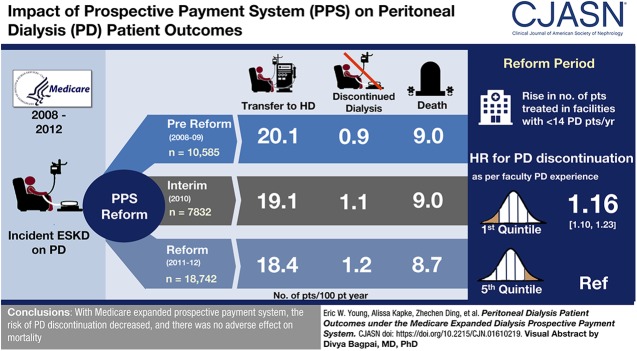
Visual Abstract
Keywords: peritoneal dialysis, outcomes, humans, United States, renal dialysis, kidney dialysis, proportional hazards models, motivation, cohort studies, prospective payment system, Medicare, Continental population groups, comorbidity
Abstract
Background and objectives
Peritoneal dialysis (PD) use increased in the United States with the introduction of a new Medicare prospective payment system in January 2011 that likely reduced financial disincentives for facility use of this home therapy. The expansion of PD to a broader population and facilities having less PD experience may have implications for patient outcomes. We assessed the impact of PD expansion on PD discontinuation and patient mortality.
Design, setting, participants, & measurements
A prospective cohort study was conducted of patients treated with PD at 90 days of ESKD. Patients were grouped by study start date relative to the Medicare payment reform: prereform (July 1, 2008 to December 31, 2009; n=10,585), interim (January 1, 2010 to December 31, 2010; n=7832), and reform period (January 1, 2011 to December 31, 2012; n=18,742). Patient characteristics and facility PD experience were compared at baseline (day 91 of ESKD). Patients were followed for 3 years for the major outcomes of PD discontinuation and mortality using Cox proportional hazards models.
Results
Patient characteristics, including age, sex, race, ethnicity, rurality, cause of ESKD, and comorbidity, were similar or showed small changes across the three study periods. There was an increasing tendency for patients on PD to be treated in facilities with less PD experience (from 34% during the prereform period being treated in facilities averaging <14 patients on PD per year to 44% in the reform period). Patients treated in facilities with less PD experience had a higher rate of PD discontinuation than patients treated in facilities with the most experience (hazard ratio [HR], 1.16; 95% confidence interval [95% CI], 1.10 to 1.23 for the first versus fifth quintile of PD experience). Nevertheless, the risk of PD discontinuation fell during the late interim period (HR, 0.88; 95% CI, 0.82 to 0.95) and most of the reform period (from HR, 0.85; 95% CI, 0.79 to 0.91 to HR, 0.94; 95% CI, 0.87 to 1.01). Mortality risk was stable across the three study periods.
Conclusions
In the context of expanding PD use and declining facility PD experience, the risk of PD discontinuation fell, and there was no adverse effect on mortality.
Podcast
This article contains a podcast at https://www.asn-online.org/media/podcast/CJASN/2019_09_12_CJN01610219.mp3
Introduction
Peritoneal dialysis (PD) has long lagged behind hemodialysis (HD) as a kidney replacement therapy for ESKD in the United States (1). Furthermore, PD usage is considerably lower in the United States compared with many other developed countries such as Australia, New Zealand, and Canada (1). PD usage in the United States is also below levels that studies of the attitudes of nephrologists have indicated would be appropriate (2,3). Several factors cited as potential barriers to self-care with PD may have been limiting use of this therapy in the United States, such as a lack of nephrologist training and experience with PD (4), a lack of patient education about PD (5,6), a lack of family support (7,8), provider awareness of Medicare coverage rules in the early months of ESKD (9), and concern that PD is associated with inferior outcomes (10,11).
Over time, there may also have been growing financial disincentives for dialysis facilities to use PD. During the 1990s and early 2000s, the prevailing fee-for-service payment approach was used for an expanding scope and volume of erythropoiesis stimulating agents and other injectable drugs that are more commonly administered to patients on HD than PD and were found to be relatively profitable for dialysis facilities (12–14). Perhaps because of a combination of factors that included such financial disincentives for using PD, PD use declined from approximately 15% of new patients on dialysis in 1990 to 6% in 2008 (1). Beginning in 2011, the Centers for Medicare & Medicaid Services (CMS) implemented a new prospective payment system (PPS) that was intended to encourage the use of PD as a home therapy by establishing the same bundled per-treatment payment rates for all dialysis modalities (15). In proximity to this change, PD use among new patients on dialysis has increased each year, reaching 10% as of 2016 (1,14,16–18).
Although no definitive comparison study of alternative maintenance dialysis modalities exists, PD offers a number of advantages in properly selected patients, including avoidance of in-center scheduling, increased patient independence, increased travel flexibility, preservation of residual kidney function, and lower overall cost (19). However, the advantages associated with PD undoubtedly depend on appropriate patient selection and facility readiness. These aspects of PD practice may be changing in the current era in which PD is being used in a broader patient population and the change in payment policy has likely incentivized the establishment of new PD programs. The results of previous studies suggest that such changes may have implications for outcomes for patients on PD. Rates of discontinuing PD have been shown to vary on the basis of characteristics such as prior treatment with HD, race, rurality, geographic region, and the dialysis facility’s level of experience with PD (20,21).
This study asked whether the policy decision to encourage the use of PD through the recent payment reform resulted in any important unintended consequences. Specifically, we studied whether the expansion in PD use among both patients and facilities was associated with adverse trends in discontinuation of PD or patient mortality. In doing so, we investigated trends in facility PD experience and the impact on patient outcomes. We also assessed whether the PD expansion selectively affected particular segments of the ESKD population in ways that could contribute to disparities in care.
Materials and Methods
Study Cohorts
The study population consisted of adult (aged ≥18 years) patients with incident ESKD who received PD as of 91 days of ESKD treatment, which allowed for a period of patient and modality stabilization during the initial months of ESKD. Data sources included the ESRD Medical Evidence Report (CMS Form 2728, which contains baseline information about all new patients with ESKD), Medicare claims, the CMS 2746 Death Notification Form, CMS Standard Information Management System Events file, and CROWNWeb.
The Medicare payment reforms were first proposed in the fall of 2009 (22) and, after a public comment period and finalization of the policy in the summer of 2010 (23), implemented on January 1, 2011. New patients on PD were grouped into three cohorts by the time period relative to implementation of the payment reform. The prereform period (July 1, 2008 to December 31, 2009) provided a baseline period for characteristics and outcomes of patients on PD. The interim period (January 1, 2010 to December 31, 2010) captured potential anticipatory changes in practice that might have occurred in response to the policy announcements about the upcoming reform but before actual implementation. The reform study period extends from the implementation date of January 1, 2011 through December 31, 2012. Each cohort was defined by new patients treated with PD on day 91 during the cohort window.
Study Variables
Patients were classified by age, race, ethnicity, pre-ESKD nephrology care, and rural/urban location as reported on the CMS 2728 Form. Patient residence was classified on a rural-urban scale by mapping the reported residence ZIP codes to Rural-Urban Commuting Area codes (24). Additional patient characteristics reported on the CMS 2728 Form were evaluated as descriptive or adjustment variables including body mass index, key laboratory values (albumin and eGFR), the primary cause of ESKD, reported comorbid conditions, current employment, insurance status, and Census region.
Patients were also characterized by the PD experience level of their dialysis facility. Facility PD experience was defined as the average number of patients on PD treated per year using data from 2003 through the year before when they reached day 90 of ESKD. For analyses, facility PD experience was divided into five groups, using quintiles.
Outcome Variables
All patients were followed for 3 years starting at 91 days of ESKD. The primary outcome measures were discontinuation of PD, death, and a combined measure of death or PD discontinuation. A patient was considered to have discontinued PD after the patient either switched to HD for at least 30 days or discontinued dialysis (25). We performed a sensitivity analysis using a 180-day discontinuation criterion (26).
Statistical Analyses
The outcome measures were modeled using multivariable Cox proportional hazards starting at 91 days of ESKD and followed to a study end point. Follow-up was censored at the earliest of a kidney transplant, recovery of kidney function, death (for analyses of PD discontinuation), switch to HD for at least 30 days (for analyses of patient mortality), discontinuation of dialysis (for analyses of patient mortality), or uncertain dialysis for at least 30 days.
We evaluated trends in outcomes of patients on PD in 6-month time intervals, adjusting for baseline patient demographic, insurance, employment, and clinical factors at onset of ESKD. Separate models were used to examine facility PD experience as a risk factor, adjusting for patient characteristics, and to ascertain the impact of adjusting for facility PD experience on hazard estimates by 6-month time period. Separate models were estimated for the prereform and reform periods to assess whether patient risk factors for poor outcomes with PD have changed under the new payment system in a way that would lead to health disparities.
Proportional hazards assumptions were tested by evaluating Kaplan–Meier curves and revealed no clear departures from these assumptions. Analyses were conducted using SAS V9.4 (SAS Institute, Cary, NC).
Results
Baseline Characteristics of Patients on PD
The study population includes 10,585, 7832, and 18,742 patients on PD in the prereform, interim, and reform periods, respectively (Table 1). The percent of new ESKD starts treated with PD increased from 7% in the baseline period to 9% after implementation of the expanded payment system. Overall, the case-mix of patients using PD at day 90 of ESKD was relatively stable, with distributions of most baseline characteristics differing by <2 percentage points across these three periods. Patients on PD were mostly white (71% and 70% in the prereform and reform periods, respectively), non-Hispanic (89% and 88%) male (56% and 57%), aged <65 years (66% and 66%), and living in an urban area (85% and 86%). There was a small increase in diabetes as the primary cause of ESKD from 42% to 44%. Small decreases were found in the prevalence of atherosclerotic disease and peripheral vascular disease. Employment at start of ESKD declined from 30% to 27% and Medicaid coverage increased from 14% to 15%. Most patients using PD at day 90 of ESKD had pre-ESKD nephrology care (which remained stable at approximately 82%). Distributions for body mass index, albumin, and GFR reported at onset of ESKD were similar for all three periods.
Table 1.
Characteristics of patients on incident PD by time period relative to implementation of the expanded ESKD prospective payment system
| Variable | Prereform Period: 2008–2009 (n=10,585)a | Interim Period: 2010 (n=7832)a | Reform Period: 2011–2012 (n=18,742)a |
|---|---|---|---|
| % PD | 7 | 8 | 9 |
| Female (%) | 44 | 43 | 43 |
| Age, yr (%) | |||
| 18–39 | 14 | 13 | 14 |
| 40–64 | 52 | 52 | 52 |
| 65–74 | 20 | 21 | 21 |
| 75+ | 13 | 14 | 14 |
| Race (%) | |||
| White | 71 | 71 | 70 |
| Black | 21 | 22 | 22 |
| Asian | 5 | 5 | 6 |
| American Indian/Alaskan Native | 1 | 1 | 1 |
| Pacific Islander | 1 | 1 | 1 |
| Other/multiracial | 1 | 0 | 0 |
| Hispanic (%) | 11 | 11 | 12 |
| Rural-urban commuting area (%) | |||
| Urban | 85 | 86 | 86 |
| Large rural | 7 | 7 | 7 |
| Small rural | 4 | 4 | 4 |
| Isolated rural | 3 | 3 | 3 |
| Unknown | 0 | 0 | 0 |
| Primary cause of ESKD (%) | |||
| Cystic kidney | 6 | 5 | 5 |
| Diabetes | 42 | 43 | 44 |
| Glomerulonephritis | 16 | 15 | 16 |
| Hypertension | 25 | 27 | 27 |
| Missing cause | 0 | 0 | 0 |
| Other cause | 7 | 6 | 6 |
| Other urologic | 1 | 1 | 1 |
| Unknown cause | 3 | 3 | 3 |
| Comorbidities (%) | |||
| Diabetes, not cause | 8 | 9 | 10 |
| Atherosclerotic disease | 15 | 15 | 13 |
| Cardiac failure | 18 | 18 | 17 |
| Cerebrovascular disease | 6 | 6 | 5 |
| Peripheral vascular disease | 9 | 9 | 8 |
| Other cardiac condition | 12 | 12 | 12 |
| Tobacco use (%) | 6 | 7 | 6 |
| Needs help with ADLs (%) | 3 | 4 | 4 |
| Current employment (%) | 30 | 27 | 27 |
| Medicaid (%) | 14 | 14 | 15 |
| Pre-ESKD nephrology care (%) | |||
| Yes | 82 | 82 | 82 |
| No | 13 | 13 | 13 |
| Unknown | 5 | 5 | 5 |
| BMI (%) | |||
| Underweight | 2 | 2 | 2 |
| Normal | 28 | 28 | 27 |
| Overweight | 31 | 31 | 31 |
| Obese | 38 | 39 | 40 |
| Missing/OOR | 1 | 1 | 1 |
| Albumin (g/dl) (%) | |||
| 1 to <3.1 | 16 | 16 | 17 |
| 3.1–3.4 | 15 | 15 | 14 |
| >3.4–5.5 | 47 | 46 | 45 |
| Missing/OOR | 22 | 23 | 23 |
| eGFR (ml/min per 1.73 m2) (%) | |||
| 2 to <5 | 6 | 6 | 8 |
| 5–10 | 41 | 39 | 41 |
| >10–20 | 49 | 50 | 48 |
| Missing/OOR | 4 | 4 | 4 |
| Facility average number of prior patients on PD per year (%) | |||
| ≤5.5 | 15 | 19 | 23 |
| 5.5 to ≤14 | 19 | 19 | 21 |
| 14 to ≤24.2 | 22 | 21 | 18 |
| 24.2 to ≤43.4 | 22 | 20 | 19 |
| >43.4 | 22 | 20 | 18 |
PD, peritoneal dialysis; ADL, activities of daily living; BMI, body mass index; OOR, out of range.
Time period corresponds to when patients reached day 90 of ESKD.
Over the study period, there was an increasing tendency for patients on PD to be treated in facilities having the least prior experience with PD. The percent of patients on PD treated in facilities averaging ≤14 patients on PD per year was 34%, 38%, and 44% of patients for the prereform, interim, and reform periods, respectively.
Primary Outcomes
The primary outcome was discontinuation of PD for at least 30 days during the 3-year follow-up window. There was a decline in the rate at which patients switched to HD over the three periods (Table 2). The trend was similar when PD discontinuation was defined using a 180-day criterion. Crude mortality was similar across the three time periods. There was a small decline in the rate of kidney transplantation. Other outcomes did not vary by study period.
Table 2.
| Outcome | Prereform Period: 2008–2009 (n=10,585) | Interim Period: 2010 (n=7832) | Reform Period: 2011–2012 (n=18,742) |
|---|---|---|---|
| Switched to HD for at least 30 d | 20.1 | 19.1 | 18.4 |
| Switched to HD for at least 180 d | 14.5 | 14.1 | 13.6 |
| Died | 9.0 | 9.0 | 8.7 |
| Discontinued dialysis | 0.9 | 1.1 | 1.2 |
| Recovered kidney function | 1.1 | 1.2 | 1.2 |
| Kidney transplant | 7.9 | 7.3 | 6.9 |
HD, hemodialysis.
Rates as number of patients per 100 patient-years.
On the basis of 3 years of follow-up starting at day 91 of ESKD.
Modeled Outcomes
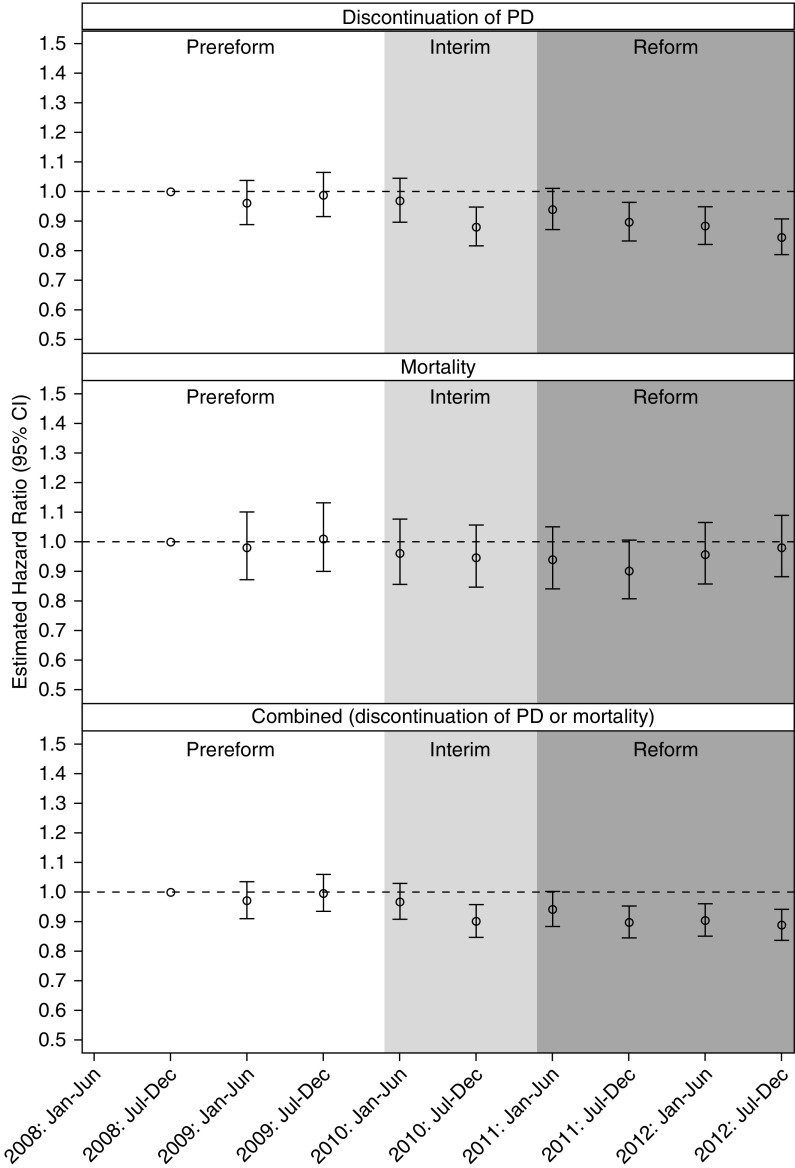
Proportional hazards modeling was employed to estimate time trends (in half-year intervals) of the primary outcomes (Figure 1). The model estimates are adjusted for the baseline patient characteristics shown in Table 1 with the exception of facility PD experience (which is examined using a separate model, as discussed below). The first time interval in the prereform period (July to December 2008) was used as the reference category. The modeled risk of PD discontinuation through 3 years of follow-up was stable through the prereform and initial interim periods, and then lower in the late interim period and over most of the reform period. Between July–December 2010 and July–December 2012, the estimated hazard ratio of PD discontinuation ranged from 0.85 (95% confidence interval, 0.79 to 0.91) to 0.94 (95% confidence interval, 0.87 to 1.01; with P<0.05 for all 6-month intervals except January to June 2011). PD patient-adjusted mortality was stable through 3 years of follow-up. The combined PD discontinuation/mortality outcome showed a downward trend in the interim and reform periods, similar to PD discontinuation as a separate outcome.
Figure 1.
Adjusted hazard ratios for PD patient outcomes, by time period. On the basis of a Cox proportional hazards model with adjustment for the factors shown in Table 3. The time period corresponds to when patients reached day 90 of ESKD.
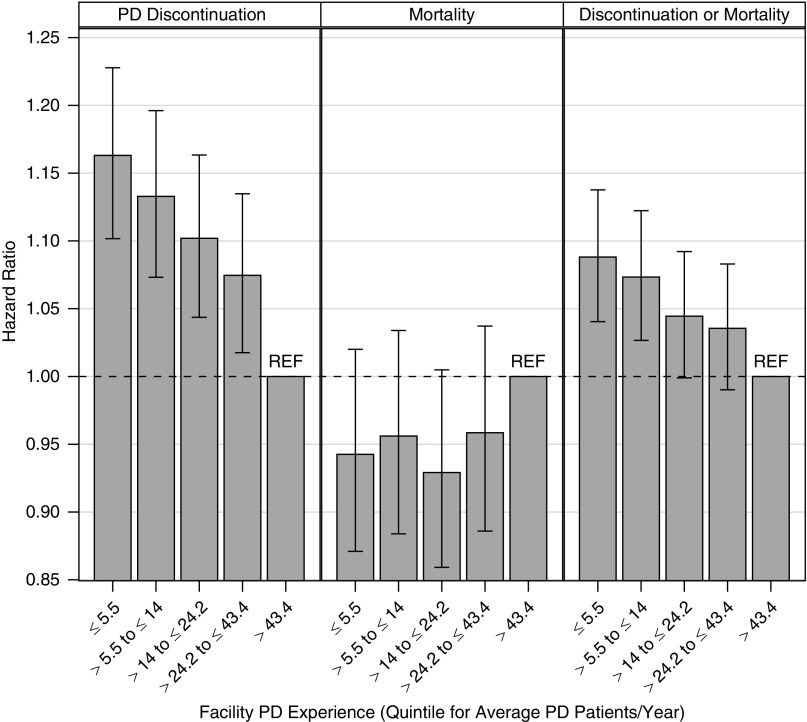
The risk of discontinuation of PD was inversely associated with facility PD experience (Figure 2). With adjustments for patient characteristics, patients on PD treated in facilities with the lowest levels of experience (5.5 or fewer patients on PD per year) had an approximately 16% higher risk of discontinuing PD compared with patients treated in facilities with the highest level of experience (>43.4 patients on PD per year). Facility PD experience did not have a statistically significant association with patient mortality. Facility PD experience was inversely related with the combined PD discontinuation/mortality outcome, similar to but less strongly than with PD discontinuation alone. When adjusting for both patient characteristics and facility PD experience, the estimated trends over the prereform, interim, and reform periods were very similar to those shown in Figure 1, for all three outcomes (see Supplemental Table 1 for detailed results).
Figure 2.
Associations between facility PD experience and PD patient outcomes. On the basis of a Cox proportional hazards model with adjustment for time period (in 6-month intervals) and the factors shown in Table 3. The error bars display 95% confidence intervals. The experience quintiles, expressed as average patients per year, are quintile 1 (0, 5.5), quintile 2 (5.5, 14), quintile 3 (14, 24.2), quintile 4 (24.2, 43.4), and quintile 5 (43.4+).
Using separate proportional hazard models by time period, we found that other risk factors for PD discontinuation and patient mortality remained similar under the PPS (Supplemental Table 2). This includes an elevated risk of discontinuing PD for patients with Medicaid insurance, while patients who are women, Asian, or Hispanic continued to be less likely to discontinue PD. In contrast, there was a higher risk of PD discontinuation among ages 18–39 (relative to ages 65–74 years) only in the reform period. There was a higher risk of discontinuing PD among black patients and those with no pre-ESKD nephrology care only in the pooled analysis (Table 3); separate estimates for the prereform and reform periods were similar but not statistically significant (Supplemental Table 2). More pronounced clinical risk factors continued to include diabetes, obesity, and assistance with activities of daily living. Similarly, patterns in PD patient mortality across demographic and socioeconomic groups largely persisted in the reform period.
Table 3.
Adjusted hazard ratios for PD patient outcomes
| Estimated Hazard Ratio (95% Confidence Interval) | |||
|---|---|---|---|
| Parameter | Discontinuation of PD | Mortality | Combined Outcome: Discontinuation of PD or Mortality |
| Female | 0.89 (0.86 to 0.93) | 0.98 (0.93 to 1.04) | 0.92 (0.90 to 0.95) |
| Age, yr (ref. 65–74 yr) | |||
| 18–39 | 1.03 (0.97 to 1.10) | 0.24 (0.21 to 0.28) | 0.78 (0.74 to 0.83) |
| 40–64 | 0.92 (0.88 to 0.97) | 0.55 (0.52 to 0.59) | 0.79 (0.76 to 0.82) |
| 75+ | 1.05 (0.99 to 1.12) | 1.61 (1.51 to 1.72) | 1.29 (1.24 to 1.35) |
| Race (ref. white) | |||
| Black | 1.05 (1.00 to 1.09) | 0.64 (0.59 to 0.69) | 0.92 (0.89 to 0.95) |
| Asian | 0.61 (0.56 to 0.67) | 0.46 (0.39 to 0.52) | 0.56 (0.52 to 0.60) |
| American Indian/Alaskan Native | 0.87 (0.75 to 1.02) | 0.76 (0.59 to 0.97) | 0.84 (0.73 to 0.95) |
| Pacific Islander | 0.77 (0.63 to 0.93) | 0.56 (0.40 to 0.77) | 0.70 (0.59–0.82) |
| Other/multiracial | 1.01 (0.73 to 1.35) | 0.60 (0.32 to 1.01) | 0.87 (0.65 to 1.12) |
| Hispanic | 0.77 (0.73 to 0.82) | 0.51 (0.46 to 0.56) | 0.69 (0.65 to 0.72) |
| Rural-urban commuting area (ref. urban) | |||
| Large rural | 1.05 (0.98 to 1.12) | 1.11 (1.01 to 1.22) | 1.07 (1.01 to 1.12) |
| Small rural | 1.02 (0.94 to 1.11) | 0.99 (0.87 to 1.11) | 1.01 (0.94 to 1.08) |
| Isolated rural | 1.07 (0.97 to 1.18) | 1.20 (1.06 to 1.35) | 1.11 (1.03 to 1.20) |
| Unknown | 0.91 (0.65 to 1.25) | 1.21 (0.80 to 1.75) | 1.05 (0.81 to 1.34) |
| BMI (ref. normal) | |||
| Underweight | 1.09 (0.96 to 1.23) | 1.38 (1.19 to 1.60) | 1.19 (1.08 to 1.31) |
| Overweight | 1.02 (0.97 to 1.07) | 0.83 (0.77 to 0.88) | 0.95 (0.92 to 0.99) |
| Obese | 1.22 (1.16 to 1.27) | 0.85 (0.80 to 0.91) | 1.09 (1.05 to 1.13) |
| Missing/OOR | 1.29 (1.06 to 1.55) | 1.05 (0.75 to 1.41) | 1.20 (1.01 to 1.41) |
| Pre-ESKD nephrology care (ref. no) | |||
| Yes | 0.94 (0.89 to 0.99) | 0.84 (0.77 to 0.91) | 0.91 (0.87 to 0.95) |
| Unknown | 0.96 (0.88 to 1.04) | 1.13 (0.99 to 1.28) | 1.01 (0.94 to 1.09) |
| Albumin (ref. 3.1–3.4 g/dl) | |||
| 1 to <3.1 | 1.18 (1.11 to 1.25) | 1.18 (1.09 to 1.29) | 1.18 (1.12 to 1.24) |
| >3.4–5.5 | 0.90 (0.86 to 0.95) | 0.78 (0.72 to 0.84) | 0.86 (0.83 to 0.90) |
| Missing/OOR | 1.02 (0.96 to 1.08) | 0.91 (0.84 to 0.99) | 0.98 (0.94 to 1.03) |
| GFR (MDRD) (ref. 5–10 ml/min per 1.73 m2) | |||
| 2 to <5 | 1.00 (0.93 to 1.07) | 0.87 (0.76 to 1.00) | 0.97 (0.91 to 1.03) |
| >10–20 | 0.99 (0.95 to 1.02) | 0.98 (0.92 to 1.03) | 0.98 (0.95 to 1.01) |
| Missing/OOR | 0.98 (0.89 to 1.07) | 1.47 (1.32 to 1.62) | 1.17 (1.09 to 1.25) |
| Diabetes | 1.28 (1.23 to 1.34) | 1.74 (1.61 to 1.87) | 1.39 (1.34 to 1.44) |
| Hypertension | 1.05 (1.00 to 1.10) | 1.21 (1.12 to 1.30) | 1.09 (1.05 to 1.13) |
| Diabetes, not cause | 1.18 (1.11 to 1.25) | 1.43 (1.31 to 1.55) | 1.25 (1.19 to 1.31) |
| Atherosclerotic disease | 0.98 (0.93 to 1.04) | 1.17 (1.10 to 1.25) | 1.06 (1.02 to 1.10) |
| Cardiac failure | 1.10 (1.05 to 1.15) | 1.57 (1.48 to 1.67) | 1.26 (1.21 to 1.30) |
| Cerebrovascular disease | 1.10 (1.02 to 1.18) | 1.16 (1.06 to 1.27) | 1.12 (1.06 to 1.19) |
| Peripheral vascular disease | 1.03 (0.97 to 1.09) | 1.09 (1.01 to 1.17) | 1.06 (1.01 to 1.11) |
| Other cardiac condition | 0.98 (0.93 to 1.03) | 1.28 (1.20 to 1.36) | 1.09 (1.05 to 1.14) |
| Tobacco use | 1.14 (1.07 to 1.22) | 1.17 (1.05 to 1.29) | 1.14 (1.08 to 1.21) |
| Needs help with ADLs | 1.19 (1.09 to 1.31) | 1.79 (1.63 to 1.96) | 1.43 (1.34 to 1.53) |
| Current employment | 0.90 (0.86 to 0.93) | 0.81 (0.75 to 0.88) | 0.88 (0.85 to 0.92) |
| Medicaid | 1.19 (1.14 to 1.25) | 1.09 (1.01 to 1.18) | 1.15 (1.11 to 1.20) |
Models were also adjusted for indicators of the 6-month time period in which patients reached day 90 of ESKD (as shown in Figure 1). For parameters with multiple groups (e.g., age), the group title row shows the P value of significance for the overall effect. PD, peritoneal dialysis; ref., reference; BMI, body mass index; OOR, out of range; MDRD, Modification of Diet in Renal Disease; ADL, activities of daily living.
Discussion
In the context of the growing use of a home dialysis therapy that was encouraged by the recent Medicare dialysis payment reform, there is no early evidence of worsening overall outcomes among patients with incident ESKD using PD. Instead, there is early evidence of a decline over time in the rate at which patients discontinue PD, and no change in patient mortality rates through 3 years of follow-up. PD discontinuation findings were similar for definitions based on either 30 or 180 days (Table 2) (26). These trends are observed both as PD was being used by an expanding patient population and as there was a decline over time in average facility experience with PD.
At the time that the Medicare payment reform was designed and implemented, the use of PD in the United States had been falling since the mid-1990s, and was low in comparison to a number of other countries with comparable socioeconomic and medical characteristics (1). PD offers potential advantages to patients in terms of independence, quality of life, patient satisfaction, and preservation of residual kidney function (19,27,28). As a continuous therapy, PD tends to avoid large swings in fluid status and exposure to uremic toxins. PD is generally less expensive to the health care system than HD (29). In the absence of detailed clinical trials, it is difficult to estimate the optimal penetration of PD but it is reasonable to assume that more patients with ESKD could benefit from a PD treatment option.
Several factors potentially contribute to low PD utilization. Many patients and family members feel overwhelmed by the self-management requirements. Complications remain a challenge, including infections and leakage. Late referral creates logistic and patient education barriers to PD. The level of PD exposure and training by nephrologists is variable. Many dialysis facilities lack experience and expertise with PD management. Technical, educational, and programmatic reforms have been advanced to address some of these issues (19,30–32).
In addition to these important patient and provider issues, there was a financial disincentive to use PD under the Medicare program that covers most patients with ESKD in the United States. In the past, Medicare provided higher-margin payments to dialysis facilities for injectable drugs such as erythropoiesis stimulating agents, iron, and vitamin D analogs (12,13). In using a home therapy, patients on PD spend less time at a dialysis facility and are less likely to receive injectable medications. After a planning and public comment process (22), in January 2011 Medicare introduced a new ESKD PPS that rolled injectable drugs into a global bundled payment per treatment (23). Furthermore, Medicare established the same bundled payment for patients on HD and PD, a policy change that was financially favorable to PD given the lower use of expensive injectable medications. As intended, the percentage of patients treated with PD grew as the new PPS was implemented (1,14,16,17).
The increase in PD use is in itself the successful fulfillment of a national policy goal. However, the increase should only be considered fully successful if it is applied to appropriate patients in a way that does not disadvantage any demographic or socioeconomic group. In addition, the policy created incentives for the formation of new PD programs (usually added to existing HD facilities) that, initially, may lack the experience found in established centers. An increase in PD use would raise important concerns if patient outcome measures declined as a result of inappropriate patient selection or inexperienced programs. This study was undertaken to carefully examine these potential unintended consequences.
We approached these questions by defining three patient cohorts on the basis of when they reached day 91 of ESKD: during a prereform period that serves as a baseline period for analysis, an interim period that potentially captures early changes in practice as facilities anticipated the new payment method, and an initial time period under the new payment system. Major decisions regarding patient and facility selection occur in this early period of ESKD. We followed patients for up to 3 years for primary outcomes of PD discontinuation and death. Although the follow-up period for the prereform and interim patient cohorts extended into the reform period, the expected major effect of the policy applies to patient and facility selection at the start of ESKD.
We found that the observed characteristics of new patients on PD were largely comparable in the three cohorts (Table 1), which is consistent with the findings of a recent study (14) and does not suggest selected targeting or exclusion of patient groups. In the context of these indications of relatively stable overall case-mix, the patient factors that are associated with PD outcomes did not show large changes between the prereform and reform periods (Supplemental Table 2, Table 3). There is consequently no evidence at this stage that the new payment system has systematically increased the risk of poor outcomes for certain subgroups of patients on PD in a way that would lead to growing health disparities. We found several patient factors that predicted outcomes that were generally consistent with prior studies (33).
The concern that expansion of PD would give rise to new PD programs with less experience was borne out by the findings. Average facility PD experience declined in the transition from prereform to interim to reform periods (Table 1). Furthermore, patients treated in facilities with lower levels of facility experience with PD have a higher risk of PD discontinuation, as shown previously (20). Despite the increase in less experienced facilities and the observed association between facility PD experience and PD discontinuation, the net observation was a lower risk of PD discontinuation. Information on the reasons for PD discontinuation were not available.
The improvements in technique survival as measured by PD discontinuation suggest that financial incentives under the new payment system may be encouraging or facilitating greater longer-term use of PD. Given the new incentive structure and potential continued growth in PD, it will be important to continue to monitor modality selection and outcomes for patients on PD. There is a need for such efforts to include longer follow-up and also consider other clinical and patient-reported outcomes that can help to inform conclusions about whether the goals for this therapy are being achieved.
These findings are limited by lack of a contemporaneous control group. We would not be able to distinguish the effect of the new payment policy from other secular trends. The patient treatment and outcome measurements relied on Medicare claims and reporting forms (Medical Evidence and Death Notification forms), which are inconsistently audited for accuracy. In addition, the claims data do not indicate the reason for PD discontinuation. Medicare claims data offer an important advantage of large sample size. Despite the limitations, the study provides strong assurance that the policy-driven expansion of PD did not adversely affect important outcomes among patients with incident ESKD using this therapy and did not create any measured patient disparities in these outcomes in the early years under the new payment system.
Disclosures
Mr. Cogan, Mr. Ding, Ms. Kapke, Dr. Mukhopadhyay, Mr. Pearson, Dr. Turenne, and Dr. Young are employed by Arbor Research Collaborative for Health (Arbor Research). Arbor Research receives research funding for the Dialysis Outcomes and Practice Patterns Study (DOPPS) Program from a consortium of more than 25 private and public sponsors that include Baxter Healthcare Corporation. Arbor Research also receives research funding from the Centers for Medicare & Medicaid Services. Mr. Cogan, Dr. Mukhopadhyay, and Mr. Pearson report participation on other projects funded through contracts from the National Institutes of Health and Centers for Medicare & Medicaid Services (CMS). Dr. Turenne reports participation in other research contracts and grants from the CMS and the Food and Drug Administration. Mr. Cogan, Dr. Mukhopadhyay, Mr. Pearson, and Dr. Turenne do not work on industry-funded projects at Arbor Research Collaborative for Health, such as the Dialysis Outcomes and Practice Patterns Studies. Dr. Baker has nothing to disclose.
Funding
This work was supported by a grant from the National Institute on Minority Health and Health Disparities of the National Institutes of Health R01MD006247. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Supplementary Material
Acknowledgments
Jennifer McCready-Maynes, an employee of Arbor Research Collaborative for Health, provided editorial assistance.
Footnotes
Published online ahead of print. Publication date available at www.cjasn.org.
Supplemental Material
This article contains the following supplemental material online at http://cjasn.asnjournals.org/lookup/suppl/doi:10.2215/CJN.01610219/-/DCSupplemental.
Supplemental Table 1. Adjusted hazard ratios for incident PD patient outcomes by time period, with and without adjustment for facility PD experience.
Supplemental Table 2. Adjusted hazard ratios for patient characteristics, prereform and reform periods.
References
- 1.United States Renal Data System : USRDS Annual Data Report: Epidemiology of Kidney Disease in the United States, Bethesda, MD, United States Renal Data System, 2017 [Google Scholar]
- 2.Thamer M, Hwang W, Fink NE, Sadler JH, Wills S, Levin NW, Bass EB, Levey AS, Brookmeyer R, Powe NR: US nephrologists’ recommendation of dialysis modality: Results of a national survey. Am J Kidney Dis 36: 1155–1165, 2000 [DOI] [PubMed] [Google Scholar]
- 3.Mendelssohn DC, Mullaney SR, Jung B, Blake PG, Mehta RL: What do American nephologists think about dialysis modality selection? Am J Kidney Dis 37: 22–29, 2001 [DOI] [PubMed] [Google Scholar]
- 4.Mehrotra R, Blake P, Berman N, Nolph KD: An analysis of dialysis training in the United States and Canada. Am J Kidney Dis 40: 152–160, 2002 [DOI] [PubMed] [Google Scholar]
- 5.Mehrotra R, Marsh D, Vonesh E, Peters V, Nissenson A: Patient education and access of ESRD patients to renal replacement therapies beyond in-center hemodialysis. Kidney Int 68: 378–390, 2005 [DOI] [PubMed] [Google Scholar]
- 6.Finkelstein FO, Story K, Firanek C, Barre P, Takano T, Soroka S, Mujais S, Rodd K, Mendelssohn D: Perceived knowledge among patients cared for by nephrologists about chronic kidney disease and end-stage renal disease therapies. Kidney Int 74: 1178–1184, 2008 [DOI] [PubMed] [Google Scholar]
- 7.Oliver MJ, Quinn RR, Richardson EP, Kiss AJ, Lamping DL, Manns BJ: Home care assistance and the utilization of peritoneal dialysis. Kidney Int 71: 673–678, 2007 [DOI] [PubMed] [Google Scholar]
- 8.Blake PG, Quinn RR, Oliver MJ: Peritoneal dialysis and the process of modality selection. Perit Dial Int 33: 233–241, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Perez JJ, Zhao B, Qureshi S, Winkelmayer WC, Erickson KF: Health insurance and the use of peritoneal dialysis in the United States. Am J Kidney Dis 71: 479–487, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Blake PG, Finkelstein FO: Why is the proportion of patients doing peritoneal dialysis declining in North America? Perit Dial Int 21: 107–114, 2001 [PubMed] [Google Scholar]
- 11.Khawar O, Kalantar-Zadeh K, Lo WK, Johnson D, Mehrotra R: Is the declining use of long-term peritoneal dialysis justified by outcome data? Clin J Am Soc Nephrol 2: 1317–1328, 2007 [DOI] [PubMed] [Google Scholar]
- 12.Office of Inspector General (OIG) : Medicare reimbursement For Existing End Stage Renal Disease Drugs (OEI-03-04-00120), Washington, DC, Department of Health and Human Services, 2004 [Google Scholar]
- 13.Office of Inspector General (OIG) : Medicare Reimbursement for New End Stage Renal Disease Drugs (OEI-03-06-00200), Washington, DC, Department of Health and Human Services, 2006 [Google Scholar]
- 14.Turenne M, Baker R, Pearson J, Cogan C, Mukhopadhyay P, Cope E: Payment reform and health disparities: Changes in dialysis modality under the new medicare dialysis payment system. Health Serv Res 53: 1430–1457, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hornberger J, Hirth RA: Financial implications of choice of dialysis type of the revised Medicare payment system: An economic analysis. Am J Kidney Dis 60: 280–287, 2012 [DOI] [PubMed] [Google Scholar]
- 16.Hirth RA, Turenne MN, Wheeler JR, Nahra TA, Sleeman KK, Zhang W, Messana JA: The initial impact of Medicare’s new prospective payment system for kidney dialysis. Am J Kidney Dis 62: 662–669, 2013 [DOI] [PubMed] [Google Scholar]
- 17.Zhang Q, Thamer M, Kshirsagar O, Zhang Y: Impact of the end stage renal disease prospective payment system on the use of peritoneal dialysis. Kidney Int Rep 2: 350–358, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.United States Renal Data System : 2018 USRDS Annual Data Report: Epidemiology of Kidney Disease in the United States, Bethesda, MD, United States Renal Data System, 2018 [Google Scholar]
- 19.Mehrotra R, Devuyst O, Davies SJ, Johnson DW: The current state of peritoneal dialysis. J Am Soc Nephrol 27: 3238–3252, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Schaubel DE, Blake PG, Fenton SS: Effect of renal center characteristics on mortality and technique failure on peritoneal dialysis. Kidney Int 60: 1517–1524, 2001 [DOI] [PubMed] [Google Scholar]
- 21.Mehrotra R, Story K, Guest S, Fedunyszyn M: Neighborhood location, rurality, geography, and outcomes of peritoneal dialysis patients in the United States. Perit Dial Int 32: 322–331, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Centers for Medicare & Medicaid Services (CMS), HHS : Medicare programs; end-stage renal disease prospective payment system; town hall meeting on end-stage renal disease prospective payment system; proposed rule and notice. Fed Regist 74: 49922–50102, 2009 [Google Scholar]
- 23.Centers for Medicare & Medicaid Services (CMS), HHS : Medicare program; end-stage renal disease prospective payment system, final rule. Fed Regist 75: 49030–49214, 2010 [PubMed] [Google Scholar]
- 24.WWAMI Rural Health Research Center : Rural-Urban Commuting Area Codes (RUCAs). 2018. Available at: http://depts.washington.edu/uwruca/index.php. Accessed XXX
- 25.Shen JI, Mitani AA, Saxena AB, Goldstein BA, Winkelmayer WC: Determinants of peritoneal dialysis technique failure in incident US patients. Perit Dial Int 33: 155–166, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lan PG, Clayton PA, Johnson DW, McDonald SP, Borlace M, Badve SV, Sud K, Boudville N: Duration of hemodialysis following peritoneal dialysis cessation in Australia and New Zealand: Proposal for a standardized definition of technique failure. Perit Dial Int 36: 623–630, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Krediet RT: Preservation of residual kidney function and urine volume in patients on dialysis. Clin J Am Soc Nephrol 12: 377–379, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Iyasere OU, Brown EA, Johansson L, Huson L, Smee J, Maxwell AP, Farrington K, Davenport A: Quality of life and physical function in older patients on dialysis: A comparison of assisted peritoneal dialysis with hemodialysis. Clin J Am Soc Nephrol 11: 423–430, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Karopadi AN, Mason G, Rettore E, Ronco C: Cost of peritoneal dialysis and haemodialysis across the world. Nephrol Dial Transplant 28: 2553–2569, 2013 [DOI] [PubMed] [Google Scholar]
- 30.Devoe DJ, Wong B, James MT, Ravani P, Oliver MJ, Barnieh L, Roberts DJ, Pauly R, Manns BJ, Kappel J, Quinn RR: Patient education and peritoneal dialysis modality selection: A systematic review and meta-analysis. Am J Kidney Dis 68: 422–433, 2016 [DOI] [PubMed] [Google Scholar]
- 31.Li PK, Chow KM: Peritoneal dialysis-first policy made successful: Perspectives and actions. Am J Kidney Dis 62: 993–1005, 2013 [DOI] [PubMed] [Google Scholar]
- 32.Ghaffari A: Urgent-start peritoneal dialysis: A quality improvement report. Am J Kidney Dis 59: 400–408, 2012 [DOI] [PubMed] [Google Scholar]
- 33.Lim WH, Dogra GK, McDonald SP, Brown FG, Johnson DW: Compared with younger peritoneal dialysis patients, elderly patients have similar peritonitis-free survival and lower risk of technique failure, but higher risk of peritonitis-related mortality. Perit Dial Int 31: 663–671, 2011 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.