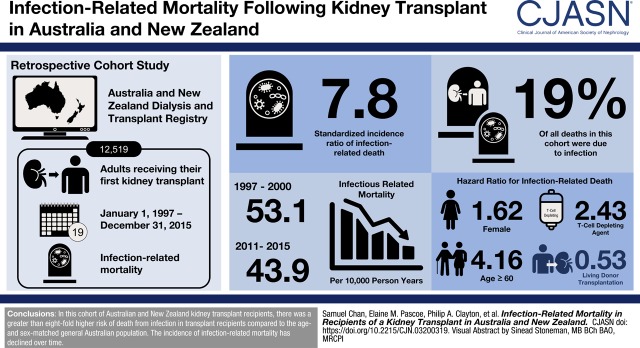
Visual Abstract
Keywords: Infection, kidney failure, kidney transplantation, mortality, survival trends, time factors, treatment outcome, humans, male, female, risk factors, incidence, New Zealand, retrospective studies, living donors, immunosuppression, registries, diabetes mellitus, t-lymphocytes, Australia
Abstract
Background and objectives
The burden of infectious disease is high among kidney transplant recipients because of concomitant immunosuppression. In this study the incidence of infectious-related mortality and associated factors were evaluated.
Design, setting, participants, & measurements
In this registry-based retrospective, longitudinal cohort study, recipients of a first kidney transplant in Australia and New Zealand between 1997 and 2015 were included. Cumulative incidence of infectious-related mortality was estimated using competing risk regression (using noninfectious mortality as a competing risk event), and compared with age-matched, populated-based data using standardized incidence ratios.
Results
Among 12,519 patients, (median age 46 years, 63% men, 15% diabetic, 6% Indigenous ethnicity), 2197 (18%) died, of whom 416 (19%) died from infection. The incidence of infection-related mortality during the study period (1997–2015) was 45.8 (95% confidence interval [95% CI], 41.6 to 50.4) per 10,000 patient-years. The incidence of infection-related mortality reduced from 53.1 (95% CI, 45.0 to 62.5) per 10,000 person-years in 1997–2000 to 43.9 (95% CI, 32.5 to 59.1) per 10,000 person-years in 2011–2015 (P<0.001) Compared with the age-matched general population, kidney transplant recipients had a markedly higher risk of infectious-related death (standardized incidence ratio, 7.8; 95% CI, 7.1 to 8.6). Infectious mortality was associated with older age (≥60 years adjusted subdistribution hazard ratio [SHR], 4.16; 95% CI, 2.15 to 8.05; reference 20–30 years), female sex (SHR, 1.62; 95% CI, 1.19 to 2.29), Indigenous ethnicity (SHR, 2.87; 95% CI, 1.84 to 4.46; reference white), earlier transplant era (2011–2015: SHR, 0.39; 95% CI, 0.20 to 0.76; reference 1997–2000), and use of T cell–depleting therapy (SHR, 2.43; 95% CI, 1.36 to 4.33). Live donor transplantation was associated with lower risk of infection-related mortality (SHR, 0.53; 95% CI, 0.37 to 0.76).
Conclusions
Infection-related mortality in kidney transplant recipients is significantly higher than the general population, but has reduced over time. Risk factors include older age, female sex, Indigenous ethnicity, T cell–depleting therapy, and deceased donor transplantation.
Podcast
This article contains a podcast at https://www.asn-online.org/media/podcast/CJASN/2019_08_27_CJN03200319.mp3
Introduction
A major challenge in kidney transplantation is the management of infectious complications, which causes substantial morbidity and mortality in this vulnerable population (1–3). With the lower risks of rejection over time, short- and longer-term infection and malignancy complications represent a greater relative challenge. Worldwide, the incidence of infectious complications among recipients of a kidney transplant has been reported to range from 49% to 80% (4,5). Consistent with disease burden, the Standardized Outcomes of Nephrology Group in Transplantation has identified infection as a core outcome that is critically important to clinicians, patients, and their caregivers (6).
However, despite being a priority outcome, evaluation of the incidence and factors associated with infectious mortality after kidney transplantation is scarce. Recently, a Finnish Registry study of 3249 adult first kidney transplant recipients between 1990 and 2012 reported that infection accounted for 21% of all deaths, mostly (89%) occurring after the first year, and were associated with older recipient age, diabetic kidney disease, longer pretransplant dialysis duration, acute rejection, and a higher serum creatinine concentration at 1 year after transplantation (7). Another key finding of this investigation was that the incidence of infectious mortality fell markedly during the study period, with infectious death in the most recent cohort (2000–2012) being half that of the earlier cohort (1990–1999) (7). In other studies, data from the United Kingdom Renal Registry have shown that infection-related mortality after kidney transplantation has remained stable at approximately 18% of all mortality between 2000 and 2011 (8,9), despite the fact that the proportion of cardiovascular deaths has progressively fallen from 34% in 2000 to 22% in 2011. Furthermore, a single-center Spanish study reported that among 1218 kidney transplant recipients analyzed between 1995 and 2004, most deaths were bacterial-related and the incidence of infection-related mortality had remained stable during this time (10).
In light of the possibility of regional and temporal variation in infection-related mortality, the aims of this study were to define the incidence of infection-related mortality among adult recipients of a first kidney transplant in Australia and New Zealand, to estimate the excess risk of infectious-related mortality compared with the general population, and to identify associated factors for infectious-related mortality to help better inform shared decision making by patients and clinicians.
Materials and Methods
Study Population and Setting
We conducted a retrospective, inception cohort study using patient records obtained from the Australia and New Zealand Dialysis and Transplant (ANZDATA) Registry. The ANZDATA Registry has collected data on all patients who commenced long-term kidney replacement therapy in all transplant units in Australia and New Zealand since 1963. Details regarding the structure and method of collection have been described previously (11). The study was conducted in accordance with Strengthening the Reporting of Observational Studies in Epidemiology guidelines (12).
All adult (aged ≥18 years) patients with ESKD who underwent first kidney transplantation in Australia and New Zealand between January 1, 1997 and December 31, 2015 were included. Recipients undergoing subsequent kidney transplants and/or multiorgan transplants were excluded.
Data Collection
Data were collected on recipient characteristics (age, weight at time of transplant, height, self-reported ethnicity, country, sex, cause of ESKD, comorbidities [stroke, hypertension, peripheral vascular disease, diabetes, chronic lung disease]), donor characteristics (age, Kidney Donor Risk Index), transplant characteristics (date, maintenance immunosuppression regimen, antirejection therapy), and transplant outcomes (graft loss and all-cause and cause-specific death). Primary kidney disease was categorized as chronic GN, cystic kidney disease, reflux nephropathy, renovascular nephrosclerosis, diabetic kidney disease, and miscellaneous. Maintenance immunosuppressive therapy was divided into three groups: tacrolimus+mycophenolate+prednisolone, cyclosporine+mycophenolate+prednisolone, or other regimen. Other therapies included azathioprine, sirolimus, and clinical trial therapies (such as Pfizer drug JAK3, fingolimod, and sotrastaurin). T cell–depleting therapy, which was used as induction and for treatment, was also collected. The ANZDATA data collection form can be found at http://www.anzdata.org.au/forms/ANZDATA/ANZDATADialysisAndTransplantSurvey2017.pdf.
Outcomes
The primary outcome measure was time to mortality owing to infection with a functioning graft after kidney transplantation. Cause of death was judged and recorded by a clinician providing care to the patient. All causes of death were grouped into five categories: infection, cardiovascular disease, withdrawal from therapy, malignancy, or miscellaneous. A map of the different causes of death to these five categories is found in Supplemental Table 2. The causes of infectious deaths were further classified on the basis of organism (bacterial, viral, fungal, protozoal, other) and site of infection (pulmonary, septicemia, central nervous system, urinary tract, and other).
Statistical Analyses
Results were expressed as frequencies (percentages) for categorical data, mean±SD for continuous normally distributed data, or median (interquartile range) for continuous non-normally distributed data.
The incidence of infection-related mortality in the ANZDATA registry was compared with the incidence in the general population by comparing the number of infectious-related deaths and the person-years at risk for each group. Kidney transplant recipients were followed from the time of transplantation until death, loss to follow-up, or the end of study (December 31, 2015), whichever came first. General population event numbers and person-years at risk were derived from whole-population data provided by the Australian Institute of Health and Welfare (AIHW) (13). The AIHW publishes yearly data on the number of infectious deaths along with general population counts. The general population data were broken down into 5-year age groups. The ratios of the observed to the expected incidences of infectious mortality were calculated using the indirect age standardization method, and their 95% confidence intervals (95% CIs) were calculated assuming a Poisson distribution.
Multivariable competing risks regression was used to identify factors associated with time to infection-related mortality. Noninfectious deaths were considered as competing events. Patients were censored at the time of loss to follow-up, kidney transplant failure, or end of study (December 31, 2015). Variables with P values <0.2 in the univariable model were selected for the multivariable model. This was independent of their P value in the multivariable model or the effect of the variable on overall model selection criteria such as the Akaike information criterion. Interactions were tested for biologically-plausible effect modification by introducing a first-order multiplicative interaction term. Data were analyzed using Stata/SE version 14.0 (StataCorp, College Station, TX). P values <0.05 were considered statistically significant.
Results
Study Population
A total of 12,519 adults with ESKD who received their first kidney transplant in Australia and New Zealand were included (Table 1). During a median follow-up of 4.1 (interquartile range, 0.8–8.8) years, there were 2197 deaths. The causes of death were infection (n=416, 19%), cardiovascular (n=711, 32%), malignancy (n=492, 22%), withdrawal (n=258, 12%), and other causes (n=320, 15%). Twenty percent of deaths were attributable to infection, of which 75% were associated with a functioning graft.
Table 1.
Characteristics of all first graft kidney transplant patients in Australia and New Zealand 1997–2015
| Characteristics | Total Population (n=12,519) | 1997–2005 (n=4838) | 2006–2015 (n=7681) |
|---|---|---|---|
| Recipient characteristics | |||
| Age (yr) | |||
| <20 | 193 (2%) | 96 (2%) | 97 (1%) |
| 20–29 | 1559 (12%) | 763 (16%) | 796 (10%) |
| 30–39 | 2361 (19%) | 1037 (21%) | 1324 (17%) |
| 40–49 | 3250 (26%) | 1341 (28%) | 1909 (25%) |
| 50–59 | 3381 (25%) | 1137 (23%) | 2244 (29%) |
| ≥60 | 1775 (14%) | 474 (10%) | 1301 (17%) |
| Sex | |||
| Men | 7827 (63%) | 2948 (61%) | 4879 (64%) |
| Ethnicity | |||
| White | 10,032 (80%) | 4023 (83%) | 6009 (79%) |
| Indigenous | 696 (6%) | 283 (6%) | 429 (6%) |
| Asian | 1138 (9%) | 362 (7%) | 776 (10%) |
| Other | 653 (5%) | 176 (4%) | 405 (5%) |
| Country | |||
| Australia | 10,715 (86%) | 4045 (84%) | 6670 (87%) |
| New Zealand | 1804 (14%) | 795 (16%) | 1009 (13%) |
| BMI categories (kg/m2) | |||
| <18.5 | 433 (4%) | 189 (4%) | 244 (3%) |
| 18.5–24 | 5253 (42%) | 2358 (49%) | 2895 (38%) |
| 25–29 | 4191 (34%) | 1583 (33%) | 2608 (34%) |
| 30–34 | 1812 (14%) | 512 (11%) | 1300 (17%) |
| 35–39 | 541 (4%) | 132 (3%) | 409 (5%) |
| ≥40 | 289 (2%) | 74 (1%) | 215 (3%) |
| End-stage kidney failure cause | |||
| Glomerulonephritis | 5490 (44%) | 2334 (48%) | 3156 (41%) |
| Cystic kidney disease | 1819 (15%) | 623 (13%) | 1196 (16%) |
| Reflux nephropathy | 1000 (8%) | 462 (10%) | 538 (7%) |
| Renovascular | 692 (5%) | 184 (4%) | 508 (7%) |
| Diabetic kidney disease | 1866 (15%) | 618 (13%) | 1248 (16%) |
| Other | 1652 (13%) | 627 (13%) | 1025 (13%) |
| Comorbidities (known or suspected) | |||
| Chronic lung disease | 822 (7%) | 251 (5%) | 571 (7%) |
| Coronary artery disease | 1993 (16%) | 579 (12%) | 1414 (18%) |
| Peripheral vascular disease | 1125 (9%) | 363 (7%) | 762 (10%) |
| Cerebrovascular disease | 619 (5%) | 197 (4%) | 425 (6%) |
| Diabetes mellitus | 2444 (20%) | 766 (16%) | 1678 (22%) |
| Donor characteristics | |||
| Donor age (yr) | |||
| <20 | 1028 (8%) | 537 (11%) | 491 (6%) |
| 20–29 | 1294 (10%) | 563 (12%) | 731 (10%) |
| 30–39 | 1652 (13%) | 720 (15%) | 932 (12%) |
| 40–49 | 2833 (23%) | 1176 (24%) | 1657 (22%) |
| 50–59 | 3203 (26%) | 1185 (24%) | 2018 (26%) |
| 60–69 | 2047 (16%) | 538 (11%) | 1509 (20%) |
| ≥70 | 462 (4%) | 131 (3%) | 331 (4%) |
| Donor source | |||
| Deceased donor | 8042 (64%) | 3103 (64%) | 4939 (64%) |
| Live donor | 4477 (36%) | 1737 (36%) | 2740 (36%) |
| Transplant characteristics | |||
| Baseline immunosuppression | |||
| Tacrolimus/mycophenolate/prednisolone | 7283 (60%) | 843 (17%) | 6440 (84%) |
| Cyclosporin/mycophenolate/prednisolone | 4347 (35%) | 2670 (55%) | 1677 (22%) |
| Other | 649 (5%) | 97 (2%) | 552 (11%) |
| Antirejection immunosuppression | |||
| T cell–depleting therapy | 982 (8%) | 635 (36%) | 347 (5%) |
Missingness of baseline characteristics (<1%): body mass index, ethnicity, chronic lung disease, coronary artery disease, peripheral vascular disease, cerebrovascular disease, and diabetes (Supplemental Table 1).
Incidence of Infectious-Related Mortality
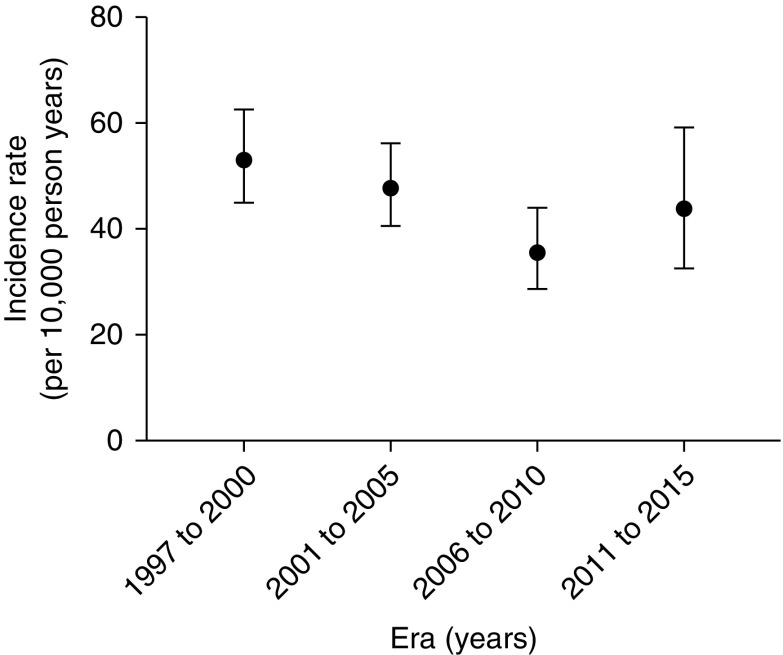
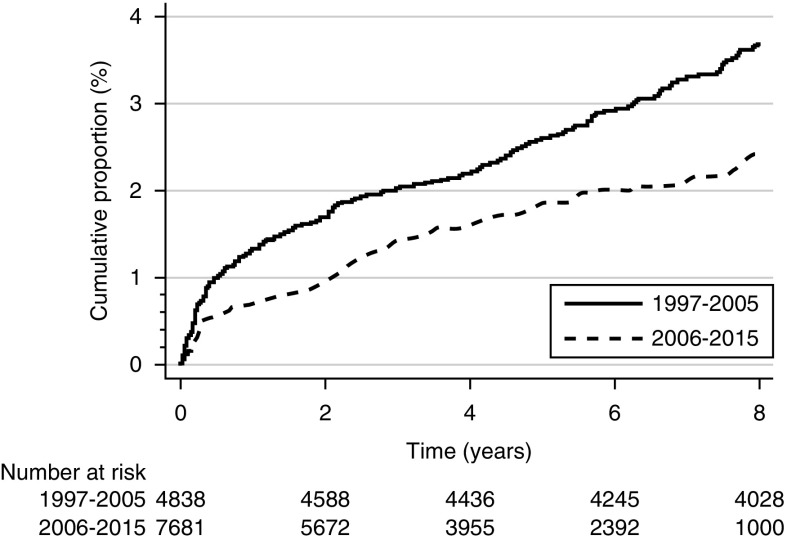
The overall incidence of infectious mortality during the study period (1997–2015) was 45.8 (95% CI, 41.6 to 50.4) per 10,000 patient-years. The incidence of infection-related mortality was 53.1 (95% CI, 45.0 to 62.5) per 10,000 person-years in 1997–2000, 47.8 (95% CI, 40.6 to 56.1) per 10,000 person-years in 2001–2005, 35.6 (95% CI, 28.7 to 44.1) per 10,000 person-years, and 43.9 (95% CI, 32.5 to 59.1) per 10,000 person-years in 2011–2015 (P<0.001) (Figure 1). The cumulative incidence of infection-related mortality by transplant era is shown in Figure 2.
Figure 1.
Incidence of infection-related mortality by transplant era. Errors bars depict the lower and upper 95% confidence limits.
Figure 2.
Cumulative incidence graph showing infection-related mortality by transplant era with competing risks of noninfectious mortality.
Organism and Site-Specific Infectious Mortality
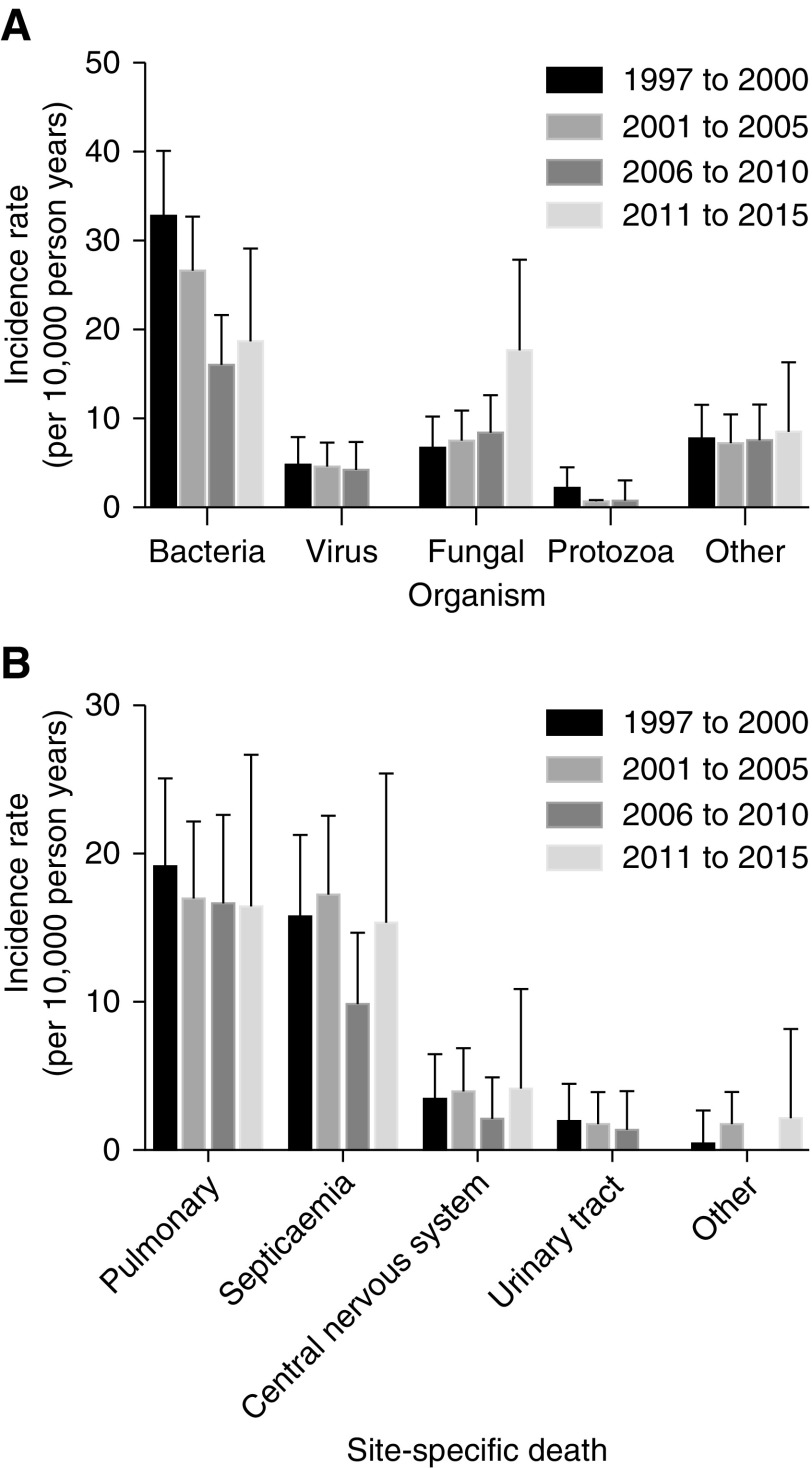
Bacterial infection accounted 223 (54%) infection-related deaths, fungi in 75 (18%), viruses in 34 (9%), protozoa in 15 (4%), and other organisms in 69 (16%). The median age at which patients died from an infection ranged between 49 and 52 years according to organism. Most patients who died from fungal and protozoal infections did so within the first 12 months after transplantation, whereas deaths from viral and bacterial infections occurred considerably later (Table 2). Deaths related to urosepsis occurred considerably later than deaths due to infection in other sites (Table 2). Supplemental Table 3 highlights the organism-specific mortality which occurred during the study period.
Table 2.
Organism and site-specific infectious death relative to median age at transplant and time after transplant
| Characteristics | n (%) | Age at Transplant (yr), Median (IQR) | Time after Transplant (mo), Median (IQR) |
|---|---|---|---|
| Organism-specific death (n=416) | |||
| Bacteria | 223 (54) | 51 (41–58) | 64 (18–119) |
| Virus | 34 (8) | 54 (42–60) | 30 (5–68) |
| Fungal | 75 (18) | 50 (41–60) | 11 (3–41) |
| Protozoa | 15 (4) | 49 (45–57) | 12 (4–81) |
| Miscellaneous | 69 (16) | 52 (42–57) | 90 (30–126) |
| Site-specific death (n=416) | |||
| Pulmonary | 158 (38) | 52 (43–60) | 50 (9–104) |
| Septicemia | 133 (32) | 49 (38–58) | 31 (4–91) |
| Central nervous system | 30 (7) | 51 (34–57) | 28 (8–59) |
| Urinary tract | 13 (3) | 55 (47–62) | 110 (42–134) |
| Miscellaneous | 82 (20) | 50 (42–58) | 68 (26–128) |
IQR, interquartile range.
The incidence of organism-specific and site-specific mortality for the entire study period (1997–2015) is shown in Figure 3, A and B, respectively. The incidence of bacterial mortality was 32.5 (95% CI, 25.3 to 40.1) per 10,000 person-years in the 1997–2000 era and 18.4 (95% CI, 11.6 to 29.1) per 10,000 person-years in the 2011–2015 era (P<0.001). The incidence of fungal mortality increased from 6.4 (95% CI, 3.9 to 10.2) per 10,000 person-years in 1997–2000 to 17.3 (95% CI, 10.8 to 27.9) per 10,000 person-years in 2006–2015 (P<0.001). The incidence of pulmonary infection-related mortality reduced from 19.1 (95% CI, 14.5 to 25.1) per 10,000 person-years in 1997–2000 to 16.3 (95% CI, 10 to 26.6) per 10,000 person-years in 2011–2015 (P<0.001).
Figure 3.
Incidence of infection-related mortality for study period (1997-2015) by transplant era. (A) Incidence of organism-specific mortality for study period by transplant era. (B) Incidence of site-specific death for study period by transplant era. Errors bars depict the upper 95% confidence limits.
Given that follow-up durations differed significantly between different eras, the incidence of organism and site-specific infectious mortality in the first 12 months after transplantation according to era were also ascertained, although this is limited by a small sample size and thus a wide confidence interval (Supplemental Figure 1, A and B).
Comparison with the General Population
Kidney transplant recipients were more likely to die from infection than the age- and sex-matched population (standardized incidence ratio [SIR], 7.8; 95% CI, 7.1 to 8.6). This excess risk was more marked in women (SIR, 11.4; 95% CI, 9.8 to 13.1) than in men (SIR, 6.2; 95% CI, 5.5 to 7.1) (Table 3).
Table 3.
Age-standardized incidence ratio of infectious-related mortality
| Characteristics | General Population Observed Events, n (Person-Years at Risk) | Transplant Population Observed Events, n (Person-Years at Risk) | SIR (95% CI) |
|---|---|---|---|
| Total population | 37,777 (395,306,413) | 416 (90,919) | 7.8 (7.1 to 8.6) |
| Men | 19,784 (196,444,645) | 232 (56,423) | 6.2 (5.5 to 7.1) |
| Women | 17,993 (198,861,768) | 184 (34,496) | 11.4 (9.8 to 13.1) |
SIR, standardized incidence ratio; 95% CI, 95% confidence interval.
Factors associated with Infection-Related Mortality
The outcomes of univariable and multivariable competing risk methods model analyses are shown in Table 4. Older recipient age, female sex, Indigenous ethnicity, earlier transplant era, use of T cell–depleting therapy, and deceased donor kidney transplantation were associated with infectious mortality. There were no interactions observed.
Table 4.
Factors associated with infection-related mortality among first graft kidney transplant recipients enrolled in Australia and New Zealand
| Demographics | No. of Events | Univariable Analysis | Multivariable Analysis | ||
|---|---|---|---|---|---|
| SHR (95% CI) | P Value | SHR (95% CI) | P Value | ||
| Recipient characteristics | |||||
| Age (yr) | <0.001 | <0.001 | |||
| <20 | 2 | 0.64 (0.15 to 2.70) | 0.55 (0.68 to 4.4) | ||
| 20–29 | 26 | 1 | 1 | ||
| 30–39 | 59 | 1.61 (1.01 to 2.56) | 1.34 (0.70 to 2.56) | ||
| 40–49 | 107 | 2.23 (1.45 to 3.42) | 1.57 (0.83 to 2.94) | ||
| 50–59 | 131 | 2.89 (1.90 to 4.42) | 2.10 (1.12 to 3.95) | ||
| ≥60 | 91 | 4.37 (2.81 to 6.77 | 4.16 (2.15 to 8.05) | ||
| Sex | <0.001 | 0.002 | |||
| Men | 232 | 1 | 1 | ||
| Women | 184 | 1.29 (1.07 to 1.56) | 1.62 (1.19 to 2.29) | ||
| Ethnicity | <0.001 | <0.001 | |||
| White | 297 | 1 | 1 | ||
| Indigenous | 66 | 3.47 (2.65 to 4.54) | 2.87 (1.84 to 4.46) | ||
| Asian | 39 | 1.33 (0.95 to 1.85) | 1.21 (0.73 to 2.01) | ||
| Other | 14 | 1.01 (0.59 to 1.72) | 1.03 (0.47 to 2.27) | ||
| Country | 0.17 | 0.40 | |||
| Australia | 343 | 1 | 1 | ||
| New Zealand | 73 | 1.19 (0.93 to 1.53) | 1.24 (0.75 to 2.05) | ||
| Body mass index (kg/m2) | 0.89 | 0.73 | |||
| <18.5 | 13 | 0.88 (0.50 to 1.56) | 1.35 (0.67 to 2.72) | ||
| 18.5–24 | 183 | 1 | 1 | ||
| 25–29 | 136 | 1.02 (0.82 to 1.27) | 0.89 (0.63 to 1.26) | ||
| 30–34 | 60 | 1.19 (0.89 to 1.60) | 0.98 (0.64 to 1.50) | ||
| 35–39 | 15 | 1.07 (0.63 to 1.81) | 0.66 (0.30 to 1.46) | ||
| ≥40 | 9 | 1.13 (0.46 to 2.73) | 0.90 (0.28 to 2.94) | ||
| Primary cause of kidney failure | <0.001 | 0.04 | |||
| Glomerulonephritis | 159 | 1 | 1 | ||
| Cystic kidney disease | 45 | 0.94 (0.68 to 1.31) | 0.52 (0.28 to 0.99) | ||
| Reflux nephropathy | 24 | 0.78 (0.51 to 1.19) | 0.93 (0.46 to 1.92) | ||
| Renovascular | 24 | 1.52 (0.99 to 2.33) | 1.64 (0.94 to 2.87) | ||
| Diabetic kidney disease | 90 | 1.95 (1.51 to 2.53) | 0.86 (0.44 to 1.68) | ||
| Other | 74 | 1.70 (1.29 to 2.23) | 1.39 (0.90 to 2.17) | ||
| Comorbidities (suspected or unknown) | <0.001 | 0.04 | |||
| Chronic lung disease | 48 | 2.17 (1.60 to 2.93) | 1.71 (1.10 to 2.65) | ||
| Coronary artery disease | 115 | 2.39 (1.93 to 2.96) | 1.34 (0.88 to 2.02) | ||
| Peripheral vascular disease | 71 | 2.37 (1.84 to 3.06) | 1.69 (1.01 to 2.82) | ||
| Cerebrovascular disease | 29 | 1.62 (1.11 to 2.36) | 1.04 (0.59 to 1.85) | ||
| Diabetes mellitus | 121 | 1.97 (1.59 to 2.43) | 1.15 (0.72 to 1.84) | ||
| Donor characteristics | |||||
| Donor age (yr) | <0.001 | 0.24 | |||
| <20 | 48 | 1 | 1 | ||
| 20–29 | 39 | 0.70 (0.46 to 1.08) | 0.62 (0.32 to 1.21) | ||
| 30–39 | 48 | 0.68 (0.46 to 1.02) | 0.57 (0.29 to 1.10) | ||
| 40–49 | 80 | 0.68 (0.47 to 0.97) | 0.58 (0.29 to 1.15) | ||
| 50–59 | 101 | 0.80 (0.57 to 1.13) | 0.67 (0.33 to 1.35) | ||
| 60–69 | 71 | 1.02 (0.71 to 1.48) | 0.69 (0.33 to 1.45) | ||
| ≥70 | 29 | 1.84 (1.16 to 2.93) | 1.33 (0.51 to 3.45) | ||
| Donor source | <0.001 | 0.003 | |||
| Deceased donor | 334 | 1 | 1 | ||
| Live donor | 82 | 0.42 (0.33 to 0.53) | 0.53 (0.37 to 0.76) | ||
| Transplant characteristics | |||||
| Era | 0.007 | 0.006 | |||
| 1997–2000 | 142 | 1 | 1 | ||
| 2001–2005 | 147 | 0.88 (0.70 to 1.11) | 0.87 (0.55 to 1.37) | ||
| 2006–2010 | 84 | 0.64 (0.49 to 0.85) | 0.61 (0.35 to 1.06) | ||
| 2011–2015 | 43 | 0.54 (0.37 to 0.77) | 0.39 (0.20 to 0.76) | ||
| Baseline immunosuppression | 0.12 | 0.49 | |||
| Tacrolimus/mycophenolate/prednisolone | 127 | 1 | 1 | ||
| Cyclosporin/mycophenolate/prednisolone | 182 | 0.88 (0.73 to 1.10) | 0.85 (0.60 to 1.22) | ||
| Other | 107 | 0.74 (0.66 to 1.01) | 0.86 (0.54 to 1.38) | ||
| Antirejection immunosuppression | <0.001 | <0.001 | |||
| No T cell–depleting therapy | 343 | 1 | 1 | ||
| T cell–depleting therapy | 73 | 2.75 (1.99 to 3.81) | 2.43 (1.36 to 4.33) | ||
SHR, subdistribution hazard ratio; 95% CI, 95% confidence interval.
Discussion
In this large, binational registry cohort of Australian and New Zealand kidney transplant recipients followed for >90,000 person-years, the incidence of infection-related mortality declined over time and occurred after a median period of 4.1 years after kidney transplantation. Bacterial infections, most commonly pneumonia followed by septicemia and central nervous system infections, accounted for most infectious deaths. Kidney transplant recipients were more likely to die from infection than the age- and sex-matched Australian population with a greater than eight-fold overall higher risk. In the modern era of immunosuppression, infection-related mortality was associated with older age, female sex, Australian Indigenous ethnicity, earlier transplant era, use of T cell–depleting therapy, and deceased donor kidney transplantation.
These findings are in line with those of a Finnish study, which showed that infectious mortality halved between 1990–1999 and 2000–2012 (7). This improvement in infectious death over time was observed despite transplanting older recipients with higher degrees of comorbidity, longer pretransplant dialysis durations, more potent immunosuppressive regimens, and greater utilization of extended criteria donor organs. Potential explanations for the falling rates of infectious death post-transplantation in the present study included changes in immunosuppressive therapies (e.g., introduction of tacrolimus-based immunosuppression from 2005 onward), improvements in infection control measures (e.g., patient charting and increased attention to hand hygiene), more widespread use of antimicrobial prophylaxis (such as cytomegalovirus prophylaxis, perioperative antibiotic administration at the time of transplantation, and long-term Pneumocystis prophylaxis), and recommendations to reduce overall immunosuppression in elderly transplant recipients (14–18).
The finding from this investigation that bacterial infection, typically involving the respiratory system, was the most frequent cause of infectious-related mortality also mirrored that of the Finnish study (7). In contrast, a single-center Spanish study of 218 kidney transplant patients between 1995 and 2004 reported that septicemia was the commonest cause of infectious death (35%), followed by pneumonia (18%) (10). The apparent disparity in findings may be explained by regional variations in infection rates and severity, or by variations in coding practices among different registries.
Compared with the age- and sex-matched general population, kidney transplant recipients exhibited an eight-fold greater incidence of infectious death, which was somewhat lower than the 32-fold excess risk reported by a United Kingdom Renal Registry study (8). This difference may relate to regional variations in infection rates and severity, or to different clinical practices with respect to immunosuppression and infection management.
An important finding of this study was the observed sex disparity in risk of infectious death. Compared with men, women experienced a 62% higher risk of infectious death. Moreover, compared with the general population, the SIR of infectious death was considerably higher in women (11.4; 95% CI, 9.8 to 13.1) than in men (6.2; 95% CI, 5.5 to 7.1). The association between female sex and infection-related mortality after kidney transplantation may be due to sex-specific differences in immune responses, sex-specific pharmacokinetic and pharmacodynamic immunosuppressive responses (19,20), and greater degrees of HLA sensitization in women, leading to different immunosuppression approaches (21,22) selection bias and sex-specific differences in the propensity for infection (e.g., greater incidence of urinary tract infections in women) (19,20).
Indigenous ethnicity was also found to be strongly associated with infection-related mortality. This may be related to socioeconomic disadvantage, remoteness of residence, overcrowded living circumstances, and poor access to health care services. There may also be ethnic differences in relation to immune system responses to infection (23). The high infectious burden has led to recent calls for evaluation of modified immunosuppressive regimens in Aboriginal and Torres Strait Islander peoples (23,24).
Another intriguing finding was that deceased donor kidney transplants were associated with higher infection-related mortality. This is consistent with the observations of a meta-analysis of four observational cohort studies involving 1721 participants in which deceased donor kidney transplant recipients had a higher odds of experiencing an infectious complication than living donor kidney transplant recipients (odds ratio, 2.65; 95% CI, 2.05 to 3.41; P<0.001) (25). Although the meta-analysis was limited by high heterogeneity (I2 93%; P<0.001) and mostly small studies with suboptimal methodological quality, it is possible that differences in ischemic times, rates of delayed graft function, and possibly immunosuppressant dosages may lead to modification of infectious risk.
The strengths of this study include its very large sample size, inclusion of all Australian and New Zealand patients who received a kidney transplant during a 19-year study period, and the use of robust statistical analyses (including competing risk regression). These greatly enhanced the study’s internal validity. These strengths should be balanced against the study’s limitations, which included limited depth of data collection. ANZDATA does not collect important information, such as individual unit management protocols, laboratory values, and severity of comorbidities. It is also important to note that there may have been important variations between different transplant units with respect to patient selection and infection prevention strategies, which could have affected the results. Although adjustment was made for many patient characteristics, the possibility of residual confounding could not be excluded. In common with other registries, ANZDATA is a voluntary registry and there is no external audit of data accuracy. Consequently, the possibilities of ascertainment bias, inaccurate coding and misclassification bias cannot be excluded. The ANZDATA registry allowed only one cause of death to be reported, and it is possible that some patients may have had several factors leading to death. Additionally, this study focused only on infectious causes of death, and no data were available about nonfatal infections, which also cause substantial morbidity in kidney transplant recipients. The ANZDATA registry does not provide any information on the immunosuppressive load post-transplant or the emergence of post-transplant diabetes, or crossvalidation of the cause of death with the national death registries of Australia or New Zealand. Moreover, there was no age- and sex-standardized information on the New Zealand population, and as such, specific New Zealand transplant recipients could not be evaluated alone. The study findings may not be generalizable outside of Australia and New Zealand. A further limitation is that this study did not look at center effects.
In conclusion, infection-related mortality among kidney transplant recipients is an important issue. Although infection-related mortality has decreased over time, approximately one fifth of kidney transplant recipients died from infection after a kidney transplant and the majority of these patients died with a functioning graft. Higher infection-related mortality was associated with older age, female sex, Indigenous ethnicity, earlier transplant era, use of antithymocyte globulin, and deceased donor kidney transplantation. This information may help better inform shared decision making by patients and clinicians.
Disclosures
Dr. Francis reports other fees from Amgen and Novartis to attend conferences. Dr. Johnson reports receiving consultancy fees, research grants, speaker honoraria, and travel sponsorships from Baxter Healthcare and Fresenius Medical Care. Dr. Johnson reports receiving consultancy fees from AstraZeneca and travel sponsorships from Amgen. Dr. Lim is supported by an investigator-originated grant from Novartis and reports receiving consultant and speaker fees from Alexion and speaker fees from Astellas. Dr. Campbell, Dr. Chan, Dr. Clayton, Dr. Isbel, Dr. McDonald, Dr. Palmer, Dr. Pascoe, and Dr. Sypek have nothing to disclose.
Funding
Dr. Chan is supported by a National Health and Medical Research Council Postgraduate Scholarship. Dr. Chan is also the recipient of a competitive Microba research grant and the Metro South Research Support Scheme outside of the submitted work. Dr. Hawley is supported by grants from the Medical Research Future Fund (Australian Government competitive grants APP1170021, APP1170281, APP1170238, APP1152390, APP1170347, and APP1159051), Metro South Research Support Scheme (Research Foundation Grant Princess Alexandra Hospital [Outcomes for Patients on RRT, TEACH-PD trial, and VALID and SWIFT trials]), National Health and Medical Research Council (Australian Government competitive grants APP1138533 and APP1162410), and PKD Foundation Australia (Research Foundation grant [IMPEDE-PKD]). Dr. Hawley is also supported by grants from Baxter Healthcare, Fresenius Medical Care, GlaxoSmithKline, Janssen, and Otsuka, outside the submitted work. Dr. Johnson is a current recipient of the Australian National Health and Medical Research Council Practitioner Fellowship.
Supplementary Material
Acknowledgments
The authors gratefully acknowledge the substantial contributions of the entire Australian and New Zealand nephrology community (physicians, surgeons, database managers, nurses, kidney operators, and patients) in providing information for and maintaining the Australia and New Zealand Dialysis and Transplant (ANZDATA) Registry database.
The data reported here were supplied by the ANZDATA Registry. The interpretation and reporting of these data are the responsibility of the authors and in no way should be seen as an official policy or interpretation of the Registry.
Footnotes
Published online ahead of print. Publication date available at www.cjasn.org.
Supplemental Material
This article contains the following supplemental material online at http://cjasn.asnjournals.org/lookup/suppl/doi:10.2215/CJN.03200319/-/DCSupplemental.
Supplemental Table 1. Total missing numbers for each baseline characteristic.
Supplemental Table 2. A map of the causes of death to the categories of death in this ANZDATA cohort study.
Supplemental Table 3. Specific pathogens associated with infection-related mortality.
Supplemental Figure 1. (A) Overall organism-specific incidence mortality rates, following 12 months of transplantation, by transplant era. (B) Overall site-specific incidence mortality rates, after 12 months of transplantation, by transplant era. Error bars depict the upper 95% confidence limit.
References
- 1.Patel R, Paya CV: Infections in solid-organ transplant recipients. Clin Microbiol Rev 10: 86–124, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Garcia GG, Harden PN, Chapman JR: World Kidney Day 2012: The global role of kidney transplantation. Am J Kidney Dis 59: 319–324, 2012 [DOI] [PubMed] [Google Scholar]
- 3.Kumar MS, Cridge P, Molavi A, Stephan R, Abouna GM: Infectious complications in the first 100 days after renal transplantation. Transplant Proc 27: 2705–2706, 1995 [PubMed] [Google Scholar]
- 4.Alangaden GJ, Thyagarajan R, Gruber SA, Morawski K, Garnick J, El-Amm JM, West MS, Sillix DH, Chandrasekar PH, Haririan A: Infectious complications after kidney transplantation: Current epidemiology and associated risk factors. Clin Transplant 20: 401–409, 2006 [DOI] [PubMed] [Google Scholar]
- 5.Pourmand G, Salem S, Mehrsai A, Taherimahmoudi M, Ebrahimi R, Pourmand MR: Infectious complications after kidney transplantation: A single-center experience. Transpl Infect Dis 9: 302–309, 2007 [DOI] [PubMed] [Google Scholar]
- 6.Tong A, Sautenet B, Poggio ED, Lentine KL, Oberbauer R, Mannon R, Murphy B, Padilla B, Chow KM, Marson L, Chadban S, Craig JC, Ju A, Manera KE, Hanson CS, Josephson MA, Knoll G; SONG-Tx Graft Health Workshop Investigators : Establishing a core outcome measure for graft health: A Standardized Outcomes in Nephrology-Kidney Transplantation (SONG-Tx) Consensus Workshop Report. Transplantation 102: 1358–1366, 2018 [DOI] [PubMed] [Google Scholar]
- 7.Kinnunen S, Karhapää P, Juutilainen A, Finne P, Helanterä I: Secular trends in infection-related mortality after kidney transplantation. Clin J Am Soc Nephrol 13: 755–762, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Vogelzang JL, van Stralen KJ, Noordzij M, Diez JA, Carrero JJ, Couchoud C, Dekker FW, Finne P, Fouque D, Heaf JG, Hoitsma A, Leivestad T, de Meester J, Metcalfe W, Palsson R, Postorino M, Ravani P, Vanholder R, Wallner M, Wanner C, Groothoff JW, Jager KJ: Mortality from infections and malignancies in patients treated with renal replacement therapy: Data from the ERA-EDTA registry. Nephrol Dial Transplant 30: 1028–1037, 2015 [DOI] [PubMed] [Google Scholar]
- 9.Pippias M, Jager KJ, Kramer A, Leivestad T, Sánchez MB, Caskey FJ, Collart F, Couchoud C, Dekker FW, Finne P, Fouque D, Heaf JG, Hemmelder MH, Kramar R, De Meester J, Noordzij M, Palsson R, Pascual J, Zurriaga O, Wanner C, Stel VS: The changing trends and outcomes in renal replacement therapy: Data from the ERA-EDTA Registry. Nephrol Dial Transplant 31: 831–841, 2016 [DOI] [PubMed] [Google Scholar]
- 10.Linares L, Cofán F, Cervera C, Ricart MJ, Oppenheimer F, Campistol JM, Moreno A: Infection-related mortality in a large cohort of renal transplant recipients. Transplant Proc 39: 2225–2227, 2007 [DOI] [PubMed] [Google Scholar]
- 11.ANZDATA Registry : 38th Report. Australia and New Zealand Dialysis and Transplant Registry, Adelaide, Australia, 2016 [Google Scholar]
- 12.von Elm E, Altman DG, Egger M, Pocock SJ, Gøtzsche PC, Vandenbroucke JP; STROBE Initiative : The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: Guidelines for reporting observational studies. Ann Intern Med 147: 573–577, 2007 [DOI] [PubMed] [Google Scholar]
- 13.Australian Institute of Health and Welfare : General Record of Incidence of Mortality (GRIM) Books, 2018. Available at: https://www.aihw.gov.au/reports/life-expectancy-death/grim-books/contents/grim-books. Accessed March 5, 2019
- 14.Ekberg H, Tedesco-Silva H, Demirbas A, Vítko S, Nashan B, Gürkan A, Margreiter R, Hugo C, Grinyó JM, Frei U, Vanrenterghem Y, Daloze P, Halloran PF; ELITE-Symphony Study : Reduced exposure to calcineurin inhibitors in renal transplantation. N Engl J Med 357: 2562–2575, 2007 [DOI] [PubMed] [Google Scholar]
- 15.Lehner LJ, Staeck O, Halleck F, Liefeldt L, Bamoulid J, Budde K: Need for optimized immunosuppression in elderly kidney transplant recipients. Transplant Rev (Orlando) 29: 237–239, 2015 [DOI] [PubMed] [Google Scholar]
- 16.Peeters LEJ, Andrews LM, Hesselink DA, de Winter BCM, van Gelder T: Personalized immunosuppression in elderly renal transplant recipients. Pharmacol Res 130: 303–307, 2018 [DOI] [PubMed] [Google Scholar]
- 17.Iwamoto H, Nakamura Y, Konno O, Tomita K, Ueno T, Yokoyama T, Kihara Y, Kawachi S, et al.Immunosuppressive therapy for elderly kidney transplant recipients. Transplant Proc 48: 799–801, 2016 [DOI] [PubMed] [Google Scholar]
- 18.Johnson DW, Nicol DL, Purdie DM, Preston JM, Brown AM, Hawley CM, Campbell SB, Wall D, Griffin AD, Isbel NM: Is mycophenolate mofetil less safe than azathioprine in elderly renal transplant recipients? Transplantation 73: 1158–1163, 2002 [DOI] [PubMed] [Google Scholar]
- 19.McClelland EE, Smith JM: Gender specific differences in the immune response to infection. Arch Immunol Ther Exp (Warsz) 59: 203–213, 2011 [DOI] [PubMed] [Google Scholar]
- 20.Rahman F, Christian HC: Non-classical actions of testosterone: An update. Trends Endocrinol Metab 18: 371–378, 2007 [DOI] [PubMed] [Google Scholar]
- 21.Sanfilippo F, Vaughn WK, Bollinger RR, Spees EK: Comparative effects of pregnancy, transfusion, and prior graft rejection on sensitization and renal transplant results. Transplantation 34: 360–366, 1982 [DOI] [PubMed] [Google Scholar]
- 22.Gore SM, Bradley BA: Renal Transplantation: Sense and Sensitization, Springer Science & Business Media, Berlin, Germany, 2007 [Google Scholar]
- 23.Boan P, Swaminathan R, Irish A: Infectious complications in indigenous renal transplant recipients in Western Australia. Intern Med J 47: 648–655, 2017 [DOI] [PubMed] [Google Scholar]
- 24.Kidney Health Australia : Charting a Comprehensive Approach to Tackling Kidney Disease, 2016. Available at: https://kidney.org.au/cms_uploads/docs/kha-pre-budget-submission-2015-16.pdf. Accessed March 2, 2019
- 25.Taminato M, Fram D, Grothe C, Pereira RR, Belasco A, Barbosa D: [Prevalence of infection in kidney transplantation from living versus deceased donor: Systematic review and meta-analysis]. Rev Esc Enferm USP 49: 509–514, 2015 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.