Abstract
Background
Women treated with chest radiation for Hodgkin lymphoma (HL) are at significantly increased risk of breast cancer and cardiovascular disease. HL survivors are recommended to have annual dual screening with mammogram (MMG) and breast magnetic resonance imaging (MRI). They are also recommended to undergo echocardiogram (echo) 5 years after completion of radiation. We performed a pilot study to characterize the women who are and are not receiving proper dual screening for breast cancer and baseline echo, and to examine the impact of a LTFU clinic consultation on screening.
Methods
A retrospective chart review of 114 women treated for HL at University of Minnesota (UMN) between 1993 and 2009 was performed. Demographics, disease and treatment history (age at diagnosis, stage, radiation dose and field, chemotherapy, recurrence) were assessed, as well as screening practices (MMG, MRI, both and echo), participation in LTFU clinic, and recommendations from providers. Data was summated in yes/no (y/n) format; statistical analysis was performed using chi- squared and Fisher’s exact tests. Breast cancer and cardiovascular screening outcomes were compared by participation in the LTFU clinic (y/n) using Fisher’s exact tests. P values < 0.05 were considered statistically significant.
Results
Forty-one of 114 women met inclusion criteria and had follow-up data for analysis. Median age at diagnosis was 29 years; 67.6% were diagnosed at stage IIa. Median dose of radiation was 3570 cGy. 56.1% participated in the LTFU clinic at the UMN. 36.6% had dual screening with both MMG and MRI, 41.5% had screening with only MMG, and 19.5% had no screening performed. Women were more likely to have dual screening if they were seen in LTFU clinic vs not seen in LTFU clinic (52.2 vs 16.7%, p = 0.02). 67.5% of women were screened with echo; women were also more likely to have screening with echo if seen in LTFU clinic vs not seen (86.4 vs 44.4%, p = 0.007).
Conclusion
Many women are not getting the proper dual screening for breast cancer despite their increased risk, with only 36.6% of our study sample getting dual screening. Having a consultation in a LTFU clinic increases dual screening for breast cancer and echo screening for cardiovascular disease. Proper screening allows for detection of secondary breast cancer at earlier stages where treatment can be local therapy. Diagnosing CV disease early could allow for proper preventative treatment or intervention.
Keywords: Radiation, Breast cancer, Screening, Recommendation
Background
Hodgkin lymphoma (HL) is a disease that typically affects approximately 10,000 young adults and children annually, with cure rates exceeding 80% [1]. Improved treatment modalities with chemotherapy and radiation have resulted in larger cohorts of patients living without cancer yet with long-term and late effects of their cancer and cancer treatment. Many of these survivors suffer from late effects of their treatment including second cancers, cardiovascular disease, pulmonary disease, hypothyroidism, and gonadal dysfunction [2].
Second cancers are a leading cause of morbidity and death in Hodgkin lymphoma survivors. The incidence of developing second cancers is 9–13% at 15–20 years and 18–26% at 30 years after completing therapy [3, 4]. The increased risk begins as soon as 8 years after chest radiation with the cumulative incidence of breast cancer increases as time from radiation increases, 13–20% incidence by age 40 to 45 [5] and a 35% incidence by age 50 [6, 7]. Other factors that influence risk of breast cancer in Hodgkin survivors include area of radiation and dose of radiation, with larger areas and higher doses being correlated to higher incidence of breast cancers [5, 7] as well as family history and other known risk factors [5]. The incidence of breast cancer in women treated with mantle zone radiation is similar to that of BRCA mutation [7] in which chemoprevention, aggressive surveillance and prophylactic mastectomies are often considered [8]. With the increased risk of breast cancer, current guidelines from the Children’s Oncology Group (COG) and the National Comprehensive Cancer Network (NCCN) recommend screening with both mammogram (MMG) and breast MRI beginning at the age of 25 or 8 years post-therapy for women who received radiation to the chest before age 40 [9, 10]. Studies suggest the sensitivity and specificity of the screening modalities were as follows: for MRI alone, 80 and 93.5%, respectively; MMG alone, 70 and 95%, respectively; both modalities combined, 100 and 88.6%, respectively [11]. If these guidelines are followed, tumors are detected at earlier stages with no lymph node involvement, predicting improvements in overall survival [11, 12].
Survivors of HL who were treated with radiation therapy are also at an increased risk of cardiovascular disease. The spectrum of complications is wide and includes coronary artery disease, congestive heart failure, vascular abnormalities, pericardial disease, conduction abnormalities and sudden cardiac death [13]. The risk of CV disease is estimated to be 17% by age 35 years [6]. While there is limited data regarding the best screening modalities for this subset of patients, the Children’s Oncology Group (COG) recommends echocardiography by 5 years for cardiovascular disease screening and then at regular intervals [14], while the National Comprehensive Cancer Network (NCCN) recommends a baseline stress echocardiogram at 10 years post-radiation [15]. Others recommend initial stress testing at 5 years post-radiation based on increased number of cardiovascular events between 5 and 10 years [16].
Given the potential risk for these long term complications in Hodgkin lymphoma survivors, the Institute of Medicine (IOM) recommends that every cancer patient receive a survivorship care plan (SCP) that includes a detailed history of their cancer diagnosis and treatment, potential consequences, and screening recommendations for surveillance and follow-up care [17]. Long-term follow-up (LTFU) or survivorship clinics have started at many centers to help patients with this transition. Little data exists, however, on how LTFU clinic or receipt of a SCP impact screening practices. Using data from the University of Minnesota, our aim was to determine the utility of a LTFU clinic and receipt of a SCP in improving breast cancer and cardiovascular screening practices in high-risk female survivors of HL.
Methods
Study design and target population
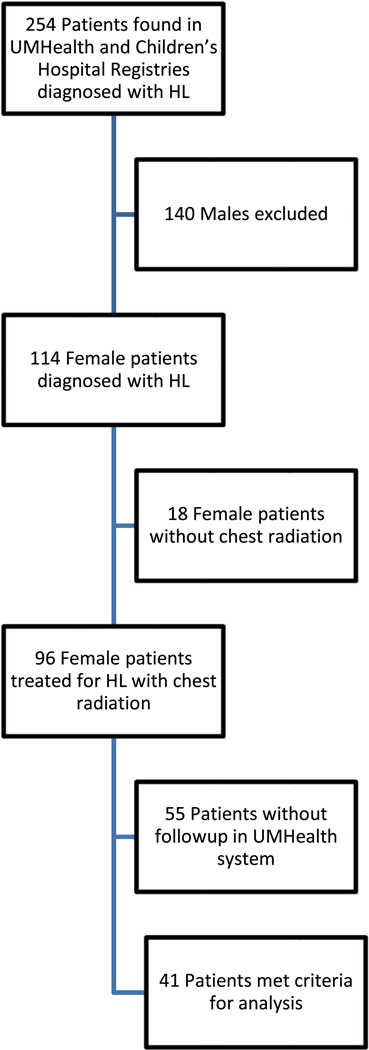
With institutional review board approval, a retrospective review of 254 patients found in the registries at University of Minnesota Health (UMHealth) and associated Children’s Hospital treated for Hodgkin Lymphoma between the years 1993 and 2009 was performed. Excluding males and patients who did not receive radiation to the chest resulted in 96 patients. Our final analysis included 41 individuals with data and follow-up in our system (Fig. 1). All of these individuals met guideline criteria for dual breast cancer screening with breast MRI and MMG, based on radiation field including the chest.We excluded younger women <25 years at the time of analysis not yet eligible for breast cancer screening per guidelines. Records were reviewed to determine age at diagnosis, stage of disease at diagnosis, radiotherapy doses and fields when available as well as risk factors for heart disease and breast cancer (Table 1). Data on screening practices, as well as participation in a long-term follow-up clinic were reviewed as outlined below.
Fig.1.
Final analysis including 41 individuals with data and follow-up
Table 1.
Summary of patient characteristics by use of LTFU clinic
| Variable | All patients N =41 |
No LTFU clinic N =18 |
LTFU clinic N =23 |
p value | |||
|---|---|---|---|---|---|---|---|
| N | % | N | % | N | % | ||
| Stage at diagnosis | 0.87 | ||||||
| 1a | 2 | 5.4 | 1 | 6.7 | 1 | 4.6 | |
| 2a | 25 | 67.6 | 9 | 60.0 | 16 | 72.7 | |
| 2b | 5 | 13.5 | 2 | 13.3 | 3 | 13.6 | |
| 3a | 2 | 5.4 | 1 | 6.7 | 1 | 4.6 | |
| 4b | 3 | 8.1 | 2 | 13.3 | 1 | 4.6 | |
| Missing | 4 | 3 | 1 | ||||
| Radiation field | 0.0004 | ||||||
| Involved field/mini-mantle | 17 | 41.5 | 10 | 55.6 | 7 | 30.4 | |
| Mantle | 19 | 46.3 | 3 | 16.7 | 16 | 69.6 | |
| Chest radiation, unsp field | 5 | 12.2 | 5 | 27.8 | 0 | 0.0 | |
| Chemotherapy | 0.57 | ||||||
| No | 3 | 7.3 | 2 | 11.1 | 1 | 4.4 | |
| Yes | 38 | 92.7 | 16 | 88.9 | 22 | 95.7 | |
| Doxorubicin | 1.00 | ||||||
| No | 2 | 5.1 | 1 | 6.3 | 1 | 4.4 | |
| Yes | 37 | 94.9 | 15 | 93.8 | 22 | 95.7 | |
| Missing | 2 | 2 | 0 | ||||
| BMT | 0.57 | ||||||
| No | 38 | 92.7 | 16 | 88.9 | 22 | 95.7 | |
| Yes | 3 | 7.3 | 2 | 11.1 | 1 | 4.4 | |
| Recurrence | 0.68 | ||||||
| No | 34 | 82.9 | 14 | 77.8 | 20 | 87.0 | |
| Yes | 7 | 17.1 | 4 | 22.2 | 3 | 13.0 | |
| Sign family Hx of BrCa | 0.38 | ||||||
| No | 32 | 84.2 | 13 | 76.5 | 19 | 90.5 | |
| Yes | 6 | 15.8 | 4 | 23.5 | 2 | 9.5 | |
| Missing | 3 | 1 | 2 | ||||
| Family Hx of CV disease | 0.09 | ||||||
| No | 13 | 34.2 | 3 | 17.7 | 10 | 47.6 | |
| Yes | 25 | 65.8 | 14 | 82.4 | 11 | 52.4 | |
| Missing | 3 | 1 | 2 | ||||
| Tobacco use | 0.41 | ||||||
| No | 30 | 73.2 | 12 | 66.7 | 18 | 78.3 | |
| Yes | 11 | 26.8 | 6 | 33.3 | 5 | 21.7 | |
| Alcohol use | 0.96 | ||||||
| No | 11 | 28.2 | 5 | 27.8 | 6 | 28.6 | |
| Yes | 28 | 71.8 | 13 | 72.2 | 15 | 71.4 | |
| Missing | 2 | 0 | 2 | ||||
| Hyperlipidemia | 0.08 | ||||||
| No | 31 | 75.6 | 11 | 61.1 | 20 | 87.0 | |
| Yes | 10 | 24.4 | 7 | 38.9 | 3 | 13.0 | |
| Hypertension | 0.64 | ||||||
| No | 36 | 87.8 | 15 | 83.3 | 21 | 91.3 | |
| Yes | 5 | 12.2 | 3 | 16.7 | 2 | 8.7 | |
| Diabetes | 1.00 | ||||||
| No | 40 | 97.6 | 18 | 100.0 | 22 | 95.7 | |
| Yes | 1 | 2.4 | 0 | 0.0 | 1 | 4.4 | |
| Hypothyroid | 0.44 | ||||||
| No | 21 | 51.2 | 8 | 44.4 | 13 | 56.5 | |
| Yes (all tx induced) | 20 | 48.8 | 10 | 55.6 | 10 | 43.5 | |
| Provider | 0.12 | ||||||
| PCP | 28 | 68.3 | 10 | 55.6 | 18 | 78.3 | |
| Onc | 13 | 31.7 | 8 | 44.4 | 5 | 21.7 | |
| Cardiovascular disease | 0.72 | ||||||
| No | 29 | 72.5 | 14 | 77.8 | 15 | 68.2 | |
| Yes | 11 | 27.5 | 4 | 22.2 | 7 | 31.8 | |
| Breast cancer | 0.20 | ||||||
| No | 38 | 95.0 | 16 | 88.9 | 22 | 100.0 | |
| Yes | 2 | 5.0 | 2 | 11.1 | 0 | 0.0 | |
| Missing | 1 | 0 | 1 | ||||
| Breast biopsies | 1.00 | ||||||
| No | 28 | 70.0 | 13 | 72.2 | 15 | 68.2 | |
| Yes | 12 | 30.0 | 5 | 27.8 | 7 | 31.8 | |
| Missing | 1 | 0 | 1 | ||||
| Other cancer | 1.00 | ||||||
| No | 37 | 92.5 | 17 | 94.4 | 20 | 90.9 | |
| Yes | 3 | 7.5 | 1 | 5.6 | 2 | 9.1 | |
| Missing | 1 | 0 | 1 | ||||
| N | Median (min, max) | N | Median (min, max) | N | Median (min, max) | ||
| Age at diagnosis | 41 | 29.0 (12–58) | 18 | 36.5 (22–58) | 23 | 24.0 (12–52) | 0.001 |
| Year of diagnosis | 41 | 2001 (1993–2009) | 18 | 2001.5 (1993–2009) | 23 | 2001 (1993–2009) | 0.69 |
| Last follow-up (year) | 41 | 2014 (2010–2014) | 18 | 2014(2011–2014) | 23 | 2014 (2010–2014) | 0.65 |
| Radiation dose | 26 | 3570 (2040–4500) | 5 | 3570 (3060–3600) | 21 | 3570 (2040–4500) | 0.97 |
Primary outcome
Our primary aim was to determine the prevalence of proper breast cancer and cardiovascular screening in women seen in follow-up either by a primary care physician or in a specialized LTFU clinic for cancer survivors. Our secondary aim was to determine whether participation in a LTFU clinic impacted prevalence of screening. Proper screening practices were defined as annual dual screening with both MRI and MMG for breast cancer and screening echocardiography between 5 and 10 years after diagnosis per COG and NCCN guidelines [9, 10, 14, 15].
Independent variables
Basic demographics (age) as well as disease characteristics (stage, age at diagnosis, use of chemotherapy, use of radiation) and other known risk factors for breast cancer and CV disease were obtained. Screening practices were defined as annual dual screening with both MRI and MMG as per recommended guidelines, those receiving only MMG screening, those who had received at least one breast MRI, and those who had not received any breast cancer screening. Characteristics were determined from documentation in physician and nursing notes, as well as demographics forms submitted by the patients to their providers, and actual imaging reports. Data was also collected on whether breast MRI was recommended by a clinician.
For CV screening, we defined the presence of cardiovascular disease screening as a hybrid of the COG and NCCN recommendations: screening echocardiography between 5 and 10 years after diagnosis. The Children’s Oncology Group guidelines recommend the frequency of echocardiography based on cumulative dose of anthracycline received as well as the presence or absence of mantle radiation and age of treatment; the National Comprehensive Cancer Network recommends screening for cardiac disease with a stress echocardiogram at 10 years following the completion of chemotherapy and radiation. Women were considered to have recommended screening with at least one screening echocardiogram up to 10 years after diagnosis.
Statistical analysis
Patient demographics and clinical data were summarized and compared by whether they attended the LTFU clinic (yes, no) using chi-squared and Fisher’s exact tests as appropriate. Breast cancer and CV screening outcomes were compared by participation in the LTFU clinic using Fisher’s exact tests. P values < 0.05 were considered statistically significant.
Results
Patient demographics
Forty-one women met inclusion criteria and had follow-up data for analysis. 56.1% participated in the LTFU clinic. Table 1 reports selected demographics and disease characteristics and treatment. Overall the majority were diagnosed at stage IIa (67.6%). Median dose of radiation was 3570 cGy (2040–4500 cGy). Between the cohorts, patients did not differ by stage at diagnosis, year of diagnosis, treatment with chemotherapy, dose of radiation. Patients did differ by age at diagnosis and field of radiation, with those attending the LTFU clinic being more likely to have a younger age at diagnosis of HL (24 vs 36.5 years; p = 0.001) and to have received mantle field radiation (69.6 vs 16.7%; p = 0.0004) as opposed to involved field or unspecified chest radiation.
Breast cancer screening
Table 2 reports the breast cancer screening practices of the women not seen and those seen in LTFU. Among all women, 80.5% had some breast cancer screening performed seen (77.8% no LTFU vs 82.6% LTFU; p = 0.71). Regardless of follow-up location, 48.8% of women had at least one MRI, and 36.6% were undergoing recommended annual dual screening with both MMG and MRI. Women seen in LTFU consultation were more likely to have had any MRI performed (69.6 vs 22.2%; p = 0.003) and were more likely to undergo recommended annual dual screening (52.2 vs 16.7%; p = 0.02). 55.6% of the women not seen in LTFU consultation received MMG screening alone as compared to 30.4% of those seen in LTFU (p = 0.11). MRI was more likely to be recommended by a LTFU clinic provider (91.3 vs 44.4%; p = 0.002), than by those having their follow-up care either through their primary care practitioner or by their primary treating oncologist.
Table 2.
Breast cancer and CV screening outcomes by use of LTFU clinic
| All patients N =41 |
No LTFU clinic N =18 |
LTFU clinic N =23 |
|||||
|---|---|---|---|---|---|---|---|
| Variable | N | % | N | % | N | % | p value |
| Screening for breast cancer | 0.71 | ||||||
| No | 8 | 19.5 | 4 | 22.2 | 4 | 17.4 | |
| Yes | 33 | 80.5 | 14 | 77.8 | 19 | 82.6 | |
| Mammogram only | 0.11 | ||||||
| No | 24 | 58.5 | 8 | 44.4 | 16 | 69.6 | |
| Yes | 17 | 41.5 | 10 | 55.6 | 7 | 30.4 | |
| Any MRIs | 0.003 | ||||||
| No | 21 | 51.2 | 14 | 77.8 | 7 | 30.4 | |
| Yes | 20 | 48.8 | 4 | 22.2 | 16 | 69.6 | |
| Dual screening | 0.02 | ||||||
| No | 26 | 63.4 | 15 | 83.3 | 11 | 47.8 | |
| Yes | 15 | 36.6 | 3 | 16.7 | 12 | 52.2 | |
| MRI recommended | 0.002 | ||||||
| No | 12 | 29.3 | 10 | 55.6 | 2 | 8.7 | |
| Yes | 29 | 70.7 | 8 | 44.4 | 21 | 91.3 | |
| CV screening | 0.007 | ||||||
| No | 13 | 32.5 | 10 | 55.6 | 3 | 13.6 | |
| Yes | 27 | 67.5 | 8 | 44.4 | 19 | 86.4 | |
Reasons documented for not obtaining MRI included the following: no insurance and high cost of MRI, pregnancy or breast feeding when due for screening, or canceled LTFU clinic appointments. One woman had dual screening, received a biopsy which returned benign and decided against aggressive screening. One primary care provider had received the consultation note from the LTFU provider but did not encourage dual screening to the patient, who ultimately only had MMG screening.
Cardiovascular screening
Among all women in the final analysis, 67.5% were getting CV screening. Women seen in LTFU consultation were more likely to have CV screening with an echocardiogram up to 10 years after completion of HL treatment (86.4 vs 44.4%; p = 0.007).
Discussion
Women who have received radiation to the chest are at increased risk of breast cancer and cardiovascular disease, however many patients and many primary care physicians do not know of this increased risk [ 18–21 ]. We investigated if participation in a LTFU clinic had an impact on screening rates. This is one of the first studies to compare screening practices of a cohort of patients followed in the same medical system by participation in LTFU clinic. There were several notable findings. First, the prevalence of breast cancer screening overall was similar to that of the general population, at 80.5% [5]. For these high-risk women in which dual screening is recommended by the national cancer organizations Children’s Oncology Group and National Comprehensive Cancer Network, only 36.6% of these women were undergoing recommended annual dual screening with MMG and MRI. Participation in a LTFU clinic did increase screening rates, with 52.2% having the recommended annual dual screening and 91.3% receiving the recommendation to have a screening MRI as compared to 16.7 and 44.4%, respectively, in those not seen in LTFU. Notably, MRI was more likely to be recommended by a LTFU clinic provider than either primary oncologist or primary care physician, which likely impacted the screening rates.
Early breast cancer screening is important for long term survival, and breast MRI improves the sensitivity of early breast cancer detection in women treated with chest radiation for HL [11]. Ng et al. found that the addition of breast MRI to MMG led to improvement in the sensitivity of breast cancer detection from 68 to 94% [22]. Early detection is important to increase the odds of cure, and to decrease the morbidity and mortality from treating advanced stage breast cancer, particularly in women who have already undergone potentially treatment limiting doses of chemotherapy or radiation. Our study demonstrates that most women are receiving some breast cancer screening. However, it appears there continues to be a barrier to understanding that Hodgkin lymphoma survivors who have received chest radiation are at high risk for breast cancer and should not be receiving “average” risk breast cancer screening recommendations with mammogram alone. If these guidelines for high-risk breast cancer screening are followed, tumors are detected at earlier stages, predicting improvements in overall survival [11].
Cardiovascular disease is increased among Hodgkin lymphoma survivors who have received mantle radiation. There appears to be a general understanding of the risk of cardiovascular disease in this population with 67.5% of all subjects receiving some screening. There was still a significantly increased prevalence of screening for cardiovascular disease for women seen in LTFU consultation. The higher rates of CV screening when compared to breast cancer screening could be explained by our definition of CV screening. CV screening requires a one-time imaging test compared to annual screening for breast cancer. We also used a hybrid recommendation of 5–10 years after completion of radiation treatment for HL, using a stricter timeline of 5 years would likely have revealed a lower rate of CV screening. It is also possible that given Hodgkin lymphoma patients often receive cardiac screening during cancer treatment due to anthracycline use, there may be more generalized awareness about cardiovascular disease. In this limited study, we were unable to comment on other cardiovascular screening measures such as blood pressure, lipid, and blood sugar management.
The transition from cancer patient to cancer survivor presents an opportunity for the health care community to provide patients education on their disease and risks for late effects of their treatment. Survivorship care plans and LTFU clinic consultations can help support this transition. The Institute of Medicine is recommending every patient be provided with a summary of their cancer treatment and recommendations for follow-up care in the form of a survivorship care plan. In our system, patients seen in a LTFU clinic 5 years after their treatment receive a SCP as well as counseling regarding long term screening. In this specialized setting, it appears the increased expertise and awareness in survivorship guidelines allow for more guideline specific care. However, there still appear to be barriers in following recommended guidelines. For example, while 91% of those seen in a LTFU received a recommendation to obtain a breast MRI, only 69.6% had actually received an MRI. Some of these barriers appeared to be lack of insurance coverage, an opposing recommendation from a primary care physician, and cost of MRI. Further work needs to be done to better understand and overcome these barriers.
At our institution during the time of this study, patients 5 years out from cancer diagnosis and treatment were referred by their primary oncologist to our LTFU clinic for cancer survivors. At that appointment, survivors receive a survivorship care plan, and counseling regarding late effects from their cancer therapy, as well as best screening practices for this patient population. Currently, we are in the process of automating this referral process so that all patients completing curative intent treatment will receive a survivorship care plan and consultation both at the completion of curative intent therapy and then again at 5 years.
There were several limitations to this pilot study. First, it was a retrospective chart review, which relies heavily on proper documentation by providers, as well as self-reports from patients on their screening practices when their MMGs were not performed through our system. This was also a small sample size, as many women were referred from neighboring areas without major medical centers, and they did not receive their follow-up care in our system. Despite these limitations, this pilot investigation provides preliminary evidence that LTFU consultation and SCPs are important in improving screening practices for secondary disease in Hodgkin lymphoma survivors.
In conclusion, consultations in a LTFU clinic can have a large impact on improving screening rates for secondary complications like breast cancer and cardiovascular disease in Hodgkin lymphoma survivors treated with mantle radiation. These findings can provide a foundation for further investigations regarding impact of LTFU clinic consultation on patients’ perceived risks and satisfaction as well as primary care physicians’ knowledge of late effects. Finally, further work is needed to better understand how to provide LTFU clinic consultations and SCPs in a cost-effective manner.
Footnotes
Compliance with ethical standards
Conflict of interest The authors declare that they have no conflict of interest.
Ethical approval All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed consent Informed consent was obtained from all individual participants included in the study.
References
- 1.Glossmann JP, Josting A, Diehl V (2002) New treatments for Hodgkin’s disease. Curr Treat Options in Oncol 3(4):283–290. 10.1007/s11864-002-0028-x [DOI] [PubMed] [Google Scholar]
- 2.Matasar MJ, Ford JS, Riedel ER, Salz T, Oeffinger KC, Straus DJ (2015) Late morbidity and mortality in patients with Hodgkin’s lymphoma treated during adulthood. J Natl Cancer Inst 107(4): djv018. 10.1093/jnci/djv018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mertens AC, Liu Q, Neglia JP, Wasilewski K, Leisenring W, Armstrong GT, Robison LL, Yasui Y (2008) Cause-specific late mortality among 5-year survivors of childhood cancer: the Childhood Cancer Survivor Study. J Natl Cancer Inst 100(19): 1368–1379. 10.1093/jnci/djn310 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hodgson DC, Gilbert ES, Dores GM, Schonfeld SJ, Lynch CF, Storm H, Hall P, Langmark F, Pukkala E, Andersson M, Kaijser M, Joensuu H, Fossa SD, Travis LB (2007) Long-term solid cancer risk among 5-year survivors of Hodgkin’s lymphoma. J Clin Oncol 25(12):1489–1497. 10.1200/JC0.2006.09.0936 [DOI] [PubMed] [Google Scholar]
- 5.Henderson TO, Amsterdam A, Bhatia S, Hudson MM, Meadows AT, Neglia JP, Diller LR, Constine LS, Smith RA, Mahoney MC, Morris EA, Montgomery LL, Landier W, Smith SM, Robison LL, Oeffinger KC (2010) Systematic review: surveillance for breast cancer in women treated with chest radiation for childhood, adolescent, or young adult cancer. Ann Intern Med 152(7):444–455; W144–54. 10.7326/0003-4819-152-7-201004060-00009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Greenfield DM, Wright J, Brown JE, Hancock BW, Davies HA, O’toole L, Eiser C, Coleman RE, Ross RJ (2006) High incidence of late effects found in Hodgkin’s lymphoma survivors, following recall for breast cancer screening. Br J Cancer 94(4):469–472. 10.1038/sj.bjc.6602974 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Moskowitz CS, Chou JF, Wolden SL, Bernstein JL, Malhotra J, Novetsky Friedman D, Mubdi NZ, Leisenring WM, Stovall M, Hammond S, Smith SA, Henderson TO, Boice JD, Hudson MM, Diller LR, Bhatia S, Kenney LB, Neglia JP, Begg CB, Robison LL, Oeffinger KC (2014) Breast cancer after chest radiation therapy for childhood cancer. J Clin Oncol 32(21):2217–2223. 10.1200/JOT.2013.54.4601 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Boucher AA, Blaes AH (2013) Prophylactic mastectomy: a treatment alternative for Hodgkin survivors? Clin Breast Cancer 13(5): 307–308. 10.1016/j.clbc.2013.06.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Landier W, Bhatia S, Eshelman DA, Forte KJ, Sweeney T, Hester AL, Darling J, Armstrong FD, Blatt J, Constine LS, Freeman CR, Friedman DL, Green DM, Marina N, Meadows AT, Neglia JP, Oeffinger KC, Robison LL, Ruccione KS, Sklar CA, Hudson MM (2004) Development of risk-based guidelines for pediatric cancer survivors: the Children’s Oncology Group Long-Term Follow-Up Guidelines from the Children’s Oncology Group Late Effects Committee and Nursing Discipline. J Clin Oncol 22(24): 4979–4990. 10.1200/JOT.2004.11.032 [DOI] [PubMed] [Google Scholar]
- 10.Bevers TB, Anderson BO, Bonaccio E, Buys S, Daly MB, Dempsey PJ, Farrar WB, Fleming I, Garber JE, Harris RE, Heerdt AS, Helvie M, Huff JG, Khakpour N, Khan SA, Krontiras H, Lyman G, Rafferty E, Shaw S, Smith ML, Tsangaris TN, Williams C, Yankeelov T (2009) NCCN clinical practice guidelines in oncology: breast cancer screening and diagnosis. J Natl Compr Cancer Netw 7(10):1060–1096. 10.6004/jnccn.2009.0070 [DOI] [PubMed] [Google Scholar]
- 11.Tieu MT, Cigsar C, Ahmed S, Ng A, Diller L, Millar BA, Crystal P, Hodgson DC (2014) Breast cancer detection among young survivors of pediatric Hodgkin lymphoma with screening magnetic resonance imaging. Cancer 120(16):2507–2513. https://doi.org/10.18.1002/cncr.28747 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hodgson DC, Cotton C, Crystal P, Nathan PC (2016) Impact of early breast cancer screening on mortality among young survivors of childhood Hodgkin’s lymphoma. J Natl Cancer Inst 108(7): djw010. 10.1093/jnci/djw01019. [DOI] [PubMed] [Google Scholar]
- 13.Jaworski C, Mariani JA, Wheeler G, Kaye DM (2013) Cardiac complications of thoracic irradiation. J Am Coll Cardiol 61(23): 2319–2328. 10.1016/jjacc.2013.01.090 [DOI] [PubMed] [Google Scholar]
- 14.Shankar SM, Marina N, Hudson MM, Hodgson DC, Adams MJ, Landier W, Bhatia S, Meeske K, Chen MH, Kinahan KE, Steinberger J, Rosenthal D, Cardiovascular Disease Task Force of the 20. Children’s Oncology Group (2008) Monitoring for cardiovascular disease in survivors of childhood cancer: report from the Cardiovascular Disease Task Force of the Children’s Oncology Group. Pediatrics 121(2):e387–e396. 10.1542/peds.2007-0575 [DOI] [PubMed] [Google Scholar]
- 15.Hoppe RT, Advani RH, Ai WZ, Ambinder RF, Bello CM, Bierman PJ, Blum KA, Dabaja B, Duron Y, Forero A, Gordon LI, Hernandez-Ilizaliturri FJ, Hochberg EP, Maloney DG, Mansur D, Mauch PM, Metzger M, Moore JO, Morgan D, Moskowitz CH, Poppe M, Pro B, Weiss L, Winter JN, Yahalom J, NCCN Hodgkin Lymphoma (2011) Hodgkin lymphoma. J Natl Compr Cancer Netw 9(9):1020–1058. 10.6004/jnccn.2011.008622. [DOI] [PubMed] [Google Scholar]
- 16.Heidenreich PA et al. (2007) Screening for coronary artery disease after mediastinal irradiation for Hodgkin’s disease. J Clin Oncol 25(1):43–49 [DOI] [PubMed] [Google Scholar]
- 17.Hewitt ME et al. (2006) From cancer patient to cancer survivor: lost in transition. The National Academies Press; xxv, Washington, DC, 506 p [Google Scholar]
- 18.Diller L, Nancarrow CM, Shaffer K, Matulonis U, Mauch P, Neuberg D, Tarbell NJ, Litman H, Garber J (2002) Breast cancer screening in women previously treated for Hodgkin’s disease: a prospective cohort study. J Clin Oncol 20(8):2085–2091. 10.1200/JCO.2002.08.031 [DOI] [PubMed] [Google Scholar]
- 19.Oeffinger KC, Ford JS, Moskowitz CS, Diller LR, Hudson MM, Chou JF, Smith SM, Mertens AC, Henderson TO, Friedman DL, Leisenring WM, Robison LL (2009) Breast cancer surveillance practices among women previously treated with chest radiation for a childhood cancer. JAMA 301(4):404–414. 10.1001/jama.2008.1039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Grunfeld E, Levine MN, Julian JA, Coyle D, Szechtman B, Mirsky D, Verma S, Dent S, Sawka C, Pritchard KI, Ginsburg D, Wood M, Whelan T (2006) Randomized trial of long-term follow-up for early-stage breast cancer: a comparison of family physician versus specialist care. J Clin Oncol 24(6):848–855. 10.1200/JTO.2005.03.2235 [DOI] [PubMed] [Google Scholar]
- 21.Lindell RB, Koh SJ, Alvarez JAM, Koyama T, Esbenshade AJ, Simmons JH, Friedman DL (2015) Knowledge of diagnosis, treatment history, and risk of late effects among childhood cancer survivors and parents: the impact of a survivorship clinic. Pediatr Blood Cancer 62(8):1444–1451. 10.1002/pbc.25509 [DOI] [PubMed] [Google Scholar]
- 22.Ng AK, Garber JE, Diller LR, Birdwell RL, Feng Y, Neuberg DS, Silver B, Fisher DC, Marcus KJ, Mauch PM (2013) Prospective study of the efficacy of breast magnetic resonance imaging and mammographic screening in survivors of Hodgkin lymphoma. J Clin Oncol 31(18):2282–2288. 10.1200/JCO.2012.46.5732 [DOI] [PubMed] [Google Scholar]