Abstract
BACKGROUND
Cytokine biomarkers have already been used to predict acute graft-versus-host disease (aGVHD) onset, nonrelapse mortality, and overall survival in human and mouse models, but the consistency of the consequences between patients and mice has not been evaluated. Furthermore, no study about any biomarker or biomarker panel for aGVHD grading or steroid sensitivity of aGVHD patients simultaneously has been reported.
METHODS
Here we established an aGVHD mouse model and explored the relation between aGVHD onset and variations of some cytokines. Based on the results and latest progress, we selected 16 cytokines and compared their serum variations in aGVHD patients and non-aGVHD patients after allogeneic hematopoietic stem cell transplantation. Using protein microarray, we explored the relation between the cytokine levels and aGVHD-related events (onset, grading, and steroid sensitivity).
RESULTS
The increase of chemokine levels in murine aGVHD was very consistent with that of patients. We found obviously higher levels of IL-2, IL-4, Elafin, sST2, TLR4, and TNF-α, and lower levels of TGF-β in both aGVHD mouse models and aGVHD patients. In addition, patients with severe aGVHD showed increased IL-6, TLR4, TNF receptor 1 (TNFR1), and Elafin and decreased TGF-β. TLR4 and TNFR1 were significantly increased in steroid-refractory aGVHD patients compared with steroid-effective patients (P < 0.05).
CONCLUSION
A combination of TLR4, TNFR1, TGF-β, and Elafin could be a new 4-biomarker panel to assist aGVHD diagnosis, grading, and evaluation of steroid sensitivity for clinical aGVHD patients.
TRIAL REGISTRATION
ChiCTR1900022292 “Clinical Research of Umbilical Cord–Derived Mesenchymal Stromal Cells in the Prophylaxis of Graft-Versus-Host Disease After HLA-Haploidentical Stem-Cell Transplantation.”
FUNDING
National Key Research Program, National Natural Science Foundation of China, Chongqing Social Career and People’s Livelihood Security Science and Technology Innovation Project, Fundamental and Frontier Research Program of Chongqing, and Foundation of Xinqiao Hospital.
Keywords: Hematology, Immunology
Keywords: Bone marrow transplantation, Cytokines
A 4-biomarker panel consisting of TLR4, TNFR1, TGF-β, and Elafin can be informative in acute graft-versus-host disease diagnosis, grading, and evaluation of steroid sensitivity in patients.
Introduction
Allogeneic hematopoietic stem cell transplantation (allo-HSCT) is one of the most effective methods to treat a variety of hematological malignancies. Acute graft-versus-host disease (aGVHD) is one of the major complications of allo-HSCT, with a high incidence of 30%–50% and a 14% fatality rate. With acute onset and rapid progression, aGVHD is one of the major factors affecting the success rate of allo-HSCT. Classically, aGVHD develops within the first 100 days after HSCT; donor T cells mediate a cytotoxic effect against multiple target organs, including the skin, gastrointestinal tract, and liver (1–3). Early diagnosis and treatment are crucial to prevent disease onset and deterioration, especially for severe aGVHD. At present, the diagnosis of aGVHD depends mainly on the clinical manifestation and pathological biopsy of target organs, which lacks disease specificity and clinical feasibility, respectively. So it is urgent to find new strategies with precise, noninvasive, and fast monitoring properties for early recognition of aGVHD.
The use of serum cytokines as aGVHD biomarkers has emerged for diagnosis and prognosis in both mouse models and human patients (4–6), but hardly any studies have discussed or explored the similarities and differences in the cytokine levels between human patients and mice. As the secretory products of specific immune cell subsets, cytokines reflect the systemic immune response as well as the functions of different immune cell populations related to aGVHD in both target organs and blood. Besides, exploring cytokine changes is also beneficial to explore aGVHD pathogenesis. Although multiple cytokines and cytokine combinations have been presented in mouse and human studies to predict aGVHD development, high risk for lethal GVHD, nonrelapse mortality, and even overall survival rate (7–9), little has been reported about a biomarker or biomarker panel for evaluating the grading and steroid sensitivity of patients with aGVHD simultaneously.
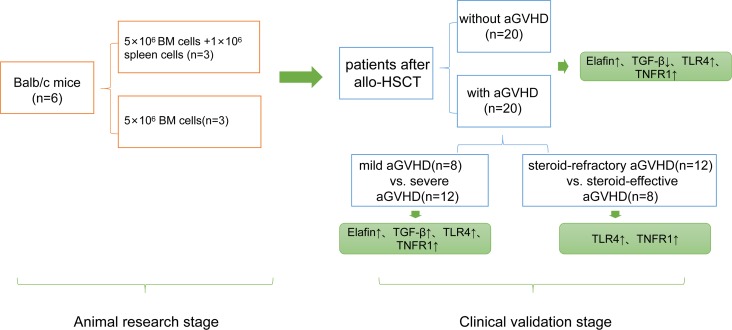
In this study, 22 common and novel cytokines were chosen as target candidates by comprehensively searching and analyzing recent articles and data (4–12). We measured the concentrations of these cytokines in mice with aGVHD and control mice with ELISA kits. Based on the results of animal experiments, we selected 16 cytokines and observed dynamically, with protein microarray technology, variations of them in blood samples of patients after allo-HSCT. We analyzed the distinctions of biomarkers for early aGVHD diagnosis of mice and patients, assessed the relation between these cytokines and aGVHD-related events (aGVHD onset, grading, and steroid sensitivity), and finally established a cocktail of cytokines with the ability to evaluate aGVHD onset, severity level, and steroid sensitivity in patients after allo-HSCT early (Figure 1).
Figure 1. Flow diagram of the study design.
Results
aGVHD evaluation in mice.
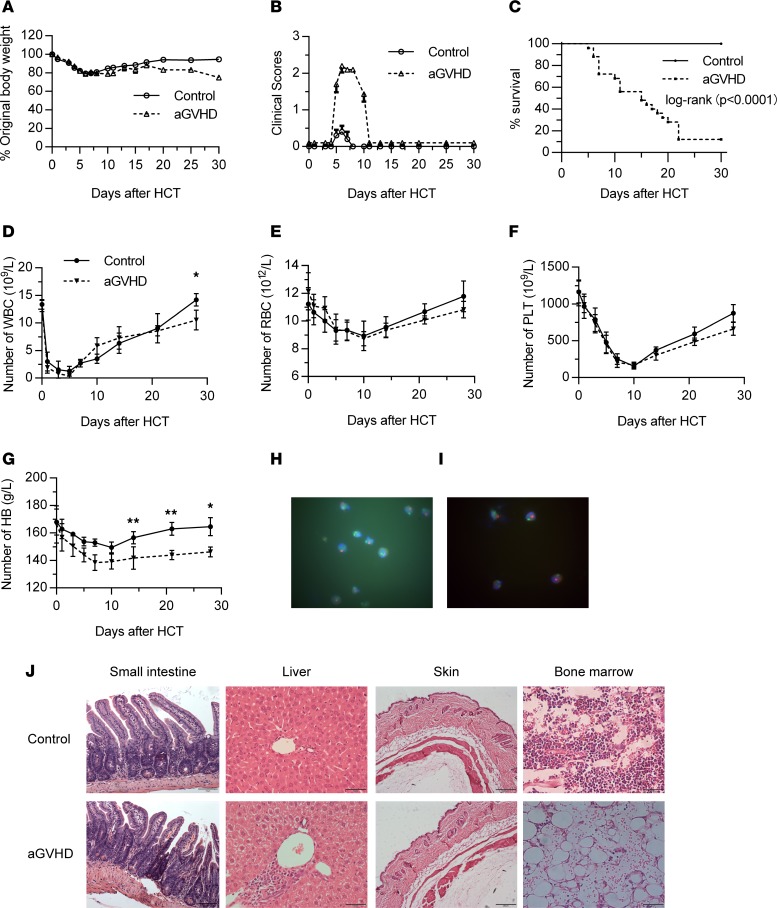
To select appropriate biomarkers to assist with aGVHD diagnosis, we first built an aGVHD mouse model with BALB/c mice as recipients and C57BL/6 mice as donors. The weight of the aGVHD group (n = 3) and control group (n = 3) experienced an acute decline after transplantation, then recovered after 7 days in both groups, but the weight of the aGVHD group did not increase to the pretransplantation level, while the control group did (Figure 2A). The clinical scores of the aGVHD group were obviously higher than those of the control group (P < 0.01), especially from day +5 to day +10 (Figure 2B). The survival rate of the control group was significantly higher than that of the aGVHD group (P < 0.0001) (Figure 2C). Besides, diarrhea, wet fur, hunch, and weakness were also observed from day +7 after allogeneic bone marrow transplantation.
Figure 2. Observation of aGVHD mouse model.
(A) Body weight loss rate (percentage) of aGVHD mice and control mice after allogeneic HSCT (allo-HSCT). (B) Clinical scores of aGVHD and control mice within 30 days after HSCT. (C) Survival curve of aGVHD and control mice within 30 days after HSCT. (D–G) Blood examination of aGVHD mice and control mice after HSCT. *P < 0.05; **P < 0.01, based on 2-tailed unpaired t test. (H) Chimeric state of donor mice in the control group by FISH. (I) Chimeric state of donor mice in the aGVHD group by FISH. Original magnification, ×1000 (H and I). (J) Pathology of different organs in aGVHD and control mice at day +14 after HSCT. Original magnification, ×200 (small intestine), ×400 (liver), ×100 (skin), ×400 (bone marrow). PLT, platelets. *P < 0.05; **P < 0.01.
Routine blood examinations of each group were conducted on days +1, +3, +5, +7, +10, +14, +21, and +28 after transplantation. Hematopoietic reconstitutions of each group started from day +7 to day +14 after transplantation. The aGVHD group recovered more slowly compared with the control group, with significant differences in hemoglobin and WBC (P < 0.05) (Figure 2, D–G). Meanwhile, we used fluorescence in situ hybridization (FISH) to evaluate the implantation of donor hematopoietic stem cells in mice of the 2 groups at day +14 after transplantation. The results showed that a large number of donor stem cells were detected in both groups, with a chimeric rate of 97.2% (control group) and 96.8% (aGVHD group) (Figure 2, H and I). Skin, liver, intestine, and bone marrow of mice in each group were obtained on day +14 after transplantation, and the fixed tissues were paraffin embedded, sectioned, and observed with H&E staining. The results showed that compared with the control group, mice in the aGVHD group had obvious villi destruction and mucosal injury in intestines. The liver showed a large number of infiltrating lymphocytes and liver cell necrosis. Bone marrow hyperplasia was inactive, with decreased parenchyma cells and increased vacuoles. No significant skin difference was observed between the 2 groups (Figure 2J). Taken together, these results indicated that the aGVHD model was successfully established.
Screening of biomarkers for aGVHD diagnosis in mouse model.
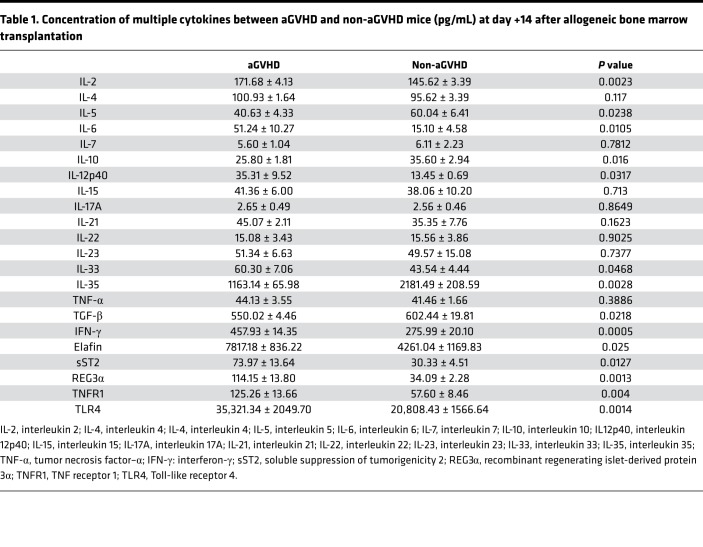
Because many cytokines have been demonstrated as being aGVHD biomarkers, we used ELISA to test serum levels of the selected 22 cytokines in blood samples of the 2 groups (Table 1). As shown in Figure 3, at day +14 after allo-HSCT, we observed significantly higher levels of IL-2, IL-4, IL-6, IL-12p40, IL-33, IFN-γ, Elafin, sST2, and REG3α and significantly lower levels of IL-5, IL-10, IL-35, and TGF-β in aGVHD mice. Among the increased cytokines, IL-6, IL-12p40, sST2, and REG3α showed a 2-fold increase compared with the control group (P < 0.05). These results demonstrated that after allo-HSCT, proinflammatory cytokines rose up quickly to a high level, while antiinflammatory cytokines slightly increased or even decreased (Figure 3).
Table 1. Concentration of multiple cytokines between aGVHD and non-aGVHD mice (pg/mL) at day +14 after allogeneic bone marrow transplantation.
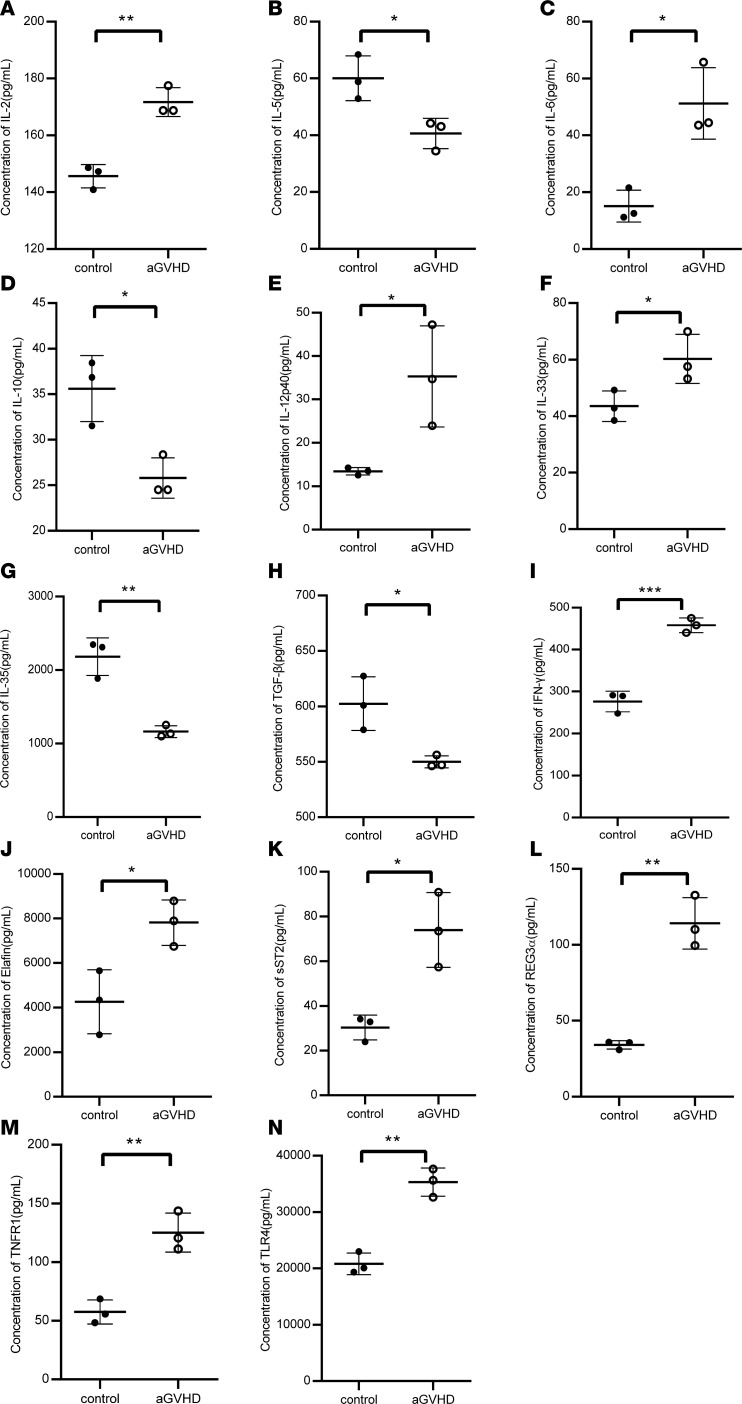
Figure 3. Concentration of 20 cytokines in control mice (n = 3) and aGVHD mice (n = 3) at day +14 after allogeneic bone marrow transplantation.
Significantly increased IL-2, IL-6, IL12p40, IL-33, IFN-γ, Elafin, sST2, REG3α, TNFR1, and TLR4 and significantly decreased IL-5, IL-10, IL-35, and TGF-β were measured in serum samples of aGVHD mice when compared with non-aGVHD mice at day +14 after transplantation based on 2-tailed, unpaired t test. (A) IL-2, P = 0.0023; (B) IL-5, P = 0.0238; (C) IL-6, P = 0.0105; (D) IL-10, P = 0.016; (E) IL-12p40, P = 0.0317; (F) IL-33, P = 0.0468; (G) IL-35, P = 0.0028; (H) TGF-β, P = 0.0218; (I) IFN-γ, P = 0.0005; (J) Elafin, P = 0.025; (K) sST2, P = 0.0127; (L) REG3α, P = 0.0013; (M) TNFR1, P = 0.004; (N) TLR4, P = 0.0014. *P < 0.05; **P < 0.01; ***P < 0.001.
Hematopoietic reconstitution for patients after allo-HSCT.
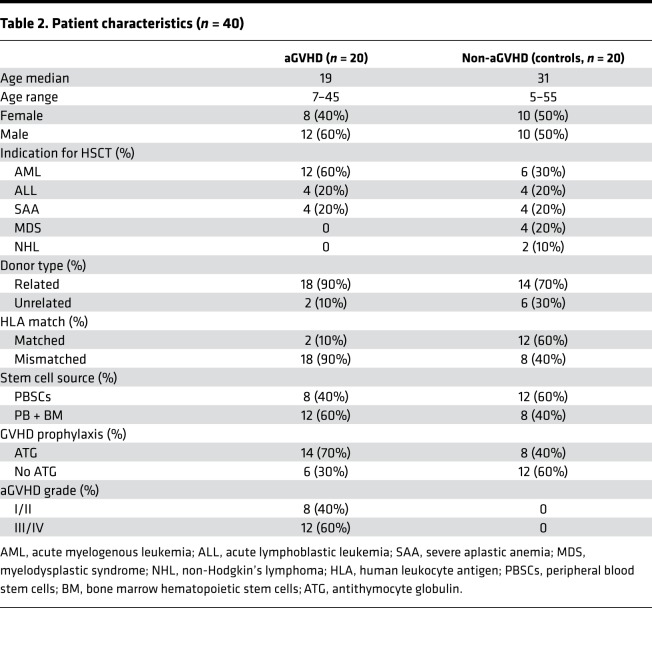
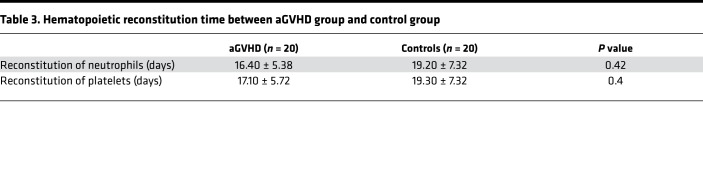
To study whether there is consistency of cytokine expression in human and mouse models of aGVHD, we enrolled patients with (n = 20) and without (n = 20) aGVHD after allo-HSCT and collected blood samples at different time points. Basic information of the patients is included in Table 2. The median time of neutrophil reconstitution greater than 0.5 × 109/L in the aGVHD group after allo-HSCT was 15.5 days (10–26 days), while in the control group the median time was 17 days (11–32 days). The median time of platelet reconstitution greater than 20 × 109/L in the aGVHD group and control group after allo-HSCT was 15.5 days (10–27 days) and 17 days (11–34 days), respectively. There was no significant difference in hematopoietic reconstitution time between the 2 groups (P > 0.05) (Table 3).
Table 2. Patient characteristics (n = 40).
Table 3. Hematopoietic reconstitution time between aGVHD group and control group.
Screening of biomarkers for aGVHD diagnosis in human patients.
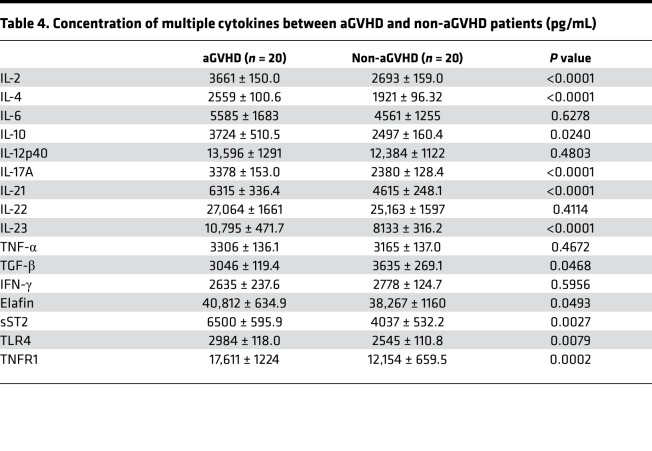
Based on the results of the animal experiment, we wanted to know whether these cytokines were also expressed in human patients. We chose 16 cytokines to be tested in human patients based on the results of the mouse experiment and the latest progressions of aGVHD, including IL-2, IL-4, IL-6, IL-10, IL-12p40, IL-17A, IL-21, IL-22, IL-2, TNF-α, TGF-β, IFN-γ, sST2, Elafin, TLR4, and TNFR1. Expression of IL-2, IL-4, IL-10, IL-21, IL-17A, IL-23, TLR4, TNFR1, sST2, and Elafin in patients with aGVHD was significantly increased, while TGF-β was decreased compared with the control group (P < 0.05) (Table 4 and Figure 4). In particular, IL-2, IL-4, IL-10, IL-21, IL-17A, and IL-23 increased by more than 30%; the result was slightly different from that in mice. IL-10 level decreased in aGVHD mice, and no statistical difference was found in IL-17A, IL-21, and IL-23 levels between aGVHD mice and control mice.
Table 4. Concentration of multiple cytokines between aGVHD and non-aGVHD patients (pg/mL).
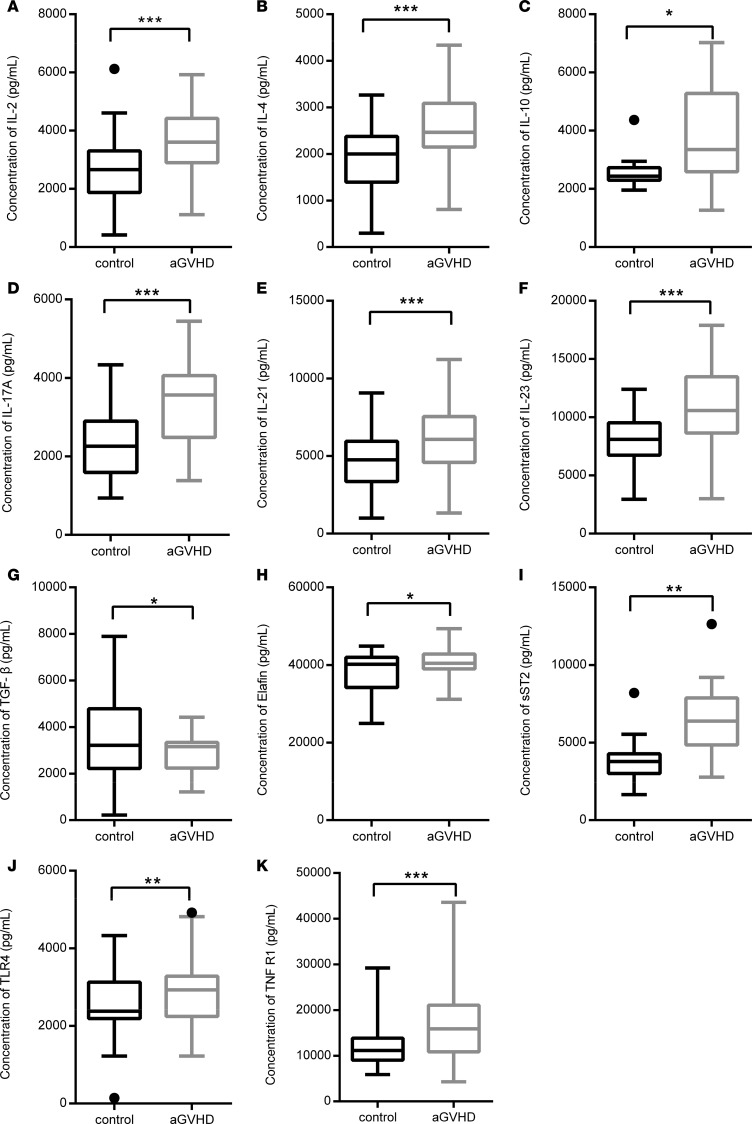
Figure 4. Statistical analysis of cytokines between allo-HSCT patients with aGVHD (n = 20) and without aGVHD (n = 20) when patients were diagnosed with aGVHD for the first time.
Significant differences of 11 serum cytokine levels between allo-HSCT patients with and without aGVHD are listed below based on 2-tailed, paired t test. (A) IL-2, P < 0.0001; (B) IL-4, P < 0.0001; (C) IL-10, P = 0.0240; (D) IL-17A, P < 0.0001; (E) IL-21, P < 0.0001; (F) IL-23, P < 0.0001; (G) TGF-β, P = 0.0468, (H) Elafin, P = 0.0493; (I) sST2, P = 0.0027; (J) TLR4, P = 0.0079; (K) TNFR1, P = 0.0002. The box plots depict the minimum and maximum values (whiskers), the upper and lower quartiles, and the median. The length of the box represents the interquartile range. *P < 0.05; **P < 0.01; ***P < 0.001.
Increased Elafin, TLR4, TNFR1, and IL-6 and decreased TGF-β were observed in patients with III/IV aGVHD.
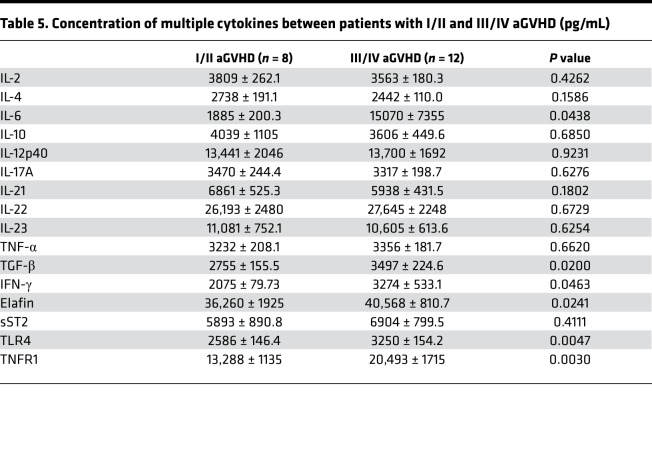
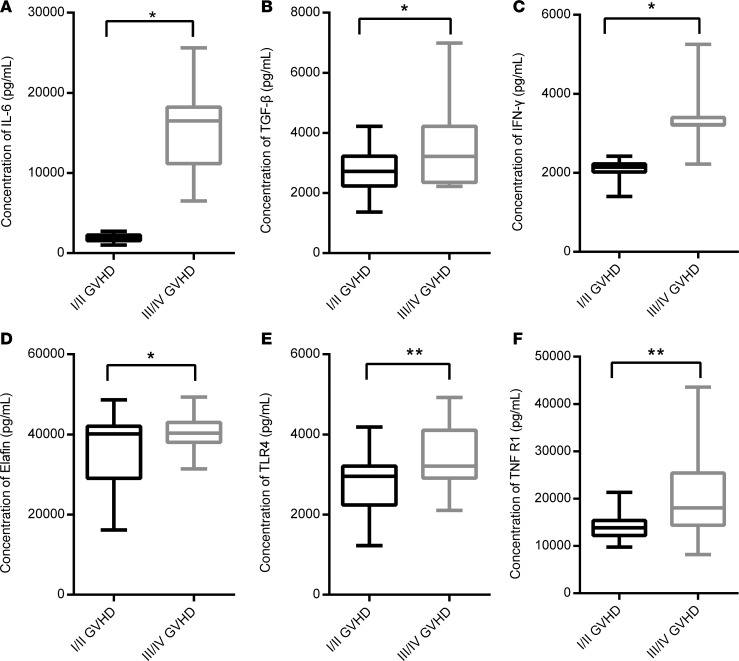
To further elucidate the role of the 16 cytokines, we explored the relation between these cytokines and aGVHD-related events. Patients with aGVHD were divided into I/II and III/IV groups according to Fred Hutchinson Cancer Research Center criteria. We found that IL-2, IL-4, IL-10, IL-17A, IL-21, and IL-23 seemed to be lower in patients with III/IV aGVHD, but there was no statistical significance. IL-6 level was extremely high in patients with III/IV aGVHD compared with I/II aGVHD (1885 ± 200.3 pg/mL vs. 15,070 ± 7355 pg/mL; P < 0.05), although high level of IL-6 is common in severe inflammatory response (13). No statistical difference was observed between patients with and without aGVHD. Interestingly, expression of TGF-β was significantly higher in patients with III/IV aGVHD compared with I/II aGVHD (3497 ± 224.6 pg/mL vs. 2755 ± 155.5 pg/mL; P < 0.05), while in patients with aGVHD the total level was decreased. Remarkable differences in TLR4, TNFR1, and Elafin were found between I/II and III/IV aGVHD patients (P < 0.05), which could be regarded as an indication of aGVHD severity (Table 5 and Figure 5).
Table 5. Concentration of multiple cytokines between patients with I/II and III/IV aGVHD (pg/mL).
Figure 5. Statistical analysis of obviously changed cytokines between patients with I/II aGVHD (n = 8) and patients with III/IV aGVHD (n = 12) at the peak of aGVHD.
Increased levels of IL-6, TGF-β, IFN-γ, Elafin, TLR4, and TNFR1 were measured in patients who were diagnosed with III/IV aGVHD based on 2-tailed, paired t test. (A) IL-6, P = 0.0438; (B) TGF-β, P = 0.0200; (C) IFN-γ, P = 0.0463; (D) Elafin, P = 0.0241; (E) TLR4, P = 0.0047; (F) TNFR1, P = 0.003. The box plots depict the minimum and maximum values (whiskers), the upper and lower quartiles, and the median. The length of the box represents the interquartile range. *P < 0.05; **P < 0.01.
TLR4 and TNFR1 were significantly increased in patients with steroid-refractory aGVHD.
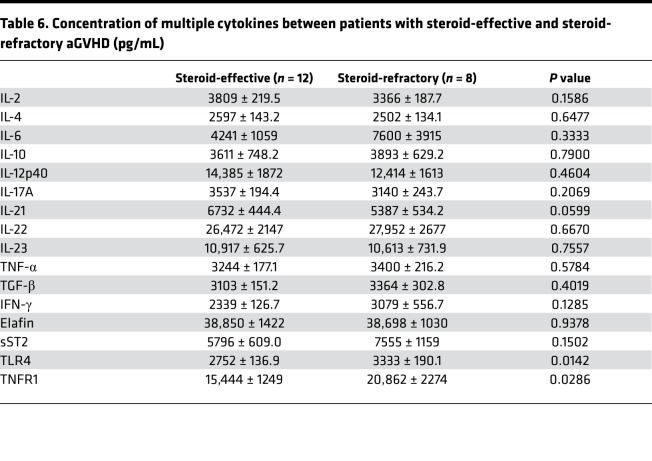
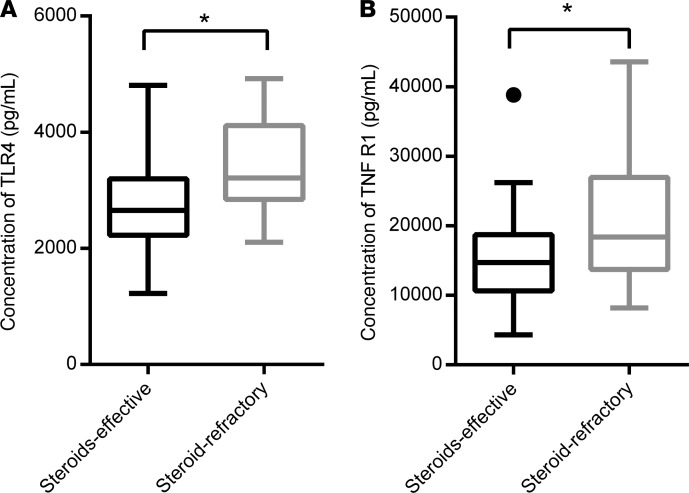
To determine whether these cytokines could be involved in the preevaluation for the standard therapeutic regimen, 20 patients with aGVHD were treated with the standard first-line regimen of 2 mg/kg/d methylprednisone. For patients who had a poor response (aGVHD was exacerbated 3 days after treatment, aGVHD could not be controlled and stabilized 7 days after treatment, or aGVHD could not be totally controlled 14 days after treatment) after standard treatment, extra anti-CD25 antibodies, tacrolimus, or mesenchymal stem cells were added. Among 20 patients with aGVHD, 12 of them (60%) were sensitive to steroid treatment, and the methylprednisone was gradually reduced and finally discontinued. Eight patients (8/20, 40%) showed poor efficacy after standard treatment. Among patients with additional drugs, 7 of them showed improved response, and only 1 patient died because of uncontrolled aGVHD (Table 2). The expression levels of TLR4 and TNFR1 in patients who were not sensitive to standard treatment were significantly increased compared with steroid-effective aGVHD patients (3333 ± 190.1 pg/mL vs. 2752 ± 136.9 pg/mL, and 20,862 ± 2274 pg/mL vs. 15,444 ± 1249 pg/mL, respectively. P < 0.05) (Table 6 and Figure 6). These data suggested that TLR4 and TNFR1 in patients can be used as a preevaluation index for drug treatment.
Table 6. Concentration of multiple cytokines between patients with steroid-effective and steroid-refractory aGVHD (pg/mL).
Figure 6. Statistical analysis of obviously changed cytokines between steroid-effective aGVHD patients (n = 8) and steroid-refractory aGVHD patients (n = 12) using 2-tailed, paired t test.
Significantly increased serum concentrations of TLR4 and TNFR1 were measured in patients with steroid-refractory aGVHD. (A) TLR4, P = 0.0142; and (B) TNFR1, P = 0.0286. The box plots depict the minimum and maximum values (whiskers), the upper and lower quartiles, and the median. The length of the box represents the interquartile range. *P < 0.05.
Variation of 4 cytokines in patients with and without aGVHD.
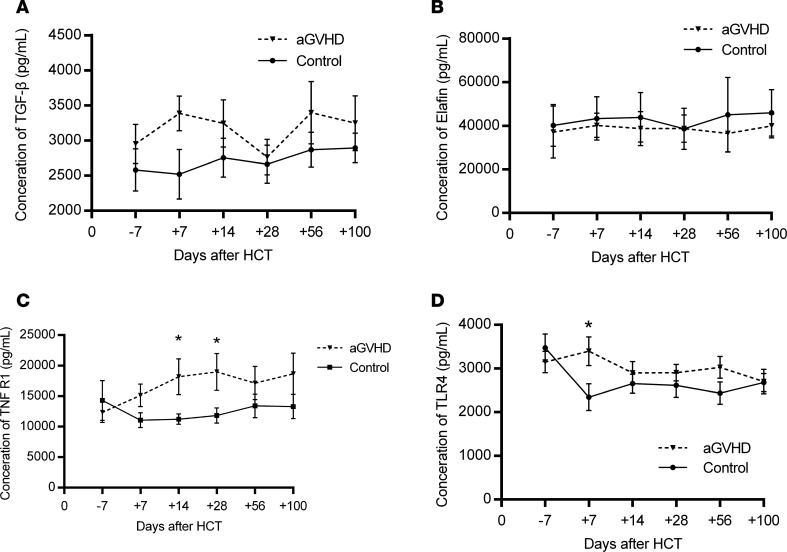
We recorded concentration changes of all cytokines at multiple time points and presented results of the 4 cytokines: Elafin, TGF-β, TLR4, and TNFR1 (Table 7 and Figure 7). As shown in Figure 7, we found there were obvious differences between patients with and without aGVHD in their serum levels of TGF-β, TLR4, and TNFR1 at the same time points within 100 days after transplantation, although the difference in the concentration of Elafin was not significant. We considered that Elafin is a specific biomarker of skin aGVHD because not all aGVHD patients had skin symptoms, and some patients without aGVHD who suffered from rashes also showed elevated Elafin levels.
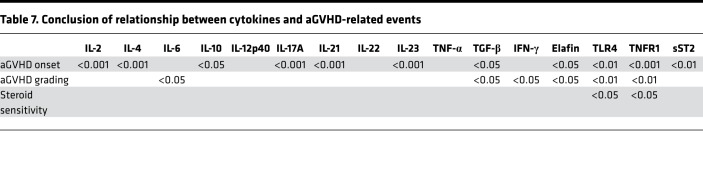
Table 7. Conclusion of relationship between cytokines and aGVHD-related events.
Figure 7. Variation of candidate aGVHD biomarkers.
Significant gaps between aGVHD patients and non-aGVHD patients were shown when comparing the serum concentrations of TNFR1 and TLR4. (A) TGF-β, (B) Elafin, (C) TNFR1, and (D) TLR4 over time in human patients using 2-tailed, paired t test. *P < 0.05.
Discussion
aGVHD, a severe complication after allo-HSCT, remains a major cause of morbidity and mortality. Although early diagnosis and prompt treatment are crucial for effective therapy, there is no unified prejudgment standard and consensus for clinical practice. In this study, we first compared the cytokine levels between a mouse model and human patients and screened 4 suitable biomarkers for both mice and humans as a combination panel for early diagnosis of aGVHD. This 4-biomarker panel can reflect aGVHD severity and efficacy of glucocorticoid treatment well for patients with aGVHD and is presented to evaluate aGVHD grading and steroid sensitivity for the first time to our knowledge in our study.
Because of the social ethics and experimental methods, it is necessary to select appropriate animal models for the study of human diseases. Because pathogenesis of aGVHD leads to a series of cytokine storms in recipients and ultimately causes organ damage, we detected the expression of cytokines in mouse and human aGVHD to show the consistency between the 2 species. In the mouse model, we found obviously higher levels of IL-2, IL-4, IL-12p40, IL-33, IFN-γ, Elafin, sST2, REG3α, TLR4, and TNF-α and lower levels of IL-10, IL-35, and TGF-β at day +14, when aGVHD develops. Based on the results of our animal research and previous articles, considering pathogenic correlation as well as novelty of cytokines, we selected 16 candidate cytokines to be tested in patient samples, including IL-2, IL-4, IL-6, IL-10, IL-12p40, IL-17A, IL-21, IL-22, IL-23, TNF-α, TGF-β, IFN-γ, Elafin, sST2, TLR4, and TNFR1.
In human patients, we found that most human cytokines showed very similar expression trends compared to aGVHD onset in mice, such as expression of IL-2, IL-4, Elafin, and TGF-β, but there still existed some differences in cytokine levels. IL-17A, IL-21, IL-22, and IL-23 were significantly increased in aGVHD patients but not in aGVHD mice (14, 15). The Th17 lineage is distinct from Th1 and Th2 with characteristic cytokines IL-17A, IL-21, IL-22, and IL-23. The role of Th17 cells is debated. Circulating Th17 cells in patients with aGVHD are increased early after allo-HSCT but decrease in the peripheral blood because they migrate into the aGVHD target tissue. Although in aGVHD mice, Th17 seemed not to increase obviously in the peripheral blood, it could be detected in target tissues, such as the lung and liver, where they trigger damage. IL-6 level in aGVHD mice grew at least twice as high as that in control mice, but there was no significant difference between patients with and without aGVHD. Because IL-6 is a proinflammatory cytokine, patients who underwent allo-HSCT were generally given immunosuppressors for aGVHD prophylaxis whereas mouse models were not. Interestingly, we detected an increased IL-10 level in patients with aGVHD; however, IL-10 is an antiinflammatory cytokine. It is secreted mainly by Th2 cells and inhibits production of IL-2 and TNF-α. We speculated that the difference may come from random error and the limited sample size.
To further explore the role of the cytokines, we analyzed the level changes of the cytokines in patients with I/II and III/IV aGVHD and found increased level of Elafin, TLR4, TNFR1, IL-6, and TGF-β in III/IV patients with statistical significance. When comparing cytokine levels in steroid-effective and steroid-refractory patients, only TLR4 and TNFR1 increased in steroid-refractory patients. Unlike some already recognized biomarkers, such as Elafin, sST2, REG3α, and classical proinflammatory cytokines, such as IL-2, IL-6, and TNF-α, TGF-β, TLR4, and TNFR1 were less reported but could be potential biomarkers for patients with aGVHD. Dynamic variations of the 4 cytokines are also shown in Figure 6. We found obvious gaps between the aGVHD group and non-aGVHD group when comparing dynamic concentration curves of TGF-β, TNFR1, and TLR4 at the same time points. This gives us some inspiration to create biomarker panels that can be tested at a fixed time point. The 4 cytokines showed similar variation in both aGVHD patients and the aGVHD mouse model, and our results were consistent with the previous reports (4, 5, 8). By comprehensive analysis, we presented a panel composed of 4 cytokines (Elafin, TLR4, TGF-β, and TNFR1) for aGVHD diagnosis and grading and glucocorticoid sensitivity evaluation in patients. In this study we used TLR4 as 1 component of this aGVHD panel for the first time to our knowledge, whereas previous clinical or animal experiments have not noticed its specificity.
The 4 cytokines have their own characteristics. Elafin (elastase inhibitor) is a classic skin aGVHD biomarker (16). It is overexpressed in inflamed epidermal tissues rather than the aGVHD effector organs (gastrointestinal tract, liver, skin), but the level is high enough to be detected in systemic circulation. Elafin is the best single discriminator for distinguishing skin aGVHD from other etiologies of rash, such as engraftment syndrome, leukemia cutis, and drug rash (17). TGF-β has already been found to be decreased in patients with aGVHD (18). It is crucial to induce proliferation of CD4+CD25+Foxp3+ regulatory T cells, and the latter are able to maintain immune homeostasis and prevent autoimmune disease (19). Although TGF-β provides much inspiration for treatment, it is seldom used as an aGVHD biomarker (20). Our data also showed a marked decrease in TGF-β concentration in patients with aGVHD. However, the expression level of TGF-β was higher in patients with severe aGVHD than that in patients with mild aGVHD, which could be due to the use of adequate steroids in patients with severe aGVHD, because steroids prompt TGF-β secretion, according to some studies. TNFR and its ligand TNF are 2 important cytokines involved in many autoimmune diseases. TNFR1 has already been reported to be used in a biomarker panel for aGVHD diagnosis (4, 8), but its ligand TNF-α was not sensitive and accurate enough to predict aGVHD and aGVHD-related events. Researchers have measured TNFR1 as a surrogate marker for TNF-α. A systemic review suggested that single TNFR1 is not adequately accurate to predict aGVHD; combining it with other biomarkers may strengthen its screening performance (17). TLRs are a series of transmembrane proteins expressed mainly in antigen-presenting cells, such as dendritic cells and macrophages (21). TLR4 seemed to be involved in the pathogenesis of gastrointestinal aGVHD (22, 23). Little research on TLR4 for aGVHD prediction has been reported in human patients. We found that the serum level of TLR4 in patients with aGVHD was increased, and it was likely to have tight correlations with severe aGVHD and steroid-refractory aGVHD. Despite the small sample size of our study, TLR4 is a potential biomarker for aGVHD prediction.
Though there are many types of biomarkers for aGVHD, including miRNA, cellular biomarkers, and proteomic biomarkers (4, 6, 24), cytokine biomarkers are preferable because of adequate foundations of clinical and fundamental research on aGVHD pathology. Generally speaking, multiple biomarkers are more informative in aGVHD diagnosis (25). We screened 4 cytokines related to the development and progression of aGVHD and helped lay the foundation for early clinical intervention and prognosis evaluation of patients with aGVHD. The 4-biomarker panel has high specificity, sensitivity, convenience, and speed, which can be adequate for aGVHD diagnosis and grading and glucocorticoid sensitivity evaluation in patients. As the next step, multicenter clinical studies would be helpful to expand the sample size to validate the clinical role of our chemokine panel. Early warning and intervention will be provided by this panel to reduce the incidence of aGVHD, improve the survival rate of patients, and ensure the safety of transplantation.
Methods
Mice preparation and bone marrow transplantation.
C57BL/6 (H-2b) male mice and BALB/c (H-2d) female mice were purchased from Laboratory Animal Center of Third Military Medical University (Chongqing, China). The induction and scoring of aGVHD were performed as previously described (26). BALB/c female mice within 8–10 weeks old were divided into the aGVHD group and control group randomly. All BALB/c mice were housed in specific pathogen–free conditions in our animal facility at a temperature of 25°C ± 1°C. Pathogen-free diet and antibiotic-containing water (gentamicin and polymyxin) were given 2 weeks before bone marrow transplantation. Male C57BL/6 (H-2b) donors were housed in a common facility with normal diet and water. BALB/c (H-2d) recipients were exposed to total body irradiation (650 cGy 60Co gamma rays) from Institution of Combined Injury, State Key Laboratory of Trauma, Burn and Combined Injury (Third Military Medical University). Mice in the aGVHD group were transplanted with 5 × 106 C57BL/6 bone marrow (BM) cells and 1 × 106 C57BL/6 spleen cells, while mice in the control group were transplanted with 5 × 106 C57BL/6 BM cells only. Body weight, clinical scores, and survival rate were monitored daily after transplantation. Hematopoietic reconstitution was monitored at days +1, +3, +5, +7, +10, +14, +21, and +28 after transplantation.
Analysis of chimerism.
The FISH was performed in BM cells from the femur and tibia of recipient mice on day +14 after transplantation. Appropriate numbers of cells were prepared and stained with mouse Y chromosome dyeing probe (orange) (Metasystems) and X chromosome dyeing probe (green) (Metasystems) in a hybridization instrument at 73°C for 5 minutes for melting and 37°C for 16 hours for hybridization. Then, 10 mL DAPI dye solution (Metasystems) was added to the sample before taking images with a fluorescence microscope (Olympus).
Histopathology.
Skin, liver, intestine, and BM were harvested from recipients of the aGVHD group and control group at day +14 after transplantation. The tissues were fixed, then paraffin embedded, sectioned, and stained with H&E (Solarbio). Images of slides were taken with an optical microscope (Olympus).
ELISA for murine model.
Blood samples of aGVHD model mice and control mice were collected by eye bleeding into a heparin-containing tube at day +14 after transplantation. The supernatant serum was separated, collected, and stored at –80°C for testing. Serum concentrations of IL-2, IL-4, IL-5, IL-6, IL-7, IL-10, IL-12p40, IL-15, IL-17A, IL-21, IL-22, IL-23, IL-33, IL-35, TNF-α, TGF-β, IFN-γ, Elafin, TNFR1, TLR4, sST2, and REG3α were measured using ELISA kits according to the manufacturers’ instructions (R&D Systems and Antibodies). ELISA kits for IL-2, IL-4, IL-5, IL-6, IL-7, IL-10, IL-12p40, IL-22, IL-23, IL-33, TNF-α, TGF-β, and IFN-γ were purchased from R&D Systems. ELISA kits for IL-15, IL-17A, IL-21, IL-35, Elafin, sST2, REG3α, TNFR1, and TLR4 were purchased from Antibodies. All serum samples were assessed simultaneously in duplicate 3 times. Absorbance was detected using Model 680 microplate reader (Bio-Rad) set to 450 nm, and results were analyzed with Microplate Manager 5.2 software (Bio-Rad).
Patient recruitment.
The patients of this study were enrolled from Xinqiao Hospital from November 2014 to November 2016. This study involved 20 patients who developed aGVHD (aGVHD group) and 20 patients who did not develop aGVHD (control group). These patients had allo-HSCT because of hematological malignancies, including AML, ALL, MDS, and severe aplastic anemia. All patients were pretreated with a conditioning regimen according to their protopathy, HSCT type, and degree of HLA match. Calcineurin inhibitor with methotrexate (MTX) and mycophenolate mofetil (MMF) for HLA-matched patients and FK506 with MTX and MMF for HLA-mismatched patients were used to prevent aGVHD. Patients who developed aGVHD were treated with 2 mg/kg/d methylprednisone, and an additional 20 mg anti-CD25 monoclonal antibody on days +1 and +4 was used while aGVHD could not be controlled. aGVHD was diagnosed and staged according to Fred Hutchinson criteria. Detailed clinical data are outlined in Table 2.
Sample preparation.
Serum samples were drawn on days –7, +7, +14, +28, +56, and +100 before and after allo-HSCT. Besides the times mentioned above, we collected serum when the disease was first diagnosed, at aGVHD peak, and after treatment from patients in the aGVHD group. All samples were collected in a sterile and dry centrifuge tube without anticoagulant. After 2 hours at room temperature, blood samples were centrifuged at 2191 g for 10 minutes to obtain serum. Supernatant liquid was transferred to an Eppendorf tube and then stored at –80°C until testing.
Preparation of protein microarray.
All samples (50 μL) were diluted with 1× PBS solution in a 1:1 ratio. Serum concentrations of these cytokines were measured by RayBiotech microarray dotted with the 16 antibodies mentioned above according to the protocols of the manufacturer. Each sample was tested 3 times.
Statistics.
We used GraphPad Prism software for statistical analysis. Statistical significance was analyzed with 2-tailed, paired t test between 2 experimental groups. All statistical data are presented as mean ± SEM. A P value less than 0.05 was considered significant. *P < 0.05; **P < 0.01; and ***P < 0.001.
Study approval.
This study was approved by the ethics committee of Third Military Medical University; all procedures were in accordance with the ethical standards of the ethics committee of Third Military Medical University and the Declaration of Helsinki.
Author contributions
XZ and QG designed this experiment. XL wrote the manuscript and prepared the figures and tables. TC enrolled patients, collected blood samples, and sent the samples to RayBiotech. RW and SY performed animal research and analyzed the data. W. Zhang, YX, W. Zhu, LZ, ZL, XW, and YF performed statistical analysis and interpreted the data.
Supplementary Material
Acknowledgments
This study was supported by National Key Research Program “Clinical Research of Umbilical Cord–Derived Mesenchymal Stromal Cells in the Prophylaxis of Graft-Versus-Host Disease After HLA-Haploidentical Stem-Cell Transplantation” (2017YFA0105502), National Natural Science Foundation of China (81400081 and 81600166), Chongqing Social Career and People’s Livelihood Security Science and Technology Innovation Project (cstc2016shms-ztzx10003), Fundamental and Frontier Research Program of Chongqing (cstc2015jcyjBX0077), and Foundation of Xinqiao Hospital (2016D413).
Version 1. 08/22/2019
Electronic publication
Footnotes
Conflict of interest: The authors have declared that no conflict of interest exists.
Role of funding source: All funding sources were used to purchase experimental reagents and mice and to pay for protein microarray analysis.
Copyright: © 2019, American Society for Clinical Investigation.
Reference information: JCI Insight. 2019;4(16):e130413.https://doi.org/10.1172/jci.insight.130413.
Contributor Information
Xiaoping Li, Email: xiaopingllli@163.com.
Ting Chen, Email: chenting1166@163.com.
Qiangguo Gao, Email: qiangguogao@163.com.
Wei Zhang, Email: 18523059047@163.com.
Yunshuo Xiao, Email: xiaoyunshuo13@163.com.
Wen Zhu, Email: zhuwen9666@163.com.
Lingyu Zeng, Email: zengly2000@163.com.
Zhenyu Li, Email: lizhenyumd@163.com.
Shijie Yang, Email: xyzysj102@163.com.
Rui Wang, Email: wangr0926@163.com.
Xiaoqi Wang, Email: xiawang@coh.org.
Yimei Feng, Email: yimeifeng@163.com.
Xi Zhang, Email: zhangxxi@sina.com.
References
- 1.Ferrara JL, Levine JE, Reddy P, Holler E. Graft-versus-host disease. Lancet. 2009;373(9674):1550–1561. doi: 10.1016/S0140-6736(09)60237-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Nassereddine S, Rafei H, Elbahesh E, Tabbara I. Acute graft versus host disease: a comprehensive review. Anticancer Res. 2017;37(4):1547–1555. doi: 10.21873/anticanres.11483. [DOI] [PubMed] [Google Scholar]
- 3.Zeiser R, Blazar BR. Acute graft-versus-host disease - biologic process, prevention, and therapy. N Engl J Med. 2017;377(22):2167–2179. doi: 10.1056/NEJMra1609337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hartwell MJ, et al. An early-biomarker algorithm predicts lethal graft-versus-host disease and survival. JCI Insight. 2017;2(3):e89798. doi: 10.1172/jci.insight.89798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.McDonald GB, Tabellini L, Storer BE, Lawler RL, Martin PJ, Hansen JA. Plasma biomarkers of acute GVHD and nonrelapse mortality: predictive value of measurements before GVHD onset and treatment. Blood. 2015;126(1):113–120. doi: 10.1182/blood-2015-03-636753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wu X, et al. Prediction of acute GVHD and relapse by metabolic biomarkers after allogeneic hematopoietic stem cell transplantation. JCI Insight. 2018;3(9):99672. doi: 10.1172/jci.insight.99672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.He FC, Holtan SG. Biomarkers in graft-versus-host disease: from prediction and diagnosis to insights into complex graft/host interactions. Curr Hematol Malig Rep. 2018;13(1):44–52. doi: 10.1007/s11899-018-0433-2. [DOI] [PubMed] [Google Scholar]
- 8.Paczesny S, et al. A biomarker panel for acute graft-versus-host disease. Blood. 2009;113(2):273–278. doi: 10.1182/blood-2008-07-167098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pierini A, et al. TNF-α priming enhances CD4+FoxP3+ regulatory T-cell suppressive function in murine GVHD prevention and treatment. Blood. 2016;128(6):866–871. doi: 10.1182/blood-2016-04-711275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Li Q, et al. Decrease of CD4(+)CD25(+) regulatory T cells and TGF-beta at early immune reconstitution is associated to the onset and severity of graft-versus-host disease following allogeneic haematogenesis stem cell transplantation. Leuk Res. 2010;34(9):1158–1168. doi: 10.1016/j.leukres.2010.03.017. [DOI] [PubMed] [Google Scholar]
- 11.Sang W, et al. MicroRNA-181a, a potential diagnosis marker, alleviates acute graft versus host disease by regulating IFN-γ production. Am J Hematol. 2015;90(11):998–1007. doi: 10.1002/ajh.24136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wu Y, et al. Essential role of interleukin-12/23p40 in the development of graft-versus-host disease in mice. Biol Blood Marrow Transplant. 2015;21(7):1195–1204. doi: 10.1016/j.bbmt.2015.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tvedt THA, Ersvaer E, Tveita AA, Bruserud Ø. Interleukin-6 in allogeneic stem cell transplantation: its possible importance for immunoregulation and as a therapeutic target. Front Immunol. 2017;8:667. doi: 10.3389/fimmu.2017.00667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Malard F, Gaugler B, Lamarthee B, Mohty M. Translational opportunities for targeting the Th17 axis in acute graft-vs.-host disease. Mucosal Immunol. 2016;9(2):299–308. doi: 10.1038/mi.2015.143. [DOI] [PubMed] [Google Scholar]
- 15.van der Waart AB, van der Velden WJ, Blijlevens NM, Dolstra H. Targeting the IL17 pathway for the prevention of graft-versus-host disease. Biol Blood Marrow Transplant. 2014;20(6):752–759. doi: 10.1016/j.bbmt.2014.02.007. [DOI] [PubMed] [Google Scholar]
- 16.Paczesny S, et al. Elafin is a biomarker of graft-versus-host disease of the skin. Sci Transl Med. 2010;2(13):13ra2. doi: 10.1126/scitranslmed.3000406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ali AM, DiPersio JF, Schroeder MA. The role of biomarkers in the diagnosis and risk stratification of acute graft-versus-host disease: a systematic review. Biol Blood Marrow Transplant. 2016;22(9):1552–1564. doi: 10.1016/j.bbmt.2016.04.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hodge GL, Hodge SJ, Nairn J, Tippett E, Holmes M, Reynolds PN. Poststorage leuko-depleted plasma inhibits T-cell proliferation and Th1 response in vitro: characterization of TGFbeta-1 as an important immunomodulatory component in stored blood. Transplantation. 2005;80(1):95–101. doi: 10.1097/01.TP.0000163866.43866.44. [DOI] [PubMed] [Google Scholar]
- 19.Gu J, et al. TGF-β-induced CD4+Foxp3+ T cells attenuate acute graft-versus-host disease by suppressing expansion and killing of effector CD8+ cells. J Immunol. 2014;193(7):3388–3397. doi: 10.4049/jimmunol.1400207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Banovic T, et al. TGF-beta in allogeneic stem cell transplantation: friend or foe? Blood. 2005;106(6):2206–2214. doi: 10.1182/blood-2005-01-0062. [DOI] [PubMed] [Google Scholar]
- 21.Brennan TV, et al. Heparan sulfate, an endogenous TLR4 agonist, promotes acute GVHD after allogeneic stem cell transplantation. Blood. 2012;120(14):2899–2908. doi: 10.1182/blood-2011-07-368720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chopra M, et al. Blocking TWEAK-Fn14 interaction inhibits hematopoietic stem cell transplantation-induced intestinal cell death and reduces GVHD. Blood. 2015;126(4):437–444. doi: 10.1182/blood-2015-01-620583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cooke KR, et al. LPS antagonism reduces graft-versus-host disease and preserves graft-versus-leukemia activity after experimental bone marrow transplantation. J Clin Invest. 2001;107(12):1581–1589. doi: 10.1172/JCI12156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lv M, et al. Ceruloplasmin is a potential biomarker for aGvHD following allogeneic hematopoietic stem cell transplantation. PLoS ONE. 2013;8(3):e58735. doi: 10.1371/journal.pone.0058735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lee SE, et al. Risk and prognostic factors for acute GVHD based on NIH consensus criteria. Bone Marrow Transplant. 2013;48(4):587–592. doi: 10.1038/bmt.2012.187. [DOI] [PubMed] [Google Scholar]
- 26.Ni X, et al. PD-L1 interacts with CD80 to regulate graft-versus-leukemia activity of donor CD8+ T cells. J Clin Invest. 2017;127(5):1960–1977. doi: 10.1172/JCI91138. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.