Abstract
Lung cancer is considered to be one of the world's deadliest diseases, with non-small cell lung cancer (NSCLC) accounting for 85% of all lung cancer cases. The present study aimed to investigate the role and underlying mechanisms of interleukin-21 (IL-21), and its receptor IL-21R, in NSCLC. Lung tissues and blood samples of NSCLC were used to measure IL-21, IL-21R and programmed death 1 ligand 1 (PD-L1) expression using ELISA, western blot and immunohistochemistry analyses. Following treatment with different doses of IL-21, the proliferation, invasion and migration of human NSCLC cell line A549 was evaluated using a cell counting kit-8, colony formation, Transwell and scratch wound healing assays, respectively. Additionally, IL-21R and PD-L1 expression in A549 cells was detected using western blot analysis and immunofluorescence. IL-21R silencing was subsequently used to investigate its effects in cell proliferation, invasion and migration. PD-L1, IL-1β and tumor necrosis factor α (TNF-α) expression were measured. Finally, Wnt/β-catenin signaling expression was evaluated using western blot analysis following treatment with IL-21. Cells were then treated with lithium chloride (LiCl), which is an agonist of Wnt/β-catenin signaling, and the levels of PD-L1, IL-1β and TNF-α were detected. The results revealed that IL-21 and IL-21R expression in the lung tissues and blood samples of patients with NSCLC were decreased, while PD-L1 expression was increased, compared with normal tissues or healthy controls. Treatment of A549 cells with IL-21 upregulated IL-21R expression, downregulated PD-L1 and inhibited cell growth and metastasis in a dose-dependent manner. Following IL-21R silencing, the effects of IL-21 treatment were reversed, suggesting that IL-21 acted on A549 cells through binding to IL-21R. In addition, the results demonstrated that IL-21 treatment reduced the expression levels of proteins associated with the Wnt/β-catenin signaling, whereas activation of Wnt/β-catenin signaling with the LiCl agonist upregulated PD-L1, IL-1β and TNF-α expression. In conclusion, the IL-21/IL-21R axis reduced the growth and invasion of NSCLC cells via inhibiting Wnt/β-catenin signaling and PD-L1 expression. The present results may provide a novel molecular target for NSCLC diagnosis and therapy.
Key words: interleukin-21, non-small cell lung cancer, invasion, programmed death 1 ligand 1
Introduction
Lung cancer is considered to be one of the leading causes of cancer-associated death worldwide (1,2). Non-small-cell lung cancer (NSCLC) accounts for ~85% of all lung cancer cases and exhibits a dismal prognosis, with a 5 year survival rate of 17.1% (3). Despite the comprehensive treatment strategies, including surgery, radiotherapy, immunotherapy and targeted therapies, the clinical outcomes of patients with NSCLC are slightly improved. However, due to recurrence and metastasis, NSCLC is still considered to be a global challenge (4,5). Similar to other cancer cell types, NSCLC cells are character-ized by their sustained proliferation (6). Therefore, there is an urgent requirement for the identification of the molecular mechanisms underlying the development and progression of NSCLC, and for the investigation into potential therapeutic targets and agents, which may improve clinical survival rates.
Interleukin (IL)-21, which is a member of the IL-2 family, is associated with the immune responses of B cells, T cells and natural killer (NK) cells (7). An accumulation of evidence has suggested that IL-21 exerts an antitumor effect in a variety of cancers, including gastric, colon and epithelial ovarian cancer (8-10). A previous study has demonstrated the association between IL-21 polymorphisms and NSCLC risk in a Chinese Han population, indicating the potential role of IL-21 in lung cancer detection and treatment (11). Notably, a recent study has suggested that IL-21 levels in the serum of patients with NSCLC were significantly decreased (12). It has also been documented that IL-21 conducts signal transduction through binding to its receptor IL-21R, and can subsequently promote antitumor function (13). However, the role of IL-21/IL-21R in NSCLC, and the underlying mechanisms, remain poorly elucidated.
Previous studies have reported that the inactivation of Wnt/β-catenin signaling protects against NSCLC (14,15). Additionally, active Wnt/β-catenin signaling can lead to T-cell exclusion and resistance to anti-programmed death 1 ligand 1 (PD-L1)/anti-cytotoxic T-lymphocyte antigen 4 (CTLA-4) monoclonal antibody therapy in melanoma (16). Emerging evidence has demonstrated that IL-21 suppresses tumor growth and metastasis through the inhibition of Wnt/β-catenin signaling in epithelial ovarian cancer (10). The present study aimed to investigate the role of IL-21/IL-21R in NSCLC. IL-21 and IL-21R expression was measured in NSCLC peripheral blood, tissues and a human NSCLC cell line. It was hypothesized that IL-21/IL-21R signaling may inhibit cell proliferation, invasion and migration in NSCLC via inhibiting Wnt/β-catenin signaling and PD-L1 expression. These results may provide a novel molecular target for use in NSCLC therapy.
Materials and methods
Patient samples
A total of 30 pairs of NSCLC tissue samples and matched adjacent normal tissues were collected from patients (15 males and 15 females; age range, 20-45) who underwent surgery at Fujian Medical University Union Hospital from 2017 to 2018. All fresh specimens were placed immediately into liquid nitrogen following surgery. Additionally, peripheral blood samples from 30 NSCLC patients (15 males and 15 females; age range, 20-45) and 30 healthy controls were obtained (15 males and 15 females; age range, 20-45), at the same time interval as the NSCLC tissues and at the same hospital. All cases were diagnosed using postoperative pathological sections, and other major organic diseases were excluded. The current study was approved by the Ethics Committee of Fujian Medical University Union Hospital and all patients provided written informed consent. All of the procedures were in compliance with the Declaration of Helsinki and relevant policies in China.
Cell culture
The human NSCLC cell line A549 was obtained from the Type Culture Collection of the Chinese Academy of Sciences. The cells were maintained in Dulbecco's modified Eagle's medium (DMEM; Gibco; Thermo Fisher Scientific, Inc.) with 10% FBS (Biological Industries, Ltd.) at 37°C in a 5% CO2 incubator.
Cell treatments
Cells were seeded into 6-well plates at a density of 1×106 cells/well. When 40-60% confluence was achieved, different doses of IL-21 (0, 10, 20, 50 and 100 ng/ml) were used to treat A549 cells for 12 h. For IL-21R silencing, cells were transfected with 50 nM IL-21R-targeting small interfering RNA (siRNA)1 (sense, 5′-CCUGCCACAUGGAUGUAUUTT-3′ and antisense, 5′-AAUACAUCCAUGUGGCAGGTT-3′), siRNA2 (sense, 5′-CCGCAAAGACUCGAGCUAUTT-3′ and antisense, 5′-AUAGCUCGAGUCUUUGCGGTT-3′) and control siRNA (sense, 5′-UUCUCCGAACGUGUCAGGUTT-3′ and antisense, 5′-ACGUGACACGUUCGGAGAATT-3′), in Opti-MEM with Lipofectamine RNAiMAX (Invitrogen; Thermo Fisher Scientific, Inc.). Following incubation for 24 h, successful transfections were determined using reverse transcription-quantitative PCR (RT-qPCR) and western blot analysis. Additionally, in order to investigate the role of Wnt/β-catenin signaling in NSCLC, the Wnt inhibitor LiCl was applied to cells for 12 h, followed by treatment with 50 ng/ml IL-21 for 24 h.
Cell Counting Kit-8 (CCK-8) assay
A549 cells were seeded into 96-well plates and incubated at 37°C in a 5% CO2 humidified incubator. Cell viability was determined using the CCK-8 reagent (Dojindo Molecular Technologies, Inc.), according to the manufacturer's protocol. After transfection for 24, 48 and 72 h, 10 μl CCK-8 solution (Dojindo Molecular Technologies, Inc.) was added to each well for 4 h, then the optical density was measured at 450 nm using a microplate reader.
Colony formation assay
To evaluate the long-term effects of IL-21, colony formation experiments were performed using A549 cells. Cells were seeded at 500 cells/well into 6-well plates and cultured for 7-12 days. Following this, the medium was removed, and the colonies were fixed with 4% paraformaldehyde for 20 min and stained with 0.2% crystal violet. Following being washed and air dried at room temperature, the colonies were visualized under a light microscope (Nikon Corporation; magnification, ×100).
Cell invasion assay
A Transwell assay was used to investigate the invasive properties of A549 cells. Cells in serum-free DMEM were seeded into the upper chamber which consisted of 8 μm-pore inserts coated with Matrigel (BD Biosciences) in culture plates. DMEM supplemented with 10% FBS was added to the lower chamber. Following a 48 h incubation, the Matrigel and the cells remaining in the upper chamber were removed with a cotton-tipped swab. Subsequently, the cells were fixed in 4% polyformaldehyde for 10 min at room temperature and stained with 0.1% crystal violet for 15 min. The number of invasive cells in five random fields (magnification, ×200) was counted using a light microscope (Olympus Corporation).
Scratch wound healing assay
Cells were seeded into 12-well plates at a density of 1×105 cells/well for adherent culture. When cells reached 80% confluence, monolayer cells were scraped off using a 10 μl sterile pipette tip. A phase-contrast microscope (IX711; Olympus Corporation) was used to monitor cells at the borders of the scratches. The degree of scratch healing was observed and images were captured in each group at 0 and 24 h.
ELISA
For measurements of patient sera, all peripheral blood samples were centrifuged at 1,000 × g for 20 min, then the supernatant was used for ELISA. For measurements of cells in vitro, A549 cells were seeded at 1×106 cells/well in 6-well plates, allowed to adhere for 18 h, then treated as indicated, and finally culture supernatant was used for ELISA. The concentrations of IL-1β (cat. no. ab2105), tumor necrosis factor (TNF)-α (cat. no. ab6671), IL-21 (cat. no. ab53655), IL-21R (cat. no. ab13268) and soluble PD-L1 (cat. no. ab237726) were measured using ELISA kits purchased from Abcam, according to the manufacturer's protocol. The experimental detection wavelength was 450 nm.
Immunohistochemistry assay
Tissues were fixed in 10% formaldehyde for 24 h at room temperature and then embedded in paraffin. Paraffin-embedded specimens were cut into 4 μm thick sections, deparaffinized and rehydrated with a graded ethanol and xylene series. They were then blocked for 30 min using Endogenous Biotin Blocking kit (Beyotime Institute of Biotechnology). Next, slides were incubated with primary antibodies targeting IL-21 (1:200; cat. no. ab53655; Abcam), IL-21R (1:200; cat. no. ab13268; Abcam) and PD-L1 (1:200; cat. no. ab237726; Abcam) overnight at 4°C. Slides were then incubated with horseradish peroxidase-secondary antibody (1:1,000; cat. no. ab181658; Abcam) for 30 min at 37°C, stained with diaminobenzidine (Beyotime Institute of Biotechnology), and counterstained with hematoxylin. Three representative sections from each patient sample were used to calculate the average staining degree for image analysis using an optical microscope. Brown nuclear staining was considered to indicate positive protein expression.
Immunofluorescence assay
A549 cells were washed with PBS, fixed with 4% paraformaldehyde for 30 min at a room temperature, permeabilized with 0.2% Triton X-100 for 5 min, and blocked with 5% BSA for 1 h at a room temperature. Cells were then incubated with primary antibodies targeting IL-21R (1:100; cat. no. ab13268; Abcam) and soluble PD-L1 (1:100; cat. no. ab237726; Abcam) overnight at 4°C. After washing with PBS, cells were incubated with fluorescein isothiocyanate (FITC)-conjugated goat anti-rabbit secondary antibody (1:2,000; cat. no. BM2004; Boster Biological Technology) for 1 h at room temperature in the dark. Images were acquired using a fluorescence microscope (Nikon Corporation) after staining the cell nuclei with DAPI (Boster Biological Technology).
RT-qPCR
Cells were seeded into 6-well plates at the density of 1×106 cells/well. Total RNA was extracted from A549 cells using TRIzol® reagent (Invitrogen; Thermo Fisher Scientific, Inc.). RNA was then reverse transcribed into cDNA using a PrimeScript™ reverse transcription reagent kit (Takara Biotechnology Co., Ltd.) according to the manufacturer's protocol. qPCR was performed using iTaq™ Universal SYBR®-Green Supermix (Bio-Rad Laboratories, Inc.) on an ABI 7500 system (Applied Biosystems; Thermo Fisher Scientific, Inc.). The amplification conditions were as follows: 95°C for 10 min, followed by 40 cycles of 95°C for 10 sec and 60°C for 60 sec. The primers used were: IL-21R, forward, 5′-ACCAGTCTGGCAACTACTCC-3′ and reverse, 5′-GGCAGGGTCTTCGTAATCTGAG-3′; GAPDH, forward, 5′-TAT GATGATATCAAGAGGGTAGT-3′ and reverse, 5′-TGTATCCAAACTCATTGTCATAC-3′. GAPDH was used as an internal control. Relative expression was analyzed using the 2-∆∆Cq method (17).
Western blot analysis
A549 cells were seeded at 1×106 cells/well in 6-well plates. Proteins were extracted from tumor tissues or A549 cells using a protein lysis buffer (RIPA; Beyotime Institute of Biotechnology). The concentration of protein was determined using a bicinchoninic acid assay protein assay kit (Beyotime Institute of Biotechnology). Proteins (25 μg/lane) were resolved using 10% SDS-PAGE and transferred to PVDF membranes (EMD Millipore). The membranes were then incubated with primary antibodies (all at 1:1,000), followed by goat anti-rabbit horseradish peroxidase-conjugated secondary antibodies (1:5,000; cat. no. ab181658; Abcam) at room temperature for 1 h. Proteins were visualized using Image Quant™ LAS 4000 (GE Healthcare Life Sciences) and quantified using Image J (version 1.46; National Institutes of Health). Anti-IL-21 (cat. no. ab5978), anti-IL-21R (cat. no. ab5980), anti-PD-L1 (cat. no. ab205921) and anti-Wnt (cat. no. ab28472) were purchased from Abcam. Anti-β-catenin (cat. no. 8480T), anti-cyclinD1 (cat. no. 3300T) and anti-GAPDH (cat. no. 5174S) antibodies were obtained from Cell Signaling Technology, Inc.
Statistical analysis
All experiments were performed for at least three independent repeats and all the data are presented as mean ± standard deviation. Statistical analysis was performed using SPSS software 16.0 (SPSS, Inc.). Statistical comparisons were made using a one-way ANOVA followed by a post hoc Dunnett's test. P<0.05 was considered to indicate a statistically significant result.
Results
Expression of IL-21, IL-21R and PD-L1 in the serum and lung tissues of patients with NSCLC
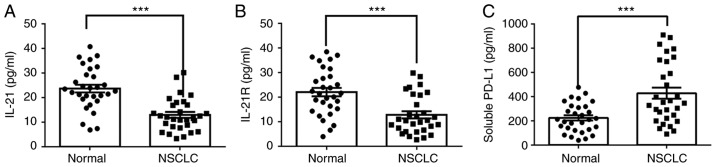
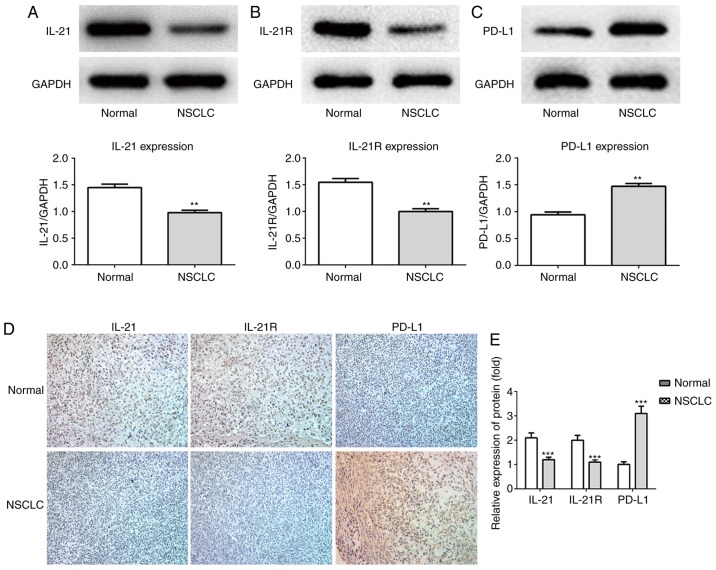
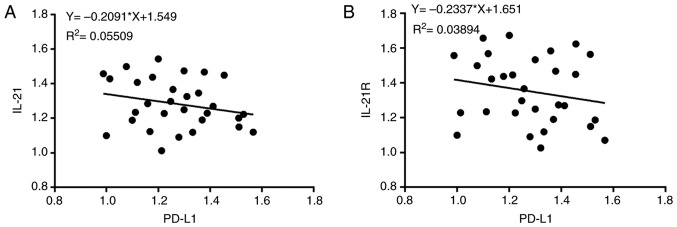
The expression levels of IL-21, IL-21R and PD-L1 in the serum and lung tissues of patients with NSCLC and healthy controls (normal) were measured in the current study. As presented in Fig. 1A-C, the protein levels of IL-21 and IL-21R were decreased, whereas PD-L1 was increased, in the serum of patients with NSCLC, compared with healthy controls. The expression levels of these proteins in the lung tissues of patients with NSCLC, as detected by western blotting (Fig. 2A-C), were in accordance with the serum ELISA results. Finally, the levels of these proteins in the lung tissues were also detected by immunohistochemistry assay, with similar results (Fig. 2D and E). Next, Pearson's correlation coefficients were used to analyze the potential correlation between the expression levels of IL-21 and PD-L1, and IL-21R and PD-L1. The results demonstrated that IL-21 (F=-0.2091; P<0.05) and IL-21R (F=-0.2337; P<0.05) were negatively correlated with PD-L1 (Fig. 3A and B). These data indicated that decreased expression of IL-21 and IL-21R, and increased expression of PD-L1, may be correlated with NSCLC progression.
Figure 1.
Levels of IL-21, IL-21R and soluble PD-L1 in serum of patients with NSCLC. The levels of (A) IL-21, (B) IL-21R and (C) soluble PD-L1 were measured using ELISA. ***P<0.001 vs. normal. IL, interleukin; R, receptor; PD-L1, programmed death 1 ligand 1; NSCLC, non-small-cell lung cancer.
Figure 2.
Protein expression of IL-21, IL-21R and PD-L1 in lung tissues of patients with NSCLC. The protein expression levels of (A) IL-21, (B) IL-21R and (C) PD-L1 were measured using western blot analysis. (D) IL-21, IL-21R and PD-L1 protein expression levels were also evaluated by immunohistochemistry (magnification, ×200). (E) Quantitative analysis of immunohistochemistry staining results. **P<0.01, ***P<0.001 vs. normal. IL, interleukin; R, receptor; PD-L1, programmed death 1 ligand 1; NSCLC, non-small-cell lung cancer.
Figure 3.
Correlation between IL-21 and PD-L1 or IL-21R and PD-L1. Pearson's correlation coefficients were used to analyze the relationship between the expression levels of (A) IL-21 and PD-L1 (P<0.05) and (B) IL-21R and PD-L1 (P<0.05). IL, interleukin; R, receptor; PD-L1, programmed death 1 ligand 1.
IL-21 treatment attenuates the proliferation, invasion and migration of A549 cells
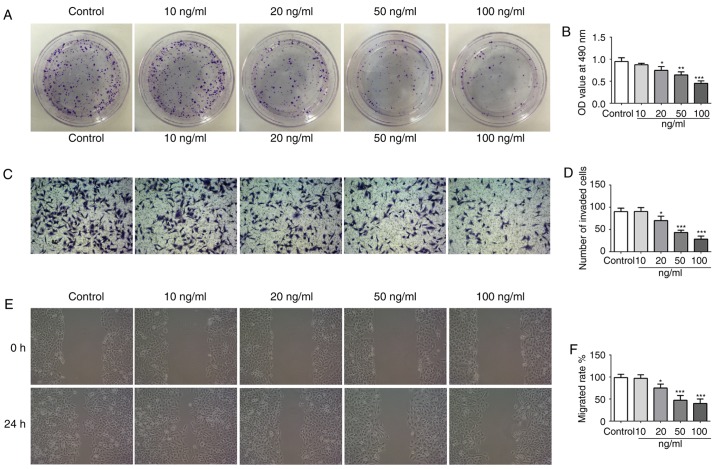
To investigate the effect of IL-21 on the NSCLC cell line A549, different doses of IL-21 (0, 10, 20, 50 and 100 ng/ml) were used to treat the cells. From the results of Fig. 4A and B, it was revealed that IL-21 treatment attenuated the proliferation of A549 cells in a dose-dependent manner. Additionally, IL-21 treatment resulted in a significant reduction of the invasive and migratory capacities of the cells (Fig. 4C-F). These results indicated that IL-21 was able to attenuate the proliferation, invasion and migration of A549 cells.
Figure 4.
IL-21 treatment inhibits the proliferation, invasion and migration of A549 cells. (A) Cell proliferation was measured using a colony formation assay. (B) Cell proliferation was also assessed by Cell Counting Kit-8 assay. (C) Representative images and (D) quantification of cell invasion, assessed using Transwell assays (magnification, ×200). (E) Representative images and (F) quantification of cell migration, assessed using a scratch assay (magnification, ×4). *P<0.05, **P<0.01 and ***P<0.001 vs. control (vehicle-treated). IL, interleukin.
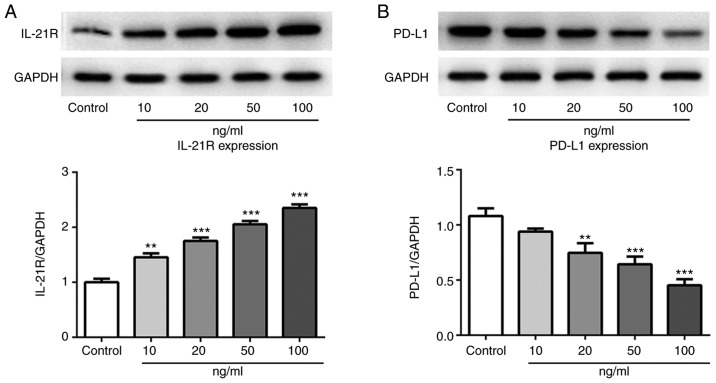
IL-21 treatment increases IL-21R and decreases PD-L1 expression in A549 cells
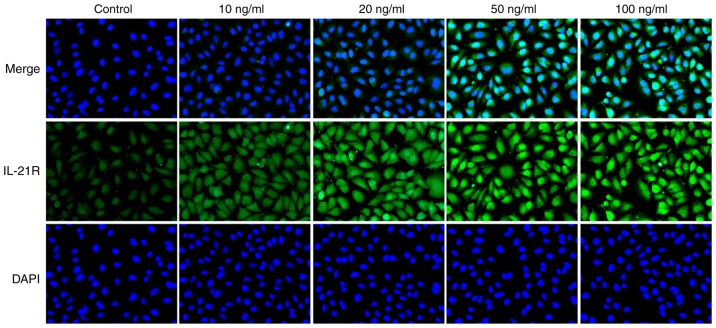
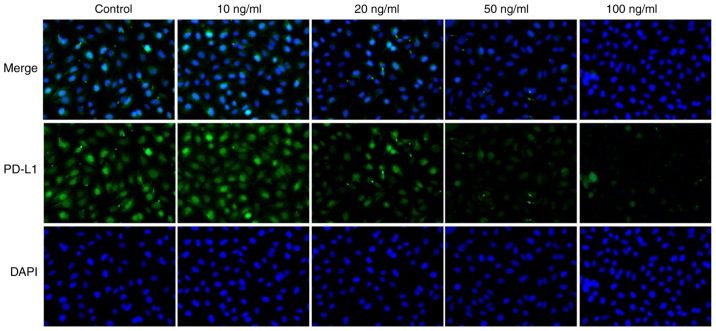
Following treatment with IL-21, the expression levels of IL-21R and PD-L1 in A549 cells were measured by western blotting in the current study. As presented in Fig. 5A and B, the protein expression levels of IL-21R were increased, while the PD-L1 expression levels were decreased, following IL-21 treatment. Results from immunofluorescence analysis for IL-21R (Fig. 6) and PD-L1 (Fig. 7) further confirmed the effects of IL-21 treatment on their expression. These findings suggested that IL-21 treatment upregulated the expression of IL-21R and downregulated the expression of PD-L1 in A549 cells.
Figure 5.
Expression levels of IL-21R and PD-L1 in A549 cells following treatment with IL-21. The protein expression levels of (A) IL-21R and (B) PD-L1 were measured by western blot analysis. **P<0.01 and ***P<0.001 vs. control. IL, interleukin; R, receptor; PD-L1, programmed death 1 ligand 1.
Figure 6.
IL-21 treatment increases the expression of IL-21R in A549 cells. Cells were treated with IL-21 and the expression levels of IL-21R in A549 cells were assessed by immunofluorescence (magnification, ×200). IL, interleukin; R, receptor.
Figure 7.
IL-21 treatment decreases the expression of PD-L1 in A549 cells. Cells were treated with IL-21 and the expression levels of PD-L1 in A549 cells were assessed by immunofluorescence (magnification, ×200). IL, interleukin; PD-L1, programmed death 1 ligand 1.
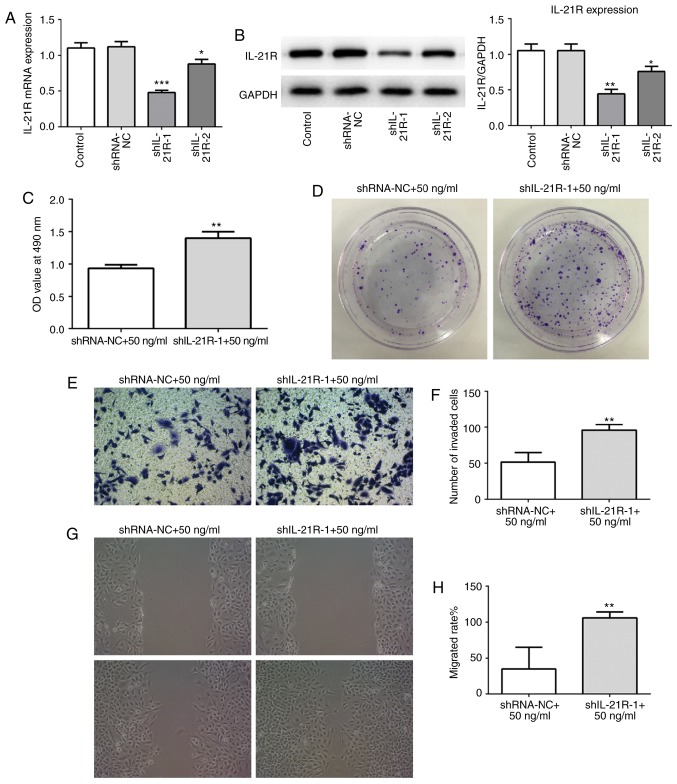
IL-21 treatment attenuates the proliferation, invasion and migration of A549 cells by binding to IL-21R
To investigate whether IL-21 was acting via binding to IL-21R, IL-21R silencing was employed. The successful silencing of IL-21R was confirmed using RT-qPCR and western blot analysis (Fig. 8A and B). Cells were treated with 50 ng/ml IL-21. As presented in Fig. 8C and D, following IL-21R silencing, the effect of IL-21 on cell proliferation of A549 cells was reversed, which indicated that IL-21 decreased proliferation by binding to IL-21R. Similar results were demonstrated for cell invasion (Fig. 8E and F) and migration (Fig. 8G and H). These results revealed that IL-21 attenuated the proliferation, invasion and migration of A549 cells by binding to IL-21R.
Figure 8.
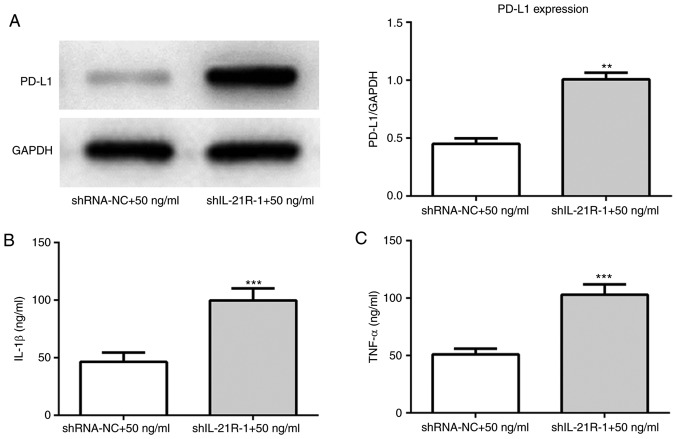
IL-21R silencing reverses the effect of IL-21 on the proliferation, invasion and migration of A549 cells. (A) Successful IL-21R silencing was confirmed by reverse transcription-quantitative PCR and (B) western blot analysis. The control groups are untransfected parental cells. *P<0.05, **P<0.01 and ***P<0.001 vs. shRNA-NC. (C) Cell proliferation was measured using a Cell Counting Kit-8 assay. **P<0.01 vs. shRNA-NC+50 ng/ml. (D) Cell proliferation was also assessed by colony formation assay. (E) Representative images and (F) quantification of cell invasion, assessed by Transwell assay (magnification, ×200). **P<0.01 vs. shRNA-NC+50 ng/ml. (G) Representative images and (H) quantification of cell migration, assessed by scratch assay (magnification, ×4). **P<0.01 vs. shRNA-NC+50 ng/ml. IL, interleukin; shRNA, short-hairpin RNA; NC, negative control.
IL-21 inhibits the Wnt/β-catenin signaling pathway and PD-L1 expression
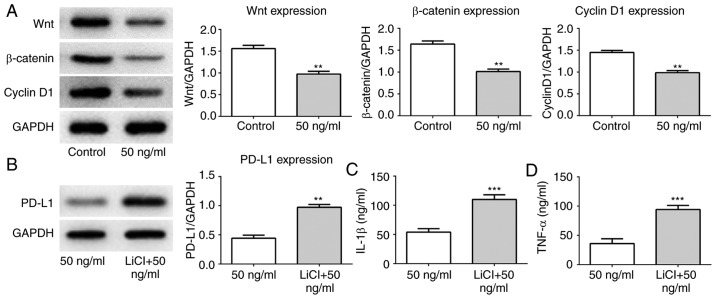
Following IL-21R silencing, IL-21 treatment increased the protein expression levels of PD-L1 (Fig. 9A). IL-1β and TNF-α expression levels were also increased (Fig. 9B and C). To investigate the regulatory mechanisms underlying this effect, the expression levels of proteins associated with the Wnt/β-catenin signaling pathway were measured. As presented in Fig. 10A, IL-21 treatment decreased the levels of Wnt, β-catenin and cyclin D1 compared with the control. Subsequently, the Wnt/β-catenin signaling agonist LiCl was used in the current study. It was demonstrated that the expression of PD-L1 was upregulated following treatment with IL-21 and LiCl compared with the IL-21 alone control (Fig. 10B). Furthermore, the levels of IL-1β and TNF-α were increased compared with the IL-21 alone control (Fig. 10C and D). These results revealed that IL-21 downregulated PD-L1 expression by inhibiting the Wnt/β-catenin signaling pathway. These results indicated that IL-21 attenuated NSCLC growth by inhibiting the Wnt/β-catenin signaling pathway and PD-L1 expression.
Figure 9.
IL-21R silencing reverses the effect of IL-21 on the levels of PD-L1, IL-1β and TNF-α. (A) The protein expression levels of PD-L1 were measured using western blot analysis. **P<0.01 vs. shRNA-NC+50 ng/ml. (B) The levels of IL-1β and (C) TNF-α were evaluated by ELISA. ***P<0.001 vs. shRNA-NC+50 ng/ml. IL, interleukin; R, receptor; PD-L1, programmed death 1 ligand 1; TNF-α, tumor necrosis factor α; shRNA, short-hairpin RNA; NC, negative control.
Figure 10.
IL-21 treatment suppresses the Wnt/β-catenin signaling pathway. (A) The protein expression levels of Wnt, β-catenin and cyclin D1 were measured using western blot analysis. **P<0.01 vs. control. (B) The protein expression levels of PD-L1 were detected using western blot analysis following treatment with LiCl. (C) The levels of IL-1β and (D) TNF-α were evaluated by ELISA. **P<0.01 and ***P<0.01 vs. 50 ng/ml (IL-21 treatment alone). IL, interleukin; PD-L1, programmed death 1 ligand 1; TNF-α, tumor necrosis factor α; shRNA, short-hairpin RNA; NC, negative control.
Discussion
The present study demonstrated that IL-21 and IL-21R were negatively correlated with PD-L1 in the lung tissues of patients with NSCLC. IL-21 was revealed to exert a protective effect on the growth and migration of NSCLC cells by binding to IL-21R. Additionally, the results indicated that IL-21 inhibited the Wnt/β-catenin signaling pathway and decreased PD-L1 expression. Following treatment with LiCl, the decreasing effect of IL-21 on PD-L1 was reversed. Therefore, the present results indicated that IL-21/IL-21R served an antitumor role in NSCLC via repression of Wnt/β-catenin signaling and PD-L1 expression.
IL-21, which is a member of the common γ-chain family, is produced by activated CD4+ T cells, NK T cells and follicular T-helper cells (18,19). Increasing evidence has indicated that IL-21 exhibits an antitumor function in a variety of tumor models (8,20). Additionally, the antitumor activity of IL-21 can be potentiated when used in combination with other immuno-stimulants, chemotherapy or with monoclonal antibodies that recognize tumor antigens (19,21,22). A previous study reported that IL-21, in combination with 5-fluorouracil, potentiated its antitumor effect in human gastric cancer. The current study demonstrated that IL-21 and IL-21R were upregulated in the peripheral blood and lung tissues of patients with NSCLC, which was in accordance with previous research (12). IL-21 treatment inhibited the growth, invasion and migration of NSCLC cells in a dose-independent manner, which revealed the antitumor effect of IL-21 on NSCLC.
PD-L1 is the major ligand of PD-1 and is expressed in a variety of tumors, including in NSCLC (23,24). Overexpression of PD-L1 is implicated in tumor immunity and inhibition of PD-L1 enhances antitumor immunity by preventing tumor cells from escaping host immune responses (25). A growing number of studies have demonstrated that PD-L1 is closely associated with tumorigenesis and invasiveness (26). In the present study, the expression of PD-L1 in lung tissues of patients with NSCLC increased, and this was negatively correlated with IL-21 and IL-21R. Upon treatment of NSCLC cells with IL-21 in vitro, the expression levels of PD-L1 were downregulated in a dose-dependent manner, which indicated that IL-21 was able to inhibit the expression of PD-L1.
It has been previously reported that the Wnt/β-catenin signaling pathway is of great importance in regulating tumor cell growth and metastasis (27,28), and inactivation of Wnt/β-catenin signaling suppresses the growth and progression of numerous cancers, including NSCLC (29). Increasing evidence has revealed that active Wnt/β-catenin signaling can lead to T-cell exclusion and resistance to anti-PD-L1/anti-CTLA-4 monoclonal antibody therapy in melanoma (16). Additionally, IL-21 can suppress tumor growth and metastasis through the inhibition of Wnt/β-catenin signaling in epithelial ovarian cancer (10). The current study demonstrated that IL-21 treatment inhibited the expression of Wnt, β-catenin and cyclin D1, while promoting PD-L1 expression. Following intervention with LiCl, the suppressing effect of IL-21 on PD-L1 expression was reversed. Therefore, the present results provided evidence that IL-21/IL-21R protected against NSCLC via repression of the Wnt/β-catenin signaling pathway and PD-L1 expression.
In conclusion, the present study demonstrated that IL-21 and IL-21R expression levels were negatively correlated with PD-L1 expression levels in the lung tissues of patients with NSCLC, and that IL-21 exerted a protective effect on the growth and invasion of NSCLC cells by binding to IL-21R. It was also revealed that IL-21/IL-21R inactivated the Wnt/β-catenin signaling pathway and decreased PD-L1 expression in NSCLC cells. These findings indicated a tumor suppressive role of IL-21 in the development of NSCLC, which may be useful for the development of novel therapies for this disease.
Acknowledgments
Not applicable.
Funding
The present study was supported by the National and Fujian Province's Key Clinical Specialty Discipline Construction Program, the Natural Science Foundation of Fujian Province,
China (grant no. 2017J01295), and the Joint Funds for the Innovation of Science and Technology, Fujian province, China (grant no. 2017Y9031).
Availability of data and materials
The analyzed data sets generated during the present study are available from the corresponding author on reasonable request.
Authors' contributions
DX and PY wrote the manuscript, interpreted the data and performed experiments. QW and XL collected the data. LL and TL searched the literature, designed the study and revised the manuscript. All authors read and approval the final manuscript.
Ethics approval and consent to participate
The study protocol was approved by the Ethics Committee of Fujian Medical University Union Hospital, and all patients provided written informed consent. All of the procedures were in compliance with The Declaration of Helsinki and relevant policies in China.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
References
- 1.Hao H, Zhou Z, Li S, Maquilan G, Folkert MR, Iyengar P, Westover KD, Albuquerque K, Liu F, Choy H, et al. Shell feature: A new radiomics descriptor for predicting distant failure after radiotherapy in non-small cell lung cancer and cervix cancer. Phys Med Biol. 2018;63:095007. doi: 10.1088/1361-6560/aabb5e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Shaw AT, Engelman JA. Ceritinib in ALK-rearranged non-small-cell lung cancer. N Engl J Med 3. 2014;70:2537–2539. doi: 10.1056/NEJMc1404894. [DOI] [PubMed] [Google Scholar]
- 3.Shroff GS, Viswanathan C, Carter BW, Benveniste MF, Truong MT, Sabloff BS. Staging lung cancer: Metastasis. Radiol Clin North Am. 2018;56:411–418. doi: 10.1016/j.rcl.2018.01.009. [DOI] [PubMed] [Google Scholar]
- 4.Legras A, Pécuchet N, Imbeaud S, Pallier K, Didelot A, Roussel H, Gibault L, Fabre E, Le Pimpec-Barthes F, Laurent-Puig P, Blons H. Epithelial-to-mesenchymal transition and microRNAs in lung cancer. Cancers (Basel) 2017;9:E101. doi: 10.3390/cancers9080101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pawlak K, Gabryel P, Kujawska A, Kasprzyk M, Piwkowski C, Kuffel B, Dyszkiewicz W. Long-term results of surgical treatment of non-small cell lung cancer in patients over 75 years of age. Kardiochir Torakochirurgia Pol. 2018;15:65–71. doi: 10.5114/kitp.2018.76470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Xu X, Chen D, Ye B, Zhong F, Chen G. Curcumin induces the apoptosis of non-small cell lung cancer cells through a calcium signaling pathway. Int J Mol Med. 2015;35:1610–1616. doi: 10.3892/ijmm.2015.2167. [DOI] [PubMed] [Google Scholar]
- 7.Monteleone I, Pallone F, Monteleone G. Interleukin-23 and Th17 cells in the control of gut inflammation. Mediators Inflamm. 2009;2009;297645 doi: 10.1155/2009/297645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fu ZQ, Zhou Q, Zhu S, Liu W. Anti-tumor mechanism of IL-21 used alone and in combination with 5-fluorouracil in vitro on human gastric cancer cells. J Biol Regul Homeost Agents. 2018;32:619–625. [PubMed] [Google Scholar]
- 9.Chen C, Liu X, Ren Y. Interleukin 21 treatment in a murine model as a novel potential cytokine immunotherapy for colon cancer. Adv Clin Exp Med. 2018;27:583–589. doi: 10.17219/acem/68703. [DOI] [PubMed] [Google Scholar]
- 10.Zhang Y, Wang J, Wu D, Li M, Zhao F, Ren M, Cai Y, Dou J. IL-21-secreting hUCMSCs combined with miR-200c inhibit tumor growth and metastasis via repression of Wnt/β-catenin signaling and epithelial-mesenchymal transition in epithelial ovarian cancer. Onco Targets Ther. 2018;11:2037–2050. doi: 10.2147/OTT.S147855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Liu L, Shi F, Li S, Liu X, Wei L, Zhang J, Ju X, Yu J. IL-21 polymorphisms rs907715 and rs2221903 are associated with decreased non-small cell lung cancer susceptibility. Int J Clin Exp Med. 2015;8:19460–19465. [PMC free article] [PubMed] [Google Scholar]
- 12.Qiu L, Yu Q, Zhou Y, Zheng S, Tao J, Jiang Q, Yuan G. Functionally impaired follicular helper T cells induce regulatory B cells and CD14+ human leukocyte antigen-DR- cell differentiation in non-small cell lung cancer. Cancer Sci. 2018;109:3751–3761. doi: 10.1111/cas.13836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kaltenmeier C, Gawanbacht A, Beyer T, Lindner S, Trzaska T, van der Merwe JA, Härter G, Grüner B, Fabricius D, Lotfi R, et al. CD4+ T cell-derived IL-21 and deprivation of CD40 signaling favor the in vivo development of granzyme B-expressing regulatory B cells in HIV patients. J Immunol. 2015;194:3768–3777. doi: 10.4049/jimmunol.1402568. [DOI] [PubMed] [Google Scholar]
- 14.Wang JY, Wang X, Wang XJ, Zheng BZ, Wang Y, Wang X, Liang B. Curcumin inhibits the growth via Wnt/β-catenin pathway in non-small-cell lung cancer cells. Eur Rev Med Pharmacol Sci. 2018;22:7492–7499. doi: 10.26355/eurrev_201811_16290. [DOI] [PubMed] [Google Scholar]
- 15.Wang XH, Cui YX, Wang ZM, Liu J. Down-regulation of FOXR2 inhibits non-small cell lung cancer cell proliferation and invasion through the Wnt/β-catenin signaling pathway. Biochem Biophys Res Commun. 2018;500:229–235. doi: 10.1016/j.bbrc.2018.04.046. [DOI] [PubMed] [Google Scholar]
- 16.Spranger S, Bao R, Gajewski TF. Melanoma-intrinsic β-catenin signalling prevents anti-tumour immunity. Nature. 2015;523:231–235. doi: 10.1038/nature14404. [DOI] [PubMed] [Google Scholar]
- 17.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 18.Santegoets SJ, Turksma AW, Powell DJ, Jr, Hooijberg E, de Gruijl TD. IL-21 in cancer immunotherapy: At the right place at the right time. Oncoimmunology. 2013;2:e24522. doi: 10.4161/onci.24522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Croce M, Rigo V, Ferrini S. IL-21: A pleiotropic cytokine with potential applications in oncology. J Immunol Res. 2015;2015;696578 doi: 10.1155/2015/696578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Moroz A, Eppolito C, Li Q, Tao J, Clegg CH, Shrikant PA. IL-21 enhances and sustains CD8+ T cell responses to achieve durable tumor immunity: Comparative evaluation of IL-2, IL-15, and IL-21. J Immunol. 2004;173:900–909. doi: 10.4049/jimmunol.173.2.900. [DOI] [PubMed] [Google Scholar]
- 21.Spolski R, Leonard WJ. Interleukin-21: A double-edged sword with therapeutic potential. Nat Rev Drug Discov. 2014;13:379–395. doi: 10.1038/nrd4296. [DOI] [PubMed] [Google Scholar]
- 22.Shao J, Xu Q, Su S, Meng F, Zou Z, Chen F, Du J, Qian X, Liu B. Engineered cells for costimulatory enhancement combined with IL-21 enhance the generation of PD-1-disrupted CTLs for adoptive immunotherapy. Cell Immunol. 2017;320:38–45. doi: 10.1016/j.cellimm.2017.09.003. [DOI] [PubMed] [Google Scholar]
- 23.Qu QX, Xie F, Huang Q, Zhang XG. Membranous and cytoplasmic expression of PD-L1 in ovarian cancer cells. Cell Physiol Biochem. 2017;43:1893–1906. doi: 10.1159/000484109. [DOI] [PubMed] [Google Scholar]
- 24.Tang Y, Fang W, Zhang Y, Hong S, Kang S, Yan Y, Chen N, Zhan J, He X, Qin T, et al. The association between PD-L1 and EGFR status and the prognostic value of PD-L1 in advanced non-small cell lung cancer patients treated with EGFR-TKIs. Oncotarget. 2015;6:14209–14219. doi: 10.18632/oncotarget.3694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mu CY, Huang JA, Chen Y, Chen C, Zhang XG. High expression of PD-L1 in lung cancer may contribute to poor prognosis and tumor cells immune escape through suppressing tumor infiltrating dendritic cells maturation. Med Oncol. 2011;28:682–688. doi: 10.1007/s12032-010-9515-2. [DOI] [PubMed] [Google Scholar]
- 26.Pang L, Han S, Jiao Y, Jiang S, He X, Li P. Bu Fei Decoction attenuates the tumor associated macrophage stimulated proliferation, migration, invasion and immunosuppression of non-small cell lung cancer, partially via IL-10 and PD-L1 regulation. Int J Oncol. 2017;51:25–38. doi: 10.3892/ijo.2017.4014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Li T, Ren J, Ma J, Wu J, Zhang R, Yuan H, Han X. LINC00702/miR-4652-3p/ZEB1 axis promotes the progression of malignant meningioma through activating Wnt/β-catenin pathway. Biomed Pharmacother. 2019;113:108718. doi: 10.1016/j.biopha.2019.108718. [DOI] [PubMed] [Google Scholar]
- 28.Jing JC, Feng Z, Chen ZH, Ji BN, Hong J, Tang N, Yu JL, Wang SY. KDM4B promotes gastric cancer metastasis by regulating miR-125b-mediated activation of Wnt signaling. J Cell Biochem. 2018 doi: 10.1002/jcb.28065. [DOI] [PubMed] [Google Scholar]
- 29.Yang Y, Liu L, Cai J, Wu J, Guan H, Zhu X, Yuan J, Li M. DEPDC1B enhances migration and invasion of non-small cell lung cancer cells via activating Wnt/β-catenin signaling. Biochem Biophys Res Commun. 2014;450:899–905. doi: 10.1016/j.bbrc.2014.06.076. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The analyzed data sets generated during the present study are available from the corresponding author on reasonable request.