Abstract
Background/Aim
The aim of this study is to determine the relationship between stromal types, PD-L1 status and clinicopathological characteristics in patients with different molecular subtypes of breast cancer.
Materials and methods
Protein expression levels of PD-L1 were determined by immunohistochemistry assay. Stromal type was classified based on the maturity of the tumor stroma.
Results
Different subtypes of breast cancer had distinct stromal types. Tumors from patients with mature stroma had lower pathological N stage and AJCC stage, more frequent high p53 expression and positive stromal PD-L1 staining. Hormone receptor negative patients had higher frequency of positive stromal PD-L1 staining. Stromal PD-L1 status was also associated with different breast cancer subtypes and EGFR expression level. Importantly, our data revealed that stromal types and stromal PD-L1 status were independent prognostic factors.
Conclusion
This study highlighted the importance of stromal types and stromal PD-L1 status in determining clinical outcomes in patients with breast cancer, and suggested that stromal type classification might be readily incorporated into routine clinical risk assessment following curative resection or optimal therapeutic design.
Introduction
Breast cancer associated stroma is consisted of fibroblasts, myofibroblasts, leukocytes, endothelial cells, macrophages, adipocytes and extracellular matrix (ECM) [1]. Current clinical management guidelines for the determination of optimal treatment of breast cancer is mainly based on the characteristics of tumor (lymph node status, pathological stage, tumor size, grade, locoregional spread, and molecular features) [2]. However, the significance of tumor stroma is often overlooked, which contains valuable information for treatment choices. Andrew H. Beck and colleagues have revealed that the stromal compartment of breast tumors contains more prognostic information than the epithelial component [3]. Based on the principal stromal tissue component, Soomin Ahn and colleague classified tumor stroma into three dominant types, including collagen dominant, fibroblast dominant and lymphocyte dominant. They found that this classifier can stratify the prognostic outcome of breast cancer [2]. Other researchers proposed that stromal regions could be characterized according to the maturity of collagen in ECM. Depending upon the qualitative characteristics of the stromal collagen in the reactive tumor area, Ueno, et al [4] histologically classified stromal types into three categories: mature—when the stroma was composed of mature collagen fibers (fine and elongated fibers into multiple layers); intermediate—when keloid-like collagen was intermingled with mature fibers; and immature—consisting of a myxoid stroma in which no mature fibers were included. This study indicated that stromal response is critical for the tumor behavior and host immune reactions in rectal cancer and would be a promising tool for prognostic outcome prediction [4]. Breast cancer is a fibrotic cancer [5], the stromal types have not been extensively studied yet, especially the relationship between molecular subtypes (luminal A, luminal B, HER-2–enriched and triple negative breast cancer). Therefore, histologically characterizing the stromal types of different subtypes of breast cancer and analyzing their correlations may provide valuable information for clinical practice.
The host immune response has been reported to play a crucial role in breast cancer progression and response to therapy [6]. It is well recognized that the immune checkpoint pathways, such as those mediated by the programmed cell death protein 1 (PD-1) and programmed death ligand 1 (PD-L1), are significant for the antitumor responses [7, 8]. PD-L1 can be expressed by tumor cells as well as stromal cells, including the infiltrating T cells, macrophages, dendritic cells and B cells [9]. The protein level of tumor expressed PD-L1 has been demonstrated to be associated with high grade, hormone receptor–negative phenotypes [10], prognostic outcome [11], lymph node status [12] and immune cell infiltration [13]. However, there were few studies focusing on the relationship between stromal PD-L1 expression and stromal types and their correlation with clinicopathological characteristics.
The tumor microenvironment is well constructed and each component should have great influences on each other. It is reasonable to speculate that different molecular subtypes of breast cancer should have different stromal types and stromal PD-L1 expression and stromal types may associate with the expression patterns of PD-L1. In this perspective, we evaluated the stromal types of 160 breast cancers samples, which were stratified into different molecular subtypes. Additionally, the tumoral and stromal expressed PD-L1 was examined by immunohistochemistry. Interestingly, we found that stromal types were significantly associated with molecular subtypes of breast cancer, pathological N stage, American Joint Committee on Cancer (AJCC) stage, PR status, p53 level and stromal PD-L1 expression status. Meanwhile, stromal PD-L1 expression status was implicated with ER status, PR status, EGFR expression level and different molecular subtypes of breast cancer. Importantly, stromal types and stromal PD-L1 status were linked to the prognostic outcome. These morphological and molecular studies extended our knowledge on the microenvironment of breast cancer and provided meaningful data for the determination of optimal treatment of breast cancer, especially for immunotherapy decision.
Materials and methods
Patient characteristics and immunohistochemistry
Serial sections of tissue microarrays (TMAs) of 160 breast cancer specimens were obtained from Shanghai Outdo Biotech Co., Ltd. (SOBC), with the approval of the Institutional Review Board. Written informed consent was obtained from all patients prior to the study. The use of the clinical specimens for research purposes was approved by the Institutional Research Ethics Committee of Beijing Cancer Hospital and the Cancer Institute and Hospital (2019KT22).
All the patients enrolled here are therapeutic naïve. Follow-up data were available and the median follow-up time was 118 months. Clinical information including age, tumor grade, tumor location, pathological T stage, pathological N stage, AJCC stage, and survival data was evaluated by reviewing medical records. Overall survival (OS) was defined as the interval between the operation and death from breast cancer or the date of final follow-up [14]. Immunohistochemistry (IHC) analysis was performed and diagnosed by two pathologists blindly on the TMAs. Hematoxylin–eosin stained was performed by Shanghai Outdo Biotech Co., Ltd. (SOBC). The immunohistochemistry assay (IHC) was performed as previous described [15]. Tissue sections were incubated with rabbit anti–PD-L1 (1:100; Cell Signaling Technology, USA), overnight at 4°C. After washing, the tissue sections were treated with goat anti-mouse/rabbit IgG HRP-polymer (Agilent Dako, California, USA) for 30 min. 3, 3’-Diaminobenzidine was used as the chromogen. IHC scores were determined by combining the intensity of staining and the proportion of positively stained tumor cells. First, the intensity was graded as follows: 0, negative; 1, weak; 2, moderate; 3, strong. Second, the proportion of positive tumor cells was graded: 0, <5%; 1, 5–25%; 2, 26–50%; 3, 51–75%; 4,>75%. A final score was derived by multiplication of these two primary scores. Final scores of 0–3 were defined as ‘Negative’ (-); scores of 4–12 as ‘Positive’ (+). IHC staining of ER (1:200; Dako, Glostrup, Denmark), PR (1:200; Dako), HER2 (1:200; Dako), AR (androgen receptor, 1:100, Dako), EGFR (1:200, Dako), p53 (1:200, Dako), Ki-67 (1:200; Dako), CK-5/6 (1:100, Dako) and Fluorescence In Situ Hybridization (FISH) of HER2 were performed and evaluated by two pathologists by Shanghai Outdo Biotech Co., Ltd. (SOBC).
Fluorescence In Situ Hybridization (FISH) of HER2
HER2 amplification was determined by FISH testing on a 4 μm formalin fixed paraffin-embedded tissue specimens using the FDA approved PathVysion HER2 DNA probe kit (Abbott Molecular, Des Plaines, IL, USA) according to the manufacturer’s instruction. HER2 FISH images were analyzed independently by two pathologists and HER2 amplification was defined by HER2-to-chromosome 17 centromere (CEP17) FISH ratio≥2.0 [16].
Molecular subtypes classification
Molecular subtypes of breast cancer were surrogated by IHC markers and were defined as below [17,18]: Luminal A: ER (+), PR (+), HER2 (−), and Ki-67 ≤14%. Luminal B: ER (+), PR (+), HER2 (−), and Ki-67 >14%; ER (+), PR (+), HER2 (high or amplification). HER2-enriched: ER (−), PR (−), HER2 (+), and Ki-67 (high). Triple-negative: ER (−), PR (−), and HER2 (−). Basal like: triple-negative with CK5/6 high expression. AR+_ER/PR-: AR (+), ER (-), PR (-). AR+_TNBC: AR (+), ER (−), PR (−), and HER2 (−).
Stromal types classification
The criteria of stromal types classification used in this study was described in the previous study [4]. Briefly, the fibrotic cancer stroma was classified into three types: mature, whose stroma was stratified into multiple layers by fine and elongated fibers and fibrocytes; intermediate, whose stroma was composed of broad bands of collagen with brightly eosinophilic hyalinization; immature, whose stroma consisted of randomly orientated keloid-like collagen bundles surrounded by myxoid stroma.
Statistical analysis
Statistical analysis was carried out using IBM SPSS Statistics 20 or GraphPad Prism 8.0 for Windows. The two tailed Pearson χ2 test or Fisher’s exact test was used to correlate the stromal types or PD-L1 expression status with clinicopathological parameters. The survival curves were plotted by using Kaplan-Meier analysis and compared by log-rank test. Univariate Cox proportional hazards regression model was used to determine the hazard ratio (HR) between different stratified groups. To evaluate whether tumor PD-L1 expression, stromal PD-L1 expression and stromal types can serve as an independent prognostic factor in our cohort, age, pathological T stage, pathological N stage and pathological grade, which were prognostic associated factors, were taken into account as covariates for multivariate Cox regression analysis. Differences were considered significant when the p value was less than 0.05.
Results
Stromal types in breast cancer
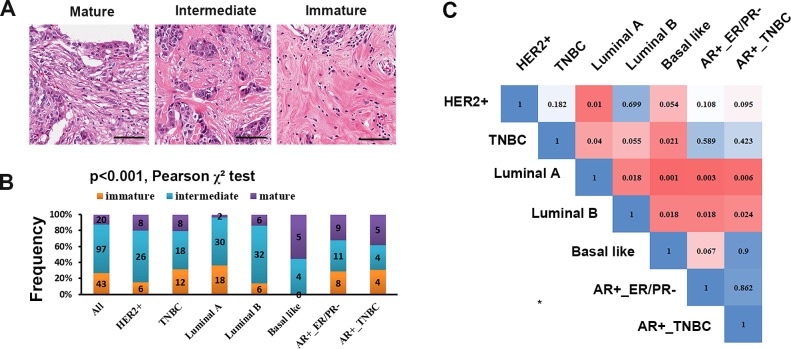
In our data set, 12.5% of patients had mature fibrotic cancer stroma whereas 60.6% had intermediate stroma and 26.9% had immature stroma (Fig 1A and 1B, Table 1). Different molecular subtypes had distinct stromal types (Pearson χ2 test, p<0.001, Fig 1B, Table 1), and the comparison between each molecular subtype by Pearson χ2 test and corrected by Bonferroni correction showed that the stromal types in Luminal A and Basal like were significantly different (Fig 1C, p = 0.001). Additionally, we observed that tumor stroma of HER2 (+) subtype had higher frequency of mature type, while lower frequency of immature type (p = 0.064). It was likely that tumors of progesterone receptor positive types (PR+) tended to have lower frequency of mature stroma (p = 0.014, Table 1). Tumors of Luminal A subtype showed lower frequency of mature stroma (p = 0.018, Table 1), however, tumors of Basal-like (p = 0.001, Table 1), AR+_ER/PR-(p = 0.004, Table 1), AR+_TNBC (p = 0.021, Table 1) subtypes had higher frequency of mature stroma. Additionally, tumors of Luminal B and Basal-like subtypes seemed to have lower frequency of immature stroma (p = 0.05 and p = 0.001, respectively. Table 1). Statistical analysis revealed that tumors with lower pathological N stage and AJCC stage accounted for higher frequency of mature stroma stromal types (p = 0.004 and p = 0.017, respectively. Table 1). Interestingly, breast cancer with mature stroma tended to have high p53 expression (p = 0.003, Table 1). However, stromal type was not correlated with the expression level of EGFR (p = 0.846, Table 1).
Fig 1. Fibrotic stromal types in different subtypes of breast cancer.
(A) Maturation of breast cancer stroma. Scale bar: 50 μm. (B) Distributions of stromal types in different molecular subtypes of breast cancer. The number in the histogram bar indicated the number of cases. Multiple comparison among different molecular subtypes was performed by Pearson χ2 test. (C) Differences between each other of stromal types constitution among seven molecular subtypes indicated by p values. The blue to red gradient ramp indicates a p value from 1 to 0.001. The comparison between each molecular subtype was conducted by Pearson χ2 test and corrected by Bonferroni correction. Because of 21 comparisons between each other among seven molecular subtypes, differences were considered significant when the Bonferroni correction p value less than 0.0024 (0.05/21).
Table 1. Patient characteristics and the inter-relationship between clinicopathological characteristics and tumor stromal types in patients breast cancer (n = 160).
| Feature | N (%) | Stromal types | ||||
|---|---|---|---|---|---|---|
| Mature (%) | Intermediate (%) | Immature (%) | p value | |||
| Age | < = 50 | 82 (51.2) | 9 (11.0) | 53 (64.6) | 20 (24.4) | 0.564 |
| >50 | 78 (48.8) | 11 (14.1) | 44 (56.4) | 23 (29.5) | ||
| Tumor location | Right side | 71 (44.4) | 5 (7.0) | 50 (70.4) | 16 (22.5) | 0.046 |
| Left side | 89 (55.6) | 15 (16.9) | 47 (52.8) | 27 (30.3) | ||
| Grade | I | 18 (11.2) | 0 (0.0) | 10 (55.6) | 8 (44.4) | 0.131 |
| II | 136 (85.0) | 19 (14.0) | 83 (61.0) | 34 (25.0) | ||
| III | 6 (3.8) | 1 (16.7) | 4 (66.7) | 1 (16.7) | ||
| pT | pT1-2 | 36 (22.8) | 2 (5.6) | 24 (66.7) | 10 (27.8) | 0.279 |
| pT3-4 | 122 (77.2) | 18 (14.8) | 71 (58.2) | 33 (27.0) | ||
| pN | pN0-1 | 111 (71.2) | 19 (17.1) | 60 (54.1) | 32 (28.8) | 0.004 |
| pN2-3 | 45 (28.8) | 1 (2.2) | 35 (77.8) | 9 (20.0) | ||
| LNM | Yes | 93 (59.6) | 11 (11.8) | 59 (63.4) | 23 (24.7) | 0.73 |
| No | 63 (40.4) | 9 (14.3) | 36 (57.1) | 18 (28.6) | ||
| AJCC stage | 1+2 | 107 (69.0) | 18 (16.8) | 58 (54.2) | 31 (29.0) | 0.017 |
| 3 | 48 (31.0) | 2 (4.2) | 36 (75.0) | 10 (20.8) | ||
| HER2 | Positive | 40 (25.0) | 8 (20.0) | 26 (65.0) | 6 (15.0) | 0.064 |
| Negative | 120 (75.0) | 12 (10.0) | 71 (59.2) | 37 (30.8) | ||
| ER | Positive | 94 (58.8) | 8 (8.5) | 61 (64.9) | 25 (26.6) | 0.17 |
| Negative | 66 (41.2) | 12 (18.2) | 36 (54.5) | 18 (27.3) | ||
| PR | Positive | 78 (48.8) | 4 (5.1) | 53 (67.9) | 21 (26.9) | 0.014 |
| Negative | 82 (51.2) | 16 (19.5) | 44 (53.7) | 22 (26.8) | ||
| AR | Positive | 102 (63.7) | 16 (15.7) | 60 (58.8) | 26 (25.5) | 0.24 |
| Negative | 58 (36.2) | 4 (6.9) | 37 (63.8) | 17 (29.3) | ||
| TNBC | Yes | 38 (23.8) | 8 (21.1) | 18 (47.4) | 12 (31.6) | 0.105 |
| No | 122 (76.2) | 12 (9.8) | 79 (64.8) | 31 (25.4) | ||
| Luminal A | Yes | 50 (31.6) | 2 (4.0) | 30 (60.0) | 18 (36.0) | 0.018 |
| No | 108 (68.4) | 18 (16.7) | 67 (62.0) | 23 (21.3) | ||
| Luminal B | Yes | 44 (27.5) | 6 (13.6) | 32 (72.7) | 6 (13.6) | 0.05 |
| No | 116 (72.5) | 14 (12.1) | 65 (56.0) | 37 (31.9) | ||
| Basal like | Yes | 9 (5.6) | 5 (55.6) | 4 (44.4) | 0 (0.0) | 0.001 |
| No | 151 (94.4) | 15 (9.9) | 93 (61.6) | 43 (28.5) | ||
| AR+_ER/PR- | Yes | 28 (17.5) | 9 (32.1) | 11 (39.3) | 8 (28.6) | 0.004 |
| No | 132 (82.5) | 11 (8.3) | 86 (65.2) | 35 (26.5) | ||
| AR+_TNBC | Yes | 13 (8.1) | 5 (38.5) | 4 (30.8) | 4 (30.8) | 0.021 |
| No | 147 (91.9) | 15 (10.2) | 93 (63.3) | 39 (26.5) | ||
| p53 | High | 36 (22.5) | 11 (30.6) | 17 (47.2) | 8 (22.2) | 0.003 |
| Low | 124 (77.5) | 9 (7.3) | 80 (64.5) | 35 (28.2) | ||
| EGFR | High | 40 (25.3) | 6 (15.0) | 23 (57.5) | 11 (27.5) | 0.846 |
| Low | 118 (74.7) | 14 (11.9) | 73 (61.9) | 31 (26.3) | ||
| Tumor PD-L1 | Positive | 11 (7.4) | 2 (18.2) | 8 (72.7) | 1 (9.1) | 0.311 |
| Negative | 138 (92.6) | 18 (13.0) | 81 (58.7) | 39 (28.3) | ||
| Stromal PD-L1 | Positive | 29 (19.5) | 12 (41.4) | 16 (55.2) | 1 (3.4) | <0.001 |
| Negative | 120 (80.5) | 8 (6.7) | 73 (60.8) | 39 (32.5) |
Correlation between stromal types and stromal PD-L1 expression status
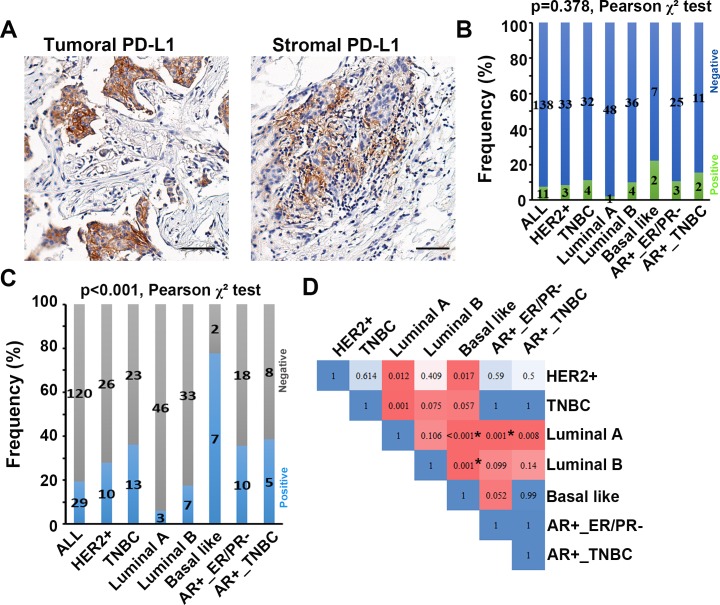
The percentage of patients with positive PD-L1 expression were relative low, which was 7.4% for tumor cells and 19.5% for stromal cells (Fig 2). Intriguingly, the stromal type was significantly associated with stromal PD-L1 status, with mature fibrotic cancer stroma having the highest rate of positive stromal PD-L1 expression (p<0.001, Table 2). However, there was no statistically significant difference between stromal types and tumoral PD-L1 status (p = 0.311, Table 2). To evaluate whether tumoral and stromal PD-L1 expression status were significantly different in different molecular subtypes of breast cancer, we performed Pearson χ2 test for multiple comparisons. The result showed that tumoral PD-L1 expression status was not significantly different among different molecular subtypes (p = 0.378, Fig 2B), while stromal PD-L1 expression status was significantly different among different molecular subtypes (p<0.001, Fig 2C). To further distinguish the difference between each molecular subtype, Pearson χ2 test and Bonferroni correction was conducted. It revealed that stromal PD-L1 expression status was significantly different between Luminal A and Basal like subtypes (p<0.001), Luminal A and AR+_ER/PR- subtypes (p = 0.001), Luminal B and Basal like (p = 0.001). Taken together, stromal subtypes might have a great influence on the PD-L1 expression in tumor microenvironmental cells.
Fig 2. Tumoral and stromal expression of PD-L1.
(A) Representative image of positive tumoral and stromal expression of PD-L1. Scale bar: 50 μm. (B, C) Distributions of tumoral(B) and stromal(C) PD-L1 expression status in different molecular subtypes of breast cancer. The number in the histogram bar indicated the number of cases. Multiple comparison among different molecular subtypes was performed by Pearson χ2 test. (D) Differences between each other of stromal PD-L1 expression status among seven molecular subtypes indicated by p values. The comparison between each molecular subtype was conducted by Pearson χ2 test and corrected by Bonferroni correction. Because of 21 comparisons between each other among seven molecular subtypes, differences were considered significant when the Bonferroni correction p value less than 0.0024 (0.05/21).
Table 2. The inter-relationship between clinicopathological characteristics and PD-L1 expression status in patients breast cancer (n = 160).
| Feature | N (%) | Tumor PD-L1 | Stromal PD-L1 | |||||
|---|---|---|---|---|---|---|---|---|
| Positive (%) | Negative (%) | p value | Positive (%) | Negative (%) | p value | |||
| Age | < = 50 | 77 (51.7) | 3 (3.9) | 74 (96.1) | 0.092 | 13 (16.9) | 64 (83.1) | 0.411 |
| >50 | 72 (48.3) | 8 (11.1) | 64 (88.9) | 16 (22.2) | 56 (77.8) | |||
| Tumor location | Right side | 65 (43.6) | 5 (7.7) | 60 (92.3) | 0.899 | 14 (21.5) | 51 (78.5) | 0.574 |
| Left side | 84 (56.4) | 6 (7.1) | 78 (92.9) | 15 (17.9) | 69 (82.1) | |||
| Grade | I | 16 (10.7) | 0 (0.0) | 16 (100.0) | 0.192 | 2 (12.5) | 14 (87.5) | 0.718 |
| II | 129 (86.6) | 11 (8.5) | 118 (91.5) | 26(20.2) | 103 (79.8) | |||
| III | 4 (2.7) | 0 (0.0) | 4 (100.0) | 1(25.0) | 3(75.0) | |||
| pT | pT1-2 | 32 (21.8) | 3 (9.4) | 29 (90.6) | 0.646 | 6 (18.8) | 26 (81.2) | 0.875 |
| pT3-4 | 115 (78.2) | 8 (7.0) | 107 (93.0) | 23 (20.0) | 92 (80.0) | |||
| pN | pN0-1 | 103 (70.5) | 8 (7.8) | 95 (92.2) | 0.869 | 23 (22.3) | 80 (77.7) | 0.248 |
| pN2-3 | 43 (29.5) | 3 (7.0) | 40 (93.0) | 6 (14.0) | 37 (86.0) | |||
| LNM | Yes | 90 (61.6) | 4 (4.4) | 86 (95.6) | 0.073 | 14 (15.6) | 76 (84.4) | 0.098 |
| No | 56 (38.4) | 7 (12.5) | 49 (87.5) | 15 (26.8) | 41 (73.2) | |||
| AJCC stage | 1+2 | 99 (68.3) | 8 (8.1) | 91 (91.9) | 0.741 | 23 (23.2) | 76 (76.8) | 0.153 |
| 3 | 46 (31.7) | 3 (6.5) | 43 (93.5) | 6 (13.0) | 40 (87.0) | |||
| HER2 | Positive | 36 (24.2) | 3 (8.3) | 33 (91.7) | 0.802 | 10 (27.8) | 26 (72.2) | 0.148 |
| Negative | 113 (75.8) | 8 (7.1) | 105 (92.9) | 19 (16.8) | 94 (83.2) | |||
| ER | Positive | 89 (59.7) | 5 (5.6) | 84 (94.4) | 0.316 | 10 (11.2) | 79 (88.8) | 0.002 |
| Negative | 60 (40.3) | 6 (10.0) | 54 (90.0) | 19 (31.7) | 41 (68.3) | |||
| PR | Positive | 71 (47.7) | 2 (2.8) | 69 (97.2) | 0.042 | 6 (8.5) | 65 (91.5) | 0.001 |
| Negative | 78 (52.3) | 9 (11.5) | 69 (88.5) | 23 (19.5) | 55 (70.5) | |||
| AR | Positive | 95 (63.8) | 6 (6.3) | 89 (93.7) | 0.509 | 16 (16.8) | 79 (83.2) | 0.284 |
| Negative | 54 (36.2) | 5 (9.3) | 49 (90.7) | 13 (24.1) | 41 (75.9) | |||
| TNBC | Yes | 36 (24.2) | 4 (11.1) | 32 (88.9) | 0.326 | 13 (36.1) | 23 (63.9) | 0.004 |
| No | 113 (75.8) | 7 (6.2) | 106 (93.8) | 16 (14.2) | 97 (85.8) | |||
| Luminal A | Yes | 49 (33.1) | 1 (2.0) | 48 (98.0) | 0.079 | 3 (6.1) | 46 (93.9) | 0.004 |
| No | 99 (66.9) | 10 (10.1) | 89 (89.9) | 26 (26.3) | 73 (73.7) | |||
| Luminal B | Yes | 40 (26.8) | 4 (10.0) | 36 (90.0) | 0.459 | 7 (17.5) | 33 (82.5) | 0.714 |
| No | 109 (73.2) | 7 (6.4) | 102 (93.6) | 22 (20.2) | 87 (79.8) | |||
| Basal like | Yes | 9 (6.0) | 2 (22.2) | 7 (77.8) | 0.079 | 7 (77.8) | 2 (22.2) | <0.001 |
| No | 140 (94.0) | 9 (6.4) | 131 (93.6) | 22 (15.7) | 118 (84.3) | |||
| AR+_ER/PR- | Yes | 28 (18.8) | 3 (10.7) | 25 (89.3) | 0.454 | 10 (35.7) | 18 (64.3) | 0.016 |
| No | 121 (81.2) | 8 (6.6) | 113 (93.4) | 19 (15.7) | 102(84.3) | |||
| AR+_TNBC | Yes | 13 (8.7) | 2 (15.4) | 11 (84.6) | 0.248 | 5 (38.5) | 8 (61.5) | 0.07 |
| No | 136 (91.3) | 9 (6.6) | 127 (93.4) | 24 (17.6) | 112 (82.4) | |||
| p53 | High | 33 (22.1) | 3 (9.1) | 30 (90.9) | 0.671 | 9 (27.3) | 24 (72.7) | 0.199 |
| Low | 116 (77.9) | 8 (6.9) | 108 (93.1) | 20 (17.2) | 96 (82.8) | |||
| EGFR | High | 37 (25.2) | 5 (13.5) | 32 (86.5) | 0.107 | 14 (37.8) | 23 (62.2) | 0.001 |
| Low | 110 (74.8) | 6 (5.5) | 104 (94.5) | 15 (13.6) | 95 (86.4) | |||
| Stromal types | mature | 20 (13.4) | 2 (10.0) | 18 (90.0) | 0.311 | 12 (60.0) | 8 (40.0) | <0.001 |
| intermediate | 89 (59.7) | 8 (9.0) | 81 (91.0) | 16 (18.0) | 73 (82.0) | |||
| immature | 40 (26.8) | 1 (2.5) | 39 (97.5) | 1 (2.5) | 39 (97.5) | |||
Stromal PD-L1 status correlated with clinicopathological characteristics
Statistical analysis revealed that tumor PD-L1 status only negatively correlated with PR status (p = 0.042), while it was not significantly associated with any other clinicopathological features. However, stromal PD-L1 status was significantly associated with many clinicopathological characteristics (Table 2), representing that higher frequency of positive stromal PD-L1 in hormone receptor negative subtypes (p = 0.002 for ER- and p = 0.001 for PR-), TNBC subtype (P = 0.004), Basal like subtype (P<0.001), AR+_ER/PR- subtype (P = 0.016) and lower frequency of positive stromal PD-L1 in Luminal A subtype (p = 0.004). Intriguingly, high EGFR expression was correlated with high frequency of positive stromal PD-L1 staining (p = 0.001). These findings suggested that stromal PD-L1 was significantly affected by host tumor characteristics and might have important clinical implications.
Stromal types and stromal PD-L1 status predicted prognostic outcome
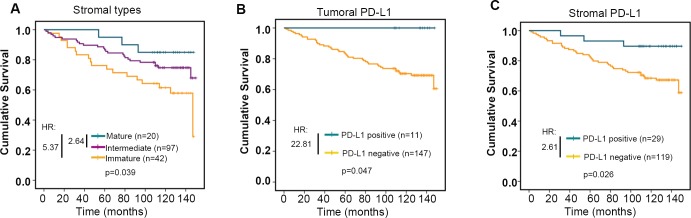
Notably, stromal types were significantly associated with overall survival of breast cancer patients, whose tumor with mature stroma had the best overall survival, and with immature stroma had the worst survival (p = 0.039, Kaplan–Meier survival analysis and log-rank test, HR: mature stroma vs immature stroma was 5.37; intermediate stroma vs immature stroma was 2.64. Fig 3A). Kaplan–Meier survival analysis also revealed that patients with tumoral or stromal PD-L1 expression were linked to better survival outcome (p = 0.047 and p = 0.026, respectively, Kaplan–Meier survival analysis and log-rank test. HR was 22.81 and 2.61, respectively. Fig 3B and 3C). Interestingly, multivariate Cox regression survival analysis adjusting for stromal types, age, pathological T stage, pathological N stage and pathological grade consistently reported strong correlation between stromal types and overall survival (p = 0.012, HR = 0.441, 95% CI 0.234 to 0.832 for mature vs immature, and p = 0.085, HR = 0.333, %95 CI 0.095 to 1.164 for intermediate vs immature. Table 3), indicating that stromal type was an independent prognostic factor for outcome in breast cancer. Similarly, multivariate Cox regression survival analysis adjusting for stromal PD-L1 status, age, pathological T stage, pathological N stage and pathological grade also revealed that stromal PD-L1 status was an independent prognostic factor for outcome of breast cancer (p = 0.033, HR = 0.278, %95 CI 0.085 to 0.902 for positive vs negative, Table 4). However, when taking all these variables into consideration, multivariate Cox regression survival analysis failed to report that stromal types and stromal PD-L1 status predicted prognosis independently (Table 5).
Fig 3. Prognostic value of stromal types and PD-L1 expression status.
Kaplan-Meier survival analysis of patients with breast cancer stratified by stromal types (A), tumoral PD-L1 expression status (B) and stromal PD-L1 expression status (C). Log-rank test was used to evaluate differences between/among different groups. Univariate Cox proportional hazards regression model was used to determine the hazard ratio (HR) between different stratified groups.
Table 3. Multivariate Cox analysis on stromal types and other prognostic factors.
| Clinical features | p value | HR | 95% CI | |
|---|---|---|---|---|
| Up | Down | |||
| Stromal type | 0.022 | |||
| Stromal type (Mature vs Immature) | 0.012 | 0.441 | 0.234 | 0.832 |
| Stromal type (Intermediate vs Immature) | 0.085 | 0.333 | 0.095 | 1.164 |
| Age | 0.035 | 1.938 | 1.049 | 3.579 |
| Grade (I vs II+III) | 0.758 | 0.857 | 0.323 | 2.279 |
| Pathological T stage (T1+T2 vs T3+T4) | 0.799 | 1.105 | 0.512 | 2.382 |
| Pathological N stage (N0+N1 vs N2+N3) | 0.003 | 0.378 | 0.198 | 0.723 |
Table 4. Multivariate Cox analysis on stromal PD-L1 and other prognostic factors.
| Clinical features | p value | HR | 95% CI | |
|---|---|---|---|---|
| Up | Down | |||
| Age | 0.021 | 2.129 | 1.120 | 4.045 |
| Grade (I vs II+III) | 0.343 | 0.620 | 0.231 | 1.666 |
| Pathological T stage (T1+T2 vs T3+T4) | 0.505 | 1.335 | 0.571 | 3.122 |
| Pathological N stage (N0+N1 vs N2+N3) | 0.017 | 0.457 | 0.240 | 0.869 |
| stromal PD-L1 | 0.033 | 0.278 | 0.085 | 0.902 |
Table 5. Multivariate Cox analysis on stromal types, stromal PD-L1 and other prognostic factors.
| Clinical features | p value | HR | 95% CI | |
|---|---|---|---|---|
| Up | Down | |||
| Age | 0.035 | 1.984 | 1.051 | 3.748 |
| Grade (I vs II+III) | 0.551 | 0.734 | 0.266 | 2.026 |
| Pathological T stage (T1+T2 vs T3+T4) | 0.439 | 1.412 | 0.589 | 3.384 |
| Pathological N stage (N0+N1 vs N2+N3) | 0.014 | 0.427 | 0.217 | 0.840 |
| stromal PD-L1 | 0.114 | 0.363 | 0.103 | 1.273 |
| Stromal types | 0.053 | |||
| Stromal type (Mature vs Immature) | 0.017 | 0.445 | 0.229 | 0.864 |
| Stromal type (Intermediate vs Immature) | 0.280 | 0.477 | 0.125 | 1.827 |
Discussion
Breast cancer is a fibrotic/desmoplastic stroma cancer [5, 19], and tumor stroma percentage (TSP) or tumor-stroma ratio (TSR) has been recently reported to have prognostic value in patients with triple negative [20] and node-negative breast cancer [21]. However, the relationship between fibrotic stromal types and clinicopathological features has not been extensive studied yet. Here, we investigated the stromal types and molecular subtypes of 160 breast cancer and explored the relationship with many important determinants of outcome such as age, grade, pathological T stage, N stage, lymph node metastasis, AJCC stage, overall survival status, tumoral and stromal PD-L1 status. Interestingly, stromal types were distinct among different molecular subtypes. Mature stromal type was significantly correlated with lower pathological N stage and AJCC stage and better prognostic outcome. Moreover, we also showed that positive stromal PD-L1 staining was enriched in patients with mature stromal type. These findings revealed that stromal type may not only be a candidate parameter for prognostication, but might also lead to the subsequent development of immuno-therapeutic strategies.
Apart from the tumor cell itself, the knowledge of the complex microenvironment will provide full understanding of the behaviors of tumor. Compelling evidence reveals that tumor stroma, one component of tumor microenvironment, remarkably affects tumor growth and progression [22, 23]. It is well studied that stroma promotes tumor proliferation and dissemination by multiple mechanisms, including remodeling extracellular matrix, recruiting of inflammatory cells, rewiring stromal regulatory pathways [24–27]. Previous studies have reported that tumor stroma was implicated with prognostic outcome in patients with colorectal [28, 29] and esophageal cancers [30]. Additionally, the percentage of tumor stroma has been recently reported to have prognostic value in patients with triple negative [20] and node-negative breast cancer [21]. However, in the basis of stromal maturity, there was just one study that reported that immature stroma of breast cancer was correlated with higher grade and positive nodes [31] Our study here showed that mature stroma of breast cancer was significantly associated with lower pathological N stage and AJCC stage, however, it did not come to statistical significance with grade and lymph node metastasis. Importantly, our results also provided evidence that different molecular subtypes of breast cancer have distinct stromal types. ER positive and PR positive tumor tended to have lower percentage of mature stroma, while the HER2 enriched subtype of tumor tended to contain a higher percentage of mature stroma. Additionally, the tumor microenvironment of Luminal A tumors consisted of less mature stroma than the other subtypes, while the tumor microenvironment of Luminal B tumors consisted of less immature stroma than the other subtypes. The percentage of mature stroma was significantly higher in basal like, AR positive-ER/PR negative, and AR positive-triple negative breast cancer. The distinct relationship between stromal types and molecular subtypes of breast cancer suggests that the behaviors of different molecular subtypes may be affected by different stromal types and stromal types should be taken into consideration when making optimal treatment choices, which is worthy and urgent need for further investigations.
Intriguingly, the percentage of tumoral p53 high expression was significantly higher in those surrounded with mature stroma. This phenomenon was consistent with previous studies which have revealed that p53 can inhibit collagen expression in fibroblast [32, 33]. Additionally, Sonja M. Wörmann et al also demonstrated that loss of p53 function in pancreatic tumor activated Shp2-JAK2–STAT3 signaling, which promoted desmoplasia [34]. The cell autonomous roles of the p53 protein have been comprehensively studied, while accumulating evidence suggests that p53 has a non-cell autonomous tumor suppressing role in the tumor stroma by regulating the expression of various secreted proteins [35]. Recently, Rong Fu et al revealed that ZEB1/p53 signaling axis in stromal fibroblasts could promote mammary epithelial tumors via enhancing FGF2/7, VEGF and IL-6 expression and secretion [36]. However, whether stromal elements interacting with tumor cells will influence the expression of p53 in tumor cells is still unclear, which is worthy to be in-depth studied.
The relationships between the gross pathological characteristics, tumor stroma and tumor microenvironment are extremely complex, however, the stromal types remained independently and strongly associated with overall survival of patients with breast cancer. These results confirmed the crucial role of tumor stroma in determining oncological outcome. Given that the determination of tumor stromal types is a relatively simple and quick procedure in risk assessment of breast cancer, it is promising that stromal maturity classification could serve as a tool for the routine pathological examination, which can be done on routine H&E sections without the necessity for further staining.
Our work also demonstrated that different molecular subtypes had different tumoral and stromal PD-L1 expression. TNBC and basal like subtypes tend to have a higher percentage of positive PD-L1 expression, while Luminal A subtype had the lowest positive staining of PD-L1, this phenomenon was consistent with the previous study [37] and suggesting further categorization of TNBC in subgroups according to the PD-L1 status will benefit immune checkpoint blockade.
In the present study, the tumor cells and stromal cells had low frequency of PD-L1 positive staining, however, the percentage of positive stromal PD-L1 staining was relatively higher than tumoral PD-L1 staining, which was consistent with the previous study in DCIS [38]. Recently, a meta-analysis including initial 4184 entries, 38 retrospective studies of breast cancer revealed that the overall pooled PD-L1 protein positivity rate was 24% (95% CI 15–64%) in tumor cells and 33% (95% CI 14–56%) in immune cells [37]. The heterogeneity in PD-L1 expression may be due to the study population, antibody used and positivity threshold, which is a major challenge that needs to be overcome before PD-L1 testing can be standardized and used in daily clinical practice. Additionally, the relatively low frequency of positive PD-L1 staining in breast cancer may be due to the limited detection technology. Heng-Huan Lee et al recently demonstrated that removal of N-linked glycosylation of PD-L1 could enhance the recognition of PD-L1 antibodies, and deglycosylated PD-L1 is a more reliable biomarker to guide immunotherapy [39]. On the other hand, the prognostic implications of PD-L1 in a large number of studies investigating breast cancer have had conflicting results. Here, we showed that positive stromal PD-L1 expression was correlated with better prognostic OS, which was consistent with the recent meta-analysis, while the correlation of tumoral PD-L1 expression status and OS was on the contrary[37]. This discrepancy may be due to the heterogeneous populations, and robust analysis and clinical validity test should be performed in a larger sample size. Meanwhile, we also found a significant correlation between stromal types and stromal PD-L1 status that positive stromal PD-L1 was strongly enriched in patients with mature stroma. Stroma types and stromal PD-L1 status were reported as independent prognostic factors when multivariate Cox regression survival analysis adjusted by age, pathological T stage, pathological N stage and pathological grade. However, when taken all these variates into consideration, stromal types or stromal PD-L1 status failed to predict prognosis independently, which indicating that stromal types had a direct effect on the expression of stromal PD-L1. These observations provided important insights for understanding immune-based treatment response, especially for the strategies of inhibitors of PD-L1 in breast cancer, and guiding the design and analysis of relevant clinical trials. Our finding also highlighted the importance of stromal types on immunological response in patients with breast cancer. Implementing this simple and reproducible parameter in routine pathological examination may help optimizing patient stratification for immune-based therapeutic strategies.
The stroma-rich tumor may hinder the penetration of monoclonal antibodies (mAbs) into the cells, and that leads to failure of the conventional cell-targeting immunoconjugate strategy. Our observation revealed that a subset of HER+ or PD-L1 positive patients, which may be benefit from anti-HER2 or anti-PD-L1 monoclonal antibodies therapy, possessed immature stroma. These patients may have no response for immunoconjugate strategy. To overcome this drawback, Masahiro Yasunaga et al designed a strategy that conjugated SN-38, a topoisomerase I inhibitor, to a mAb to collagen 4, a plentiful component of the tumor stroma via ester-bond, which was effective to stroma-rich pancreatic cancer[40]. Toward Hypervascular stroma-poor tumor, the authors generated an anti-CD20 mAb-PEG-SN-38 via carbamate-bond as conventional immunoconjugate, which promoted SN-38 to be released by a carboxylesterase inside of the tumor cell following the internalization, showed strong anti-tumor activity [40]. Another promising example is that Samaresh Sau et al conjugated PD-L1 antibody to Doxorubicin (Dox) through a hydrazone linker containing a polyethylene glycol (PEG) spacer. Dox was used to disrupt the tumor extracellular environment so that PD-L1 antibody can penetrate the tumor core. PD-L1-Dox demonstrates significant antitumor activity in a breast cancer spheroid model [41]. These studies raise the possibility that targeting tumor stroma via immunoconjugate strategy may be a promising choice for increasing treatment efficacy.
In conclusion, our study demonstrates the importance of stromal types and stromal PD-L1 status in determining clinical outcomes in patients with breast cancer. Due to its simple and quick assessment procure, stromal type classification may be readily incorporated into routine clinical risk stratification following curative resection or optimal therapeutic design. These findings also highlighted that stromal types should be taken into consideration in the determinant of different therapeutic strategies for different molecular subtypes, especially for immune-based therapy.
Acknowledgments
We are grateful to Dr. Rongzhen Luo from Department of Pathology, Sun Yat-sen University Cancer Center (SYSUCC) and Dr. Wei Sun from Department of Pathology, Beijing Cancer Hospital for pathological analysis supporting.
Data Availability
All relevant data are within the manuscript.
Funding Statement
This work was supported by National Natural Science Foundation of China (81602342, 81802780), Natural Science Foundation of Guangdong Province, China (2017A030313466).
References
- 1.Shimoda M, Mellody KT, Orimo A. Carcinoma-associated fibroblasts are a rate-limiting determinant for tumour progression. Semin Cell Dev Biol. 2010;21(1):19–25. 10.1016/j.semcdb.2009.10.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ahn S, Cho J, Sung J, Lee JE, Nam SJ, Kim KM, et al. The prognostic significance of tumor-associated stroma in invasive breast carcinoma. Tumour Biol. 2012;33(5):1573–80. 10.1007/s13277-012-0411-6 [DOI] [PubMed] [Google Scholar]
- 3.Beck AH, Sangoi AR, Leung S, Marinelli RJ, Nielsen TO, van de Vijver MJ, et al. Systematic analysis of breast cancer morphology uncovers stromal features associated with survival. Sci Transl Med. 2011;3(108):108ra13. [DOI] [PubMed] [Google Scholar]
- 4.Ueno H, Jones AM, Wilkinson KH, Jass JR, Talbot IC. Histological categorisation of fibrotic cancer stroma in advanced rectal cancer. Gut. 2004;53(4):581–6. 10.1136/gut.2003.028365 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kauppila S, Stenback F, Risteli J, Jukkola A, Risteli L. Aberrant type I and type III collagen gene expression in human breast cancer in vivo. J Pathol. 1998;186(3):262–8. [DOI] [PubMed] [Google Scholar]
- 6.Santa-Maria CA, Nanda R. Immune Checkpoint Inhibitor Therapy in Breast Cancer. J Natl Compr Canc Netw. 2018;16(10):1259–68. 10.6004/jnccn.2018.7046 [DOI] [PubMed] [Google Scholar]
- 7.Emens LA. Breast cancer immunobiology driving immunotherapy: vaccines and immune checkpoint blockade. Expert Rev Anticancer Ther. 2012;12(12):1597–611. 10.1586/era.12.147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cimino-Mathews A, Foote JB, Emens LA. Immune targeting in breast cancer. Oncology (Williston Park). 2015;29(5):375–85. [PubMed] [Google Scholar]
- 9.Dong H, Zhu G, Tamada K, Chen L. B7-H1, a third member of the B7 family, co-stimulates T-cell proliferation and interleukin-10 secretion. Nat Med. 1999;5(12):1365–9. 10.1038/70932 [DOI] [PubMed] [Google Scholar]
- 10.Dill EA, Gru AA, Atkins KA, Friedman LA, Moore ME, Bullock TN, et al. PD-L1 Expression and Intratumoral Heterogeneity Across Breast Cancer Subtypes and Stages: An Assessment of 245 Primary and 40 Metastatic Tumors. Am J Surg Pathol. 2017;41(3):334–42. 10.1097/PAS.0000000000000780 [DOI] [PubMed] [Google Scholar]
- 11.Stovgaard ES, Dyhl-Polk A, Roslind A, Balslev E, Nielsen D. PD-L1 expression in breast cancer: expression in subtypes and prognostic significance: a systematic review. Breast Cancer Res Treat. 2019. [DOI] [PubMed] [Google Scholar]
- 12.Zhang M, Sun H, Zhao S, Wang Y, Pu H, Wang Y, et al. Expression of PD-L1 and prognosis in breast cancer: a meta-analysis. Oncotarget. 2017;8(19):31347–54. 10.18632/oncotarget.15532 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tawfik O, Kimler BF, Karnik T, Shehata P. Clinicopathological correlation of PD-L1 expression in primary and metastatic breast cancer and infiltrating immune cells. Hum Pathol. 2018;80:170–8. 10.1016/j.humpath.2018.06.008 [DOI] [PubMed] [Google Scholar]
- 14.Gao Z, Wang C, Cui Y, Shen Z, Jiang K, Shen D, et al. Efficacy and Safety of Complete Mesocolic Excision in Patients With Colon Cancer: Three-year Results From a Prospective, Nonrandomized, Double-blind, Controlled Trial. Ann Surg. 2018. [DOI] [PubMed] [Google Scholar]
- 15.Zhang W, Hong R, Xue L, Ou Y, Liu X, Zhao Z, et al. Piccolo mediates EGFR signaling and acts as a prognostic biomarker in esophageal squamous cell carcinoma. Oncogene. 2017;36(27):3890–902. 10.1038/onc.2017.15 [DOI] [PubMed] [Google Scholar]
- 16.Press MF, Sauter G, Buyse M, Fourmanoir H, Quinaux E, Tsao-Wei DD, et al. HER2 Gene Amplification Testing by Fluorescent In Situ Hybridization (FISH): Comparison of the ASCO-College of American Pathologists Guidelines With FISH Scores Used for Enrollment in Breast Cancer International Research Group Clinical Trials. J Clin Oncol. 2016;34(29):3518–+. 10.1200/JCO.2016.66.6693 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Guiu S, Michiels S, Andre F, Cortes J, Denkert C, Di Leo A, et al. Molecular subclasses of breast cancer: how do we define them? The IMPAKT 2012 Working Group Statement. Ann Oncol. 2012;23(12):2997–3006. 10.1093/annonc/mds586 [DOI] [PubMed] [Google Scholar]
- 18.de Kruijf EM, Bastiaannet E, Ruberta F, de Craen AJM, Kuppen PJK, Smit VTHBM, et al. Comparison of frequencies and prognostic effect of molecular subtypes between young and elderly breast cancer patients. Mol Oncol. 2014;8(5):1014–25. 10.1016/j.molonc.2014.03.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Huijbers IJ, Iravani M, Popov S, Robertson D, Al-Sarraj S, Jones C, et al. A role for fibrillar collagen deposition and the collagen internalization receptor endo180 in glioma invasion. PLoS One. 2010;5(3):e9808 10.1371/journal.pone.0009808 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mohammed ZM, Going JJ, Edwards J, Elsberger B, Doughty JC, McMillan DC. The relationship between components of tumour inflammatory cell infiltrate and clinicopathological factors and survival in patients with primary operable invasive ductal breast cancer. Br J Cancer. 2012;107(5):864–73. 10.1038/bjc.2012.347 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dekker TJ, van de Velde CJ, van Pelt GW, Kroep JR, Julien JP, Smit VT, et al. Prognostic significance of the tumor-stroma ratio: validation study in node-negative premenopausal breast cancer patients from the EORTC perioperative chemotherapy (POP) trial (10854). Breast Cancer Res Treat. 2013;139(2):371–9. 10.1007/s10549-013-2571-5 [DOI] [PubMed] [Google Scholar]
- 22.Tlsty TD, Hein PW. Know thy neighbor: stromal cells can contribute oncogenic signals. Curr Opin Genet Dev. 2001;11(1):54–9. 10.1016/s0959-437x(00)00156-8 [DOI] [PubMed] [Google Scholar]
- 23.Lukashev ME, Werb Z. ECM signalling: orchestrating cell behaviour and misbehaviour. Trends Cell Biol. 1998;8(11):437–41. [DOI] [PubMed] [Google Scholar]
- 24.Kim JB, Stein R, O'Hare MJ. Tumour-stromal interactions in breast cancer: the role of stroma in tumourigenesis. Tumour Biol. 2005;26(4):173–85. 10.1159/000086950 [DOI] [PubMed] [Google Scholar]
- 25.Hu M, Polyak K. Microenvironmental regulation of cancer development. Curr Opin Genet Dev. 2008;18(1):27–34. 10.1016/j.gde.2007.12.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cirri P, Chiarugi P. Cancer-associated-fibroblasts and tumour cells: a diabolic liaison driving cancer progression. Cancer Metastasis Rev. 2012;31(1–2):195–208. 10.1007/s10555-011-9340-x [DOI] [PubMed] [Google Scholar]
- 27.Chen IX, Chauhan VP, Posada J, Ng MR, Wu MW, Adstamongkonkul P, et al. Blocking CXCR4 alleviates desmoplasia, increases T-lymphocyte infiltration, and improves immunotherapy in metastatic breast cancer. Proc Natl Acad Sci U S A. 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mesker WE, Junggeburt JM, Szuhai K, de Heer P, Morreau H, Tanke HJ, et al. The carcinoma-stromal ratio of colon carcinoma is an independent factor for survival compared to lymph node status and tumor stage. Cell Oncol. 2007;29(5):387–98. 10.1155/2007/175276 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Park JH, Richards CH, McMillan DC, Horgan PG, Roxburgh CS. The relationship between tumour stroma percentage, the tumour microenvironment and survival in patients with primary operable colorectal cancer. Ann Oncol. 2014;25(3):644–51. 10.1093/annonc/mdt593 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wang K, Ma W, Wang J, Yu L, Zhang X, Wang Z, et al. Tumor-stroma ratio is an independent predictor for survival in esophageal squamous cell carcinoma. J Thorac Oncol. 2012;7(9):1457–61. 10.1097/JTO.0b013e318260dfe8 [DOI] [PubMed] [Google Scholar]
- 31.Walker RA. The complexities of breast cancer desmoplasia. Breast Cancer Res. 2001;3(3):143–5. 10.1186/bcr287 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Shi J, Xiao H, Li J, Zhang J, Li Y, Zhang J, et al. Wild-type p53-modulated autophagy and autophagic fibroblast apoptosis inhibit hypertrophic scar formation. Lab Invest. 2018;98(11):1423–37. 10.1038/s41374-018-0099-3 [DOI] [PubMed] [Google Scholar]
- 33.Vennin C, Melenec P, Rouet R, Nobis M, Cazet AS, Murphy KJ, et al. CAF hierarchy driven by pancreatic cancer cell p53-status creates a pro-metastatic and chemoresistant environment via perlecan. Nat Commun. 2019;10(1):3637 10.1038/s41467-019-10968-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wormann SM, Song L, Ai J, Diakopoulos KN, Kurkowski MU, Gorgulu K, et al. Loss of P53 Function Activates JAK2-STAT3 Signaling to Promote Pancreatic Tumor Growth, Stroma Modification, and Gemcitabine Resistance in Mice and Is Associated With Patient Survival. Gastroenterology. 2016;151(1):180–93 e12. 10.1053/j.gastro.2016.03.010 [DOI] [PubMed] [Google Scholar]
- 35.Stein Y, Aloni-Grinstein R, Rotter V. Mutant p53—a potential player in shaping the tumor-stroma crosstalk. J Mol Cell Biol. 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Fu R, Han CF, Ni T, Di L, Liu LJ, Lv WC, et al. A ZEB1/p53 signaling axis in stromal fibroblasts promotes mammary epithelial tumours. Nat Commun. 2019;10(1):3210 10.1038/s41467-019-11278-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Matikas A, Zerdes I, Lovrot J, Richard F, Sotiriou C, Bergh J, et al. Prognostic Implications of PD-L1 Expression in Breast Cancer: Systematic Review and Meta-analysis of Immunohistochemistry and Pooled Analysis of Transcriptomic Data. Clin Cancer Res. 2019. [DOI] [PubMed] [Google Scholar]
- 38.Thompson E, Taube JM, Elwood H, Sharma R, Meeker A, Warzecha HN, et al. The immune microenvironment of breast ductal carcinoma in situ. Mod Pathol. 2016;29(3):249–58. 10.1038/modpathol.2015.158 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lee HH, Wang YN, Xia W, Chen CH, Rau KM, Ye L, et al. Removal of N-Linked Glycosylation Enhances PD-L1 Detection and Predicts Anti-PD-1/PD-L1 Therapeutic Efficacy. Cancer Cell. 2019;36(2):168–78 e4. 10.1016/j.ccell.2019.06.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yasunaga M, Manabe S, Tarin D, Matsumura Y. Tailored immunoconjugate therapy depending on a quantity of tumor stroma. Cancer Sci. 2013;104(2):231–7. 10.1111/cas.12062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sau S, Petrovici A, Alsaab HO, Bhise K, Iyer AK. PDL-1 Antibody Drug Conjugate for Selective Chemo-Guided Immune Modulation of Cancer. Cancers (Basel). 2019;11(2). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All relevant data are within the manuscript.