patients and patients with chronic kidney disease. Recommendations of the Advisory Committee on Immunization Practices (ACIP).Available at: https://www.cdc.gov/dialysis/pdfs/vaccinating_dialysis_patients_and_patients_dec2012.pdf. Accessed February 17, 2019
- 4.Grohskopf LA, Sokolow LZ, Broder KR, Walter EB, Fry AM, Jernigan DB: Prevention and control of seasonal influenza with vaccines: Recommendations of the Advisory Committee on Immunization Practices-United States, 2018-19 Influenza season. MMWR Recomm Rep 67: 1–20, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Noh JY, Song JY, Choi WS, Lee J, Seo YB, Kwon YJ, Ko GJ, Cha DR, Kang YS, Lee YK, Cheong HJ, Kim WJ: Immunogenicity of trivalent influenza vaccines in patients with chronic kidney disease undergoing hemodialysis: MF59-adjuvanted versus non-adjuvanted vaccines. Hum Vaccin Immunother 12: 2902–2908, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Miskulin DC, Weiner DE, Tighiouart H, Lacson EK Jr, Meyer KB, Dad T, Manley HJ: High-Dose seasonal influenza vaccine in patients undergoing dialysis. Clin J Am Soc Nephrol 13: 1703–1711, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Schillie S, Vellozzi C, Reingold A, Harris A, Haber P, Ward JW, Nelson NP: Prevention of hepatitis B virus infection in the United States: Recommendations of the advisory committee on immunization practices. MMWR Recomm Rep 67: 1–31, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Foy MC, Thio CL, Hwang HS, Saulynas M, Hamilton JP, Fine DM, Atta MG: False-negative hepatitis B virus (HBV) surface antigen in a vaccinated dialysis patient with a high level of HBV DNA in the United States. Clin Vaccine Immunol 19: 820–822, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rhea S, Moorman A, Pace R, Mobley V, MacFarquhar J, Robinson E, Hayden T, Thai H, Drobeniuc J, Brooks JT, Moore Z, Patel PR: Hepatitis B reverse seroconversion and transmission in a hemodialysis center: A public health investigation and case report. Am J Kidney Dis 68: 292–295, 2016 [DOI] [PubMed] [Google Scholar]
- 10.Lacson E, Teng M, Ong J, Vienneau L, Ofsthun N, Lazarus JM: Antibody response to Engerix-B and Recombivax-HB hepatitis B vaccination in end-stage renal disease. Hemodial Int 9: 367–375, 2005 [DOI] [PubMed] [Google Scholar]