Abstract
Context
GH activates agouti-related protein (AgRP) neurons, leading to orexigenic responses in mice. The relationship between serum GH and plasma AgRP, which has been shown to reflect hypothalamic AgRP, has not been evaluated in humans.
Objective
To test the hypothesis that central stimulatory actions of GH on hypothalamic AgRP could be reflected in plasma AgRP in acromegaly.
Methods
We studied 23 patients with active acromegaly before and for ≤2 years after surgical (n = 13) or GH receptor antagonist therapy with pegvisomant (n = 10), and 100 healthy subjects with morning fasting blood samples for AgRP, leptin, GH, and IGF-1 and anthropometric measurements.
Results
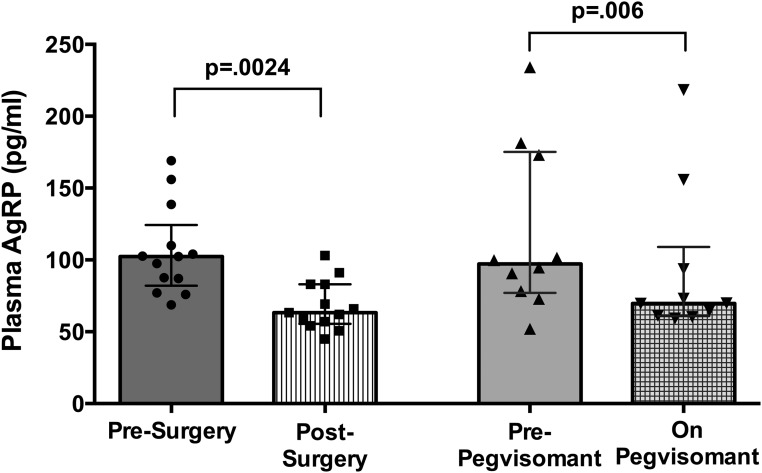
The plasma AgRP levels were higher in those with active acromegaly than in the matched healthy subjects [median, 100 pg/mL; interquartile range (IQR), 78 to 139 pg/mL vs median, 63 pg/mL; IQR, 58 to 67 pg/mL; P < 0.0001]. Plasma AgRP decreased from before to after surgery (median, 102 pg/mL; IQR, 82 to 124 pg/mL vs median, 63 pg/mL; IQR, 55.6 to 83 pg/mL; P = 0.0024) and from before to during pegvisomant therapy (median, 97 pg/mL; IQR, 77 to 175 pg/mL vs median, 63; IQR, 61 to 109 pg/mL; P = 0.006). The plasma AgRP level correlated with GH (r = 0.319; P = 0.011) and IGF-1 (r = 0.292; P = 0.002). In repeated measure analysis, AgRP was significantly associated with IGF-1.
Conclusions
Our data have provided evidence of a stimulatory effect of GH on plasma AgRP in humans. The levels were greater in active acromegaly and decreased in parallel with GH and IGF-1 decreases with acromegaly treatment. Data from mice suggest that AgRP may mediate some of the known effects of GH on energy metabolism. This warrants further study in patients with acromegaly and other populations.
Plasma AgRP levels are elevated in active acromegaly and decrease with treatment with surgery or pegvisomant, providing evidence of a stimulatory effect of GH on plasma AgRP in humans.
GH is an important component of the homeostatic response to change in nutritional state. Pituitary GH secretion increases and circulating GH levels increase in response to acute and chronic nutrient deprivation (1–3). Also, in states of nutrient excess, GH secretion is suppressed and levels decrease (4–7). GH has acute and chronic effects on substrate metabolism in peripheral tissues (8). In the fasted state, GH’s lipolytic and insulin antagonistic effects contribute to the maintenance of sufficient circulating glucose concentrations (9, 10). GH also stimulates IGF-1 production and directly, and via IGF-1, exerts protein anabolic effects to protect the lean body mass (8). Emerging data have also suggested that GH acts centrally in the hypothalamus to affect energy metabolism (7, 11).
The arcuate nucleus of the hypothalamus is a focus for the integration of central pathways and peripheral signals that regulate energy homeostasis and growth and reproduction (12). Located within the arcuate nucleus are the anorexigenic proopiomelanocortin-expressing and the orexigenic agouti-related peptide (AgRP)-expressing neurons (13). The neuropeptide AgRP promotes food intake and inhibits energy expenditure by antagonizing the proopiomelanocortin-derived peptide α-melanocyte-stimulating hormone at melanocortin receptors (14). GH-releasing hormone neurons are also located in the arcuate nucleus (15), and GH receptors are widely expressed in the hypothalamus, including the arcuate nucleus (16) and on AgRP neurons (17). Recently, in mice, GH has been shown to activate AgRP neurons to produce orexigenic responses and antagonism at the GH receptor with pegvisomant can attenuate these responses (11). These data point to an important action of GH to signal nutrient status via AgRP neurons (11).
Acromegaly is characterized by elevated circulating levels of GH and IGF-1. Serum IGF-1 levels reflect the degree of GH excess across a spectrum of GH levels up to a mean concentration of ∼20 µg/L, above which the IGF-1 levels will plateau (18). With acromegaly treatment, the GH and IGF-1 levels will decrease. Thus, acromegaly provides a human model in which to investigate the in vivo relationships among GH, IGF, and AgRP. Recent rodent and human data have supported that plasma AgRP is a marker of hypothalamic AgRP (19–23). In humans, plasma AgRP levels have increased after both acute and chronic caloric restriction (20) and are related to the nutritional state and adiposity (19–21) in the pattern expected for hypothalamic AgRP (14). Therefore, we hypothesized that the central actions of GH on hypothalamic AgRP could be reflected in the plasma AgRP levels in acromegaly. To test this hypothesis, we investigated the plasma levels of AgRP in patients with acromegaly before and after surgical or GH receptor agonist treatment and examined the relationship of plasma AgRP levels with those of GH and IGF-1 levels in acromegaly.
Materials and Methods
Study subjects
Acromegaly subjects
We studied one group of 13 subjects with active, untreated acromegaly before and after surgery (Table 1). Acromegaly was diagnosed by an IGF-1 level greater than the age-adjusted normal range, a nadir GH level after oral glucose of >1 µg/L, the presence of the clinical characteristics of acromegaly, and pathological confirmation of a GH-secreting pituitary tumor removed by transsphenoidal surgery. After surgery, 11 had normalization of IGF-1 levels and 2 had persistent active disease (elevated IGF-1 levels). None received additional therapy during the study period. One patient had panhypopituitarism treated with stable replacement doses of levothyroxine, hydrocortisone, and testosterone for 3 months before baseline testing. In the men, the pre- and postoperative serum testosterone levels were normal in seven and low to normal in one. In three women, menses were regular pre- and postoperatively, and two women were postmenopausal and not receiving hormone replacement therapy. One patient had type 2 diabetes treated during the study period with similar doses of insulin and metformin.
Table 1.
Characteristics of Those With Acromegaly Before and After Surgery or Pegvisomant Therapy
| Characteristic | Acromegaly Surgery Group | Acromegaly Pegvisomant Group | ||||
|---|---|---|---|---|---|---|
| Before Surgery | After Surgerya | P Value | Before Pegvisomant | After Pegvisomanta | P Value | |
| Sex, n | ||||||
| Male | 8 | 8 | 6 | 6 | ||
| Female | 5 | 5 | 4 | 4 | ||
| Age, y | 44 (22–62) | 45 (24-63) | 0.001 | 42.5 (21–62) | 44 (21–64) | 0.004 |
| Previous therapy | S (n = 10), C (n = 3), SA (n = 10), RT (n = 4) | |||||
| Fasting GH, µg/L | 11 (5.9–87.7) | 0.43 (0.05–2) | 0.0002 | 5.6 (2.3–75.8) | 11.3 (6.6–133) | 0.03 |
| IGF-I, µg/L | 603 (482–994) | 200 (110–533) | 0.0002 | 555 (406–785) | 263 (156–422) | 0.002 |
| BMI, kg/m2 | 29.5 (22.4–44) | 31 (22.4–44) | 0.22 | 30.3 (27.5–35.6) | 30.9 (25.4–66.2) | 0.85 |
Data presented as median (range).
Abbreviations: C, cabergoline; RT, radiotherapy; S, transsphenoidal surgery; SA, long-acting somatostatin analog.
Data from the last visit included in the present study.
We also studied a second group of 10 patients with acromegaly before and during pegvisomant therapy (Table 1). Previous acromegaly medical therapies had been discontinued ≥3 months before baseline testing. Two patients had type 2 diabetes; one treated with insulin and one with pioglitazone and metformin; all at doses that were unchanged during the study period. Throughout the study period, one woman had regular menses, one was treated with an oral contraceptive, and two were postmenopausal and not receiving hormone replacement therapy. Pegvisomant therapy was begun at 10 mg daily and escalated monthly during the study period. The final daily dose was 30 mg for one patient, 25 mg for one, 20 mg for four, 10 mg for two, 25 mg every other day for one, and 15 mg every other day for one patient. Patients were tested on a day of medication administration. The IGF-1 levels remained mildly elevated in 4 of 10 pegvisomant-treated patients at the final study visit.
Healthy subjects
We studied 100 healthy subjects (40 men and 60 women) across a spectrum of ages (range, 18 to 78 years) and body mass index (BMI) levels (range, 17 to 41 kg/m2). They were weight stable, nonsmokers, and taking no medications other than vitamins. All 100 subjects were ambulatory with normal renal function and no liver disease. The institutional review board of Columbia University approved the present study. All the subjects gave informed consent before participation.
Study Design
The subjects underwent morning peripheral blood sampling between 9:00 and 11:00 am after an overnight fast. EDTA plasma and serum samples were obtained. The samples were centrifuged and stored in multiple aliquots at −80°C until assayed. GH, IGF-1, leptin, and AgRP were measured in the patients with acromegaly, and IGF-1 and AgRP were measured in the healthy subjects. The body weight and height of all participants was measured using a digital scale to the nearest 0.01 kg and nearest 0.5 cm, respectively.
The patients with acromegaly were studied at visits conducted before and during a 2-year follow-up period after surgery or during pegvisomant therapy. Eight subjects had six visits, five subjects each had had three, four, or five visits because of a persistently elevated IGF-1 level necessitating transition to a different form of therapy in six or one to three missed visits in nine subjects. The healthy subjects were studied once.
Hormone assays
GH and IGF-1 were measured using the IMMULITE® chemiluminescent immunometric assay (Siemens). In our laboratory, functional sensitivity for GH was 0.05 µg/L. IGF-I levels were compared with their age-appropriate normal ranges. AgRP was assayed using two-site ELISA (R&D Systems, Minneapolis, MN) using a recombinant full-length human AgRP standard. The cross-reactivity with the C-terminal peptide, AgRP83-132 is 17% (24). Leptin was measured using the human RIA kit (Millipore, Billerica, MA).
Statistical analysis
The variables for those with acromegaly were compared before and after surgery and before and during pegvisomant treatment and with those from the matched healthy subjects using the Wilcoxon signed rank test. Each subject with acromegaly was matched to three healthy subjects by sex, age ±3 years, and BMI ±3 kg/m2 at their pretreatment visit and separately at the last evaluation of the study after surgery or during pegvisomant therapy. The Spearman correlation analysis was used to assess the relationships between AgRP and IGF-I, GH, and leptin in those with acromegaly. For the repeated measures, the generalized estimating equation (GEE) with working independence correlation structure and linear link was used to assess the relationships between AgRP and IGF-I, GH, age, and BMI in those with acromegaly. All acromegaly data points were used in the correlation and GEE analysis, except for the postpegvisomant GH levels. Multivariable GEE analysis was performed with predictors that were statistically significant at the 0.1 level in the univariable GEE models. The relationships between AgRP, IGF-1, BMI, and age in the healthy subjects were assessed using the Spearman correlation analysis and univariable and multivariable regression with the variables log-transformed before analysis. P values < 0.05 were considered to indicate statistical significance. Data are presented as median and interquartile range or median and range, as indicated.
Results
GH and IGF-1
The characteristics of the patients with acromegaly before and after surgery and before and during pegvisomant therapy are presented in Table 1. IGF-1 levels normalized in 11 of 13 patients after surgery and 6 of 10 patients during pegvisomant treatment. GH levels decreased after surgery but rose with pegvisomant therapy. The BMI did not change with either of the acromegaly treatments.
Plasma AgRP
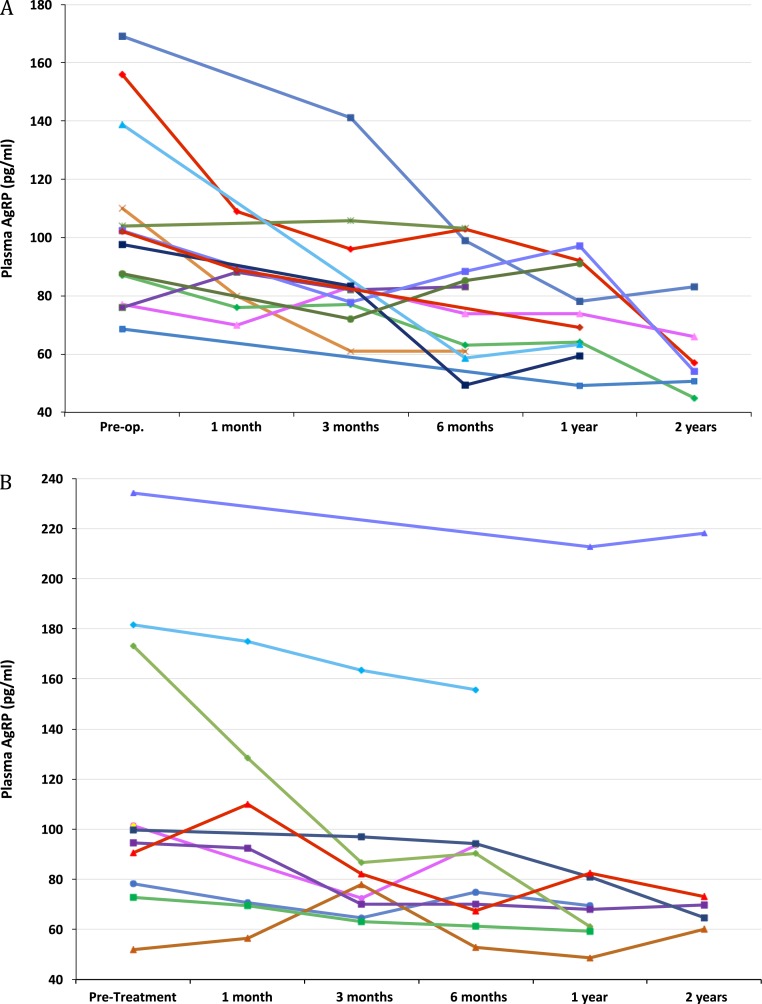
The plasma AgRP levels were higher in the subjects with acromegaly before treatment compared with the levels for matched healthy subjects (Table 2). The plasma AgRP levels decreased after surgery and pegvisomant therapy (Fig. 1). The plasma AgRP levels were substantially lower after acromegaly therapy compared with the pretreatment levels (Fig. 2). These results remained statistically significant after exclusion of the subjects with diabetes. The first to last post-therapy plasma AgRP levels did not differ in either treatment group. The plasma AgRP levels after acromegaly therapy did not differ from those of the matched controls (Table 2).
Table 2.
Plasma AgRP, IGF-1, Sex, Age, and BMI for Patients With Acromegaly (Surgery and Pegvisomant Groups Combined) vs Matched Healthy Subjects
| Variable | Acromegaly Before Treatment | Matched Healthy Subjects | P Value | Acromegaly After Treatment | Matched Healthy Subjects | P Value |
|---|---|---|---|---|---|---|
| Plasma AgRP, pg/mL | 100 (78–139) | 62.9 (58–67) | < 0.0001 | 66 (59–83) | 63 (57–70) | 0.08 |
| IGF-I, µg/L | 600 (499–735) | 142.3 (132–159) | < 0.0001 | 228 (182–312) | 145 (134–158) | < 0.0001 |
| Sex, n | NA | NA | ||||
| Male | 14 | 42 | 14 | 42 | ||
| Female | 9 | 27 | 9 | 27 | ||
| Age, y | 44.1 (22–62) | 44.8 (22–61.7) | 0.68 | 45 (21–64) | 45.3 (24–61.7) | 0.39 |
| BMI, kg/m2 | 29.6 (27.6–33) | 29.1 (27.8–32) | 0.22 | 29 (26.6–33) | 29 (27.8–32) | 0.32 |
Data presented as median (interquartile range), unless otherwise specified.
Abbreviation: NA, not applicable.
Figure 1.
Plasma AgRP levels in each study subject before and at each follow-up visit after (A) surgery and (B) pegvisomant treatment.
Figure 2.
Plasma AgRP levels in subjects with acromegaly (Left) before vs after surgery and (Right) before vs after pegvisomant treatment. Data shown as median, interquartile range, and individual subjects’ values before and at final visit after treatment for each subject.
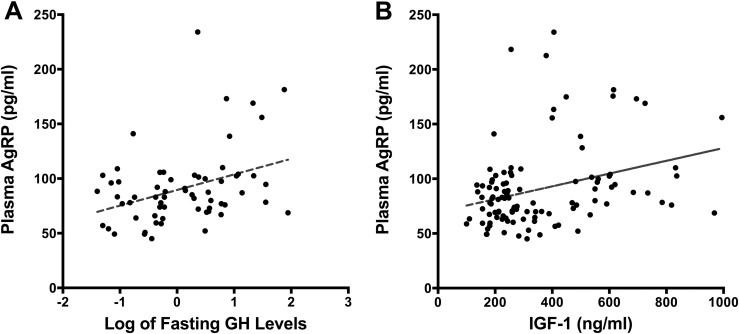
In the subjects with acromegaly, the fasting plasma AgRP levels correlated positively with the fasting serum GH (r = 0.319; P = 0.011) and IGF-1 (r = 0.292; P = 0.002) levels (Fig. 3) but not with the plasma leptin levels (P = 0.75). The significance of these results did not change after exclusion of the subjects with diabetes. On univariable GEE analysis, the change in IGF-1 level was significantly associated with the change in AgRP (β = 0.06; SE = 0.02; P < 0.001) with acromegaly treatment. However, the changes in GH (β = 0.600; SE = 0.415; P = 0.148), age (β = −0.798; SE = 0.622; P = 0.199), and BMI (β = −0.704; SE = 0.880; P = 0.424) were not. The IGF-1 level remained a significant predictor of AgRP after adjustment for observation period for those with acromegaly (β = 0.049; SE = 0.021; P = 0.017).
Figure 3.
Scatter plots of (A) fasting GH (log transformed) and plasma AgRP levels and (B) IGF-1 and plasma AgRP levels in patients with acromegaly.
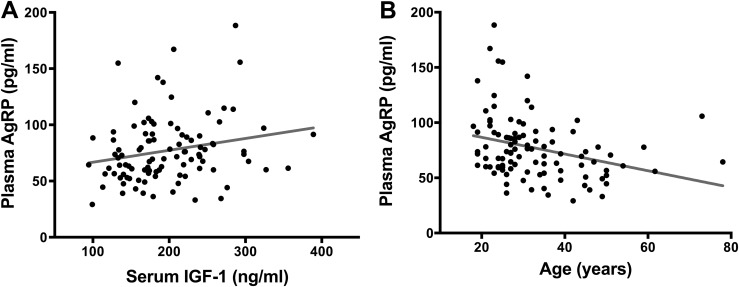
In healthy subjects, plasma AgRP correlated positively with IGF-1 (r = 0.237; P = 0.017) and inversely with age (r = −0.35; P < 0.001; Fig. 4) and BMI (r = −0.253; P = 0.01). On univariable analysis, the statistically significant predictors of the AgRP level were IGF-1 (β = 0.1936; SE = 0.081; P = 0.019), age (β = −0.3121; SE = 0.084; P = 0.0004), and BMI (β = −0.126; SE = 0.051; P = 0.016). On multivariable analysis, age (β = −0.589; SE = 0.285; P = 0.042) remained a statistically significant predictor of the AgRP level, but BMI (β = −0.845; SE = 0.601; P = 0.16) and IGF-1 (β = 0.048; SE = 0.055; P = 0.39) did not.
Figure 4.
Bivariate regression plots of (A) serum IGF-1 levels and plasma AgRP levels and (B) age and AgRP levels in healthy subjects.
Discussion
GH has well-known direct effects on metabolism in peripheral tissues (8), and recent studies have also found that GH acts in the brain to control energy metabolism (11, 25). In mice, GH activates hypothalamic AgRP neurons, leading to increased food intake and decreased energy expenditure, effects characteristic of the orexigenic peptide AgRP (11). We have extended these findings in mice to humans and have provided data relating the circulating levels of AgRP to those of GH and IGF-1 in patients with acromegaly, supporting a stimulatory effect of GH on AgRP in humans. To the best of our knowledge, our data are the first evidence in humans that circulating GH might act centrally at the hypothalamus to stimulate AgRP. These data suggest, based on studies of mice and what is known about the effects of AgRP, that this interaction might be a key mechanism by which GH modulates energy metabolism in humans.
Our data provide evidence that GH excess increases plasma AgRP levels in patients with acromegaly. We found higher plasma AgRP levels in patients with active acromegaly than in the sex-, age-, and BMI-matched healthy subjects. We also found that the plasma AgRP levels were lowered with surgical treatment of acromegaly, which reduced the GH and IGF-1 levels. The plasma AgRP levels in patients with treated acromegaly were similar to those of matched healthy subjects, suggesting that normalization of circulating GH and IGF-1 levels returned the AgRP levels to the expected levels. We did find a trend toward higher AgRP levels in those with treated acromegaly compared with healthy subjects, which might be explained by a degree of persistent GH excess as evidenced by mildly elevated IGF-1 levels in six patients with acromegaly at the final study visit and higher IGF-1 levels in the those with treated acromegaly compared with healthy subjects. Because the within-subject post-treatment AgRP levels did not differ significantly, that samples were not available from all measurement points for some subjects (Fig. 1) is unlikely to have affected the results. Data from mice support our human data. In mice, systemic administration of GH activated AgRP neurons (11), and transgenic overexpression of GH in the central nervous system increased hypothalamic expression of AgRP and neuropeptide Y and increased food intake (26).
We also found that AgRP levels were lowered by treatment of acromegaly with the GH receptor antagonist pegvisomant. Pegvisomant lowered the IGF-1 levels, reflecting peripheral GH receptor blockade and reduced GH-stimulated IGF-1 production in the liver and other tissues. The GH levels increased with pegvisomant treatment, as expected (27). Our observation that the plasma AgRP levels were lowered despite increasing GH levels suggests that central blockade of GH receptors on AgRP neurons by pegvisomant occurs, leading to reduced AgRP centrally and peripherally. In mice, pegvisomant administration produced effects on energy metabolism consistent with a blockade of the stimulatory effects of GH on hypothalamic AgRP neurons (11). In our study, the AgRP levels were significantly reduced by pegvisomant; however, the changes seemed less marked than those that occurred after surgical therapy. However, our study was not designed to directly compare these two therapies, and obvious mechanistic differences between surgery and pegvisomant therapies does not permit a direct comparison of GH lowering between them. Additional studies, potentially matching subjects for IGF-1 decreases, which might estimate the effectiveness of GH receptor blockade, are needed to examine the differential effects of acromegaly treatment types on plasma AgRP levels.
We also found that the plasma AgRP levels correlated positively with the GH and IGF-1 levels in acromegaly and the reduction in IGF-1 with acromegaly therapy was associated with the reduction in AgRP levels, further supporting a relationship between GH and AgRP in humans. In healthy subjects, the IGF-1 levels correlated positively with those of AgRP. However, after adjustment for age and BMI, this relationship was no longer statistically significant. In patients with acromegaly, however, the IGF-1 levels were a more important predictor of AgRP levels than age or BMI. In patients with acromegaly, the contribution of GH to IGF-1 production and, thus, to circulating IGF-1 levels is greater than that in healthy subjects, in whom GH, age, nutritional status, and other factors contribute greater shares to determining the circulating IGF-1 levels (28). Thus, IGF-1 levels might not reflect the GH status in settings of normal or low GH levels (29), and IGF-1 levels might not be an ideal surrogate for testing the contribution of circulating GH to that of AgRP in healthy humans. Because age is an important determinant of IGF-1 levels, reflecting the age-related decline in GH secretion, some component of the inverse relationship between age and plasma AgRP that we found in healthy subjects could have resulted from declining GH with advancing age that was not detected in the serum IGF-1 levels. However, random or single fasting GH levels are unlikely to be informative of the total daily GH secretion in healthy subjects. Thus, larger studies examining the relationship of AgRP levels to detailed GH secretory patterns are needed to further understand the relationships among age, GH, and AgRP in healthy humans.
Our data have also confirmed the data from others demonstrating an inverse relationship between BMI and AgRP in healthy subjects (21). In the patients with acromegaly, we did not observe this correlation. This might be explained by the known altered body composition of patients with acromegaly, which renders BMI less predictive of adiposity in patients with acromegaly (30). We also found, as has been reported previously in healthy humans (21), that fasting, morning plasma AgRP levels were similar over time, for ≤2 years, in those with treated acromegaly (Fig. 1). A diurnal rhythm for AgRP levels has also been noted previously in healthy humans (31). Additional detailed measurements of AgRP levels are needed to determine whether a diurnal rhythm is preserved in patients with acromegaly. Leptin inhibits AgRP neurons (32), and plasma leptin levels correlate inversely with those of AgRP in humans, in line with the negative correlation of AgRP with the percentage of body fat in lean and obese subjects (20). In patients with acromegaly, the plasma leptin and AgRP levels do not correlate. We, and others, have shown that leptin levels are lower in patients with acromegaly and increase as acromegaly is treated (33), likely in parallel with the increase in fat mass with acromegaly treatment. However, changes in leptin levels have not correlated well with the changes in adiposity with acromegaly treatment (34), suggesting a dysregulation of leptin in acromegaly, which might explain our current findings. Our study included three subjects with diabetes; however, our results were similar after excluding their data. The subjects with diabetes had patterns of change in AgRP levels similar to those of the subjects without diabetes. However, additional study is needed to further characterize the plasma AgRP levels in patients with diabetes and acromegaly.
Recent data from mice have suggested that GH stimulation of AgRP is an important mechanism by which GH restores energy homeostasis in settings of nutrient deprivation (11). GH receptor ablation specifically in AgRP neurons had no effect on energy balance or metabolism in the baseline fed state (11). However, when the mice were food restricted, the metabolic and neuroendocrine adaptations to weight loss were impaired (11). The expected decrease in energy expenditure during food restriction was blunted, as were the effects on the thyroid, gonadal, and adrenal axes (11). Because plasma AgRP levels increased with caloric restriction in humans (20) and hypothalamic AgRP promotes food intake and weight gain and reduces energy expenditure (35), AgRP might have a role in the metabolic effects of the increase in GH that occurs with nutrient deprivation and in physiologic settings in which increased nutritional intake is important. Other previous data have also pointed to an interaction of AgRP neurons with GH releasing hormone neurons in the hypothalamus that might couple nutritional status with linear growth (36). These data and the age-related decline in AgRP levels we found in healthy subjects suggest a possible physiologic role for GH, via AgRP, to promote food intake and, thus, growth in youth. Further studies are warranted to elucidate the relationship of changes in GH and AgRP to those of metabolism across the age spectrum.
Acromegaly presents a unique constellation of body composition and metabolic abnormalities with lower adipose tissue, especially visceral adipose tissue, despite the presence of substantial hyperinsulinemia, insulin resistance, and glucose intolerance (33, 37). The mechanisms for this acromegaly-specific lipodystrophy have not yet been fully elucidated (34). Data from mice have shown AgRP to affect peripheral glucose and lipid metabolism, including the latter in the liver (38–42). Also, plasma AgRP levels in healthy humans were found to reflect differences in adiposity, leptin, insulin levels, and homeostatic model assessment score (19–21). Although AgRP prevents fat loss during starvation, in the fed state, overexpression of AgRP or a central infusion of AgRP will lead to increased adiposity, fatty liver, and impaired glucose tolerance (43, 44). However, in acromegaly, despite elevated AgRP levels, there is a marked decrease in adiposity reflecting the dominant effects of GH on fat metabolism. Despite the decrease in adiposity characteristic of acromegaly, glucose metabolism is impaired (33, 34). Thus, it is intriguing to speculate on the possible role of GH excess acting centrally on hypothalamic AgRP in the metabolic disorders, in particular the altered glucose and lipid metabolism, characteristic of acromegaly. This warrants further study.
In conclusion, we found higher levels of plasma AgRP levels in patients with active acromegaly. These levels decreased with acromegaly therapy, paralleling the decrease in GH and IGF-1 levels that occurs with acromegaly treatment. These data provide evidence of a stimulatory effect of GH excess on AgRP in humans. Further studies are needed to examine the relationship between AgRP and GH and the influence of AgRP on the metabolic and body composition abnormalities of acromegaly.
Acknowledgments
Financial Support: This study was funded by the National Institutes of Health (NIH) Grants R01 DK 110771 and DK064720 to P.U.F. and, in part, by Columbia University Clinical and Translational Science Awards and NIH Grant UL1 TR000040.
Clinical Trial Information: ClinicalTrials.gov no. NCT01809808 (registered 13 March 2013).
Disclosure Summary: P.U.F. has received grant support and honoraria from Pfizer, Inc. The remaining authors have nothing to disclose.
Data Availability: All data generated or analyzed during this study are included in this published article or in the data repositories listed in References.
Glossary
Abbreviations:
- AgRP
agouti-related protein
- BMI
body mass index
- GEE
generalized estimating equation
- IQR
interquartile range
References and Notes
- 1. Roth J, Glick SM, Yalow RS, Bersonsa. Hypoglycemia: a potent stimulus to secretion of growth hormone. Science. 1963;140(3570):987–988. [DOI] [PubMed] [Google Scholar]
- 2. Nørrelund H. The metabolic role of growth hormone in humans with particular reference to fasting. Growth Horm IGF Res. 2005;15(2):95–122. [DOI] [PubMed] [Google Scholar]
- 3. Hunter WM, Willoughby JM, Strong JA. Plasma insulin and growth hormone during 22-hour fasts and after graded glucose loads in six healthy adults. J Endocrinol. 1968;40(3):297–311. [DOI] [PubMed] [Google Scholar]
- 4. Cornford AS, Barkan AL, Horowitz JF. Rapid suppression of growth hormone concentration by overeating: potential mediation by hyperinsulinemia. J Clin Endocrinol Metab. 2011;96(3):824–830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Veldhuis JD, Iranmanesh A, Ho KK, Waters MJ, Johnson ML, Lizarralde G. Dual defects in pulsatile growth hormone secretion and clearance subserve the hyposomatotropism of obesity in man. J Clin Endocrinol Metab. 1991;72(1):51–59. [DOI] [PubMed] [Google Scholar]
- 6. Rasmussen MH, Hvidberg A, Juul A, Main KM, Gotfredsen A, Skakkebaek NE, Hilsted J, Skakkebae NE. Massive weight loss restores 24-hour growth hormone release profiles and serum insulin-like growth factor-I levels in obese subjects. J Clin Endocrinol Metab. 1995;80(4):1407–1415. [DOI] [PubMed] [Google Scholar]
- 7. Steyn FJ, Tolle V, Chen C, Epelbaum J. Neuroendocrine regulation of growth hormone secretion. Compr Physiol. 2016;6(2):687–735. [DOI] [PubMed] [Google Scholar]
- 8. Jørgensen JO, Møller L, Krag M, Billestrup N, Christiansen JS. Effects of growth hormone on glucose and fat metabolism in human subjects. Endocrinol Metab Clin North Am. 2007;36(1):75–87. [DOI] [PubMed] [Google Scholar]
- 9. Luque RM, Gahete MD, Cordoba-Chacon J, Childs GV, Kineman RD. Does the pituitary somatotrope play a primary role in regulating GH output in metabolic extremes? Ann N Y Acad Sci. 2011;1220(1):82–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Vijayakumar A, Novosyadlyy R, Wu Y, Yakar S, LeRoith D. Biological effects of growth hormone on carbohydrate and lipid metabolism. Growth Horm IGF Res. 2010;20(1):1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Furigo IC, Teixeira PDS, de Souza GO, Couto GCL, Romero GG, Perelló M, Frazão R, Elias LL, Metzger M, List EO, Kopchick JJ, Donato J Jr. Growth hormone regulates neuroendocrine responses to weight loss via AgRP neurons. Nat Commun. 2019;10(1):662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Hill JW, Elmquist JK, Elias CF. Hypothalamic pathways linking energy balance and reproduction. Am J Physiol Endocrinol Metab. 2008;294(5):E827–E832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Baldini G, Phelan KD. The melanocortin pathway and control of appetite-progress and therapeutic implications. J Endocrinol. 2019;241(1):R1–R33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Zagmutt S, Mera P, Soler-Vázquez MC, Herrero L, Serra D. Targeting AgRP neurons to maintain energy balance: Lessons from animal models. Biochem Pharmacol. 2018;155:224–232. [DOI] [PubMed] [Google Scholar]
- 15. Bluet-Pajot MT, Tolle V, Zizzari P, Robert C, Hammond C, Mitchell V, Beauvillain JC, Viollet C, Epelbaum J, Kordon C. Growth hormone secretagogues and hypothalamic networks. Endocrine. 2001;14(1):1–8. [DOI] [PubMed] [Google Scholar]
- 16. Furigo IC, Metzger M, Teixeira PD, Soares CR, Donato J Jr. Distribution of growth hormone-responsive cells in the mouse brain. Brain Struct Funct. 2017;222(1):341–363. [DOI] [PubMed] [Google Scholar]
- 17. Kamegai J, Minami S, Sugihara H, Hasegawa O, Higuchi H, Wakabayashi I. Growth hormone receptor gene is expressed in neuropeptide Y neurons in hypothalamic arcuate nucleus of rats. Endocrinology. 1996;137(5):2109–2112. [DOI] [PubMed] [Google Scholar]
- 18. Barkan AL, Beitins IZ, Kelch RP. Plasma insulin-like growth factor-I/somatomedin-C in acromegaly: correlation with the degree of growth hormone hypersecretion. J Clin Endocrinol Metab. 1988;67(1):69–73. [DOI] [PubMed] [Google Scholar]
- 19. Page-Wilson G, Meece K, White A, Rosenbaum M, Leibel RL, Smiley R, Wardlaw SL. Proopiomelanocortin, agouti-related protein, and leptin in human cerebrospinal fluid: correlations with body weight and adiposity. Am J Physiol Endocrinol Metab. 2015;309(5):E458–E465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Page-Wilson G, Nguyen KT, Atalayer D, Meece K, Bainbridge HA, Korner J, Gordon RJ, Panigrahi SK, White A, Smiley R, Wardlaw SL. Evaluation of CSF and plasma biomarkers of brain melanocortin activity in response to caloric restriction in humans. Am J Physiol Endocrinol Metab. 2017;312(1):E19–E26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Page-Wilson G, Peters JB, Panigrahi SK, Jacobs TP, Korner J, Otten M, Bruce JN, Wardlaw SL. Plasma agouti-related protein and cortisol levels in Cushing disease: evidence for the regulation of agouti-related protein by glucocorticoids in humans. J Clin Endocrinol Metab. 2019;104(3):961–969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Li JY, Finniss S, Yang YK, Zeng Q, Qu SY, Barsh G, Dickinson C, Gantz I. Agouti-related protein-like immunoreactivity: characterization of release from hypothalamic tissue and presence in serum. Endocrinology. 2000;141(6):1942–1950. [DOI] [PubMed] [Google Scholar]
- 23. Shen CP, Wu KK, Shearman LP, Camacho R, Tota MR, Fong TM, Van der Ploeg LH. Plasma agouti-related protein level: a possible correlation with fasted and fed states in humans and rats. J Neuroendocrinol. 2002;14(8):607–610. [DOI] [PubMed] [Google Scholar]
- 24. Page-Wilson G, Reitman-Ivashkov E, Meece K, White A, Rosenbaum M, Smiley RM, Wardlaw SL. Cerebrospinal fluid levels of leptin, proopiomelanocortin, and agouti-related protein in human pregnancy: evidence for leptin resistance. J Clin Endocrinol Metab. 2013;98(1):264–271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Arámburo C, Alba-Betancourt C, Luna M, Harvey S. Expression and function of growth hormone in the nervous system: a brief review. Gen Comp Endocrinol. 2014;203:35–42. [DOI] [PubMed] [Google Scholar]
- 26. Bohlooly-Y M, Olsson B, Bruder CE, Lindén D, Sjögren K, Bjursell M, Egecioglu E, Svensson L, Brodin P, Waterton JC, Isaksson OG, Sundler F, Ahrén B, Ohlsson C, Oscarsson J, Törnell J. Growth hormone overexpression in the central nervous system results in hyperphagia-induced obesity associated with insulin resistance and dyslipidemia. Diabetes. 2005;54(1):51–62. [DOI] [PubMed] [Google Scholar]
- 27. van der Lely AJ, Hutson RK, Trainer PJ, Besser GM, Barkan AL, Katznelson L, Klibanski A, Herman-Bonert V, Melmed S, Vance ML, Freda PU, Stewart PM, Friend KE, Clemmons DR, Johannsson G, Stavrou S, Cook DM, Phillips LS, Strasburger CJ, Hackett S, Zib KA, Davis RJ, Scarlett JA, Thorner MO. Long-term treatment of acromegaly with pegvisomant, a growth hormone receptor antagonist. Lancet. 2001;358(9295):1754–1759. [DOI] [PubMed] [Google Scholar]
- 28. Clemmons DR. Value of insulin-like growth factor system markers in the assessment of growth hormone status. Endocrinol Metab Clin North Am. 2007;36(1):109–129. [DOI] [PubMed] [Google Scholar]
- 29. Mukherjee A, Shalet SM. The value of IGF1 estimation in adults with GH deficiency. Eur J Endocrinol. 2009;161(Suppl 1):S33–S39. [DOI] [PubMed] [Google Scholar]
- 30. Reid TJ, Jin Z, Shen W, Reyes-Vidal CM, Fernandez JC, Bruce JN, Kostadinov J, Post KD, Freda PU. IGF-1 levels across the spectrum of normal to elevated in acromegaly: relationship to insulin sensitivity, markers of cardiovascular risk and body composition. Pituitary. 2015;18(6):808–819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Panigrahi SK, Hicks TJ, Lucey BP, Wardlaw SL. Characterization of the diurnal rhythm for cortisol and cortisone in human cerebrospinal fluid as related to the diurnal rhythm in blood and to changes in agouti-related protein (AgRP) levels. In: Proceedings of the Endocrine Society Annual Meeting; 17–20 June 2018; Chicago, IL. Abstract SUN-043. [Google Scholar]
- 32. Pinto S, Roseberry AG, Liu H, Diano S, Shanabrough M, Cai X, Friedman JM, Horvath TL. Rapid rewiring of arcuate nucleus feeding circuits by leptin. Science. 2004;304(5667):110–115. [DOI] [PubMed] [Google Scholar]
- 33. Reyes-Vidal C, Fernandez JC, Bruce JN, Crisman C, Conwell IM, Kostadinov J, Geer EB, Post KD, Freda PU. Prospective study of surgical treatment of acromegaly: effects on ghrelin, weight, adiposity, and markers of CV risk. J Clin Endocrinol Metab. 2014;99(11):4124–4132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Reyes-Vidal CM, Mojahed H, Shen W, Jin Z, Arias-Mendoza F, Fernandez JC, Gallagher D, Bruce JN, Post KD, Freda PU. Adipose tissue redistribution and ectopic lipid deposition in active acromegaly and effects of surgical treatment. J Clin Endocrinol Metab. 2015;100(8):2946–2955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Krashes MJ, Koda S, Ye C, Rogan SC, Adams AC, Cusher DS, Maratos-Flier E, Roth BL, Lowell BB. Rapid, reversible activation of AgRP neurons drives feeding behavior in mice. J Clin Invest. 2011;121(4):1424–1428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Lee B, Kim J, An T, Kim S, Patel EM, Raber J, Lee SK, Lee S, Lee JW. Dlx1/2 and Otp coordinate the production of hypothalamic GHRH- and AgRP-neurons. Nat Commun. 2018;9(1):2026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Freda PU, Shen W, Heymsfield SB, Reyes-Vidal CM, Geer EB, Bruce JN, Gallagher D. Lower visceral and subcutaneous but higher intermuscular adipose tissue depots in patients with growth hormone and insulin-like growth factor I excess due to acromegaly. J Clin Endocrinol Metab. 2008;93(6):2334–2343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Könner AC, Janoschek R, Plum L, Jordan SD, Rother E, Ma X, Xu C, Enriori P, Hampel B, Barsh GS, Kahn CR, Cowley MA, Ashcroft FM, Brüning JC. Insulin action in AgRP-expressing neurons is required for suppression of hepatic glucose production. Cell Metab. 2007;5(6):438–449. [DOI] [PubMed] [Google Scholar]
- 39. Ren H, Cook JR, Kon N, Accili D. Gpr17 in AgRP neurons regulates feeding and sensitivity to insulin and leptin. Diabetes. 2015;64(11):3670–3679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Maier MT, Vilhelmsson A, Louie SM, Vagena E, Nomura DK, Koliwad SK, Xu AW. Regulation of hepatic lipid accumulation and distribution by agouti-related protein in male mice. Endocrinology. 2018;159(6):2408–2420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Bell BB, Harlan SM, Morgan DA, Guo DF, Cui H, Rahmouni K. Differential contribution of POMC and AgRP neurons to the regulation of regional autonomic nerve activity by leptin. Mol Metab. 2018;8:1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Nogueiras R, Wiedmer P, Perez-Tilve D, Veyrat-Durebex C, Keogh JM, Sutton GM, Pfluger PT, Castaneda TR, Neschen S, Hofmann SM, Howles PN, Morgan DA, Benoit SC, Szanto I, Schrott B, Schürmann A, Joost HG, Hammond C, Hui DY, Woods SC, Rahmouni K, Butler AA, Farooqi IS, O’Rahilly S, Rohner-Jeanrenaud F, Tschöp MH. The central melanocortin system directly controls peripheral lipid metabolism. J Clin Invest. 2007;117(11):3475–3488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Korner J, Wissig S, Kim A, Conwell IM, Wardlaw SL. Effects of agouti-related protein on metabolism and hypothalamic neuropeptide gene expression. J Neuroendocrinol. 2003;15(12):1116–1121. [DOI] [PubMed] [Google Scholar]
- 44. Graham M, Shutter JR, Sarmiento U, Sarosi I, Stark KL. Overexpression of Agrt leads to obesity in transgenic mice. Nat Genet. 1997;17(3):273–274. [DOI] [PubMed] [Google Scholar]