Abstract
Isosteviol sodium (STVNa), which is a derivate of the natural sweet-tasting glycoside stevioside, has recently been developed and it has been determined that this compound exhibits neuro- and cardio-protective properties. In the current study, whether STVNa interferes with the development of cardiac hypertrophy, which is induced by isoprenaline (Iso), was investigated in an experimental rat model. Rats were treated with a vehicle (0.9% NaCl; control), isoprenaline (Iso; 5 mg/kg) or Iso (5 mg/kg) with STVNa (4 mg/kg; Iso + STVNa). Cardiomyocytes were isolated using enzymatic dissociation and were treated with 5 µM Iso for 24 h and co-treated with 5 µM STVNa. Brain natriuretic peptide (BNP) mRNA expression was determined using PCR analysis. Cell surface area, intracellular reactive oxygen species (ROS), mitochondrial transmembrane potential (ΔΨm), cytoplasmic Ca2+ and Ca2+ and contractile function were examined using a laser scanning confocal microscope. The current study demonstrated that STVNa inhibited Iso-induced cardiac hypertrophy by inhibiting cardiomyocyte size. STVNa significantly reduced cell surface area and decreased BNP mRNA expression in ventricular cardiomyocyte Iso-induced hypertrophy. STVNa was also revealed to restore ΔΨm and reduce ROS generation and intracellular Ca2+ concentration when compared with the Iso-treated group. Additionally, STVNa preserved Ca2+ transients in hypertrophic cardiomyocytes. In conclusion, the present study demonstrated that STVNa protects against Iso-induced myocardial hypertrophy by reducing oxidative stress, restoring ΔΨm and maintaining Ca2+ homeostasis.
Key words: isosteviol, cardiac hypertrophy, reactive oxygen species, mitochondria, Ca2+
Introduction
Stevioside, a natural sweet-tasting glycoside, is found in Stevia rebaudiana. Isosteviol, a derivate of stevioside, has been demonstrated to exhibit a variety of beneficial pharmacological effects (1-5). Isosteviol sodium salt (STVNa), which is a beyerane diterpene, a more soluble and injectable form of isosteviol, has recently been synthesized via acid hydrolysis of stevioside, and it has been determined that STVNa exhibits neuro- and cardio-protective properties (1-5). It has also been indicated that STVNa attenuates right ventricular hypertrophy and pulmonary artery remodeling in an experimental model of transverse aortic constriction and ameliorates diabetic cardiomyopathy (6,7). However, whether STVNa exhibits an effect on the development of left ventricular hypertrophy (LVH) is, to the best of our knowledge, yet to be determined.
LVH is defined as the enlargement and thickening of the left ventricle walls, which form the main contractile chamber of the heart. LVH is the ultimate outcome in a variety of pathological states, including hypertension, valvular disease, myocardial infarction and cardiomyopathy (8). This condition is usually associated with the activation of β-adrenergic signaling and the consequent increase in oxidative stress, protein synthesis, proto-oncogene expression and the stimulation of mitogen activated protein kinases and phosphatidyl inositol-3 kinases (9). The development of pathological LVH is initially beneficial as it allows the heart to maintain its cardiac pump function despite abnormal pressure and/or volume load. However, this ultimately leads to depression of the intrinsic contractile state of the myocardium and subsequent heart failure (8). Additional therapeutic strategies, which prevent LVH and heart failure are urgently required (10).
Isoprenaline (Iso), a non-selective β-adrenoceptor agonist, is widely used to induce LVH in animal experimental models of cardiac hypertrophy (11-13). This model successfully mimics sustained adrenergic stimulation, which is a major mechanism in the pathogenesis of maladaptive cardiac hypertrophy (14). In the current study, this particular model was used to assess whether STVNa modifies the development of myocardial hypertrophy and if it does, to determine the underlying mechanism governing this.
Materials and methods
Materials
All chemicals (including caffeine) used in the current study were purchased from Sigma-Aldrich; Merck KGaA, unless otherwise stated. H2DCFDA, JC-1 and Fluo-4 ester were purchased from Invitrogen; Thermo Fisher Scientific, Inc. mitoTEMPO was purchased from Enzo Life Sciences, Inc. Medium-199 (M199) was purchased from Thermo Fisher Scientific, Inc. PCR reagent kit, primers and markers were purchased from Takara Biotechnology Co., Ltd. STVNa, which is the sodium salt of isosteviol and is a beyerane diterpene, was obtained via acid hydrolysis of stevioside, and was synthesized by the Chemical Synthesis Group of Institute of Biomedical and Pharmaceutical Sciences, Guangdong University of Technology (Guangzhou, China).
Rats and experimental protocol
Sixty male Sprague-Dawley rats (weight, 200-250 g; age, 6 weeks) were obtained from the Experimental Animal Center of Guangzhou University of Chinese Medicine (Guangzhou, China). All animal experimental protocols complied with the Guide for the Care and Use of Laboratory Animals, which was published by the National Institutes of Health. The current study was approved by the Institutional Animal Research Committee of South China University of Technology (Guangzhou, China). Sprague-Dawley rats were housed in a room maintained at 24°C and 50% humidity with a 12-h light/dark cycle and provided with standard food and water ad libitum. Rats were randomly divided into three groups (60 in total): Control group, treated with vehicle (0.9% NaCl; control) (n=20); Iso group, treated with isoprenaline (5 mg/kg; Iso) (n=20); Iso + STVNa group, treated with isoprenaline (5 mg/kg) with isosteviol sodium (4 mg/kg; Iso + STVNa) (n=20). Vehicle and compounds were injected intraperitoneally daily for 7 days, as previously described (15).
Heart weight index measurement
Rats were weighed (body weight, BW), anesthetized by sodium pentobarbital [intra-peritoneal (IP), 50 mg/kg] and heparinized (IP, 1,000 U/kg). Rats were sacrificed by overdose of sodium pentobarbital (>150 mg/kg). The thoracic cavity was subsequently opened and the heart was harvested in a clean glass dish, washed with cold saline solution and weighed [heart weight, (HW)]. The atrium was cut off and the ventricle was separated and weighed [left ventricle weight (LW)]. The tibia length was also measured (Tibia). The heart weight indexes are represented by ratios of HW/BW, HW/Tibia and LW/Tibia.
Histological analysis
Rat hearts were fixed in 10% formalin at 25°C for 8 h. Transverse sections were embedded in paraffin and were cut into 5 mm sections. Hematoxylin and eosin (H&E; hematoxylin staining for 5 min, eosin staining for 2 min, at 25°C) were used to assess the cardiomyocyte cross-sectional area. Images were captured with a light microscope and analyzed using ImageJ 1.48 (National Institutes of Health). A total of >50 cells were counted in each independent heart from each group.
Isolation of cardiomyocytes and cells treatment
Ventricular myocytes were isolated from untreated, wild-type male Sprague-Dawley rats (200-250 g) as described previously (16), with some modifications. Heparinized (IP; 1,000 U/kg) animals were anesthetized using sodium pentobarbital (IP, 50 mg/kg). Excised hearts were transferred to a Langendorf perfusion apparatus and perfused with Ca2+-free Tyrode's solution (NaCl 137 mM; KCl 5.4 mM; NaH2PO4 1.2 mM; MgCl2. 6H2O 1.2 mM; HEPES 20 mM; taurine 30 mM; glucose 20 mM; pH 7.4) for 5 min. The perfusion solution was then switched to Ca2+-free Tyrode's solution containing collagenase II (0.4 mg/ml) and protease (Sigma-Aldrich; Merck KGaA; 0.1 mg/ml). After 30 min, ventricles were cut into small pieces, incubated in a 37°C water bath and separated into individual cardiomyocytes via slow pipetting. The cells were filtered through a 200 nm mesh and settled in Tyrode's solution containing 1.2 mM Ca2+ and bovine serum albumin (BSA; 0.1%). Cells were subsequently re-suspended in M199 (Invitrogen; Thermo Fisher Scientific, Inc.) containing 10% FBS supplemented with BSA (0.1%) and transferred to laminin coated culture dishes. After a 1.5 h of incubation in a CO2 incubator (5% CO2; 95% O2), the medium was replaced with serum free M199 (pH 7.4) supplemented with 0.1% BSA. To induce hypertrophy, cells were treated with 5 µM Iso (Sigma-Aldrich; Merck KGaA) for 24 h. To investigate the effect of STVNa on Iso-induced hypertrophy, Iso (5 µM)-treated cells were co-treated with a variety of STVNa concentrations (1, 5, 10 and 20 µM). The most effective STVNa concentration (5 µM; Fig. S1) was used for subsequent experimentation.
Measurement of ROS, mitochondrial membrane potential and calcium
For ROS measurement, cardiomyocytes were loaded with 10 µM H2DCFDA in serum-free medium at 37°C for 20 min in the dark and then resuspended in 1 mM Ca2+ Tyrode's solution to wash out residues of the dye. DCFDA was excited at 480 nm and measured at 525 nm. For mitochondrial membrane potential measurement, cardiomyocytes were incubated with 5 µM JC-1 at 37°C in the dark for 30 min. Cells were washed twice in 1 mM Ca2+ Tyrode's solution. Red fluorescence was exited at 585 nm and measured at 590 nm. Green fluorescence was exited at 514 nm and measured at 529 nm. For calcium measurement, cardiomyocytes were exposed to 1 µM Fluo-4 AM at 37°C for 40 min for loading and then washed twice in 1 mM Ca2+ Tyrode's solution. Fluo-4 was excited at 488 nm and measured at >520 nm. For analysis, intensity of fluorescence for targeted cells was directly read using the Zeiss LSM 710 confocal software (ZEN version 2011, Carl Zeiss Meditec AG).
Measurement of cell surface area
Phase contrast images that were captured using a light Olympus IX83 microscope (Olympus Corporation) were used to measure the surface area of different groups using Image Pro-Plus 6.0 (National Institutes of Health) software. A total of 120 cells from six different animals were analyzed to determine the morphological changes that were induced by Iso.
Measurement of mRNA levels
The effect of STVNa on the hypertrophic response of cardiomyocytes to Iso stimulus was assessed by monitoring BNP mRNA expression using reverse transcription (RT)-quantitative (q) PCR. Total RNA was extracted from cells using RNAiso Plus (Takara Biotechnology Co., Ltd.; Total RNA extraction reagent) according to the manufacturer's protocol. The concentration was determined by measuring the absorbance at 260 nm and RNA purity was determined by measuring 260/280 ratio using a NanoDrop 2000c Spectrophotometer (Thermo Fisher Scientific, Inc.). Total RNA (0.5 µg) was used for RT with the PrimeScript II First Strand cDNA synthesis kit (cat. no. 6210A; Takara Biotechnology Co., Ltd.), following the manufacturer's protocol. qPCR was performed by using ChamQ SYBR PCR kit Q311-01 (Vazyme Biotech Co., Ltd.). Relative quantification of gene expression was normalized to GAPDH. The nucleotide sequences of the primers used were: BNP forward, 5′-CTG TGA CGG GCT GAG GTT-3′ and reverse, 5′-GCA AGT TTG TGC TGG AAG-3′; GAPDH forward, 5′-GCA AGT TCA ACG GCA CAG-3′, and reverse, 5′-CGC CAG TAG ACT CCA CGA C-3′.
Analysis of cardiomyocyte contractile function
Cell contraction was recorded in the frame-scanning mode and time-series mode using a Zeiss LSM 710 confocal microscope. Cells were stimulated to contract at 1 Hz and scanned for 5 min (100 ms/Frame). The rate of contraction and shortening were measured and analyzed using the Zeiss LSM Imaging processing software (ZEN version 2011; Carl Zeiss Meditec AG).
Statistical analysis
Data are expressed as the mean ± standard error of the mean from three experimental repeats. Statistical analysis was performed using a one-way analysis of variance followed by the Tukey post-hoc test (SigmaPlot v14; Jandel Corporation). P<0.05 was considered to indicate a statistically significant difference. For the analysis of HW/BW, cross-sectional area of H&E staining, cell surface area of cardiomyocytes, BNP mRNA expression, ROS fluorescence intensity and Ca2+ fluorescence intensity, the raw mean value of the control group was used as a reference value, and the raw value of the control group was divided by this value. The raw mean value of the control group was set at 1 and the data of the other groups were presented as the fold-change of the control.
Results
STVNa prevents the development of Iso-induced cardiac hypertrophy
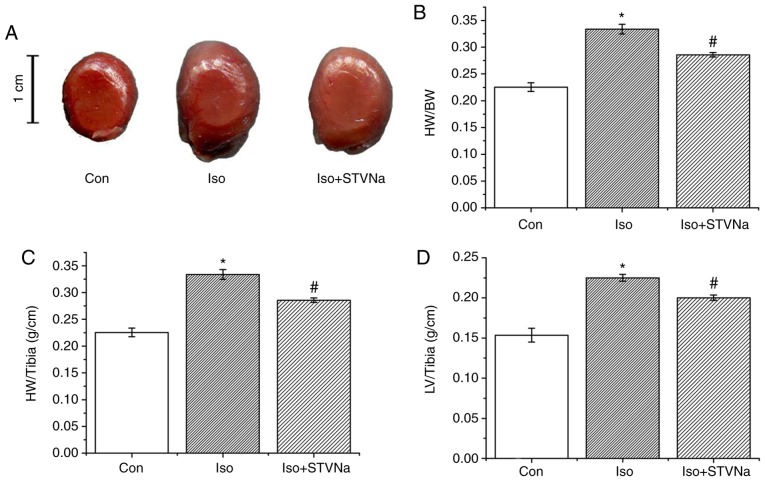
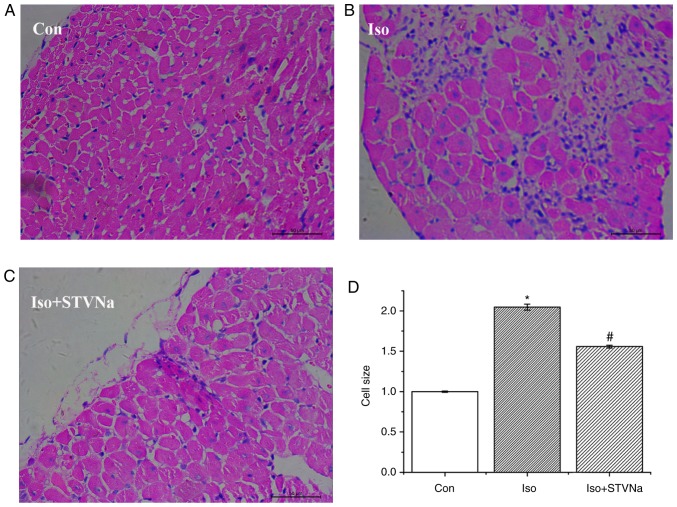
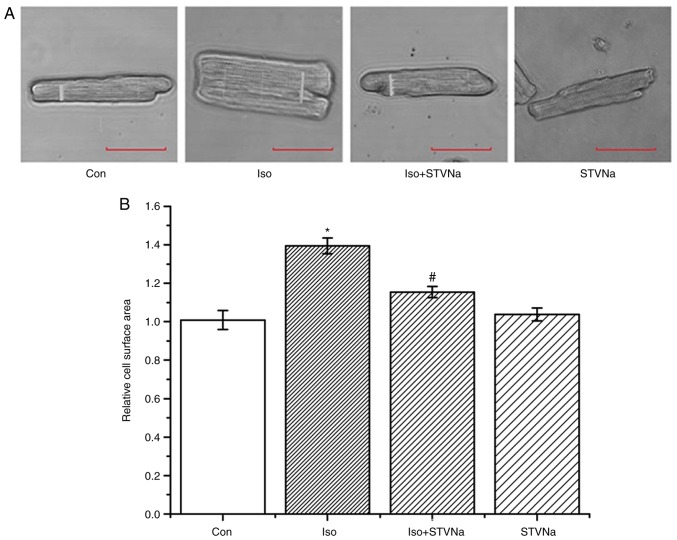
Experiment were carried out using rat heart tissues. Treatment with Iso significantly increased HW/BW (1.00±0.02 vs. 1.53±0.05; P<0.05; n=6; Fig. 1), HW/tibia (0.23±0.01 g/cm vs. 0.33±0.01 g/cm; P<0.05; n=6; Fig. 1) and LV/tibia (0.15±0.01 g/cm vs. 0.23±0.01 g/cm; P<0.05; n=6; Fig. 1) ratios. This effect was inhibited by STVNa (Fig. 1; HW/BW, 1.53±0.05 vs. 1.35±0.06; n=6; P=0.05; HW/tibia, 0.33±0.01 g/cm vs. 0.29±0.01 g/cm; n=6; P<0.05; LV/tibia, 0.23±0.01 g/cm vs. 0.20±0.01 g/cm; n=6; P=0.01). The histological analysis of myocardial tissues demonstrated that cardiomyocyte cross sectional areas were significantly increased in mice treated with Iso (1.00±0.01 fold-change vs. 2.05±0.04 fold-change; n=6; P<0.05; Fig. 2) and this increase was partly inhibited by STVNa (1.56±0.02 fold-change; n=6; P>0.05 vs. control; Fig. 2). Similar results were obtained subsequent to the examination of the effects of Iso and STVNa treatments on cardiomyocytes size in vitro, which were carried out using cardiomyocytes isolated from rats. Although STVNa did not solely affect cardiomyocyte surface area (control, 1.01±0.05 fold-change; STVNa, 1.04±0.03 fold-change, n=7 for each; P=0.639; Fig. 3) it prevented an increase in this parameter that was induced by Iso (Iso, 1.39±0.04 fold-change; Iso + STVNa, 1.15±0.03 fold-change; n=7 for each; P<0.05; Fig. 3).
Figure 1.
STVNa prevents Iso-induced cardiac hypertrophy. (A) Original images of hearts from different experimental groups as labelled. Graphs depicting (B) HW/Tibia (C) HW/BW and (D) LV/Tibia. Each bar represents the mean ± standard error of the mean. *P<0.05 vs. the Con group; #P<0.05 vs. Iso group (n=6 for each). HW, heart weight; Tibia, tibia length; BW, body weight; LV, left ventricular weight; Iso, isoprenaline; STVNa, isosteviol sodium; Con, control.
Figure 2.
STVNa prevents Iso-induced increase in cardiomyocyte size. Experiment were carried out using rat cardiac tissue. Original images of hematoxylin and eosin staining from the left ventricle (Scale bar=50 µm) in (A) Con group, (B) Iso group and (C) Iso + STVNa group. (D) Graph corresponding to the conditions in A, B and C. Each bar represents the mean ± standard error of the mean. *P<0.05 vs. the Con group; #P<0.05 vs. Iso group (n=6 for each). Iso, isoprenaline; STVNa, isosteviol sodium; Con, control.
Figure 3.
STVNa prevents the increase in cardiomyocyte surface area induced by Iso treatment in vitro. Experiments were carried out using cardiomyocytes isolated from rats. Original images of cardiomyocytes under (A) labelled conditions and the (B) corresponding graph. Each bar represents mean ± standard error of the mean. *P<0.05 vs. the Con group; #P<0.05 vs. Iso group (n=7 for each). Iso, isoprenaline; STVNa, isosteviol sodium; Con, control.
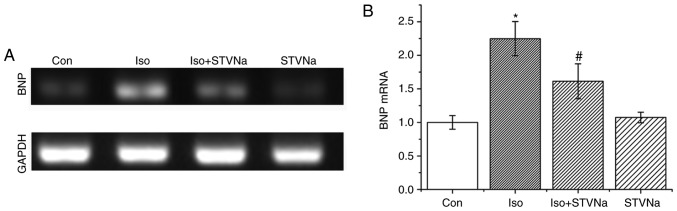
BNP mRNA is a well-established biomarker for cardiac hypertrophy and heart failure (17). mRNA measurement was carried out using cardiomyocytes isolated from untreated, wild-type rats. Iso treatment significantly increased BNP mRNA levels in cardiomyocytes (control, 1.00±0.10 fold-change; Iso, 2.25±0.25 fold-change; n=5 for each; P<0.05; Fig. 4). STVNa prevented this increase (1.61±0.25 fold-change; n=5; P<0.05 vs. control; Fig. 4) although it did not exhibit any effect on BNP mRNA levels when used on its own (1.07±0.08 fold-change; n=5; P>0.05 vs. control; Fig. 4).
Figure 4.
STVNa prevents the increase in cardiomyocytes BNP mRNA level induced by Iso treatment. Experiments were carried out using cardiomyocytes isolated from rats. Original images of reverse transcription PCR products under (A) labelled conditions and the (B) corresponding graph. Each bar represents the mean ± standard error of the mean. *P<0.05 vs. the Con group; #P<0.05 vs. Iso group (n=5 for each). BNP, brain natriuretic peptide; Iso, isoprenaline; STVNa, isosteviol sodium; Con, control.
STVNa reduces ROS production in cardiomyocytes treated with Iso
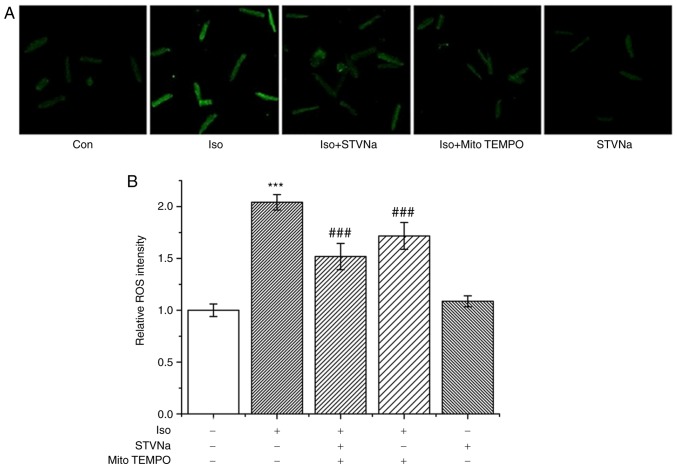
Iso treatment significantly increased ROS production in cardiomyocytes as indicated by DCFH fluorescence (from 1.00±0.06 fold-change under control conditions to 2.04±0.07 fold-change when treated with Iso; n=6 for each; P<0.001; Fig. 5). STVNa did not solely affect ROS production (1.09±0.05 fold-change; n=6; P=0.524 vs. the control; Fig. 5). However, STVNa prevented the effect exhibited by Iso (1.52±0.13 fold-change; n=6; P=0.004 vs. Iso-treated group; Fig. 5) in a similar manner to mitoTEMPO, a mitochondrial-targeted antioxidant (1.72±0.13 fold-change; n=6; P=0.274 vs. Iso + STVNa-treated group; Fig. 5). Experiments were carried out using cardiomyocytes isolated from untreated, wild-type rats.
Figure 5.
STVNa reduces ROS production in cardiomyocytes treated with Iso. Experiments were carried out using cardiomyocytes isolated from rats. Original images under (A) labelled conditions and the (B) corresponding graph. The level of ROS was detected using DCF. Scale bar is 100 µm. Each bar represents the mean ± standard error of the mean. ***P<0.01 vs. Con group; ###P<0.01 vs. Iso group (n=6 for each). ROS, reactive oxygen species; Iso, isoprenaline; STVNa, isosteviol sodium; Con, control.
STVNa prevents mitochondrial membrane depolarization induced by Iso treatment
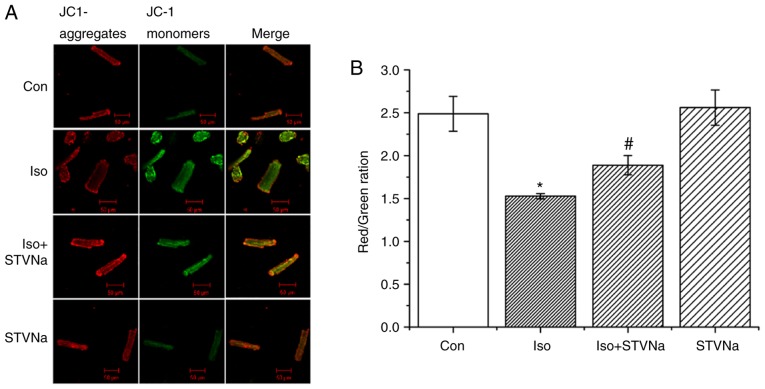
Treatment with Iso led to mitochondrial membrane depolarization in wild-type cardiomyocytes (isolated from untreated wild-type rats) as indicated by the significant decrease that was observed in channels ratio (from 2.49±0.20 under control conditions to 1.53±0.03 following Iso treatment; n=6 for each; P<0.05; Fig. 6). STVNa did not solely affect mitochondrial membrane potential (2.56±0.20; n=6; P=0.746 vs. control; Fig. 6). However, STVNa inhibited the effect of Iso treatment (1.89±0.11; n=6; P=0.011 vs. Iso group; Fig. 6).
Figure 6.
STVNa prevents mitochondrial membrane depolarization induced by Iso treatment. Experiments were carried out using cardiomyocytes isolated from rats. Original images under (A) labelled conditions and the (B) corresponding graph. Mitochondrial membrane potential was measured using JC-1. Scale bar is 100 µm. Each bar represents the mean ± standard error of the mean. *P<0.05 vs. Con; #P<0.05 vs. Iso group (n=6 for each). Iso, isoprenaline; STVNa, isosteviol sodium; Con, control.
STVNa prevents Ca2+ loading and impaired Ca2+ dynamics induced by Iso treatment
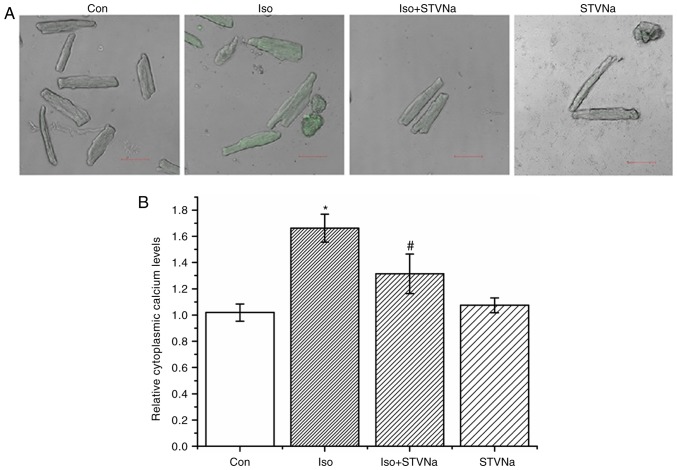
Cardiomyocytes were isolated from untreated wild-type rats. Treatment with Iso induced intracellular Ca2+ loading as reflected by the significant increase observed in Fluo-4 fluorescence (from 1.02±0.07 fold-change under control conditions to 1.66±0.11 fold-change when treated with Iso; n=6 for each; P<0.05; Fig. 7). STVNa did not solely affect intracellular Ca2+ (1.07±0.06 fold-change; n=6; P=0.707 vs. control; Fig. 7). However, STVNa inhibited the effect of Iso treatment (1.31±0.15 fold-change; n=6; P=0.04 vs. Iso group alone; Fig. 7).
Figure 7.
STVNa prevents Ca2+ loading that is induced by Iso treatment. Experiments were carried out using cardiomyocytes isolated from rats. Original images captured by the frame-scanning mode of the confocal microscope under (A) labelled conditions and the (B) corresponding graph. The level of Ca2+ was detected using Fluo-4. Scale bar is 50 µm. Each bar represents the mean ± standard error of the mean. *P<0.05 vs. the Con group; #P<0.05 vs. Iso group (n=6 for each). Iso, isoprenaline; STVNa, isosteviol sodium; Con, control.
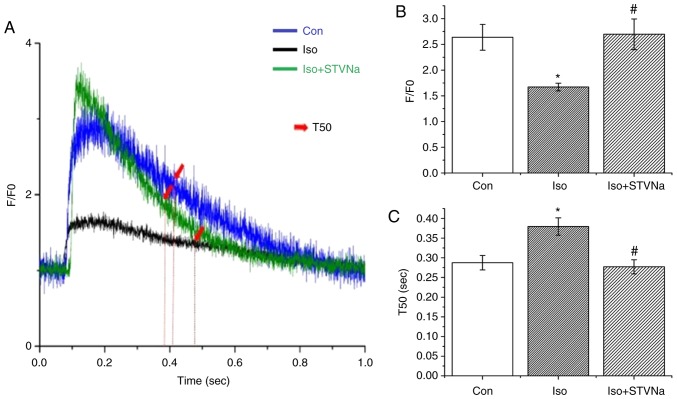
To examine any potential changes in Ca2+ dynamics, the transient Ca2+ was assessed in cardiomyocytes. The amplitude of calcium transient (F/F0) significantly decreased (from 2.64±0.25 under control conditions to 1.67±0.07 when treated with Iso; n=9 for each; P=0.013; Fig. 8) and the time of Ca2+ uptake was significantly extended (T50 values were 0.29±0.02 sec under control conditions and 0.38±0.02 sec when treated with Iso; n=9 for each; P=0.006; Fig. 8). Co-treatment with STVNa prevented the effects of Iso treatment (F/F0, 2.67±0.30; n=8; P=0.860 vs. control; T50, 0.28±0.02 S; n=9; P=0.711 vs. control; Fig. 8).
Figure 8.
STVNa prevents impairment in Ca2+ dynamics induced by Iso treatment. Experiments were carried out using cardiomyocytes isolated from rats. (A) Original tracing of Ca2+ transients. Intracellular Ca2+ was monitored using Fluo-4. Bar graph showing (B) F/F0 and (C) T50 for the groups corresponding in A. Each bar represents the mean ± standard error of the mean. *P<0.05 vs. the Con group; #P<0.05 vs. Iso group (n=9 for each). Iso, isoprenaline; STVNa, isosteviol sodium; Con, control.
STVNa prevents the sarcoplasmic reticulum (SR) Ca2+ depletion that is induced by Iso
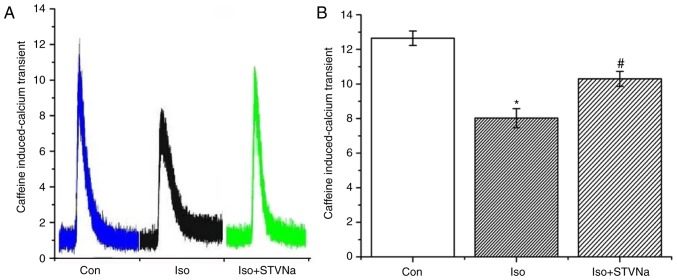
Caffeine (10 mM) was used to measure the quantities of Ca2+ stored in SR. Iso significantly decreased the quantity of Ca2+ in SR (from 12.65±0.42 under control conditions to 8.03±0.55 when treated with Iso; n=6 for each; P<0.05; Fig. 9). STVNa inhibited this effect (10.30±0.43; n=6; P=0.004 vs. the Iso group; Fig. 9).
Figure 9.
STVNa prevents SR calcium depletion induced by Iso. Caffeine induced Ca2+ transients indicating Ca2+ content of SR. Experiments were carried out using cardiomyocytes isolated from rats. Representative traces of Fluo-4 fluorescence following application of (A) caffeine with the (B) corresponding graph. Each bar represents the mean ± standard error of the mean. *P<0.05 vs. the Con group; #P<0.05 vs. Iso group (n=6 for each). Iso, isoprenaline; STVNa, isosteviol sodium; Con, control; SR, sarcoplasmic reticulum.
STVNa prevents the Iso-induced impairment of cardiomyocytes contractile function
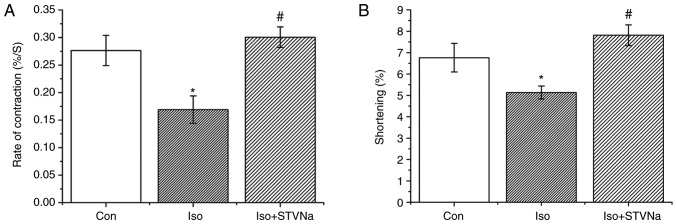
Treatment with Iso decreased shortening and rate of contraction of wild-type cardiomyoctyes (shortening, from 6.76±0.67% under control conditions to 5.13±0.30% when treated with Iso; n=8 for each; P=0.067; rate of contraction, from 0.28±0.03%.S-1 under control conditions to 0.17±0.02%.S-1 when treated with Iso; n=8 for each; P=0.009; Fig. 10). STVNa inhibited both effects exhibited by Iso treatment (shortening, 7.81±0.49%; n=8; P=0.004 vs. Iso; rate of contraction, 0.30±0.02%.S-1; n=8; P=0.003 vs. Iso; Fig. 10).
Figure 10.
STVNa prevents Iso-induced impairment of cardiomyocytes contractile function. Experiments were carried out using cardiomyocytes isolated from rats. Bar graphs depicting (A) rate of contraction (%/S) and (B) shortening of cardiomyocytes (%). Each bar represents the mean ± standard error of the mean. *P<0.05 vs. the Con group; #P<0.05 vs. Iso group (n=8 for each). Iso, isoprenaline; STVNa, isosteviol sodium; Con, control.
Discussion
A chronic increase in sympathetic activation occurs during hypertension, obesity, sleep apnea and mental stress, and this can promote the development of cardiac hypertrophy and heart failure through the sustained stimulation of β-adrenergic receptors (18). The results of the current study demonstrated that sustained stimulation with Iso induces cardiac hypertrophy, which is in agreement with the well-established features of the experimental model used (11). The current study revealed that the induction of cardiac hypertrophy by Iso was associated with i) increased ROS, ii) mitochondrial membrane depolarization, iii) intracellular Ca2+ loading, iv) impaired Ca2+ transients and v) impaired cardiac contractility.
A central mechanism that is associated with the development of cardiac hypertrophy is an increase in ROS and the subsequent oxidative stress (19). The activation of β-adrenoreceptors has been specifically linked with ROS generation and cardiac hypertrophy (20,21). Oxidative stress has been indicated to activate extracellular signal regulated kinase 1/2 and stimulate protein synthesis in ventricular remodeling (13,22). It has also been suggested that compounds attenuating oxidative stress may also attenuate cardiac hypertrophy (23). In the present study, it was demonstrated that STVNa did not solely affect ROS levels, but prevented an Iso-induced increase in ROS, making this compound a potential therapeutic candidate for use in the prevention of cardiac hypertrophy.
In addition to ATP synthesis, the electron transport chain of mitochondria is a significant source of ROS (24), which, in turn, can damage mitochondria and affect the activity and function of mitochondrial ion channels. Any alterations in mitochondrial ion channel function and mitochondrial homeostasis is reflected in the mitochondrial membrane potential. Mitochondrial membrane depolarization is a well-established indicator of disturbed mitochondrial homeostasis (25). The results of the current study indicated that Iso-treatment induced mitochondrial membrane depolarization, which is supported by previous studies (26-28) that have used this experimental model. STVNa did not solely affect mitochondrial membrane potential but prevented the mitochondrial membrane depolarization that was induced by Iso. These results suggested that STVNa prevented increases in ROS and consequently, mitochondrial damage.
Intracellular Ca2+ homeostasis is crucial for cardiac contractile function (29). Intracellular Ca2+ levels have been demonstrated to reflect the overall metabolic condition of cardiomyocytes (30,31). In the present study, Iso-pretreatment was revealed to increase intracellular Ca2+ and impair intracellular Ca2+ transients and cardiomyocytes contractility. These effects exhibited by Iso were expected, due to the results of multiple studies that indicated that sustained stimulation with β-agonists increased intracellular Ca2+ levels and impaired contractility (32-34). The sustained increase in Ca2+ activated the protein phosphatase calcineurin and its target, the NFAT family of transcription factors, which are critical mediators of pathological hypertrophy (35). Links between mitochondrial impairment, intracellular Ca2+ loading, impaired contractility and cardiac hypertrophy resulting in heart failure are well established (36). STVNa was revealed to prevent all negative events associated with sustained activation of β-adrenoceptors, including cardiac hypertrophy, ROS production, mitochondrial membrane depolarization, impaired Ca2+ homeostasis and cardiomyocytes contractility.
A recent study has demonstrated that STVNa sensitizes ATP-sensitive K+ (KATP) channels, in the mitochondria and sarcolemma, to KATP channel openers (37). The results of this aforementioned study, which revealed that STVNa did not affect mitochondrial membrane potential, is in agreement with the consensus that STVNa does not solely activate KATP channels (37), but rather makes channels more sensitive to endogenous channel openers. It has also been previously established that lactate, a product of anaerobic metabolism in the heart, activates KATP channels irrespective of high intracellular ATP levels (38,39). The activation of mitochondrial and sarcolemmal KATP channels has been demonstrated to regulate intracellular Ca2+ homeostasis (30,31). Therefore, the regulation of Ca2+ homeostasis by STVNa corresponds with its ability to sensitize KATP channels to KATP channel openers.
In conclusion, the current study demonstrated that STVNa prevents the development of cardiac hypertrophy, which is induced by Iso by preventing ROS generation, protecting mitochondrial function and regulating intracellular Ca2+ homeostasis. These results suggest that STVNa should be a potential therapeutic strategy against cardiac hypertrophy and heart failure in the future.
In the current study, the therapeutic effect of STVNa and the underlying mechanism by matching in vitro and in vivo experiments was defined. However, ex vivo experiments were not performed, which would provide another layer of tests for the present hypothesis. This could be viewed as a limitation of the present study although the in vitro and in vivo experiments match each other very well and strongly support the conclusions.
Supplementary Data
Acknowledgments
Not applicable.
Funding
The present study was supported by a grant from the 'Major Science and Technology Projects', Bureau of Science, Technology & Information, Guangzhou City, 2013 (Category reference number 164; grant. no. 201300000051) and the National Natural Science Foundation of China (grant. no. 31300940). AJ was supported by the University of Nicosia Medical School.
Availability of data and materials
The dataset used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Authors' contributions
YC and WT designed the experiments. YC, HB, HS, FF, ZF and NL performed the experiments and YC, HS and AJ analyzed the data. AJ and YC wrote the manuscript. All authors read and approved the final manuscript.
Ethics approval and consent to participate
All animal experimental protocols complied with the Guide for the Care and Use of Laboratory Animals, published by the United States National Institutes of Health. The current study was approved by the Institutional Animal Research Committee of South China University of Technology.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
References
- 1.Hu H, Sun Xo, Tian F, Zhang H, Liu Q, Tan W. Neuroprotective effects of isosteviol sodium injection on acute focal cerebral ischemia in rats. Oxid Med Cell Longev. 2016;2016;1379162 doi: 10.1155/2016/1379162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zhang H, Sun X, Xie Y, Zan J, Tan W. Isosteviol sodium protects against permanent cerebral ischemia injury in mice via inhibition of NF-κB-mediated inflammatory and apoptotic responses. J Stroke Cerebrovasc Dis. 2017;26:2603–2614. doi: 10.1016/j.jstrokecerebrovasdis.2017.06.023. [DOI] [PubMed] [Google Scholar]
- 3.Zan J, Zhang H, Lu MY, Beng HM, Zhong KL, Sun XO, Tan W. Isosteviol sodium injection improves outcomes by modulating TLRs/NF-κB-dependent inflammatory responses following experimental traumatic brain injury in rats. Neuroreport. 2018;29:794–803. doi: 10.1097/WNR.0000000000001033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zhong KL, Lu MY, Liu F, Mei Y, Zhang XJ, Zhang H, Zan J, Sun XO, Tan W. Isosteviol sodium protects neural cells against hypoxia-induced apoptosis through inhibiting MAPK and NF-κB pathways. J Stroke Cerebrovasc Dis. 2019;28:175–184. doi: 10.1016/j.jstrokecerebrovasdis.2018.09.020. [DOI] [PubMed] [Google Scholar]
- 5.Sun X, Yang Y, Xie Y, Shi X, Huang L, Tan W. Protective role of STVNa in myocardial ischemia reperfusion injury by inhibiting mitochondrial fission. Oncotarget. 2017;9:1898–1905. doi: 10.18632/oncotarget.22969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Liu Q, Hu H, Hu T, Han T, Wang A, Huang L, Tan Q, Tan W. STVNa attenuates right ventricle hypertrophy and pulmonary artery remodeling in rats induced by transverse aortic constriction. Biomed Pharmacother. 2018;101:371–378. doi: 10.1016/j.biopha.2018.02.078. [DOI] [PubMed] [Google Scholar]
- 7.Tang SG, Liu XY, Ye JM, Hu TT, Yang YY, Han T, Tan W. Isosteviol ameliorates diabetic cardiomyopathy in rats by inhibiting ERK and NF-κB signaling pathways. J Endocrinol. 2018;238:47–60. doi: 10.1530/JOE-17-0681. [DOI] [PubMed] [Google Scholar]
- 8.Dini FL, Galeotti GG, Terlizzese G, Fabiani I, Pugliese NR, Rovai I. Left ventricular mass and thickness: Why does it matter? . Heart Fail Clin. 2019;15:159–166. doi: 10.1016/j.hfc.2018.12.013. [DOI] [PubMed] [Google Scholar]
- 9.Bertero E and Maack C: Metabolic remodelling in heart failure Nat Rev Cardiol. 2018;15:457–470. doi: 10.1038/s41569-018-0044-6. [DOI] [PubMed] [Google Scholar]
- 10.Haselhuhn LR, Brotman DJ, Wittstein IS. Heart failure guidelines: What you need to know about the 2017 focused update. Cleve Clin J Med. 2019;86:123–139. doi: 10.3949/ccjm.86a.18022. [DOI] [PubMed] [Google Scholar]
- 11.Tse J, Powell JR, Baste CA, Priest RE, Kuo JF. Isoproterenol-induced cardiac hypertrophy: Modifications in characteristics of beta-adrenergic receptor, adenylate cyclase, and ventricular contraction. Endocrinology. 1979;105:246–255. doi: 10.1210/endo-105-1-246. [DOI] [PubMed] [Google Scholar]
- 12.Morisco C, Zebrowski DC, Vatner DE, Vatner SF, Sadoshim J. Beta-adrenergic cardiac hypertrophy is mediated primarily by the beta(1)-subtype in the rat heart. J Mol Cell Cardiol. 2001;33:561–573. doi: 10.1006/jmcc.2000.1332. [DOI] [PubMed] [Google Scholar]
- 13.Osadchii OE. Cardiac hypertrophy induced by sustained beta-adrenoreceptor activation: Pathophysiological aspects. Heart Fail Rev. 2007;12:66–86. doi: 10.1007/s10741-007-9007-4. [DOI] [PubMed] [Google Scholar]
- 14.Nichtova Z, Novotova M, Kralova E, Stankovicova T. Morphological and functional characteristics of models of experimental myocardial injury induced by isoproterenol. Gen Physiol Biophys. 2012;31:141–151. doi: 10.4149/gpb_2012_015. [DOI] [PubMed] [Google Scholar]
- 15.Krenek P, Kmecova J, Kucerova D, Bajuszova Z, Musil P, Gazova A, Ochodnicky P, Klimas J, Kyselovic J. Isoproterenol-induced heart failure in the rat is associated with nitric oxide-dependent functional alterations of cardiac function. Eur J Heart Fail. 2009;11:140–146. doi: 10.1093/eurjhf/hfn026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Claycomb WC, Palazzo MC. Culture of the terminally differentiated adult cardiac muscle cell: A light and scanning electron microscope study. Dev Biol. 1980;80:466–482. doi: 10.1016/0012-1606(80)90419-4. [DOI] [PubMed] [Google Scholar]
- 17.Sergeeva IA, Christoffels VM. Regulation of expression of atrial and brain natriuretic peptide, biomarkers for heart development and disease. Biochem Biophys Acta. 18322013:2403–2412. doi: 10.1016/j.bbadis.2013.07.003. [DOI] [PubMed] [Google Scholar]
- 18.Shin E, Ko KS, Rhee BD, Han J, Kim N. Different effects of prolonged β-adrenergic stimulation on heart and cerebral artery. Integr Med Res. 2014;3:204–210. doi: 10.1016/j.imr.2014.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhang GX, Kimura S, Nishiyama A, Shokoji T, Rahman M, Yao L, Nagai Y, Fujisawa Y, Miyatake A, Abe Y. Cardiac oxidative stress in acute and chronic isoproterenol-infused rats. Cardiovasc Res. 2005;65:230–238. doi: 10.1016/j.cardiores.2004.08.013. [DOI] [PubMed] [Google Scholar]
- 20.Srivastava S, Chandrasekar B, Gu Y, Luo J, Hamid T, Hill BG, Prabhu SD. Downregulation of cuzn-superoxide dismutase contributes to beta-adrenergic receptor-mediated oxidative stress in the heart. Cardiovasc Res. 2007;74:445–455. doi: 10.1016/j.cardiores.2007.02.016. [DOI] [PubMed] [Google Scholar]
- 21.Takimoto E, Kass DA. Role of oxidative stress in cardiac hypertrophy and remodeling. Hypertension. 2007;49:241–248. doi: 10.1161/01.HYP.0000254415.31362.a7. [DOI] [PubMed] [Google Scholar]
- 22.Vidal M, Wieland T, Lohse MJ, Lorenz K. β-Adrenergic receptor stimulation causes cardiac hypertrophy via a Gβγ/Erk-dependent pathway. Cardiovasc Res. 2012;96:255–264. doi: 10.1093/cvr/cvs249. [DOI] [PubMed] [Google Scholar]
- 23.Cha HN, Choi JH, Kim YW, Kim JY, Ahn MW, Park SY. Metformin inhibits isoproterenol-induced cardiac hypertrophy in mice. Korean J Physiol Pharmacol. 2010;14:377–384. doi: 10.4196/kjpp.2010.14.6.377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Murphy MP. How mitochondria produce reactive oxygen species. Biochem J. 2009;417:1–13. doi: 10.1042/BJ20081386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jovanović A. Cardioprotective signaling: Past, present and future. Eur J Pharmacol. 2018;833:314–319. doi: 10.1016/j.ejphar.2018.06.029. [DOI] [PubMed] [Google Scholar]
- 26.Zhou B, Wu LJ, Li LH, Tashiro S, Onodera S, Uchiumi F, Ikejima T. Silibinin protects against isoproterenol-induced rat cardiac myocyte injury through mitochondrial pathway after up-regulation of sirt1. J Pharmacol Sci. 2006;102:387–395. doi: 10.1254/jphs.FPJ06005X. [DOI] [PubMed] [Google Scholar]
- 27.Zhang Y, Xu J, Long Z, Wang C, Wang L, Sun P, Li P, Wang T. Hydrogen (H2) inhibits isoproterenol-induced cardiac hypertrophy via antioxidative pathways. Front Pharmacol. 2016;7:392. doi: 10.3389/fphar.2016.00392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Remondino A, Kwon SH, Communal C, Pimentel DR, Sawyer DB, Singh K, Colucci WS. Beta-Adrenergic receptor-stimulated apoptosis in cardiac myocytes is mediated by reactive oxygen species/c-Jun NH2-terminal kinase-dependent activation of the mitochondrial pathway. Circ Res. 2003;92:136–138. doi: 10.1161/01.RES.0000054624.03539.B4. [DOI] [PubMed] [Google Scholar]
- 29.Eisner DA, Caldwell JL, Kistamás K, Trafford AW. Calcium and excitation-contraction coupling in the heart. Circ Res. 2017;121:181–195. doi: 10.1161/CIRCRESAHA.117.310230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Du Q, Jovanović S, Clelland A, Sukhodub A, Budas G, Phelan K, Murray-Tait V, Malone L, Jovanović A. Overexpression of SUR2A generates a cardiac phenotype resistant to ischaemia. FASEB J. 2006;20:1131–1141. doi: 10.1096/fj.05-5483com. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sukhodub A, Sudhir R, Du Q, Jovanović S, Reyes S, Jovanović A. Nicotinamide-rich diet improves physical endurance by up-regulating SUR2A in the heart. J Cell Mol Med. 2011;15:1703–1712. doi: 10.1111/j.1582-4934.2010.01156.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.de Lucia C, Eguchi A, Koch WJ. New insights in cardiac β-adrenergic signaling during heart failure and aging. Front Pharmacol. 2018;9:904. doi: 10.3389/fphar.2018.00904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rengo G, Lymperopoulos A, Koch WJ. Future g protein-coupled receptor targets for treatment of heart failure. Curr Treat Options Cardiovasc Med. 2009;11:328–338. doi: 10.1007/s11936-009-0033-5. [DOI] [PubMed] [Google Scholar]
- 34.Bristow MR, Ginsburg R, Umans V, Fowler M, Minobe W, Rasmussen R, Zera P, Menlove R, Shah P, Jamieson S, et al. Beta 1- and beta 2-adrenergic-receptor subpopulations in nonfailing and failing human ventricular myocardium: Coupling of both receptor subtypes to muscle contraction and selective beta 1-receptor downregulation in heart failure. Circ Res. 1986;59:297–309. doi: 10.1161/01.RES.59.3.297. [DOI] [PubMed] [Google Scholar]
- 35.Dorn GW, II, Force T. Protein kinase cascades in the regulation of cardiac hypertrophy. J Clin Invest. 2005;115:527–537. doi: 10.1172/JCI24178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bertero E, Maack C. Calcium signaling and reactive oxygen species in mitochondria. Circ Res. 2018;122:1460–1478. doi: 10.1161/CIRCRESAHA.118.310082. [DOI] [PubMed] [Google Scholar]
- 37.Fan Z, Wen T, Chen Y, Huang L, Lin W, Yin C, Tan W. Isosteviol sensitizes sarcKATP channels towards pinacidil and potentiates mitochondrial uncoupling of diazoxide in Guinea Pig ventricular myocytes. Oxid Med Cell Longev. 2016;2016;6362812 doi: 10.1155/2016/6362812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Jovanović S, Du Q, Sukhodub A, Jovanović A. M-LDH physically associated with sarcolemmal K channels mediates cytoprotection in heart embryonic H9C2 cells. Int J ATP Biochem Cell Biol. 2009;41:2295–2301. doi: 10.1016/j.biocel.2009.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Jovanović S, Du Q, Sukhodub A, Jovanović A. Dual mechanism of cytoprotection afforded by M-LDH in embryonic heart H9C2 cells. Biochim Biophys Acta. 2009;1793:1379–1386. doi: 10.1016/j.bbamcr.2009.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The dataset used and/or analyzed during the current study are available from the corresponding author on reasonable request.