i. Summary
The packaging and shipment of biospecimens is a multistep process for which a distinct set of regulations needs to be followed, depending on whether a biospecimen is shipped domestically or internationally and whether the shipment contains hazardous materials. Shipments may be delayed if these regulations are not followed. Once learned, the process is straightforward. Major principles include double or triple packaging, adequate absorbent material, appropriate coolant, accurate labeling and complete documentation. Training in packaging and shipping is often offered at major biomedical institutions and is a requirement for shipping biohazards.
Keywords: Biohazard, biobank, biospecimen, shipping, IATA, dry ice, frozen
1. Introduction
The shipment of biological samples is a well-regulated procedure with set rules and protocols that must be followed with strict adherence in order to ship materials in a safe and efficient manner. Failure to comply with these shipping guidelines may result in delays or failures in delivery. Within these guidelines, there are variations in regulations specific to the type of biospecimen being shipped and whether or not the sample is hazardous. Infectious substances are categorized into one of nine International Air Transit Association (IATA) types (1):
Category A infectious substances
Category B biological substances
Patient specimens
Exempt human or animal specimens
Genetically modified organisms
Exempt substances
Biological products
Infected animals
Medical waste
The majority of shipped substances are classified as either Category A infectious substances or Category B biological substances. This categorization scheme will determine how a particular substance is packaged and shipped. A Category A infectious substance is one that according to the International Air Transit Association, is “transported in a form that, when exposure to it occurs, is capable of causing permanent disability, life-threatening and/or fatal disease in otherwise healthy humans or animals (2).” Category B biological substances “are infectious substances which do not meet the criteria for inclusion in Category A (2).” There are separate guidelines for shipping frozen and non-frozen Category A infectious substances and Category B biological substances. While packaging is similar, documentation and labeling is different. These regulations must be meticulously observed to best preserve biological sample quality and to ensure the safety and health of all individuals who may come in contact with it. The protocols described here apply in the United States and will likely vary in other nations as local regulations may vary. However, many of the principles are universally applicable.
2. Materials
Category A infectious substances
Category B biological substances
Leak-proof primary receptacle
Leak-proof secondary receptacle
Rigid outer packaging, cannot measure less than 100 mm at its smallest side
Absorbent material (Paper towels etc.)
Ice
Dry ice
Liquid nitrogen
Cool packs
Itemized list of contents
Class 9 label of Miscellaneous Dangerous Goods/UN1845 Dry Ice label
Insulation
Blue “C” stickers
Air Waybill
Declaration of Dangerous Goods
Customs invoice
Centers for Disease Control and Prevention permit
UN3373 label
3. Methods
3.1. Department of Transportation (DOT) and International Air Transit Association (IATA) Regulations
In the U.S. the shipping of biospecimens requires observance of Department of Transportation (DOT) and International Air Transit Association (IATA) regulations. UN IDs, otherwise known as UN numbers, are four-digit United Nations hazardous materials identifiers. For Category A infectious substances, shipments must either be classified as UN2814 or UN2900 (3). The identifier UN2814 signifies an infectious substance that affects humans, whereas UN2900 denotes an infectious substance that affects animals (4). Category A substances are substances most hazardous to individuals and communities. The party responsible for shipping Category A specimens must adhere to either the Code of Federal Regulations (CFR) title 49 CFR, part 173.196 (5, 6), or to IATA packing instruction 602 (7).
3.2. General principles of packaging and shipping infectious substances
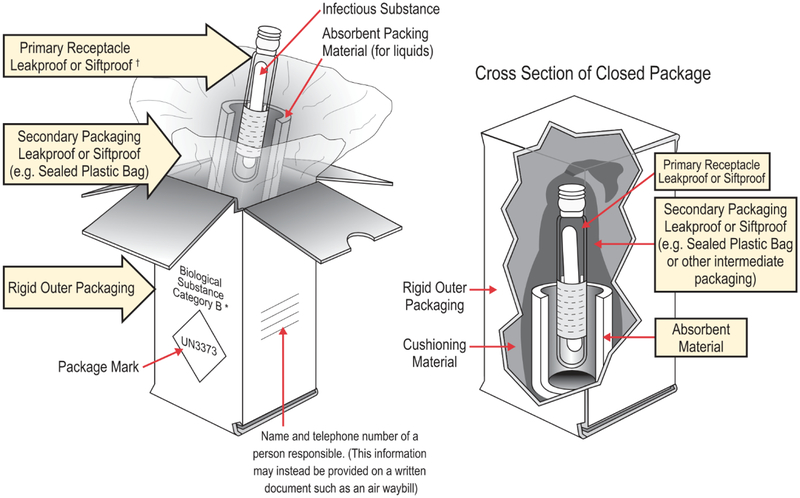
Shipping of infectious substances require triple packaging, which consists of a leak-proof primary receptacle, a leak-proof secondary packaging, and a rigid outer packaging material (8) (Fig. 1).
Figure 1.
Triple packaging container (8).
The person preparing and packaging the materials must undergo training regarding the shipment of dangerous goods. The appropriate shipping containers are readily available from commercial vendors but should meet the below requirements (9–12).
1. To avoid damage in shipment, any fragile primary receptacle packaged with one or more additional primary receptacles must be wrapped individually within the secondary packaging. This separation will prevent contact between the primary receptacles and minimize the possibility of damage and/or leakage.
2. The outer packaging must be of acceptable strength compared to the total mass of the primary and secondary receptacles and cannot measure less than 100 mm at its smallest external side length.
3. If the substance is liquid, an absorbent material able to absorb the entire contents of the receptacle(s) should be placed between the primary receptacle and secondary packaging.
4. There needs to be a list of the entire contents of the package between the outer and secondary packaging.
5. The primary receptacle or the secondary packaging needs to be capable of withstanding a difference in pressure not exceeding 95 kPa without leaking.
6. As well as withstanding pressure, the primary receptacle or secondary packaging needs to be capable of withstanding temperature changes of −40°C to 55°C.
7. An itemized list of the contents of the shipment must be included with the secondary container.
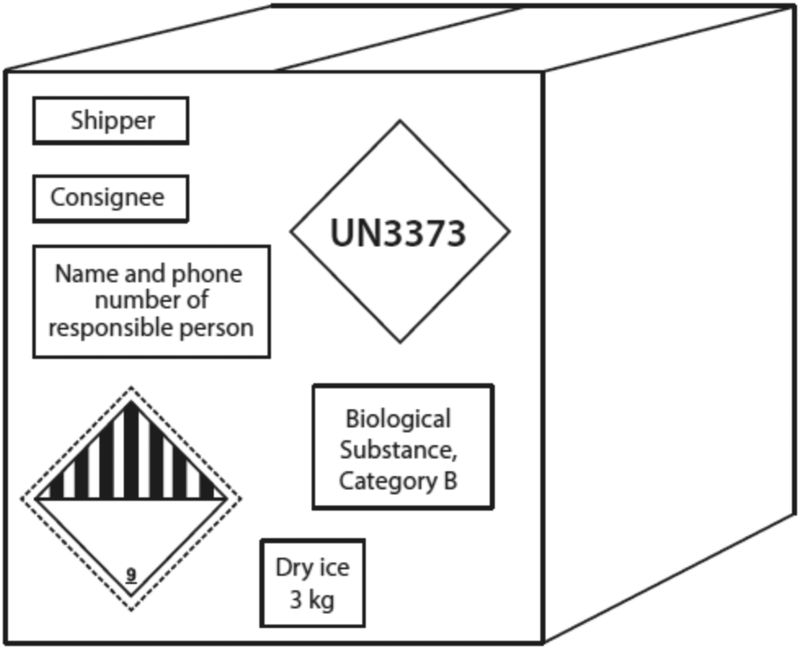
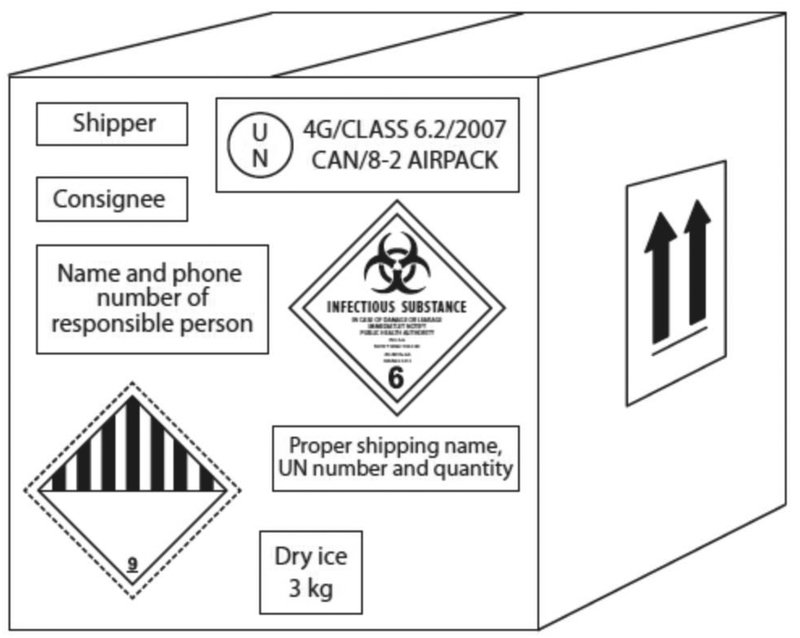
8. The shipment must also be labeled appropriately on the outside (13) (Fig. 2 and 3).
Figure 2.
A completely labeled outer package for a Category B biological substance (13).
Figure 3.
A completely labeled outer package for a Category A infectious substance (13).
9. Except for shipments that contain body parts, organs, and/or whole bodies, the outer packaging should not hold more than 4 kg in contents, excluding the added weight of ice, dry ice, or liquid nitrogen, if applicable.
10. The shipment must be able to pass a drop test from a height of 1.2 meters.
11. If shipping by air freight, an Air Waybill and copies of the Declaration of Dangerous Goods must be distributed to the airline. An Air Waybill is a contract between the shipper and the airline carrier (14). If the forms are incomplete, the shipment will be rejected.
12. Documentation should be retained by the shipper for a period of two years.
13. Category A infectious substances should not be packaged together with Category B biological substances.
14. Ambient specimens and refrigerated specimens can be packaged together as long as there is insulation between them.
3.3. Ambient temperature shipping considerations
1. When shipping an infectious substance at ambient temperature or higher, the primary receptacle must be made of glass, metal, or plastic, and a secured leak-proof seal must be provided.
2. If the primary receptacle uses a screw top, the top must be secured with material such as adhesive tape.
3. If a lyophilized substance is to be transported in a primary receptacle, the receptacle should be flame-sealed with glass ampoules or rubber-stoppered glass vials with metal seals.
4. Glass slides can be placed in slide holders and then shipped in padded envelopes or boxes.
5. Paraffin blocks should be placed in a small sealed plastic bag so that, if the blocks melts, tissue will remain trapped in the bag. In addition, if shipping through hot climate areas, the plastic bag-enclosed block should be taped or attached with rubber bands to an ice pack. All of this can be placed within an outer container.
3.4. Cold or frozen sample shipments
1. When infectious substances are shipped refrigerated or frozen, the secondary packaging should be surrounded by ice, dry ice, or another refrigerant. The ice, dry ice, or other refrigerant must be placed in an outer packaging with at least one complete package marked in accordance with marking regulations.
2. Supports must also be used to keep the secondary packaging oriented in the same way after any ice or dry ice has dissipated.
3. The use of ice requires a leak-proof outer packaging.
4. For dry ice, the packaging must allow the escape of carbon dioxide, and the outer packaging must be labeled as either “Dry Ice” or “Carbon Dioxide, Solid” along with “UN1845”. Labeling should include the net quantity of dry ice in kilograms, and the Class 9 label of Miscellaneous Dangerous Goods.
5. Similar to shipping dry ice, when shipping liquid nitrogen, the packaging must be able to hold their form at the temperature of the liquid nitrogen and the temperature and pressure of the aircraft. The packaging for liquid nitrogen must be metal vacuum insulated vessels or flasks which are vented to the atmosphere. This ventilation prevents any increase in pressure within the packaging. To maintain the internal pressure, fill and discharge openings must be protected from the possible entrance of foreign objects that may cause a change in internal pressure. It is prohibited to use any safety relief valves, check valves, frangible discs, or other similar devices in vent lines. To further protect the shipment, orientation markings must be specified, and the packaging should be designed to not allow the release of refrigerated liquid nitrogen regardless of the orientation of the package.
6. The secondary container is sealed and labeled with a blue “C” sticker if the shipment contains cultures before being placed inside the external container.
7. An Air Waybill, a form that is specific to each airline, is necessary, as well. Different waybills are available for shipments that are transported with dry ice and those that are not transported with dry ice.
8. A Declaration of Dangerous Goods form must also be completed in order to ensure delivery of biological samples to an international address. This document must provide a description of the contents that are being shipped, along with the names and addresses of the shipper and recipient, flight details, and the shipper’s signature. Also, the appropriate boxes must be checked regarding the amount of the specimen and the radioactivity as well as the proper shipping name of the infectious material, specimen class and the UN number, quantity of infectious substance(s), the type of packaging, an emergency contact number, and the date labeled.
3.5. Live animals, organs, body parts, and bodies
Infectious substances transported in other forms, besides specimen samples, requires added precaution (8). The use of live animals as a form of shipment for infectious diseases is prohibited unless the substance cannot be sent in any other form. If a transported animal contains or is contaminated with an infectious substance, the conditions of transport must be approved by the Associate Administrator for Hazardous Materials Safety, who administers the Pipeline and Hazardous Materials Safety Administration for the Department of Transportation. For body parts, organs, or whole bodies, defined as 6.2 materials or infectious substances, the shipper must follow packaging guidelines for shipping infectious substances as well as the Code of Federal Regulations 173.197, regulated medical waste packaging requirements (6, 8).
3.6. International Shipping
1. Unlike domestic shipping, international shipping takes additional planning to prevent possible problems (15). Also, shipments must be planned according to the day that biosamples are shipped. International shipments should be sent on either Monday or Tuesday to prevent the shipment from being held unnecessarily over the weekend or at customs (also a good practice for domestic shipping). Also, do not ship the day before a holiday because customs may hold the shipment one day.
2. When shipping, be careful to verify specimens and have two personal identifiers. If there is a mismatch in personal identifiers, the shipment will be classified as mislabeled.
3. Contact a freight forwarder, a company which organizes shipments, and request the appropriate documentation, including documents regarding possible local regulations.
4. Typically, a customs invoice needs to be completed and should include a detailed description of the volume in milliliters of the specimen and the weight of dry ice being shipped in kilograms, typed on official letterhead. A customs invoice is required for any shipment containing biospecimens to be cleared through United States customs.
5. An Airway Bill and Dangerous Goods declaration should be filled out an enclosed.
6. Three copies of this declaration must be printed, and one copy must be kept for at least two years following each particular shipment.
7. For infectious materials, international shipments may require a Centers for Disease Control and Prevention (CDC) permit.
8. All documentation should be placed in a plastic document pouch, which in turn is affixed to the external surface of the corresponding container(s) to be shipped across international borders.
9. Frozen specimens requires approximately ten pounds of dry ice, a layer of pellet dry ice approximately 4–6 cm should line the bottom of the secondary container, upon which the labeled frozen specimen bags, or the primary receptacle(s), are placed. These bags are then covered with dry ice. Gel packs can also be included and may last an additional day should the dry ice completely evaporate. The secondary container is sealed and placed within the shipping container, the outer packaging, which itself is sealed.
10. A UN1845 Class 9 dry ice label must be completed to include the weight in kilograms of dry ice within the shipment; this label is affixed to the external surface of the tertiary container.
11. Shipping refrigerated and ambient specimens requires either a five or ten pound container, the latter of which is a requisite if biological samples contain cultured materials. Either frozen or cold cool packs should be placed within the secondary container in order to maintain a suitable temperature for biospecimen preservation.
3.7. Shipping from other Countries
The International Air Transit Association (IATA) operates within a large number of countries around the world, including nations in North and South America, Europe, and Eastern Asia (16). Shipping requirements from these countries to the United States are similar but may have variations that differ from common IATA rules. Shipments originating from Canada must follow IATA regulations as well as additional guidelines (17, 18). Shipping from Canada also requires compliance with Canadian General Standards Board (CGSB) 43.125 regulations. The CGSB issues the national standards for packaging infectious substances, diagnostic specimen, biological products, and medical waste. Shipments of biological materials from the United Kingdom (UK) have adhered to European Union packaging and shipping standards (19) but may change with its exit from the European Union. Depending on the level of danger, substances are assigned class types and further packing groups (PGs), designated as PGI, PGII, or PGIII. The class and packing group tell the shipper how a substance should be packed, including specific labelling, materials for packaging, and transport. Transport guidelines for samples is dependent on EU agreements and legislature in the UK at the time of shipment.
3.8. Timing of shipping and communications between shipper and recipient
The timing of shipping frozen samples is very important. We prefer to ship frozen specimens by overnight express either on a Monday or Tuesday. In case of delay, the receiving institution will likely still receive the materials during a weekday when the receiving institution will be well staffed. The possibility of delay emphasizes the importance of using sufficient dry ice in the package to last more than one day. We are aware of anecdotal instances of materials delivered on the weekend and sitting in a loading dock over the course of the weekend resulting in thawed specimens. In addition, our laboratory communicates with the recipients as to the best date for shipping. Some smaller research laboratories may be closed if all the personnel are away at a meeting or on a retreat. During holidays such as Christmas and New Year holidays or the Lunar New Year, the recipients may ask for shipping to be delayed until they have returned. Upon shipping, we provide the tracking number to the recipient so that they can track the samples. This step is particularly important for international shipping. On occasion, materials may be held up in customs and the intended recipient may have to work with their country’s customs office to get the package released. Also, while the shipping company may deliver to the correct institution, it is not a guarantee that the package has arrived at the recipient’s laboratory. On occasion, particularly in large, sprawling companies or universities, the package may end up at an incorrect office or laboratory where it has the potential to “rest” for days. Expeditious recovery of the misrouted package is desirable before the dry ice is evaporated. We request that the intended recipient notify us when they receive the package in their laboratory.
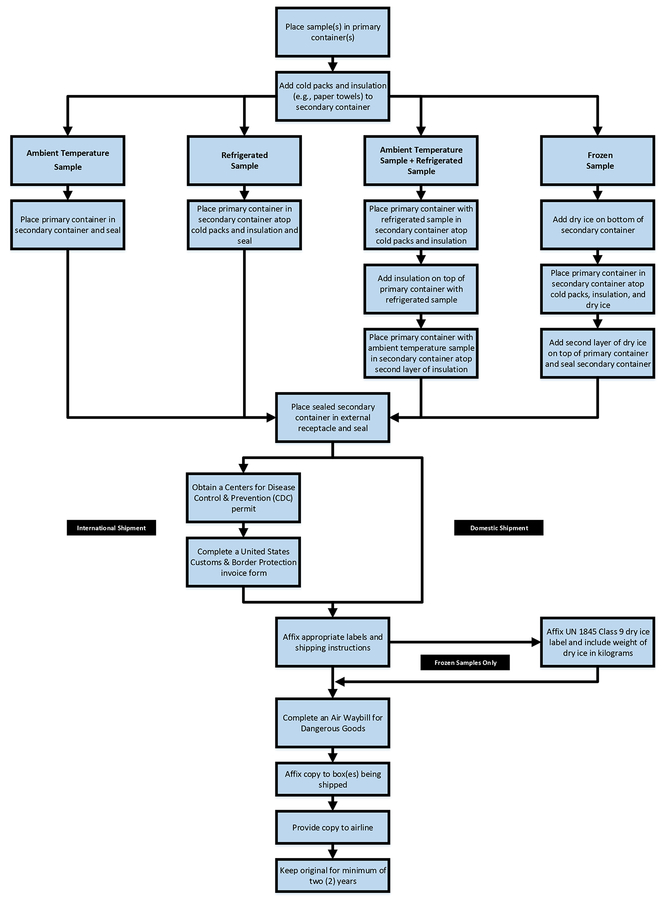
Figure 4.
Schematic diagram for domestic and international shipping.
Acknowledgement
This work was supported in part by NIH:NCI P50-CA211015, NIH:NIMH U24 MH100929, the Art of the Brain Foundation, and the Henry E. Singleton Brain Cancer Research Program.
4. References
- 1.American Society for Microbiology (2011) Packaging and Shipping Infectious Substances. http://dhmh.maryland.gov/laboratories/docs/ASM_Packing_and_Shipping_Infectious_Substances.pdf. Accessed 3 July 2015
- 2.International Air Transit Association (2017) Dangerous Goods Regulations, 58th Editions. http://www.iata.org/whatwedo/cargo/dgr/Documents/infectious-substance-classification-DGR56-en.pdf. Accessed 20 Sept 2017
- 3.AZoM (2006) CAS Numbers and UN Numbers – Identifications Systems for Materials and Chemical. https://www.azom.com/article.aspx?ArticleID=3506. Accessed 20 Sept 2017
- 4.UN3373 Medical Packaging (2016) Regulations for UN3373. http://www.un3373.com/info/regulations/. Accessed 20 Sept 2017
- 5.US Government Publishing Office (2011) Code of Federal Regulations. http://www.gpo.gov/fdsys/granule/CFR-2011-title49-vol2/CFR-2011-title49-vol2-sec173-196. Accessed 20 Aug 2015
- 6.Authenticated U.S. Government Information GPO (2014) 49 CFR Ch. 1 (10-1-11 Edition). http://www.gpo.gov/fdsys/pkg/CFR-2011-title49-vol2/pdf/CFR-2011-title49-vol2-sec173-196.pdf. Accessed 20 Aug 2015
- 7.Gruber AG (2010) IATA Transport of Biological Samples: Air Transport View. International Air Transport Association. http://www.oie.int/fileadmin/Home/eng/Conferences_Events/sites/VETO2010/Session%203/Session_3_3_Andrea_Graf-Gruber.pdf. Accessed 20 Sept 2017
- 8.US Department of Transportation Pipeline and Hazardous Materials Safety Administration (2006) Transporting Infectious Substances Safely. https://www.phmsa.dot.gov/staticfiles/PHMSA/DownloadableFiles/Files/Transporting_Infectious_Substances_brochure.pdf. Accessed 20 Sept 2017
- 9.Code of Federal Regulations (1998) General Requirements for Shipments and Packagings. http://www.gpo.gov/fdsys/pkg/CFR-1998-title49-vol2/xml/CFR-1998-title49-vol2-part173.xml#seqnum173.3. Accessed 29 Aug 2015
- 10.International Air Transit Association (2017) Packing Instruction 650 - Dangerous Goods Regulations. https://www.iata.org/whatwedo/cargo/dgr/Documents/packing-instruction-650-DGR56-en.pdf Accessed 22 Sept 2017
- 11.Mayo Clinic (2017) Specimen Transport. http://www.mayomedicallaboratories.com/specimen/transport/index.php. Accessed 20 Sept 2017
- 12.Mayo Clinic (2017) United States Shipping Guide. http://www.mayomedicallaboratories.com/specimen/transport/index.php. Accessed 20 Sept 2017
- 13.Miller JM et al. (2012) Guidelines for Safe Work Practices in Human and Animal Medical Diagnostic Laboratories. Centers for Disease Control and Prevention. https://www.cdc.gov/mmwr/preview/mmwrhtml/su6101a1.htm. Accessed 20 Sept 2017
- 14.International Air Transit Association (2017) Air Waybill. http://www.iata.org/whatwedo/cargo/e/eawb/Pages/index.aspx. Accessed 22 Sept 2017
- 15.Mayo Clinic (2017) International Shipping Guide. http://www.mayomedicallaboratories.com/mediax/specimen/international-shipping-guide.pdf. Accessed 20 Sept 2017
- 16.International Air Transit Association (2015) IATA by Region. http://www.iata.org/about/worldwide/Pages/index.aspx. Accessed 1 Oct 2015
- 17.Transport Canada (2015) Means of Containment. http://www.tc.gc.ca/eng/tdg/clear-part5-300.htm. Accessed 29 Aug 2015
- 18.Transport Canada (2015) List of Safety Standards (CSA or CGSB standards). https://www.tc.gc.ca/eng/tdg/moc-listofstandards-279.html. Accessed 5 Sept 2015
- 19.Gov.UK (2012) Moving Dangerous Goods. https://www.gov.uk/guidance/moving-dangerous-goods. Accessed 5 Sept 2015