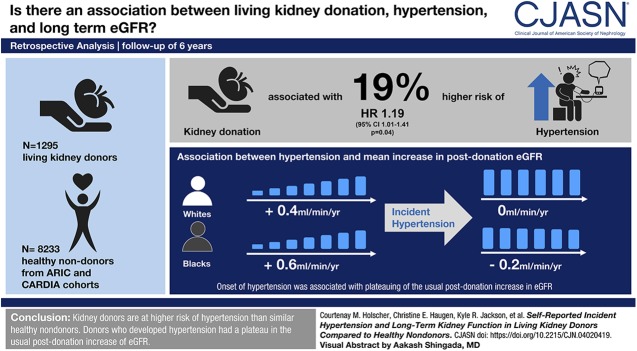
Visual Abstract
Keywords: kidney donation, hypertension, United States, confidence intervals, linear models, follow-up studies, self report, living donors, kidney, tissue and organ harvesting, glomerular filtration rate
Abstract
Background and objectives
The risk of hypertension attributable to living kidney donation remains unknown as does the effect of developing postdonation hypertension on subsequent eGFR. We sought to understand the association between living kidney donation, hypertension, and long-term eGFR by comparing donors with a cohort of healthy nondonors.
Design, setting, participants, & measurements
We compared 1295 living kidney donors with median 6 years of follow-up with a weighted cohort of 8233 healthy nondonors. We quantified the risk of self-reported hypertension using a parametric survival model. We examined the association of hypertension with yearly change in eGFR using multilevel linear regression and clustering by participant, with an interaction term for race.
Results
Kidney donation was independently associated with a 19% higher risk of hypertension (adjusted hazard ratio, 1.19; 95% confidence interval, 1.01 to 1.41; P=0.04); this association did not vary by race (interaction P=0.60). For white and black nondonors, there was a mean decline in eGFR (−0.4 and −0.3 ml/min per year, respectively) that steepened after incident hypertension (−0.8 and −0.9 ml/min per year, respectively; both P<0.001). For white and black kidney donors, there was a mean increase in eGFR after donation (+0.4 and +0.6 ml/min per year, respectively) that plateaued after incident hypertension (0 and −0.2 ml/min per year, respectively; P=0.07 and P=0.01, respectively, after hypertension).
Conclusions
Kidney donors are at higher risk of hypertension than similar healthy nondonors, regardless of race. Donors who developed hypertension had a plateau in the usual postdonation increase of eGFR.
Introduction
Living kidney donation is associated with an increased risk of ESKD compared with in healthy nondonors (1), with hypertension as one of the most frequent concomitant etiologies (2,3). Because prior studies have mainly been cross-sectional or lacking comparison with healthy nondonors, the timeline over which kidney donors develop hypertension and how this compares with nondonors have not been elucidated. As such, our ability to individualize ESKD risk prediction for potential donors is currently coarse and lacks grounding in models of disease progression (4). To improve informed consent and physiologic understanding, it is critical that we not only clarify the risk of hypertension attributable to donation and the timeline of incident postdonation hypertension but also, clarify the effect of postdonation hypertension on subsequent eGFR.
It is also possible that race affects the risk of developing postdonation hypertension. For example, black donors were 1.5-fold more likely to have hypertension than white donors in a study of 4650 living kidney donors using linked private insurance claims data (5), with similar findings from a study of 4007 donors using linked Medicare claims (6). However, these studies lacked comparison with a selected healthy nondonor cohort, and therefore, they could not describe incidence of hypertension or the risk of hypertension attributable to donation. One longitudinal study of 338 black kidney donors and matched nondonors reported a 2.4-fold higher risk of hypertension in donors, but it did not examine white donors or nondonors (7). A meta-analysis examining BP in 5145 living kidney donors concluded that donors may have a 5-mm Hg greater increase in BP than the increase anticipated with normal aging, but it did not consider the effect of race or the association of hypertension with subsequent eGFR (8).
To understand the association between living kidney donation, race, hypertension, and long-term eGFR, we conducted a longitudinal multicenter study of 1295 living kidney donors. We compared the living kidney donors with a cohort of healthy nondonors drawn from two national prospective cohort studies: the Atherosclerosis Risk in Communities (ARIC) cohort and the Coronary Artery Risk Development in Young Adults (CARDIA) cohort. We examined the long-term risk of hypertension as well as the association between incident hypertension and eGFR trajectory in both kidney donors and healthy nondonors, with race as a possible effect modifier.
Materials and Methods
Study Population
Living Kidney Donors.
This study was part of the Wellness and Health Outcomes in the Live Donor study, an ongoing multicenter cohort study of living kidney donors enrolled with at least 2 years postdonation follow-up. We studied black and white donors ages 18–66 years old at donation who had at least one postdonation serum creatinine measurement. These included donors from six transplant centers. Age at donation, sex, race, education level, and smoking history were self-reported. Baseline body mass index (BMI) and serum creatinine were abstracted from preoperative medical records. GFR was estimated using the Chronic Kidney Disease Epidemiology Collaboration equation (9). There were 16 donors (1%) who reported having hypertension at baseline; these donors were excluded from estimates of risk of incident postdonation hypertension, but they were included in estimates of eGFR trajectory. This study was approved by the Johns Hopkins Medicine Institutional Review Board (NA_00044282), and all donor participants provided written informed consent to participate. The clinical and research activities being reported are consistent with the Principles of the Declaration of Istanbul as outlined in the Declaration of Istanbul on Organ Trafficking and Transplant Tourism.
Healthy Nondonors.
A cohort of healthy nondonor controls was constructed from the ARIC and CARDIA prospective cohort studies. The ARIC study began in 1987 (10) and enrolled approximately 16,000 adults ages 45–65 years old across four United States communities. The CARDIA study began in 1985 (11) and enrolled approximately 5000 adults ages 18–30 years old across four different United States communities. To cover as wide an age spectrum as possible from the CARDIA study, we randomized 20% of CARDIA study participants to have the year 0 visit considered baseline (at ages 18–30 years old), 40% to have the year 10 visit considered baseline (at ages 28–40 years old), and 40% to have the year 15 visit considered baseline (at ages 33–45 years old).
Both the ARIC study and the CARDIA study obtained demographic characteristics and medical history through survey questionnaires and included physical examination and laboratory components under study protocols described elsewhere (10–14). Specifically, age at baseline, sex, race, education level, and smoking history were self-reported as part of survey questionnaires; however, height and weight were measured and BMI was calculated at study visits. We excluded the ARIC and CARDIA study participants with absolute contraindications for living kidney donation, including diabetes mellitus, history of significant cardiovascular disease, cancer, chronic lung disease, kidney disease or eGFR<60 ml/min per 1.73 m2, BMI>40, or current pregnancy (15). We excluded participants with baseline hypertension for the purpose of estimating the risk of incident hypertension associated with kidney donation. We also excluded participants who had no follow-up serum creatinine after their baseline visit.
Statistical Analyses
Nondonor Weighting.
We used a propensity score method of “weighting by the odds” to weight the nondonor population to achieve better balance in baseline characteristics with the donor population, allowing for estimation of the average treatment effect on the treated (16,17). To do this, we first used a logistic regression model to calculate a propensity score of odds of being a donor on the basis of baseline age category (in deciles) with an interaction with race given the larger baseline prevalence of blacks in the CARDIA study. We also included terms for sex, baseline eGFR, high school education, and ever smoker status in this propensity score model. Missing indicators were used for missing baseline eGFR, education level, and smoking status.
We then weighted the population as follows: all kidney donors were assigned a weight of one. Nondonors were weighted by the odds of being a donor on the basis of the propensity score model described above. Although 99% of nondonors had an odds below one and thus, were down-weighted in the sample, there were nondonors who had a higher odds of having been a donor and were up-weighted in the nondonor sample. The maximum weight that any nondonor was assigned was 1.32: that is, no nondonor was weighted >1.4-fold. Balance was demonstrated by all weighted baseline characteristics having a standardized bias <0.25 (17).
Incident Hypertension.
For kidney donors, the year of incident hypertension was self-reported as part of the enrollment survey questionnaire. In the ARIC study, hypertension was ascertained through the use of antihypertensive medications as self-reported at each study visit. In the CARDIA study, hypertension was self-reported at each study visit. Kaplan–Meier curves were used to estimate cumulative incidence of hypertension in the weighted study population. Interval censoring (for example, time between study visits in the ARIC and CARDIA studies) was handled using a parametric regression model for interval-censored survival time data specified by a Weibull survival distribution. We selected a Weibull distribution to minimize the Akaike information criterion, a relative indicator of model quality (18). As a sensitivity analysis, we examined the unweighted population using a parametric regression model for interval-censored data; the direction of our inferences remained the same, and thus, we report results from the weighted model.
eGFR over Time.
We used multilevel linear regression to model eGFR using our weighted population with clustering by participant and allowing a random intercept. We included a term for time after baseline with an interaction term for race to examine how the eGFR trajectory varied within each group. To include time-varying hypertension in the model, we created a term for the number of days that a participant had hypertension at each eGFR measurement starting at the first eGFR measurement with recorded hypertension. We included interactions for race with the time-varying hypertension term to examine slope of eGFR after incident hypertension, but we did not model an intercept to allow hypertension to be associated with a different eGFR trajectory but not a different baseline eGFR. We specified an exponential covariance structure to minimize Akaike information criterion, and we specified a robust variance to account for the use of a weighted population. As a sensitivity analysis, we examined the unweighted population using multilevel linear regression with clustering by participant; our inferences remained the same; thus, we report results from the weighted model. As an additional sensitivity analysis, we limited our analysis to participants with at least three follow-up creatinine measurements; our inferences remained the same, and thus, we report results from the primary analysis.
The 95% confidence intervals (95% CIs) are reported as per the method of Louis and Zeger (19). All analyses were performed using Stata/SE 15.1 for Windows (College Station, TX).
Results
Study Population
We studied 1295 kidney donors and 8233 healthy nondonors. There were differences in characteristics at baseline between kidney donors and healthy nondonors, but these differences were mitigated by our weighting strategy (Table 1). Kidney donors had a median of six postdonation eGFR measurements reported (interquartile range [IQR], 3–10) over a median of 6 years (IQR, 2–11 years; maximum, 27 years); healthy nondonors had a median of five eGFR measurements reported (IQR, 5–5) over a median of 23 years (IQR, 9–24 years; maximum, 27 years).
Table 1.
Baseline characteristics of healthy nondonors and living kidney donors before and after weighting
| Characteristic | Healthy Nondonors | Kidney Donors | Standardized Bias |
|---|---|---|---|
| Unweighted study population | |||
| N | 8233 | 1295 | |
| Baseline age, median yr (IQR) | 49 (40–55) | 46 (38–53) | |
| Men, % | 46 | 36 | |
| Black, % | 24 | 14 | |
| Baseline BMI, median (IQR) | 26 (23–28) | 26 (24–30) | |
| Missing baseline BMI, % | 0 | 35 | |
| Baseline eGFR, median (IQR) | 93 (83–103) | 96 (83–107) | |
| Missing baseline eGFR, % | 0 | 22 | |
| High school education, % | |||
| Yes | 88 | 94 | |
| No | 12 | 1 | |
| Missing | 0 | 5 | |
| Some college education, % | |||
| Yes | 59 | 64 | |
| No | 41 | 32 | |
| Missing | 0 | 5 | |
| Ever smoker, % | |||
| Yes | 52 | 36 | |
| No | 38 | 59 | |
| Missing | 10 | 5 | |
| Weighted study population | |||
| N | 935 | 1295 | |
| Baseline age, mean yr ±SD | 45±10 | 45±11 | 0.04 |
| Men, % | 37 | 36 | 0.02 |
| Black, % | 11 | 14 | −0.09 |
| Obese, % | 15 | 23 | −0.18 |
| Baseline eGFR, mean ml/min ±SD | 95±14 | 96±17 | −0.06 |
| High school education, % | 98 | 99 | −0.02 |
| Some college education, % | 68 | 67 | 0.01 |
| Ever smoker, % | 40 | 38 | 0.03 |
Unweighted healthy nondonors are compared with donors using Wilcoxon signed-rank sum tests and chi-squared tests, whereas weighted healthy nondonors are compared with donors using standardized bias. Balance through weighting is demonstrated by all baseline characteristics having a standardized bias <0.25 (17). IQR, interquartile range; BMI, body mass index.
Incident Hypertension
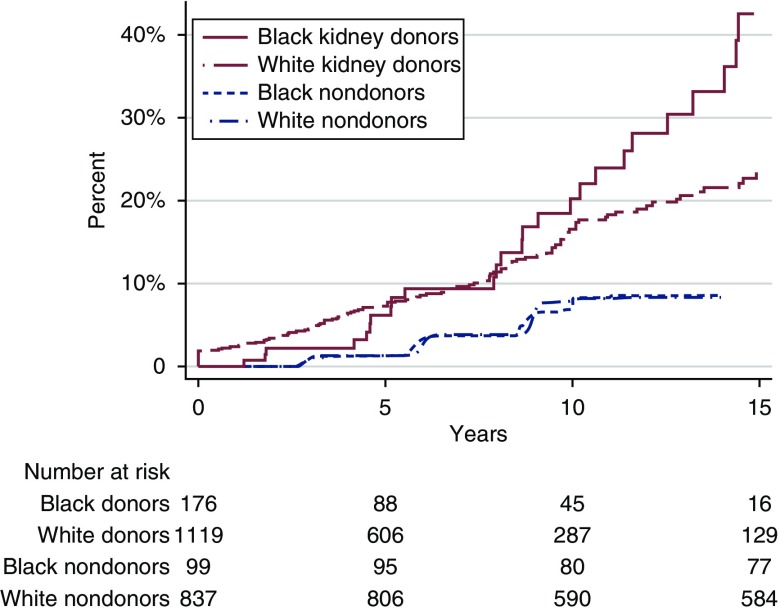
Kidney donors had a higher incidence of hypertension than healthy nondonors, with black donors at higher risk than white donors (Figure 1). At 15 years, 8% of white nondonors, 9% of black nondonors, 23% of white kidney donors, and 42% of black kidney donors had hypertension. Kidney donation was associated with a 19% higher risk of hypertension (adjusted hazard ratio, 1.19; 95% CI, 1.01 to 1.41; P=0.04), regardless of race; separately, black race was associated with a 27% higher risk of hypertension (adjusted hazard ratio, 1.27; 95% CI, 1.13 to 1.42; P<0.001), regardless of whether the individual donated a kidney. Although black kidney donors were at highest risk, the association between kidney donation and hypertension did not vary by race (interaction P=0.60); in other words, although the baseline risk of hypertension was higher for blacks, the effect of donating a kidney on the development of hypertension was the same for blacks as for whites.
Figure 1.
Donors have a higher incidence of hypertension compared to nondonors. Cumulative incidence of self-reported hypertension in black and white kidney donors and weighted nondonors over 15 years.
eGFR over Time
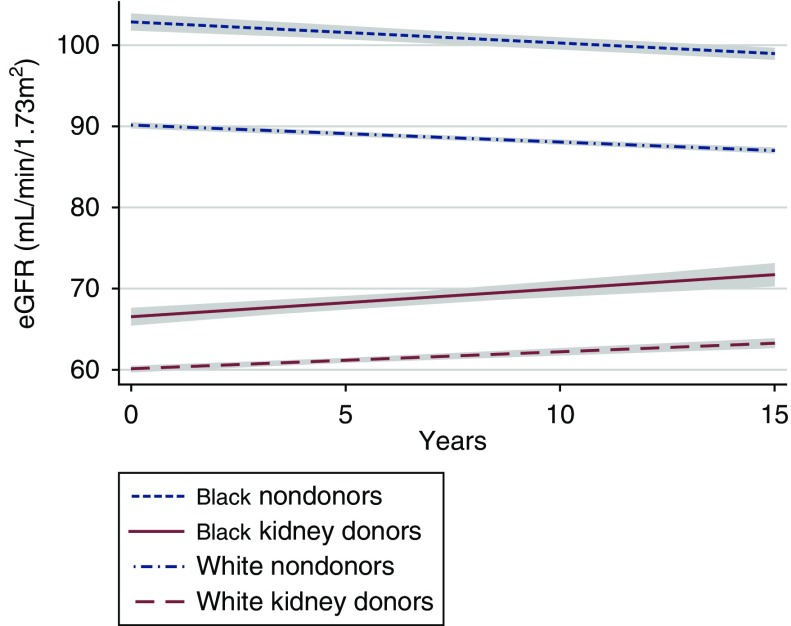
For nondonors, we found a decline in eGFR over time regardless of race (Figure 2). For white healthy nondonors, we found a mean yearly decline in eGFR (−0.38 ml/min per 1.73 m2; 95% CI, −0.41 to −0.35 ml/min per 1.73 m2) that steepened with incident hypertension (−0.76 ml/min per 1.73 m2; 95% CI, −0.90 to −0.62 ml/min per 1.73 m2 per year; P<0.001 for difference after incident hypertension) (Figure 3, Table 2). Similarly, for black healthy nondonors, we found a mean yearly decline in eGFR (−0.32 ml/min per 1.73 m2; 95% CI, −0.38 to −0.25 ml/min per 1.73 m2) that steepened with incident hypertension (−0.91 ml/min per 1.73 m2; 95% CI, −1.20 to −0.62 ml/min per 1.73 m2 per year; P<0.001 for difference after incident hypertension).
Figure 2.
Donors have an increase in eGFR while nondonors have a decrease in eGFR over time. eGFR trajectory in black and white kidney donors and weighted nondonors. Trajectories are shown with 95% confidence intervals (gray).
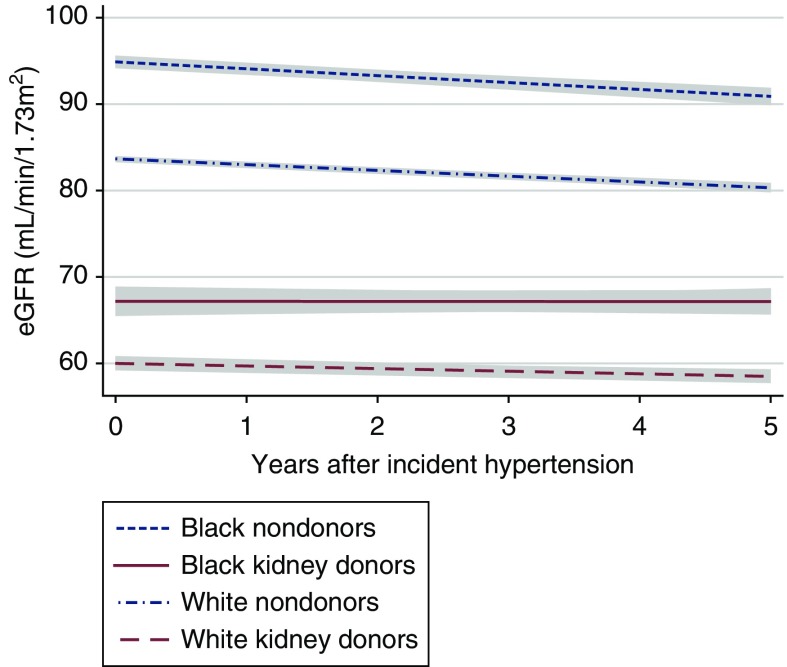
Figure 3.
Donors have a plateau in eGFR associated with hypertension while nondonors have a steepened decline in eGFR associated with hypertenion. eGFR trajectory in black and white kidney donors and nondonors after incident hypertension. Trajectories are shown with 95% confidence intervals (gray).
Table 2.
Yearly change in eGFR in kidney donors and healthy nondonors by race and hypertension
| Study Group | Yearly Change in eGFR, ml/min | P Value for Hypertension |
|---|---|---|
| White nondonor, no hypertension | −0.38 (−0.41 to −0.35) | <0.001 |
| White nondonor with hypertension | −0.76 (−0.90 to −0.62) | |
| Black nondonor, no hypertension | −0.32 (−0.38 to −0.25) | <0.001 |
| Black nondonor with hypertension | −0.91 (−1.20 to −0.62) | |
| White kidney donor, no hypertension | +0.42 (+0.34 to +0.50) | 0.07 |
| White kidney donor with hypertension | 0.00 (−0.45 to +0.45) | |
| Black kidney donor, no hypertension | +0.64 (+0.36 to +0.92) | 0.01 |
| Black kidney donor with hypertension | −0.20 (−0.72 to +0.33) |
Yearly change in eGFR shown with 95% confidence intervals. Negative values indicate a decrease in eGFR over time, whereas positive values indicate an increase.
For kidney donors, we found an increase in eGFR over time regardless of race (Figure 2). For white kidney donors, we found a mean yearly increase in eGFR after donation of +0.42 (95% CI, +0.34 to +0.50) ml/min per 1.73 m2. With incident hypertension, however, eGFR plateaued (mean change per year with hypertension, 0.00; 95% CI, −0.45 to +0.45 ml/min per 1.73 m2; P=0.07 for difference after incident hypertension) (Figure 3, Table 2). Similarly, for black kidney donors, we found a mean yearly increase in eGFR after donation of +0.64 (95% CI, +0.36 to +0.92) ml/min per 1.73 m2. With incident hypertension, however, eGFR plateaued (mean change per year with hypertension, −0.20; 95% CI, −0.72 to +0.33 ml/min per 1.73 m2; P=0.01 for difference after incident hypertension).
Discussion
In this multicenter study of 1295 living kidney donors compared with 8233 healthy nondonor controls drawn from the ARIC study and the CARDIA study, we found that kidney donation was associated with a 19% higher risk of hypertension, regardless of race. Although black kidney donors had the highest incidence of hypertension, the association between kidney donation and hypertension did not vary by race (interaction P=0.60). Importantly, although kidney donors experienced an increase in eGFR over time after donation, after they developed hypertension, their eGFR plateaued. This identifies incident hypertension as a risk factor in eGFR after kidney donation.
Our finding that black kidney donors had higher incidence of hypertension compared with white donors reaffirms and expands on several studies (5,6,20). Our inclusion of a comparison nondonor cohort provides additional insight into the risk associated with donation, and our longitudinal study design provides insight into the timeline of incident hypertension. Specifically within black participants, our finding that black kidney donors had higher risk than healthy black nondonors reaffirms the findings of Doshi et al. (7) in their study of hypertension in black donors and matched nondonors, which reported an absolute risk difference of 22.9% (95% CI, 12.2% to 33.6%) at a mean of 7 years after donation or cohort entry. In fact, our study extends the findings of Doshi et al. (7) to conclude that this extra risk of hypertension in black donors is attributable to donation; in other words, both black and white donors had 19% higher risk of hypertension than their healthy nondonor counterparts.
Our finding that, overall, living kidney donors experience an increase in eGFR over time reaffirms and expands on findings from previous studies (21–23). In a prospective study of 182 predominantly white donors and selected paired controls, GFR measured by iohexol clearance declined 0.36 ml/min per year in controls but increased 1.47 ml/min per year in donors (24,25). Ibrahim et al. (21) measured GFR in a cohort of 255 donors, 99% of whom were white, and found a similar yearly increase in GFR of mean 0.20 ml/min. Matas et al. (22) found that the increase in eGFR continued until at least 20 years postdonation in their study of 2002 predominantly white donors. In 136 black living kidney donors, Doshi et al. (23) actually found a declining trajectory of eGFR that was similar to matched nondonors; however, 41% of their donor cohort had hypertension, and those patients could have overwhelmed the postdonation eGFR trends. No previous studies reported eGFR trajectories stratified by hypertension, and therefore, our study clarifies the association between hypertension and eGFR in donors by examining eGFR trajectory before and after incident hypertension.
The biologic mechanism for the plateau in eGFR after incident hypertension in living kidney donors merits some discussion. Lenihan et al. (26) studied 21 living kidney donors with median 6.3 years follow-up and found that the increase in single-kidney GFR was associated with an increase in kidney plasma flow and glomerular hypertrophy rather than glomerular hypertension, and they considered this hyperfiltration to be adaptive. However, in nondonors with diabetes, polycystic kidney disease, sickle cell anemia, or obesity, glomerular hyperfiltration has been associated with kidney damage, although whether glomerular hypertrophy and hypertension are causes or consequences of kidney damage is less clear (27). In animal studies, uninephrectomy and associated lower GFR lead to higher BP (28–30), reaffirming our findings of higher risk of hypertension in kidney donors.
Our study has several limitations that deserve mention. First, we abstracted serum creatinine values from donor medical records using values from multiple laboratories. Although this can introduce variation to eGFR estimates, our use of multilevel regression with clustering by participant and our large number of both black and white donors help mitigate this variation. Second, although our use of propensity score weighting achieved balance in measured confounders, there is the possibility that unmeasured confounders might bias our weighting method to create a nondonor population that is “healthier” than the donor population. This scenario would bias our findings away from the null. However, this is unlikely, because our estimates of the risk of hypertension associated with donation reaffirm prior studies. Additionally, we rely on self-report for the outcome of hypertension without consideration of treatment required, and we are limited by recall errors. Importantly, our estimates are similar to those obtained when hypertension was ascertained from pharmacy billing records (20). We do not study BP measurements, the degree of BP control, or quality of care for participants with hypertension, which might be expected to impact the effect of hypertension on eGFR. Future investigation of our cohort of donors will include examination of granular BP data. We also do not study the association of antihypertensive drugs with eGFR. Lentine et al. (31) recently found an association between postdonation eGFR and antihypertensive medication use, supporting the concept that eGFR, hypertension, and antihypertensive medications are closely linked. Additional prospective studies may be needed to elucidate the effect of antihypertensive medications on eGFR in living kidney donors. Finally, because of sample size considerations, we did not include participants of races other than black or white; thus, our conclusions must be limited to black and white donors. Despite the size of our donor cohort, we may not have had power to demonstrate significance of some interactions. Strengths of our study include our use of a comparable healthy nondonor cohort and the long-term follow-up period for both kidney donors and nondonors.
In conclusion, we found that kidney donation was associated with a 19% higher risk of hypertension without effect modification by race. Notably, however, over 40% of black kidney donors developed hypertension by 15 years postdonation. We identified incident hypertension as a risk factor in postdonation eGFR, which merits aggressive preventive measures and careful management, because it is associated with cessation of the increase in eGFR after donation. Although intuitive, this finding strengthens our understanding of kidney physiology after living kidney donation. Additional work is needed to identify opportunities and best practices for preventing, recognizing, and managing hypertension in living kidney donors.
Disclosures
Dr. Friedewald reports grants, personal fees, and other fees from Transplant Genomics, Inc.; grants from Shire; grants and personal fees from Abbvie; personal fees from Viela Bio; personal fees from Novartis; personal fees from Sanofi; grants from Vaiteris; and personal fees from American Society of Nephrology, outside the submitted work. Dr. Gupta has received honoraria from CareDx, Mallinckrodt Pharmaceuticals, and One Lambda/Thermo Fisher for sponsored presentations and research support from Gilead Pharmaceuticals, outside the submitted work. Dr. Locke reports honorarium, payment for development of educational presentations, and payment for lectures, including service on a speakers bureau, from Sanofi outside the submitted work. Dr. Weir reports personal fees from Relypsa and personal fees from ZS Pharma during the conduct of the study. Dr. Weir reports personal fees from AbbVie, Akebia, Amgen, AstraZeneca, Boston Scientific, Janssen, MSD, Novartis, and Sandoz, outside the submitted work. The remaining authors have nothing to disclose.
Funding
This work was supported by grants from the National Institute of Diabetes and Digestive and Kidney Diseases (F32DK109662, F32DK113719, K01DK101677, K23DK103918, K24DK101828, and R01DK096008); and the National Institute on Aging (F32AG053025) and an American College of Surgeons Resident Research Scholarship.
Acknowledgments
This manuscript was prepared using Atherosclerosis Risk in Communities (ARIC) and Coronary Artery Risk Development in Young Adults (CARDIA) research materials obtained from the National Heart, Lung, and Blood Institute (NHLBI) Biologic Specimen and Data Repository Information Coordinating Center and does not necessarily reflect the opinions or views of the ARIC, the CARDIA, or the NHLBI.
The analyses described here are the responsibility of the authors alone and do not necessarily reflect the views or policies of the Department of Health and Human Services nor does mention of trade names, commercial products, or organizations imply endorsement by the US Government.
Footnotes
Published online ahead of print. Publication date available at www.cjasn.org.
See related editorial, “Is Hypertension Following Donor Nephrectomy Cause For Elevated Living Donor Kidney Function Concern?” on pages 1427–1429.
References
- 1.Muzaale AD, Massie AB, Wang MC, Montgomery RA, McBride MA, Wainright JL, Segev DL: Risk of end-stage renal disease following live kidney donation. JAMA 311: 579–586, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Anjum S, Muzaale AD, Massie AB, Bae S, Luo X, Grams ME, Lentine KL, Garg AX, Segev DL: Patterns of end-stage renal disease caused by diabetes, hypertension, and glomerulonephritis in live kidney donors. Am J Transplant 16: 3540–3547, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Matas AJ, Berglund DM, Vock DM, Ibrahim HN: Causes and timing of end-stage renal disease after living kidney donation. Am J Transplant 18: 1140–1150, 2018 [DOI] [PubMed] [Google Scholar]
- 4.Grams ME, Sang Y, Levey AS, Matsushita K, Ballew S, Chang AR, Chow EK, Kasiske BL, Kovesdy CP, Nadkarni GN, Shalev V, Segev DL, Coresh J, Lentine KL, Garg AX; Chronic Kidney Disease Prognosis Consortium : Kidney-failure risk projection for the living kidney-donor candidate. N Engl J Med 374: 411–421, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lentine KL, Schnitzler MA, Xiao H, Saab G, Salvalaggio PR, Axelrod D, Davis CL, Abbott KC, Brennan DC: Racial variation in medical outcomes among living kidney donors. N Engl J Med 363: 724–732, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lentine KL, Schnitzler MA, Xiao H, Axelrod D, Garg AX, Tuttle-Newhall JE, Brennan DC, Segev DL: Consistency of racial variation in medical outcomes among publicly and privately insured living kidney donors. Transplantation 97: 316–324, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Doshi MD, Goggins MO, Li L, Garg AX: Medical outcomes in African American live kidney donors: A matched cohort study. Am J Transplant 13: 111–118, 2013 [DOI] [PubMed] [Google Scholar]
- 8.Boudville N, Prasad GV, Knoll G, Muirhead N, Thiessen-Philbrook H, Yang RC, Rosas-Arellano MP, Housawi A, Garg AX; Donor Nephrectomy Outcomes Research (DONOR) Network : Meta-analysis: Risk for hypertension in living kidney donors. Ann Intern Med 145: 185–196, 2006 [DOI] [PubMed] [Google Scholar]
- 9.Levey AS, Stevens LA, Schmid CH, Zhang YL, Castro AF 3rd, Feldman HI, Kusek JW, Eggers P, Van Lente F, Greene T, Coresh J; CKD-EPI (Chronic Kidney Disease Epidemiology Collaboration) : A new equation to estimate glomerular filtration rate. Ann Intern Med 150: 604–612, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.The ARIC investigators: The Atherosclerosis Risk in Communities (ARIC) study: Design and objectives. The ARIC investigators. Am J Epidemiol 129: 687–702, 1989 [PubMed] [Google Scholar]
- 11.Friedman GD, Cutter GR, Donahue RP, Hughes GH, Hulley SB, Jacobs DR Jr., Liu K, Savage PJ: CARDIA: Study design, recruitment, and some characteristics of the examined subjects. J Clin Epidemiol 41: 1105–1116, 1988 [DOI] [PubMed] [Google Scholar]
- 12.Coresh J, Astor BC, McQuillan G, Kusek J, Greene T, Van Lente F, Levey AS: Calibration and random variation of the serum creatinine assay as critical elements of using equations to estimate glomerular filtration rate. Am J Kidney Dis 39: 920–929, 2002 [DOI] [PubMed] [Google Scholar]
- 13.Manjunath G, Tighiouart H, Ibrahim H, MacLeod B, Salem DN, Griffith JL, Coresh J, Levey AS, Sarnak MJ: Level of kidney function as a risk factor for atherosclerotic cardiovascular outcomes in the community. J Am Coll Cardiol 41: 47–55, 2003 [DOI] [PubMed] [Google Scholar]
- 14.Peralta CA, Lin F, Shlipak MG, Siscovick D, Lewis C, Jacobs DR Jr., Bibbins-Domingo K: Race differences in prevalence of chronic kidney disease among young adults using creatinine-based glomerular filtration rate-estimating equations. Nephrol Dial Transplant 25: 3934–3939, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lentine KL, Kasiske BL, Levey AS, Adams PL, Alberú J, Bakr MA, Gallon L, Garvey CA, Guleria S, Li PK, Segev DL, Taler SJ, Tanabe K, Wright L, Zeier MG, Cheung M, Garg AX: Summary of Kidney Disease: Improving Global Outcomes (KDIGO) clinical practice guideline on the evaluation and care of living kidney donors. Transplantation 101: 1783–1792, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hirano K, Imbens GW, Ridder G: Efficient estimation of average treatment effects using the estimated propensity score. Econometrica 71: 1161–1189, 2003 [Google Scholar]
- 17.Harder VS, Stuart EA, Anthony JC: Propensity score techniques and the assessment of measured covariate balance to test causal associations in psychological research. Psychol Methods 15: 234–249, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Posada D, Buckley TR: Model selection and model averaging in phylogenetics: Advantages of Akaike information criterion and bayesian approaches over likelihood ratio tests. Syst Biol 53: 793–808, 2004 [DOI] [PubMed] [Google Scholar]
- 19.Louis TA, Zeger SL: Effective communication of standard errors and confidence intervals. Biostatistics 10: 1–2, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lentine KL, Schnitzler MA, Garg AX, Xiao H, Axelrod D, Tuttle-Newhall JE, Brennan DC, Segev DL: Understanding antihypertensive medication use after living kidney donation through linked national registry and pharmacy claims data. Am J Nephrol 40: 174–183, 2014 [DOI] [PubMed] [Google Scholar]
- 21.Ibrahim HN, Foley R, Tan L, Rogers T, Bailey RF, Guo H, Gross CR, Matas AJ: Long-term consequences of kidney donation. N Engl J Med 360: 459–469, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Matas AJ, Vock DM, Ibrahim HN: GFR ≤25 years postdonation in living kidney donors with (vs. without) a first-degree relative with ESRD. Am J Transplant 18: 625–631, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Doshi MD, Ortigosa-Goggins M, Garg AX, Li L, Poggio ED, Winkler CA, Kopp JB: APOL1 genotype and renal function of black living donors. J Am Soc Nephrol 29: 1309–1316, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kasiske BL, Anderson-Haag T, Ibrahim HN, Pesavento TE, Weir MR, Nogueira JM, Cosio FG, Kraus ES, Rabb HH, Kalil RS, Posselt AA, Kimmel PL, Steffes MW: A prospective controlled study of kidney donors: Baseline and 6-month follow-up. Am J Kidney Dis 62: 577–586, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kasiske BL, Anderson-Haag T, Israni AK, Kalil RS, Kimmel PL, Kraus ES, Kumar R, Posselt AA, Pesavento TE, Rabb H, Steffes MW, Snyder JJ, Weir MR: A prospective controlled study of living kidney donors: Three-year follow-up. Am J Kidney Dis 66: 114–124, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lenihan CR, Busque S, Derby G, Blouch K, Myers BD, Tan JC: Longitudinal study of living kidney donor glomerular dynamics after nephrectomy. J Clin Invest 125: 1311–1318, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Helal I, Fick-Brosnahan GM, Reed-Gitomer B, Schrier RW: Glomerular hyperfiltration: Definitions, mechanisms and clinical implications. Nat Rev Nephrol 8: 293–300, 2012 [DOI] [PubMed] [Google Scholar]
- 28.Moritz KM, Wintour EM, Dodic M: Fetal uninephrectomy leads to postnatal hypertension and compromised renal function. Hypertension 39: 1071–1076, 2002 [DOI] [PubMed] [Google Scholar]
- 29.Carlström M, Sällström J, Skøtt O, Larsson E, Persson AE: Uninephrectomy in young age or chronic salt loading causes salt-sensitive hypertension in adult rats. Hypertension 49: 1342–1350, 2007 [DOI] [PubMed] [Google Scholar]
- 30.Rodríguez-Gómez I, Wangensteen R, Pérez-Abud R, Quesada A, Del Moral RG, Osuna A, O’Valle F, de Dios Luna J, Vargas F: Long-term consequences of uninephrectomy in male and female rats. Hypertension 60: 1458–1463, 2012 [DOI] [PubMed] [Google Scholar]
- 31.Lentine K, Segev D, Lam N, Garg A, Naik A, Axelrod D, Xiao H, Henderson M, Massie A, Kasiske B, Hess G, Hsu C, Brennan D, Schnitzler M: Post-Donation eGFR Levels Predicts New-Onset Antihypertensive Medication Use after Living Kidney Donation, Hoboken, NJ, Wiley, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]