Background
In the United States, lung cancer is the second most common diagnosed cancer and the leading cause of cancer-related death. Though tobacco smoking is the major risk factor accounting for 80 to 90% of all lung cancer diagnoses, there are numerous other risk factors that have been identified as casually associated with lung cancer etiology. However, there are few causally-linked risk factors for lung cancer diagnosed among never smokers which, if considered a unique reportable category, is the 11th most common cancer and the 7th leading cause of cancer-related death. Lung cancer survival has only marginally improved over the last several decades, but the availability of screening and early detection by low-dose computer tomography and advances in targeted treatments and immunotherapy will likely decrease mortality rates and improve patient survival outcomes in the near future.
Keywords: Lung cancer, epidemiology, statistics, disparities, risk factors, prevention early detection, lung cancer screening, epidemiology of lung cancer
Descriptive Epidemiology
Incidence
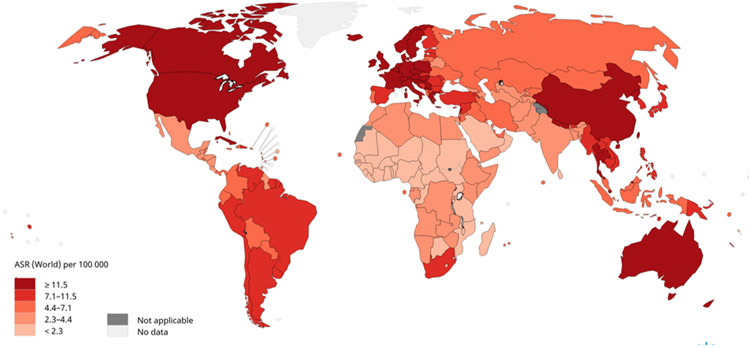
Globally, lung cancer has been the most common diagnosed cancer for the last several decades [1, 2]. In 2018, there was an estimated 2.1 million new lung cancer diagnoses accounting for 12% of the global cancer burden [1, 2]. Among men, lung cancer remains the most common cancer diagnosis with approximately 1.37 million diagnoses in 2018, with the highest incidence rates in Micronesia (54.1 per 100,000), Polynesia (52.0 per 100,000), Central and Eastern Europe (49.3 per 100,000) and Eastern Asia (47.2 per 100,000). Among women, incidence rates are generally lower than men with approximately over 725,000 new lung cancer diagnoses in 2018. Geographical variations in incidence rates differ for women compared to men (Figures 1a and 1b) which are attributed to historical differences in cigarette smoking. Among women, the highest incidence rates occur in North America (30.7 per 100,000), Northern Europe (26.9 per 100,000), and Western Europe (25.7 per 100,000).
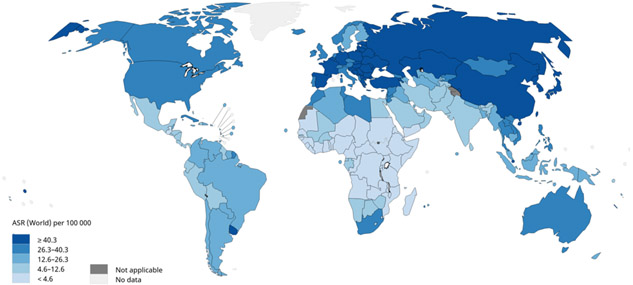
Figure 1.
Age-standardized rates (ASR) for lung cancer incidence worldwide. Figure 1a shows age-standardized incidence rates for lung cancer among males using data from GLOBOCAN, 2018. Lung cancer incidence among males is highest in Micronesia, Polynesia, Central and Eastern Europe, and Eastern Asia and lowest in most of Africa. Figure 1b shows age-standardized incidence rates for lung cancer among females using data from GLOBOCAN, 2018. Lung cancer incidence among females is highest in North America, Northern Europe, Western Europe, and Australia/New Zealand and lowest in most of Africa. Data source: GLOBOCAN 2018. Graph production: IARC (http://gco.iarc.fr/today), World Health Organization.
In the United States, lung cancer is the second most common cancer in men after prostate cancer and the second most common cancer in women after breast cancer [3, 4]. In 2019 an estimated 228,150 new cases of lung cancer are expected. The incidence rate among men is 71.3 per 100,000 and for women it is 52.3 per 100,000. Although the incidence rate has been declining in men since the mid-1980s, incidence rates did not start declining for women until the mid-2000s because of historical sex-specific differences of smoking uptake and cessation. The decline in incidence has gained momentum in the past decade with rates decreasing from 2011 to 2015 by nearly 3% per year in men and 1.5% per year in women. Geographically, lung cancer incidence is higher the Midwest, East, and South with highest rates observed in the South for both men and women (Figures 2a and 2b).
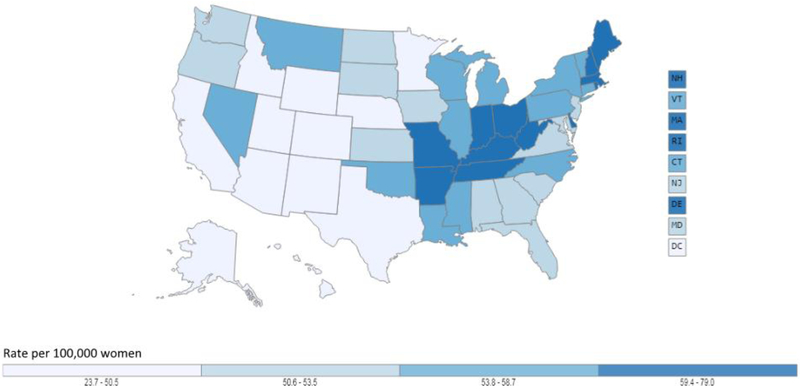
Figure 2.
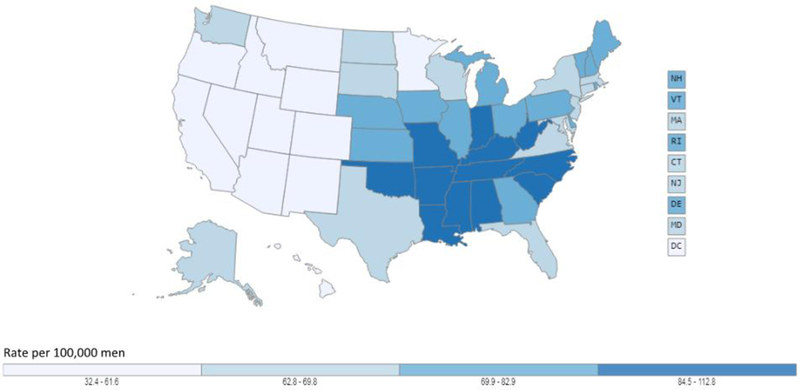
Age-adjusted lung cancer incidence rates in the United States. Figure 2a shows age-adjusted lung cancer incidence rates for males in the United States, 2011 −2015, using data from U.S. Cancer Statistics Working Group. Figure 2b shows age-adjusted lung cancer incidence rates for females in the United States, 2011 −2015, using data from U.S. Cancer Statistics Working Group. Among both males and females, lung cancer incidence is higher in the Midwest and East and the highest rates are observed in the South while the lowest rates are generally found in Western states. Data source: U.S. Cancer Statistics Working Group. U.S. Cancer Statistics Data Visualizations Tool, based on November 2017 submission data (1999-2015): U.S. Department of Health and Human Services, Centers for Disease Control and Prevention and National Cancer Institute (www.cdc.gov/cancer/dataviz), June 2018
Mortality
The global geographical patterns in lung cancer deaths closely follow those in incidence because of poor survival and the high fatality rate of this disease (Figure 3a and 3b). Worldwide, lung cancer is the leading cause of cancer death in men and the second-leading cause in women. In 2018, with an estimated 1.8 million deaths occurred (1.2 million in men and 576,100 in women), accounting for 1 in 5 cancer deaths worldwide [1, 2]. The geographical variations by country/region and between men and women are largely attributed to historical patterns in tobacco smoking and maturity of the tobacco epidemic [2].
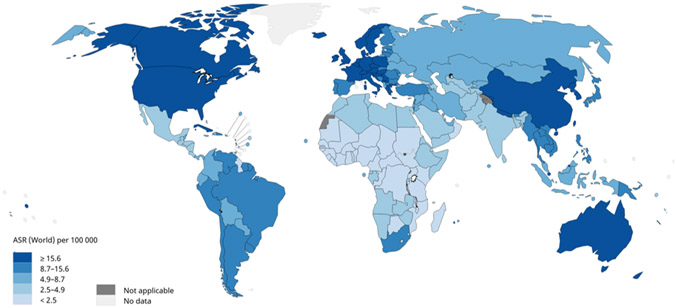
Figure 3.
Estimated age-standardized rates (ASR) for lung cancer mortality worldwide. Figure 3a shows age-standardized mortality rates for lung cancer among males using data from GLOBOCAN, 2018. Lung cancer mortality among males is highest in Eastern Europe, Western Asia, Northern Africa, and specific countries in Eastern Asia and lowest in most of Africa. Figure 3b shows age-standardized mortality rates for lung cancer among females using data from GLOBOCAN, 2018. Lung cancer mortality among females in North America, Northern Europe, Western Europe, and Australia/New Zealand and lowest in most of Africa. Data source: GLOBOCAN 2018. Graph production: IARC (http://gco.iarc.fr/today), World Health Organization.
In the United States, lung cancer is the leading cause of cancer-related death among both men and women [3, 4]. In 2019, an estimated 142,670 deaths are expected to occur, or about 23.5% of all cancer deaths. The mortality rate among men is 51.6 per 100,000 and 34.4 per 100,000 for women. Due to reductions in smoking, the lung cancer death rate has declined 48% since 1990 in men and by 23% since 2002 in women. From 2012 to 2016, the death rate dropped by about 4% per year in men and 3% per year in women. Geographically, lung cancer mortality follows a pattern similar to incidence including the highest rates observed in the South (Figures 4a and 4b).
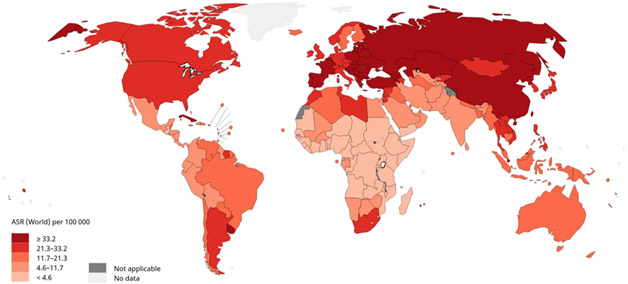
Figure 4.
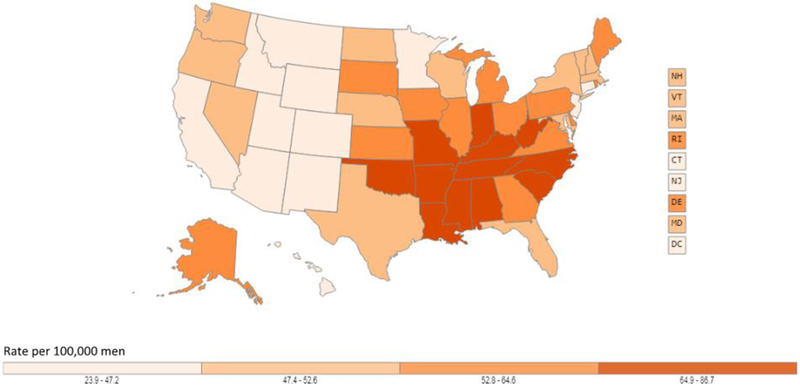
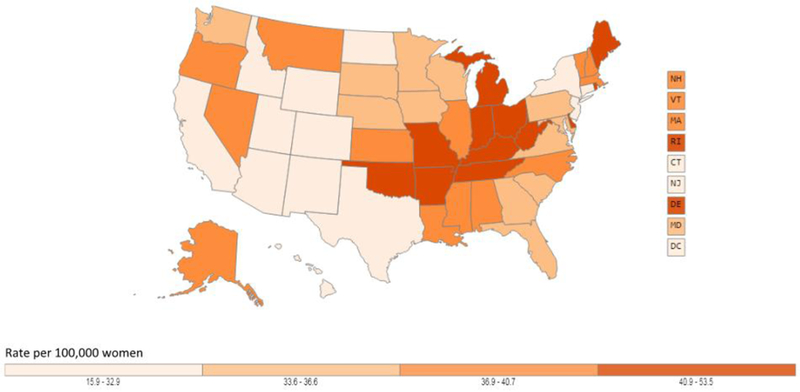
Age-adjusted lung cancer mortality rates in the United States. Figure 4a shows age-adjusted lung cancer mortality rates for males in the United States, 2011 −2015, using data from U.S. Cancer Statistics Working Group. Figure 4b shows age-adjusted lung cancer mortality rates for females in the United States, 2011 −2015, using data from U.S. Cancer Statistics Working Group. Among both males and females, lung cancer morality is higher in the Midwest, East, and South and lowest in most Mountain states and California. Data source: U.S. Cancer Statistics Working Group. U.S. Cancer Statistics Data Visualizations Tool, based on November 2017 submission data (1999-2015): U.S. Department of Health and Human Services, Centers for Disease Control and Prevention and National Cancer Institute (www.cdc.gov/cancer/dataviz), June 2018.
Survival
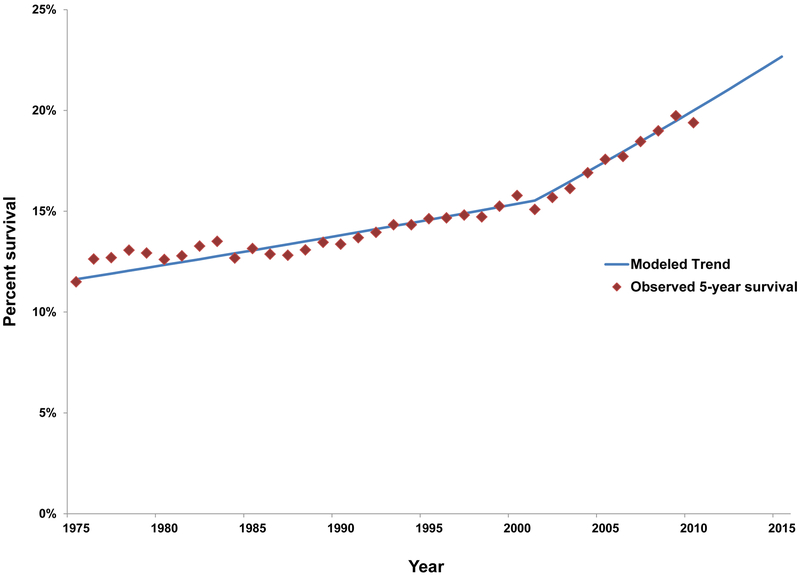
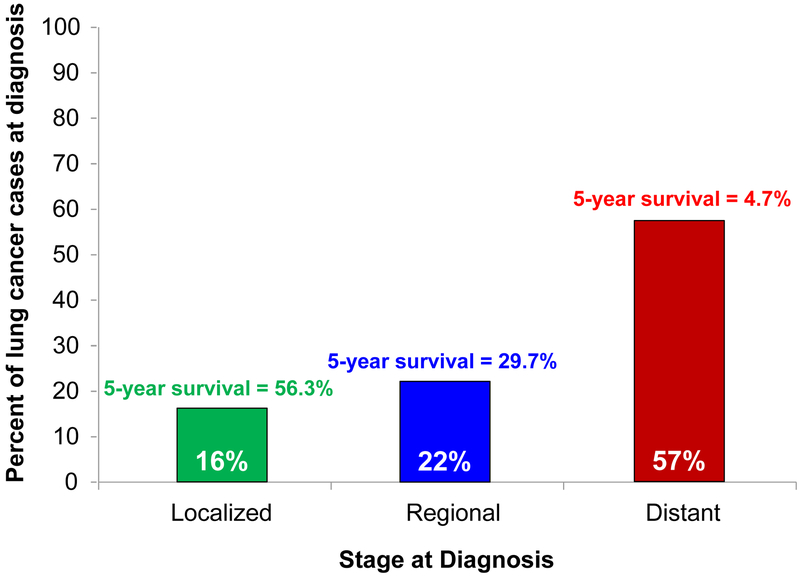
Despite substantial improvements in survival in recent years for most other cancer types in the United States, there have only been small improvements in 5-year survival among patients diagnosed with lung cancer (Figure 5). This lack of improvement is primarily due to the majority of patients are diagnosed with last stage disease where the survival rates are dismal (Figure 6). The five-year relative survival rate for all lung cancers (non-small cell lung cancer [NSCLC] and small cell lung cancer combined) is 19% and the five-year survival is higher for non-small cell lung cancer (23%) than small cell lung cancer (6%) [3, 4].
Figure 5.
Temporal trends in 5-year relative percent survival for lung and bronchus cancer. Figure 5 shows observed and modeled trends in lung and bronchus cancer 5-year survival from 1975-2015 using data from SEER 18 (https://seer.cancer.gov/statfacts/html/lungb.html).
Figure 6.
Percent of lung cancer cases at diagnosis and 5-year relative survival by stage. Figure 6 shows the percentage of lung cancer cases diagnosed in the U.S. by stage and their respective 5-year survival rates using data from SEER 18 (https://seer.cancer.gov/statfacts/html/lungb.html). “Localized” is confined to the primary sites, “regional” has spread to the regional lymph nodes, and “distant” is a cancer that has metastasized. “Unknown”, which accounts for 4% of diagnoses and has an 8.2% 5-year survival, is not shown.
Despite the high mortality rates and poor survival outcomes associated with a lung cancer diagnosis, the next-generation of targeted therapies and the emergence of immune checkpoint inhibitors have demonstrated durable long-term survival in subsets of patients. As such, these therapies may hold the key in improving lung cancer patient outcomes leading to curable lung cancer among early-stage diagnoses and a chronic and manageable disease for patients with advanced and metastatic disease.
Histological Classification
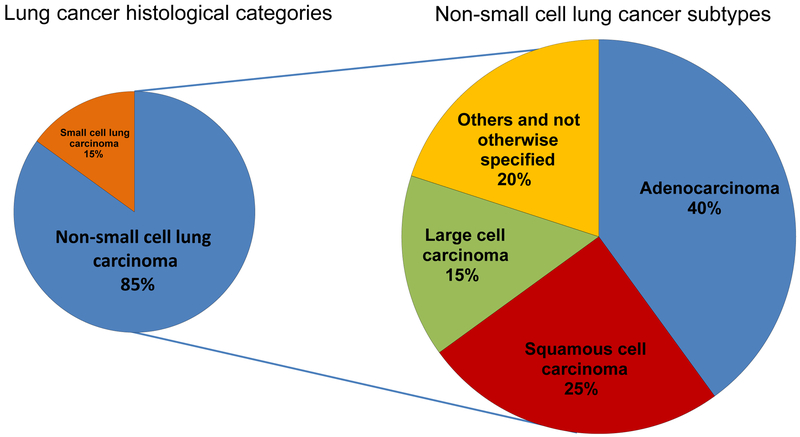
Lung cancer tumors are divided into two broad histological categories: non-small cell lung carcinoma (NSCLC) and small-cell lung carcinoma (SCLC). NSCLC represents more than 80 to 85% of lung cancers of which approximately 40% are adenocarcinoma, 25 to 30% are squamous cell carcinoma, and 10 to 15% are large cell carcinomas (Figure 7) [5-7]. Bronchioloalveolar carcinoma (BAC) was a distinct histologic classification representing a sub-group of adenocarcinomas and has been replaced with adenocarcinoma in situ, minimally invasive adenocarcinoma, and invasive adenocarcinoma of the lung [8]. Other less common histologic subtypes include adenosquamous carcinoma, pleomorphic sarcomatoid carcinoma, large cell neuroendocrine carcinoma, and carcinoid tumor.
Figure 7.
Histological classification of lung cancer. Figure 7 shows the two major lung cancer histological categories (non-small cell lung carcinoma [NSCLC] and small-cell lung carcinoma) and the most common histological subtypes among NSCLC (adenocarcinoma, squamous cell carcinoma, and large cell carcinoma).
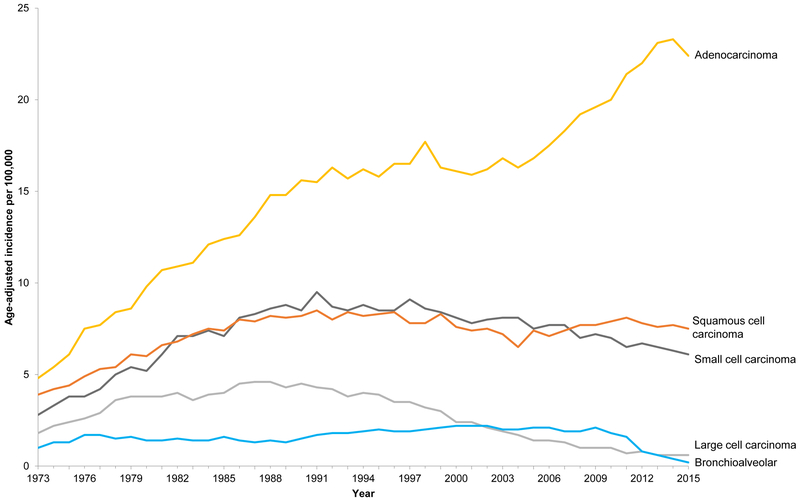
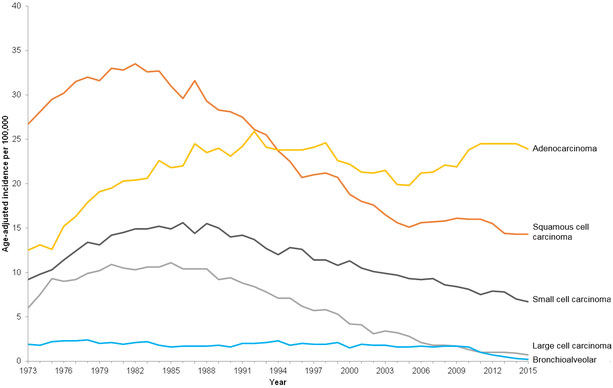
Among women, adenocarcinoma has been the most frequently diagnosed histological subtype since at least the 1970s (Figure 8a). Among men, the incidence rate of lung adenocarcinoma has been on the rise since the 1970s and the incidence rate for lung adenocarcinoma surpassed squamous cell carcinoma around 1994 (Figure 8b). The incidence rate for squamous cell carcinomas has been on the decline since the early 1980s. This temporal shift in histological diagnoses is largely attributed to the widespread use of filtered cigarettes and increasing amounts of tobacco-specific nitrosamines in tobacco [9]. Regarding the former, earlier in the 20th century, most mass-produced cigarettes were non-filtered which discouraged deep inhalation and combusted tobacco smoke exposed primarily the trachea and bronchus resulting in observed higher rates of squamous cell carcinoma diagnoses especially among men [10]. When filtered cigarettes were introduced, combusted tobacco smoke dispersed deeper into the respiratory tree due to deeper inhalation resulting in adenocarcinomas with a more peripheral distribution [11]. The introduction of so-called “light” filtered cigarettes and changing tobacco blends, which decreased nicotine but increased nitrate and N‐nitrosamines, had the paradoxical effect of increasing, rather than decreasing, lung cancer risk due to promotion of deeper and more frequent inhalation of combusted tobacco smoke [10, 11].
Figure 8.
Age-adjusted incidence rates (per 100,000) for lung and bronchus cancer by year of diagnosis and histology. Figure 8a shows age-adjusted incidence rates among females for lung and bronchus cancer by year of diagnosis and histology using SEER 9, 1973-2015. Figure 8b shows age-adjusted incidence rates among males for lung and bronchus cancer by year of diagnosis and histology using SEER 9, 1973-2015. The incidence rates are age-adjusted to the 2000 U.S. population.
Although the binary division of lung cancer into NSCLC and SCLC is still widely applied and relevant, advances in genomic profiling has resulted in a paradigm shift whereby lung cancers are also characterized and classified by tumor biomarkers and genetic alterations, such as gene expression, mutations, amplifications, and rearrangements (Table 1), that are critical to tumor growth and survival and can be exploited with specific targeted agents or immune-checkpoint blockades [12-14].
Table 1.
Frequency of Somatic Mutations and Alterations in NSCLC
| Gene | Alteration type | Frequency in NSCLC |
|---|---|---|
| EGFR | Mutation | 10–35% |
| KRAS | Mutation | 15–25% |
| FGFR1 | Amplification | 20% |
| PTEN | Mutation | 4–8% |
| DDR2 | Mutation | ~4% |
| ALK | Rearrangement | 3–7% |
| HER2 | Mutation | 2–4% |
| MET | Amplification | 2–4% |
| BRAF | Mutation | 1–3% |
| PIK3CA | Mutation | 1–3% |
| AKT1 | Mutation | 1% |
| MEK1 | Mutation | 1% |
| NRAS | Mutation | 1% |
| RET | Rearrangement | 1% |
| ROS1 | Rearrangement | 1% |
Disparities
Males vs. females
Though the terms “sex” and “gender” have been historically interchangeable in medical research, their uses are distinct as sex is conventionally based on anatomy and physiology while gender typically refers to identity, behavior, or socially constructed roles. As such, research in potential lung cancer disparities has not disentangled sex vs. gender. Nonetheless, the established differences in lung cancer incidence and mortality rates between males and females are attributed to historical patterns in tobacco smoking as noted above. To address potential sex-specific differences in lung cancer risk, O’Keeffe et al [15] conducted a systematic review and meta-analysis of prospective cohort studies on the sex-specific association of smoking with the risk of fatal and non-fatal lung cancer. By restricting the analyses to cohort studies, the goal was to minimize bias often present in case-control studies. Data from 99 cohort studies representing more than 7 million individuals and over 50 000 incident cases of lung cancer found no evidence for sex-specific differences for risk of smoking-related lung cancer. Specifically, the authors reported a pooled adjusted lung cancer relative risk of 6.99 for females and 7.33 for males and found no evidence of publication bias or differences across major pre-defined participant and study subtypes. The female-to-male ratio of relative risk was 0.99, 1.11, and 0.94, for light, moderate and heavy smoking, respectively. The authors acknowledge that “…these data may yet underestimate the true relative risk of smoking-related lung cancer in women, given later uptake and lower intensity of smoking in women.”
Regarding sex-specific lung cancer among never smokers, there is compelling historic evidence [16-18] that suggests a higher risk, incidence, and mortality among never-smoking females versus never-smoking males. Conversely, a multi-institutional registry-based study [19] of over 12,000 lung cancer patients found that the proportion of lung cancer patients who reported themselves as never smokers increased over time, but the observed increase was independent of sex.
Race and Ethnicity
Racial and ethnic differences in lung cancer incidence, mortality, and survival outcomes are well-documented and are largely attributed to inequalities in wealth (i.e., socioeconomic status) leading to differences in risk factor exposures and barriers to high‐quality prevention, early detection, and treatment [4]. Analyses from the American Cancer Society [4] revealed that lung cancer incidence for non-Hispanic Black men (85.4 per 100,000) is higher than non-Hispanic White men (74.3 per 100,000) and Hispanic men (39.2 per 100,000). However, the incidence for non-Hispanic Black women (49.2 per 100,000) and Hispanic women (24.6 per 100,000) are lower than non-Hispanic White women (57.4 per 100,000). Similar trends were noted for lung cancer mortality. Black lung cancer patients (16%) have overall lower 5‐year relative survival rate than Whites (19%) which is consistent for localized (52% vs. 56%) and regional disease (27% vs 30%), but not for distant disease (5% vs. 5%). Black lung cancer patients are more frequently diagnosed with distant disease compared to White patients (61% vs. 57%) and less frequently diagnosed with localized disease (13% vs. 17%).
Socioeconomic Status
Socioeconomic status (SES) is a broad term for the social standing or “class” of an individual or group of people and is often measured based on highest attained education, income, and occupation. SES is associated with health and disease through multiple interacting pathways in terms of resources, physical and psychosocial stressors, and health-related behaviors and risk factors. SES is strongly associated with some lung cancer risk factors including tobacco smoking behavior, whereby uptake may be higher among those with low SES and quit attempts are less likely to be successful [20]. Results from the American Cancer Society found that cancer mortality is 28% higher overall in poor counties than affluent counties in the United States and >40% higher among men in poor counties [4]. A pooled analysis of 17,021 cases and 20,885 controls found, after adjusting for smoking, low SES based on International Socio-Economic Index was associated with an 84% increased risk of lung cancer among men and a 54% increased risk among women [21]. Lung cancer risk was still elevated but somewhat attenuated when SES was assessed using the European Socio-economic Classification. The authors concluded that the strong associations emphasizes the need for further exploration of the pathways from SES to lung cancer and “clarifying these pathways could then contribute to further understanding of lung cancer etiology and shape prevention approaches”.
LGBTQ Individuals
The lesbian, gay, bisexual, transgender, and queer/questioning (LGBTQ) community, also referred to as sexual and gender minorities, is a diverse and medically underserved population that has been historically marginalized [22-25]. The sparse but growing body of evidence demonstrates the LGBTQ population may an ignored epidemic [26] associated with increased risk and poorer outcomes for certain cancers including lung cancer [27-32]. Prior studies linking Surveillance, Epidemiology and End Results (SEER) data with the United States Census [31] and California Cancer Registry with the California Health Interview Survey [30] provided evidence that gay men have higher incidence and mortality rates for lung cancer and lesbian females have lower incidence and mortality rates from lung cancer compared to the general population. In the bisexual community, men have a lower incidence of lung cancer, whereas bisexual women have higher incidence of lung cancer. The lung cancer disparities among LGBTQ individuals may be attributed, in part, to higher prevalence of tobacco smoking among this population [33-35]. To date there are no published risk estimates for the association between tobacco smoking and lung cancer among LGBTQ individuals. Another potential risk modifier is HIV infection where gay and bisexual individuals account for over 67% of all HIV diagnoses [36] and incidence of lung cancer among HIV-infected patients is significantly higher than the general population [37]. HIV and lung cancer is discussed below.
Risk Factors
A summary of causative and putative lung cancer risk factors that are discussed below are summarized in Table 2.
Table 2.
Established and putative risk lung cancer risk factors
| Risk factor | Magnitude of association |
|---|---|
| Tobacco smoking | 20-fold increased risk vs. never smoker |
| Secondhand smoke | 25% to 28% increased risk vs. never smoker |
| Electronic cigarettes | Presently unknown |
| Other tobacco use (cigars, pipes, water pipes) | 1.9 to 4.6-fold increased risk |
| Smoked cannabis | Presently no known risk |
| Radon | 14% to 29% increased risk |
| Asbestos | 12% to 24% increased risk |
| History of COPD, emphysema, or chronic bronchitis | 2 to 3-fold increased risk |
| History of asthma | 28% to 44% increased risk |
| History of pneumonia | 30% to 57% increased risk |
| History of Chlamydia pneumoniae | 1.2 to 2.4-fold increased risk |
| History of tuberculosis | 48% to 76% increased risk |
| HIV | 2-fold increased risk |
Tobacco smoking
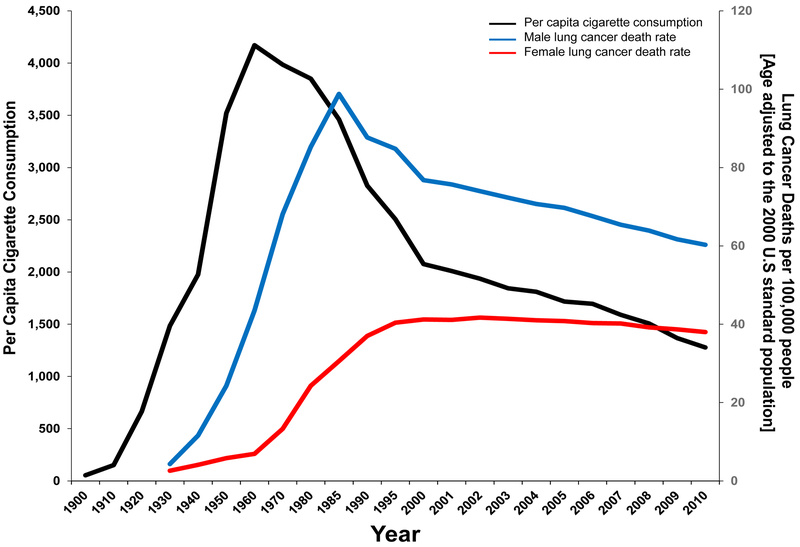
Unequivocally, tobacco smoking is the most important and prevalent lung cancer risk factor. A rare disease at the beginning of the 20th century, lung cancer was one of the first diseases to be causally linked to tobacco smoking [38]. Throughout most of the 20th century in the United States, lung cancer incidence and mortality increased as the per capita in cigarette consumption increased (Figure 9) and as successive generations of first male and then female smokers began smoking at earlier ages. Men predominantly began smoking manufactured cigarettes earlier in the 20th century, during and after World War II. Though few women smoked regularly before World War II, average age at initiation continued to decrease and per capital in cigarette consumption increased through the 1960s [39]. Tobacco consumption fell drastically in the United States following publication of the landmark 1964 U.S. Surgeon General’s Report that concluded cigarette smoking is causally related to lung cancer in men [40].
Figure 9.
Trends in cigarette and lung cancer death rates. Figure 9 shows the temporal trends in cigarette use versus lung cancer death rates for both males and females in the U.S. using data from the Centers for Disease Control and Prevention. Data sources from: National Center for Health Statistics (NCHS), Centers for Disease Control and Prevention, 2017 and CDC Report on Consumption of Combustible and Smokeless Tobacco — United States, 2000-2015, page 1359.
Tobacco smoke contains more than 7,000 chemicals including at least 69 established carcinogens and other toxicants associated with major diseases [20]. Although only around 15% of smokers develop lung cancer, 80 to 90% of lung cancer diagnoses are attributed to tobacco smoking in the United States [3]. The relative risk of lung cancer is estimated to be about 20-fold higher than that of a lifetime never smoker and the magnitude of lung cancer risk is related to smoking intensity (i.e., cigarettes smoked per day and number of years smoked) [40-42]. Numerous lung cancer risk models [43-48] are available as web-based tools [49] that provide risk assessment based on demographic information including smoking history and intensity.
Exposure to secondhand smoke
Secondhand smoke, or side-stream smoke, is an indirect carcinogenic exposure resulting from the burning of tobacco products. From 1988 to 2014, secondhand smoke exposure among never smokers in the United States significantly declined from 87.5% to 25.2% attributed to tobacco control efforts and smoke-free laws and policies in workplaces and public places [50]. However, there has been no change in secondhand smoke exposure between 2011 to 2012 and between 2013 to 2014 with an estimated 1 in 4 never smokers, or about 58 million people, exposed to secondhand smoke from 2013 to 2014 [50]. Carcinogens that have been measured in secondhand smoke include polycyclic aromatic hydrocarbons, nitrosamines, and aromatic amines. Studies have shown that nicotine and its metabolite cotinine as well as DNA adducts from tobacco carcinogens are present the urine of never smokers who are exposed to secondhand smoke [51]. A 2006 report from the United States Surgeon General on The Health Consequences of Involuntary Exposure to Tobacco Smoke [52] concluded there is no safe level of exposure to secondhand tobacco smoke and stated, “The evidence is sufficient to infer a causal relationship between secondhand smoke exposure and lung cancer among lifetime nonsmokers. This conclusion extends to all secondhand smoke exposure, regardless of location.” A meta-analysis published in 2018 of twelve studies found that secondhand smoke exposure compared to never smokers without such exposure was associated with a 25% increased risk of lung cancer [53]. A separate meta-analysis that assessed the association between secondhand smoke and lung cancer in Japanese non-smokers found a 28% increased risk [54].
Electronic-cigarettes
Electronic nicotine delivery systems, also referred to as electronic-cigarettes and E-cigarettes, allow for the delivery of nicotine to the lung epithelium via an electronic device. Though a patent for this type of device was first issued in 1965, mass production of electronic-cigarettes did not occur until 2003 and became widely available in 2005 in the United States. Today in the United States, there are over 460 different brands on the market with over 7,700 flavors [55, 56] and prevalence of e‐cigarette use among adults is estimated to be between 2.6% and 4.5% [57-61]. Of particular concern is the uptake of e-cigarette use among youth ages 12-18, with the 2017 National Youth Tobacco Survey reporting 11.7% of high school students and 3.3% of middle school students using e-cigarettes within the last month. One year later, 20.8% of high school students and 4.9% of middle school students reported using e-cigarettes within the last month, representing increases of 78% and 48%, respectively [62]. Additionally, e-cigarette use among US youths is associated with increased risk of initiation of traditional cigarette use [63, 64]. Within the next 10 years, it is anticipated that total sales of e-cigarettes are anticipated to exceed tobacco products [65]. Although there are various configurations, these devices typically include a mouthpiece and a battery-operated heating element to heat fluid contained in a replaceable cartridge or reservoir that contains a mixture of liquid nicotine, flavorings, and other chemical solvents [66]. Propylene glycol and vegetable glycerin are the two major solvents in electronic-cigarettes and studies have shown that vapors from these solvents contain toxic and carcinogenic carbonyl compounds including formaldehyde, acetaldehyde, acetone, and acrolein [62, 67]. Studies have also shown that e-cigarette use is associated with increased oxidative stress, which seems to mediate the adverse effects of e-cigarettes. Oxidative stress develops in e-cigarette-exposed human bronchial and lung epithelial cell which can result adverse intermediate events including inflammation, cytotoxicity and increased endothelial cell permeability [68, 69]. A model has been proposed for the role of oxidative stress in mediating adverse effects of electronic-cigarettes leading to cancer, cardiopulmonary pathogenesis, and neurodegenerative disorders [65]. Furthermore, studies have demonstrated that e-cigarettes generate have acute deleterious effects on lung function [70, 71]. Cumulatively, data suggests that vapor produce from electronic-cigarettes contains potentially harmful compounds and may lead to adverse effects on human health. Although data suggest that electronic-cigarettes may less harmful alternative to conventional cigarettes, at present there are no data regarding the long-term cancer risk associated with low-level exposure to the detected carcinogens [72].
Other Tobacco Use
Although cigarettes remain the most prevalent form of tobacco use in the United States, other tobacco products including pipes, cigars, and water pipes (e.g., hookah) are still common and have been associated with increased risk and mortality of lung cancer. Christensen et al [73] identified 357,420 individuals who were never, current or former users of cigars, pipes and cigarettes by linking data from the National Longitudinal Mortality Study and the Tobacco Use Supplement of the Current Population Survey. After excluding nearly 49,000 individuals who reported multiple tobacco product use, risk of lung cancer death among daily users was highest among cigarette users (12.7-fold increased risk), followed by daily cigar use (4.2-fold increased risk), and then daily pipe users (1.7-fold increased risk). A meta-analysis [74] of 287 epidemiological studies of lung cancer found that pipe use only was associated with a 3.3-fold increased risk of lung cancer and cigar use only was associated with a 2.95-fold increased risk. A recent pooled-analysis [75] of five prospective cohort studies from U.S. National Cancer Institute (NCI) Cohort Consortium and that had collected data on cigar and pipe smoking found 2.7-fold increased risk of lung cancer cigar use only and 1.9-fold increased risk for pipe use only. A meta-analysis [76] of 13 case-control studies reported a 4.6-fold increased risk of lung cancer among those using water pipes only. While risk of lung cancer and death is lower for individuals using these products compared to those who smoke cigarettes, it should be noted that these are not safer alternatives to cigarette smoking as the lower point estimates are likely attributed to lower smoking intensity and perhaps lesser degrees of inhalation of these products.
Cannabis
Though the terms cannabis and marijuana are frequently used interchangeably, cannabis is the generic term that includes cannabinoids, hemp, and marijuana derived from the Cannabis sativa plant [77]. In the United States, smoked cannabis is estimated to be the most commonly inhaled drug after tobacco with an estimated 7,000 new users a day [78]. As of early 2019, thirty states, the District of Columbia, Guam, and Puerto Rico have legalized marijuana use for medical purposes and twenty states and the District of Columbia have decriminalized the possession of small amounts of marijuana for personal use [79]. However, smoked cannabis contains many of the same chemical toxins and carcinogens as tobacco smoke including acetaldehyde, acrolein, ammonia, carbon monoxide, formaldehyde, phenols, nitrosamines, and polycyclic aromatic hydrocarbons [80]. Additionally, regular smoking of marijuana alone is associated with adverse effects on the respiratory system similar to that of cigarette smoking [81, 82]. However, despite the evidence of adverse biological effects, to date there is no conclusive evidence that suggests cannabis smoking is associated with an increased incidence of lung cancer. A pooled analysis from [83] the International Lung Cancer Consortium of 2,159 lung cancer cases and 2,985 controls found little evidence for an increased risk of lung cancer among habitual or long‐term cannabis smokers. However, it should be noted that studies to date have been limited by sample size, self-report, and confounding (e.g., many marijuana users also report tobacco use). Marijuana use is prevalent among youth in the United States as data from the National Survey on Drug Use and Health reported that prevalence of past-year use was between 12% and 16% for adolescents ages 12 to 17 between 2002 and 2014 [84]. In addition, over the last decade, fewer adolescents perceive “moderate” or “regular” use of marijuana as a health risk [85, 86]. As the association between smoked cannabis and lung cancer is still undefined and marijuana use is prevalent, more research will be required in the future to characterize the association between smoked cannabis and risk of lung cancer and for other diseases.
Radon
Because tobacco smoking is a potent and prevalent risk factor, secondary causes of lung cancer are often diminished in perceived importance. However, there are numerous other exposures that are causally linked to lung cancer risk. Radon is an invisible, odorless, tasteless radioactive gas that is found in soil and produced naturally during the radioactive decay of thorium and uranium. All humans are exposed to radon gas and there are substantial geographic variations globally and throughout the United States. Worldwide, 3% to 14% of lung cancers are attributed to radon exposure and the variance is attributed to geographic differences in radon concentration and on the method of calculation [87]. In the United States, radon exposure is estimated to be the second leading cause of lung cancer and responsible for over 21,000 or 13% of lung cancer deaths each year [87, 88]. Published meta-analyses have reported that indoor radon exposure is associated with a 14 to 29% increased risk of lung cancer [89-91].
Occupational Exposures
Occupational exposure to carcinogens is estimated to account for 5 to 10% of lung cancers [41, 88, 92, 93] of which asbestos exposure is historically the most common. Asbestos is a commercial term for a group of naturally occurring mineral silicate fibers, including amphiboles (crocidolite, amosite, tremolite, anthophyllite, and actinolite) and chrysotile (the sole serpentine fiber). Asbestos is found on all continents, use has been used commercially since the 19th century, and is still used in some countries today in numerous applications including insulation, textile, cement, and roofing [94]. Although the mechanisms involved in asbestos-associated diseases are complex and the molecular pathways involved are not fully established, direct and indirect cellular and molecular effects likely contribute to lung cancer etiology including oxidative stress, chronic inflammation, genetic and epigenetic alterations, and cellular toxicity and fibrosis [95]. A meta-analysis of 14 case-control studies conducted in Europe and Canada that included 17,705 lung cancer cases and 21,813 controls found ever-exposure to asbestos was associated with a 24% increased risk in men and 12% increased risk in women [96]. There is substantial synergistic effects [97] between asbestos exposure and tobacco smoking on lung cancer risk and morality [96, 98, 99].
The International Agency for Research on Cancer (IARC) evaluates carcinogenicity for a wide range of human exposures. Agents classified as “carcinogenic to humans” (Group 1) [100] that have sufficient evidence of causing lung cancer in humans include numerous occupational-related exposures including arsenic, beryllium, cadmium, chromium, and diesel exhaust and specific occupations including aluminum production, coal gasification, coke production, underground hematite mining, iron and steel founding, painting, and rubber production (reviewed in [101]).
History of Non-Infectious Related Respiratory Diseases
History of chronic obstructive pulmonary disease (COPD), which includes emphysema, or chronic bronchitis, is an irreversible chronic inflammatory condition that leads to fixed narrowing of small airways and alveolar wall destruction. The long-standing inflammatory reaction in the bronchi is accompanied by a continual cycle of injury and repair and therefore could play a key role in lung carcinogenesis. In the United States, over 15 million people reported ever receiving a diagnosis of COPD in 2015 and is the third leading cause of death behind heart disease and cancer [102]. Tobacco smoking is the major risk factor for COPD [103] so it is unexpected to find a positive association between COPD and lung cancer. Published meta-analyses have reported a 2 to 3-fold risk of lung associated with a history of COPD, emphysema, or chronic bronchitis [104-106]. A pooled analysis from the International Lung Cancer Consortium found that a history of emphysema conferred a 2.44-fold increased risk of lung cancer [107].
Asthma is a common childhood disease affecting approximately 300 million people worldwide [108]. Asthma is characterized by chronic inflammation of the lungs and presents with airway hyper-reactivity, excessive mucous formation, and respiratory obstruction. Asthma has been suspected as a potential risk factor for lung cancer since inflammation also plays a pivotal role in the lung cancer pathogenesis. A pooled analysis published in 2012 of 16 studies in the International Lung Cancer Consortium concluded that increased risk between asthma and lung cancer may not reflect a causal effect since the increased incidence of was largely observed in small cell and squamous cell lung carcinomas, primarily within 2 years of asthma diagnosis, and the association was weak among never smokers [109]. However, a meta-analysis published in 2017 that included 18 studies with over 16 million individuals [110] found that asthma was significantly associated with a 44% increased risk of lung cancer and a 28% increased risk among never smokers. Subgroup analyses also demonstrated significant increases for non-Hispanic Whites, Asians, males, and females.
History of Infectious-Related Respiratory Diseases
Pneumococcal disease is an umbrella term for a group of syndromes caused by a variety of organisms resulting in varied manifestations and sequelae [111, 112]. Most commonly, pneumococcal disease is an infection caused by the Streptococcus pneumoniae bacterium which can infect the lungs (pneumonia), bloodstream (bacteremia), and tissues and fluids surrounding the brain and spinal cord (meningitis). In the United States, approximately 400,000 hospitalizations from pneumococcal pneumonia occur annually [113]. Pneumonia is a putative lung cancer risk factor through several possible mechanisms from mediators of chronic local inflammation including elevated reactive oxygen species that can cause DNA damage and somatic mutations, anti-apoptotic signaling, and increased angiogenesis [113]. Published meta-analyses have reported a history pneumonia was associated with a 30 to 40% increased risk of lung cancer risk [105, 114] and a pooled analysis from the International Lung Cancer Consortium reported a 57% increased risk [107]. However, such findings should be interpreted with caution since reverse causality cannot be ruled out since pulmonary infections can be a result of a weakened immune system due to lung cancer [105]. Furthermore, the timing of a pneumonia diagnosis can coincide with or confound the diagnosis of lung cancer and pneumonia may be a complication of lung cancer such as post-obstructive pneumonia [115].
Chlamydia pneumonia (C. pneumoniae) is the most commonly occurring intracellular bacterial pathogen and is responsible for sinusitis, pharyngitis, and pneumonia [116]. It is transmitted occurs via respiratory secretions and may increase risk of lung cancer through mediators of inflammation similar to those speculated for pneumonia [117] as described above. A meta-analysis [118] of 12 studies including 2,595 lung cancer cases and 2,585 controls reported that C. pneumoniae infection was associated with a 1.5-fold increased risk of lung cancer. C. pneumoniae infection was significantly associated with a 1.2-fold increased risk of lung cancer in prospective studies and 2.2-fold increased risk in retrospective studies. When definition of chronic infection was defined by antibody titre, the IgA ≥ 16 cutoff group was associated with a 1.2-fold increased risk and the IgA ≥ 64 cutoff group was associated with a 2.4-fold increased risk. Tuberculosis is a communicable infectious disease transmitted by cough aerosol and is caused by the Mycobacterium tuberculosis bacterium. Although tuberculosis primarily affects the lungs, it can affect other parts of the body. Worldwide incidence of tuberculosis has slowly declined over the past decade; in 2013 an estimated 9 million incident cases of tuberculosis (126 cases per 100,000) were reported with more than 60% of the burden concentrated in the 22 high-burden countries [119]. The United States is a low-incidence country with an annual incidence of 30 tuberculosis cases per 1 million [120]. Tuberculosis can induce chronic inflammation and pulmonary fibrosis leading to higher rates of genetic alterations and mutations which have been suggested mechanisms regarding the role of tuberculosis on lung cancer risk [121]. A pooled analysis from the International Lung Cancer Consortium and a meta-analysis reported that previous history of tuberculosis was associated with a 48% and 76% increased risk of lung cancer, respectively [105, 107].
Human Immunodeficiency Virus (HIV)
Individuals who are infected with HIV are at increased for many cancers, attributed to many factors including HIV-related immunosuppression which impairs control of oncogenic viral infections, mediators of inflammation, and co-infection with oncogenic viruses such as Hepatitis B and C [122-124]. Lung cancer is a leading non-Acquired Immunity Deficiency Syndrome (AIDS) defining cancer (NADC) and is the most frequent cause of cancer-related death among persons infected with HIV [125]. Though adults with HIV are more likely to smoke cigarettes than the general adult population [126], when accounting for smoking elevated incidence of lung cancer among HIV-infected persons has been observed [127]. The HIV/AIDS Cancer Match (HACM) Study used linked data collected by US HIV and cancer registries to describe cancer risk in HIV-infected people in the United States relative to the general population [128]. Standardized incidence ratios (SIRs) were used to test for differences by AIDS status and over time. Among 448,258 HIV-infected people, lung cancer was the second common individual cancer type (11.6%) was lung cancer risk was elevated 2-fold.
Other Lifestyle Factors
There is also compelling evidence that other factors may be associated with an increased risk of lung cancer for both smokers and never smokers including poor diet and low body mass index [129-137].
Inherited Genetics
In 2004, the Genetic Epidemiology of Lung Cancer Consortium revealed the first evidence for a major susceptibility locus influencing lung cancer risk to a region on 6q23–25 [138]. With the arrival of genome-wide association (GWA) studies about 17 years ago, it is now possible to interrogate the human genome more comprehensively for associations between inherited single-nucleotide polymorphisms (SNPs) and human disease. GWA studies have successfully identified genetic factors significantly associated with lung cancer susceptibility with varying strengths of association evidence and some loci have been refined to specific subgroups including sex, ethnicity, smoking status, and histological subtypes [139, 140]. Data from these large GWA studies could be leveraged towards development of risk models based on polygenic risk scores defined by the combination of SNPs that yield the best predictive model [141].
Lung Cancer among Never Smokers
Globally, approximately 25% of lung cancer diagnoses are among never smokers (LCANS) [142] and approximately 60 to 80% of women diagnosed with NSCLC are never smokers. In East and South Asia, a high proportion of female lung cancers occur among never smokers [143]. In the United States, although smoking rates and the incidence of lung cancer has declined over the last several decades, the incidence of lung cancer among never smokers (LCANS) has been on the rise. Approximately 10 to 20% of all lung cancer diagnoses occur in never smokers in the United States, and if considered a separate reportable category, LCANS is the 11th most common cancer and the 7th leading cause of cancer-related deaths.
Many of the exposures associated with lung cancer risk have been found to be risk factors for both smokers and never smokers. Nonetheless, risk factors found to be associated with LCANS include second-hand smoke, cooking fumes, ionizing radiation, radon gas, inherited genetic susceptibility, occupational exposures, preexisting lung disease, and oncogenic viruses (reviewed in [144]). Among all risk factors, advanced age is the most significant contributor to LCANS. Even if the incidence of LCANS remains constant over time, the number of lung cancer deaths among never smokers is expected to increase significantly in the following decades as the prevalence of smoking continues to decline as the population age-structure continues to shift to older ages [144]. Thus, lung cancer will continue to be a substantial public health burden in the United States in spite of the significant improvements in tobacco control and early detection.
The histology of LCANS is most likely to be adenocarcinoma and molecular profiling studies have found that the tumor genome of LCANS is significantly different from the genome of lung cancers arising in smokers. Mutations in TP53, KRAS, and STK11 are more frequent in tobacco smokers with lung cancer, whereas EGFR and HER2 mutations and the ALK-ELM4 fusion are more common among LCANS [145]. Revealing and understanding differences at the molecular level among LCANS may identify the etiological processes involved in tumorigenesis and reveal important therapeutic strategies for targeting key oncogenic events. As such, the National Cancer Institute has launched “Sherlock Lung: A Molecular Epidemiologic Study of Lung Cancer in Never Smokers” [146] with the goal of tracing lung cancer etiology in never smokers by analyzing molecular data in conjunction with histological features to develop an integrated molecular, histological, and radiological classification of LCANS.
Prevention
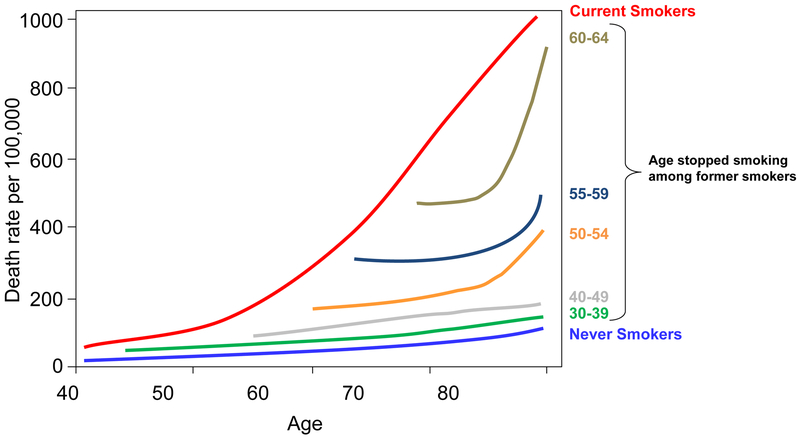
Most lung cancers are preventable and could be mitigated by reducing smoking initiation among adolescents and increasing smoking cessation among adults. Fortunately, smoking rates have steadily declined in the United States since the 1960s [10]. In 2016, the prevalence of current cigarette smoking among adults was 15.5%, which was substantially declined from 20.9% in 2005 [147]. Though primary prevention (smoking prevention and cessation) mitigates risk and mortality, former smokers remain at significant risk of dying from lung cancer (Figure 10) [148]. As such, early detection is currently the only option for those who have already quit smoking and among those individuals who are at high-risk. Lung cancer will likely remain a major public health burden globally throughout the 21st century and advances in risk assessment, early detection, diagnosis, and treatment will be imperative in improving outcomes of this disease [149].
Figure 10.
Lung cancer mortality by smoking status. Figure 10 shows lung cancer mortality (per 100,000) among current smokers, former smokers, and never smokers based on published figures that were adapted from Halpern et al ([reference 148] J Natl Cancer Inst 1993; 85:457-464). Former smokers are presented by age-at-quit.
Screening and Early Detection
As described earlier, the majority of lung cancer patients are diagnosed with advanced stage disease, where the prospects for cure are limited. However, local therapy for early stage disease is associated with substantially improved overall survival. Until recently, a modality for the successful detection of early stage lung cancer has been elusive. In 2011, results from the National Lung Screening Trial (NLST) demonstrated a 20% relative reduction in lung cancer mortality for individuals screened by low-dose helical computed tomography (LDCT) compared to standard chest radiography in a high-risk population of 53,454 current and former smokers aged 55–74 years [150]. Screen-detected incidence lung cancers diagnosed following a positive screen at 1 or 2 years after the baseline screen accounted for 58% of all LDCT-detected lung cancers in the NLST, were 2.7-fold higher in the LDCT arm versus the chest radiography arm, and were associated with a favorable stage shift from advanced to more early stage lung cancers [150]. Additionally, in the LDCT arm, a subset of screen-detected incidence lung cancers where their antecedent screens were positive prior to the screen of the cancer diagnosis were associated with improved 5-year survival compared to prevalent lung cancers [151] that are usually diagnosed when patients develop symptoms in a “real world setting”. Following publication of the NLST results, the United States Preventive Services Task Force (USPSTF) in December 2013 and the Centers for Medicare and Medicaid (CMS) in February 2015 issued recommendations for annual LDCT screening for eligible high-risk individuals. [152, 153]. Both the USPSTF and CMS guidelines recommend smoking cessation interventions for individuals who enter a lung cancer screening program. Novel smoking cessation strategies tailored to the lung cancer screening setting will likely amplify the survivorship gains expected from screening alone [154].
Despite the conclusive benefits shown by the NLST and the recommendations and implementation of lung cancer screening in the United States, European nations have not yet issued similar recommendations because of the absence of proven benefit in randomized clinical trials conducted in Europe [155]. However, in 2018 the initial results of the Nederlands-Leuvens Longkanker Screenings ONderzo ek (NELSON) trial [156] were presented at the 19th World Conference on Lung Cancer of the International Association for the Study of Lung Cancer and indicated significant reductions in lung cancer mortality. Moreover, two additional randomized trials conducted in Italy [157] and Germany [158] were published in 2019 providing additional confirmation of lung cancer screening efficacy. The Multicentric Italian Lung Detection (MILD) trial [157], in which 4,099 participants aged 49–75 years with a smoking history of ≥ 20 pack-years were prospectively randomized to undergo LDCT screening for a median period of 6 years (n = 2,376) or to a control arm with no screening intervention (n = 1,723). Landmark analysis that considered only individuals alive with no lung cancer diagnosis after 5 years from randomization revealed a 58% reduction in lung cancer mortality and a 32% reduction in all-cause mortality after the fifth year of screening. The German Lung cancer Screening Intervention (LUSI) was a randomized trial [158] of 4,052 long‐term smokers aged 50–69 years comparing five annual rounds of LDCT screening (n = 2,029) versus a control arm without screening (n = 2,023) found a 26% in lung cancer mortality over an average observation time of 8.8 years after randomization. The cumulative evidence based on the results of the NLST, the MILD trial, the LUSI trial, and anticipated publication of the NELSON trial, has demonstrated substantial beneficial mortality reductions associated with LDCT screening.
Future Directions
Over the last several decades substantial progress has been made across the cancer control continuum in terms of etiology, prevention, early detection, diagnosis, treatment, survivorship, and end of life; however, lung cancer is still a major public health burden globally and in the United States. Etiologically, concerted efforts are needed to identify causal risk factors for lung cancer among never smokers and identify never smokers at the greatest risk for lung cancer that perhaps can benefit from a lung cancer screening program. Additionally, the impact of marijuana and electronic-cigarettes on lung cancer risk needs to be clarified. From a prevention standpoint, additional research is needed to identify potential agents that can reduce lung cancer risk especially among former smokers. Precision-based risk and screening should be explored to identify individuals who would benefit most from entering a lung cancer screening program. Advancements in screening technology and biomarkers in the screening setting could reduce false positives and overdiagnosis and improve nodule management. Further, research is needed on the feasibility and efficacy of providing smoking cessation treatment in the lung cancer screening setting. Biomarkers that are highly predictive of negative responses to targeted therapies and immunotherapy are a significant unmet clinical since there are subgroups of that may not respond to these specific treatments. This is particularly salient in the subsets of patients that may experience treatment-induced rapid disease progression which can be rapid and lethal. Finally, more research is needed to personalize treatment plans that minimize adverse survivorship issues and lead to improved quality of life for lung cancer survivors.
Acknowledgements:
This manuscript is dedicated to the memory of Grace C. Schabath (1941-2019). This work was funded in part by the National Institutes of Health (NIH) grant U01 CA200464 (M.B.S.).
Footnotes
Disclosures: None
References
- 1.American Cancer Society. Global Cancer Facts & Figures 4th Edition. Atlanta: American Cancer Society; 2018. [Google Scholar]
- 2.Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 2018; 68:394–424. [DOI] [PubMed] [Google Scholar]
- 3.American Cancer Society. Cancer Facts & Figures 2019. Atlanta: American Cancer Society; 2019. [Google Scholar]
- 4.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2019. CA Cancer J Clin 2019; 69:7–34. [DOI] [PubMed] [Google Scholar]
- 5.Rami-Porta R, Bolejack V, Giroux DJ, Chansky K, Crowley J, Asamura H, et al. The IASLC lung cancer staging project: the new database to inform the eighth edition of the TNM classification of lung cancer. J Thorac Oncol 2014; 9:1618–1624. [DOI] [PubMed] [Google Scholar]
- 6.Chansky K, Detterbeck FC, Nicholson AG, Rusch VW, Vallieres E, Groome P, et al. The IASLC Lung Cancer Staging Project: External Validation of the Revision of the TNM Stage Groupings in the Eighth Edition of the TNM Classification of Lung Cancer. J Thorac Oncol 2017; 12:1109–1121. [DOI] [PubMed] [Google Scholar]
- 7.Wahbah M, Boroumand N, Castro C, El-Zeky F, Eltorky M. Changing trends in the distribution of the histologic types of lung cancer: a review of 4,439 cases. Ann Diagn Pathol 2007; 11:89–96. [DOI] [PubMed] [Google Scholar]
- 8.Travis WD, Brambilla E, Riely GJ. New pathologic classification of lung cancer: relevance for clinical practice and clinical trials. J Clin Oncol 2013; 31:992–1001. [DOI] [PubMed] [Google Scholar]
- 9.Song MA, Benowitz NL, Berman M, Brasky TM, Cummings KM, Hatsukami DK, et al. Cigarette Filter Ventilation and its Relationship to Increasing Rates of Lung Adenocarcinoma. J Natl Cancer Inst 2017; 109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.United States. Public Health Service. Office of the Surgeon General: The health consequences of smoking--50 years of progress : a report of the surgeon general. Rockville, MD: U.S. Department of Health and Human Services, Public Health Service, Office of the Surgeon General; 2014. [Google Scholar]
- 11.Lewis DR, Check DP, Caporaso NE, Travis WD, Devesa SS. US lung cancer trends by histologic type. Cancer 2014; 120:2883–2892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pao W, Girard N. New driver mutations in non-small-cell lung cancer. Lancet Oncol 2011; 12:175–180. [DOI] [PubMed] [Google Scholar]
- 13.Hirsch FR, Scagliotti GV, Mulshine JL, Kwon R, Curran WJ Jr., Wu YL, et al. Lung cancer: current therapies and new targeted treatments. Lancet 2017; 389:299–311. [DOI] [PubMed] [Google Scholar]
- 14.Zhu QG, Zhang SM, Ding XX, He B, Zhang HQ. Driver genes in non-small cell lung cancer: Characteristics, detection methods, and targeted therapies. Oncotarget 2017; 8:57680–57692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.O’Keeffe LM, Taylor G, Huxley RR, Mitchell P, Woodward M, Peters SAE. Smoking as a risk factor for lung cancer in women and men: a systematic review and meta-analysis. BMJ Open 2018; 8:e021611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Thun MJ, Hannan LM, Adams-Campbell LL, Boffetta P, Buring JE, Feskanich D, et al. Lung cancer occurrence in never-smokers: an analysis of 13 cohorts and 22 cancer registry studies. PLoS Med 2008; 5:e185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Freedman ND, Leitzmann MF, Hollenbeck AR, Schatzkin A, Abnet CC. Cigarette smoking and subsequent risk of lung cancer in men and women: analysis of a prospective cohort study. Lancet Oncol 2008; 9:649–656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zang EA, Wynder EL. Differences in lung cancer risk between men and women: examination of the evidence. J Natl Cancer Inst 1996; 88:183–192. [DOI] [PubMed] [Google Scholar]
- 19.Pelosof L, Ahn C, Gao A, Horn L, Madrigales A, Cox J, et al. Proportion of Never-Smoker Non-Small Cell Lung Cancer Patients at Three Diverse Institutions. J Natl Cancer Inst 2017; 109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hiscock R, Bauld L, Amos A, Fidler JA, Munafo M. Socioeconomic status and smoking: a review. Ann N Y Acad Sci 2012; 1248:107–123. [DOI] [PubMed] [Google Scholar]
- 21.Hovanec J, Siemiatycki J, Conway DI, Olsson A, Stucker I, Guida F, et al. Lung cancer and socioeconomic status in a pooled analysis of case-control studies. PLoS One 2018; 13:e0192999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fredriksen-Goldsen KI, Kim HJ, Barkan SE, Muraco A, Hoy-Ellis CP. Health disparities among lesbian, gay, and bisexual older adults: results from a population-based study. Am J Public Health 2013; 103:1802–1809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lisy K, Peters MDJ, Schofield P, Jefford M. Experiences and unmet needs of lesbian, gay, and bisexual people with cancer care: A systematic review and meta-synthesis. Psychooncology 2018. [DOI] [PubMed] [Google Scholar]
- 24.Matthews AK, Breen E, Kittiteerasack P. Social Determinants of LGBT Cancer Health Inequities. Semin Oncol Nurs 2018; 34:12–20. [DOI] [PubMed] [Google Scholar]
- 25.Simoni JM, Smith L, Oost KM, Lehavot K, Fredriksen-Goldsen K. Disparities in Physical Health Conditions Among Lesbian and Bisexual Women: A Systematic Review of Population-Based Studies. J Homosex 2017; 64:32–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Boehmer U, Elk R. LGBT Populations and Cancer: Is It an Ignored Epidemic? LGBT Health 2015. [DOI] [PubMed] [Google Scholar]
- 27.Quinn GP, Sanchez JA, Sutton SK, Vadaparampil ST, Nguyen GT, Green BL, et al. Cancer and lesbian, gay, bisexual, transgender/transsexual, and queer/questioning (LGBTQ) populations. CA Cancer J Clin 2015; 65:384–400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Waterman L, Voss J. HPV, cervical cancer risks, and barriers to care for lesbian women. The Nurse practitioner 2015; 40:46–53; quiz 53-44. [DOI] [PubMed] [Google Scholar]
- 29.Graham R, Berkowitz B, Blum R, Bockting W, Bradford J, de Vries B, et al. : The Health of Lesbian, Gay, Bisexual, and Transgender People: Building a Foundation for Better Understanding. In. Washington, DC: The National Institute of Health; 2011. [PubMed] [Google Scholar]
- 30.Boehmer U, Miao X, Maxwell NI, Ozonoff A. Sexual minority population density and incidence of lung, colorectal and female breast cancer in California. BMJ open 2014; 4:e004461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Boehmer U, Ozonoff A, Miao X. An ecological approach to examine lung cancer disparities due to sexual orientation. Public Health 2012; 126:605–612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gonzales G, Zinone R. Cancer diagnoses among lesbian, gay, and bisexual adults: results from the 2013-2016 National Health Interview Survey. Cancer Causes Control 2018; 29:845–854. [DOI] [PubMed] [Google Scholar]
- 33.Conron KJ, Mimiaga MJ, Landers SJ. A population-based study of sexual orientation identity and gender differences in adult health. Am J Public Health 2010; 100:1953–1960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Dilley JA, Spigner C, Boysun MJ, Dent CW, Pizacani BA. Does tobacco industry marketing excessively impact lesbian, gay and bisexual communities? Tob Control 2008; 17:385–390. [DOI] [PubMed] [Google Scholar]
- 35.Smoking and health: report of the Advisory Committee to the Surgeon General of the Public Health Service. Washington, DC: Department of Health, Education, and Welfare; 1964. (PHS publication no. 1103.). [Google Scholar]
- 36.Centers for Disease Control and Prevention. HIV Surveillance Report, 2016; vol. 28 http://www.cdc.gov/hiv/library/reports/hiv-surveillance.html. Published November 2017. Accessed June 2019. [Google Scholar]
- 37.Hou W, Fu J, Ge Y, Du J, Hua S. Incidence and risk of lung cancer in HIV-infected patients. J Cancer Res Clin Oncol 2013; 139:1781–1794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Thun MJ. Early landmark studies of smoking and lung cancer. Lancet Oncol 2010; 11:1200. [DOI] [PubMed] [Google Scholar]
- 39.Thun MJ, Carter BD, Feskanich D, Freedman ND, Prentice R, Lopez AD, et al. 50-year trends in smoking-related mortality in the United States. N Engl J Med 2013; 368:351–364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.U.S. Department of Health and Human Services. The Health Consequences of Smoking—50 Years of Progress: A Report of the Surgeon General, 2014. Atlanta, GA: U.S. Department of Health and Human Services, Centers for Disease Control and Prevention, National Center for Chronic Disease Prevention and Health Promotion, Office on Smoking and Health, 2014. [Google Scholar]
- 41.Alberg AJ, Brock MV, Ford JG, Samet JM, Spivack SD. Epidemiology of lung cancer: Diagnosis and management of lung cancer, 3rd ed: American College of Chest Physicians evidence-based clinical practice guidelines; Chest 2013; 143:e1S–e29S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Peto J That the effects of smoking should be measured in pack-years: misconceptions 4. Br J Cancer 2012; 107:406–407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Spitz MR, Hong WK, Amos CI, Wu X, Schabath MB, Dong Q, et al. A risk model for prediction of lung cancer. J Natl Cancer Inst 2007; 99:715–726. [DOI] [PubMed] [Google Scholar]
- 44.Markaki M, Tsamardinos I, Langhammer A, Lagani V, Hveem K, Roe OD. A Validated Clinical Risk Prediction Model for Lung Cancer in Smokers of All Ages and Exposure Types: A HUNT Study. EBioMedicine 2018; 31:36–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hoggart C, Brennan P, Tjonneland A, Vogel U, Overvad K, Ostergaard JN, et al. A risk model for lung cancer incidence. Cancer Prev Res (Phila) 2012; 5:834–846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Marcus MW, Chen Y, Raji OY, Duffy SW, Field JK. LLPi: Liverpool Lung Project Risk Prediction Model for Lung Cancer Incidence. Cancer Prev Res (Phila) 2015; 8:570–575. [DOI] [PubMed] [Google Scholar]
- 47.Tammemagi MC, Church TR, Hocking WG, Silvestri GA, Kvale PA, Riley TL, et al. Evaluation of the lung cancer risks at which to screen ever- and never-smokers: screening rules applied to the PLCO and NLST cohorts. PLoS Med 2014; 11:e1001764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.National Cancer Institute, Division of Cancer Control & Population Sciences. Lung Cancer Risk Prediction Models. URL at: https://epi.grants.cancer.gov/cancer_risk_prediction/lung.html Accessed May 21, 2019.
- 49.Lung Cancer Risk Assessment Tool. URL at: http://lungcancerrisk.s3-website-us-east-1.amazonaws.com/ Accessed May 21, 2019
- 50.Tsai J, Homa DM, Gentzke AS, Mahoney M, Sharapova SR, Sosnoff CS, et al. Exposure to Secondhand Smoke Among Nonsmokers - United States, 1988-2014. MMWR Morb Mortal Wkly Rep 2018; 67:1342–1346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Hackshaw AK. Lung cancer and passive smoking. Stat Methods Med Res 1998; 7:119–136. [DOI] [PubMed] [Google Scholar]
- 52.United States. Public Health Service. Office of the Surgeon General.: The health consequences of involuntary exposure to tobacco smoke : a report of the Surgeon General. Rockville, MD: U.S. Dept. of Health and Human Services, Public Health Service, Office of the Surgeon General; 2006. [Google Scholar]
- 53.Kim AS, Ko HJ, Kwon JH, Lee JM. Exposure to Secondhand Smoke and Risk of Cancer in Never Smokers: A Meta-Analysis of Epidemiologic Studies. Int J Environ Res Public Health 2018; 15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Hori M, Tanaka H, Wakai K, Sasazuki S, Katanoda K. Secondhand smoke exposure and risk of lung cancer in Japan: a systematic review and meta-analysis of epidemiologic studies. Jpn J Clin Oncol 2016; 46:942–951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Villanti AC, Johnson AL, Ambrose BK, Cummings KM, Stanton CA, Rose SW, et al. Flavored Tobacco Product Use in Youth and Adults: Findings From the First Wave of the PATH Study (2013-2014). Am J Prev Med 2017; 53:139–151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Zhu SH, Sun JY, Bonnevie E, Cummins SE, Gamst A, Yin L, et al. Four hundred and sixty brands of e-cigarettes and counting: implications for product regulation. Tobacco Control 2014; 23:3–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Mirbolouk M, Charkhchi P, Kianoush S, Uddin SMI, Orimoloye OA, Jaber R, et al. Prevalence and Distribution of E-Cigarette Use Among US Adults: Behavioral Risk Factor Surveillance System, 2016. Ann Intern Med 2018; 169:429–+. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Jaber RM, Mirbolouk M, DeFilippis AP, Maziak W, Keith R, Payne T, et al. Electronic Cigarette Use Prevalence, Associated Factors, and Pattern by Cigarette Smoking Status in the United States From NHANES (National Health and Nutrition Examination Survey) 2013-2014. J Am Heart Assoc 2018; 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Bao W, Xu GF, Lu JC, Snetselaar LG, Wallace RB. Changes in Electronic Cigarette Use Among Adults in the United States, 2014-2016. Jama-J Am Med Assoc 2018; 319:2039–2041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Jaber RM, Saxena A, Nasir K, Mirbolouk M, Maziak W, DeFilippis A, et al. Electronic Cigarette Use Prevalence and Pattern by Cigarette Smoking Status Among United States Adults: Findings From NHANES 2013-2014. Circulation 2017; 136. [Google Scholar]
- 61.Syamlal G, Jamal A, King BA, Mazurek JM. Electronic Cigarette Use Among Working Adults - United States, 2014. Mmwr-Morbid Mortal W 2016; 65:557–561. [DOI] [PubMed] [Google Scholar]
- 62.Kosmider L, Sobczak A, Fik M, Knysak J, Zaciera M, Kurek J, et al. Carbonyl compounds in electronic cigarette vapors: effects of nicotine solvent and battery output voltage. Nicotine Tob Res 2014; 16:1319–1326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Leventhal AM, Strong DR, Kirkpatrick MG, Unger JB, Sussman S, Riggs NR, et al. Association of Electronic Cigarette Use With Initiation of Combustible Tobacco Product Smoking in Early Adolescence. Jama-J Am Med Assoc 2015; 314:700–707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Soneji S, Barrington-Trimis JL, Wills TA. Association Between Initial Use of e-Cigarettes and Subsequent Cigarette Smoking Among Adolescents and Young Adults: A Systematic Review and Meta-analysis (vol 171, pg 788, 2017). Jama Pediatr 2018; 172:98–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Cai H, Wang C. Graphical review: The redox dark side of e-cigarettes; exposure to oxidants and public health concerns. Redox Biol 2017; 13:402–406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Unger M, Unger DW. E-cigarettes/electronic nicotine delivery systems: a word of caution on health and new product development. J Thorac Dis 2018; 10:S2588–S2592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Dautzenberg B, Garelik D. Patients with lung cancer: Are electronic cigarettes harmful or useful? Lung Cancer 2017; 105:42–48. [DOI] [PubMed] [Google Scholar]
- 68.Scheffler S, Dieken H, Krischenowski O, Forster C, Branscheid D, Aufderheide M. Evaluation of E-cigarette liquid vapor and mainstream cigarette smoke after direct exposure of primary human bronchial epithelial cells. Int J Environ Res Public Health 2015; 12:3915–3925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Lerner CA, Sundar IK, Yao H, Gerloff J, Ossip DJ, McIntosh S, et al. Vapors produced by electronic cigarettes and e-juices with flavorings induce toxicity, oxidative stress, and inflammatory response in lung epithelial cells and in mouse lung. PLoS One 2015; 10:e0116732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Flouris AD, Chorti MS, Poulianiti KP, Jamurtas AZ, Kostikas K, Tzatzarakis MN, et al. Acute impact of active and passive electronic cigarette smoking on serum cotinine and lung function. Inhal Toxicol 2013; 25:91–101. [DOI] [PubMed] [Google Scholar]
- 71.Vardavas CI, Anagnostopoulos N, Kougias M, Evangelopoulou V, Connolly GN, Behrakis PK. Short-term pulmonary effects of using an electronic cigarette: impact on respiratory flow resistance, impedance, and exhaled nitric oxide. Chest 2012; 141:1400–1406. [DOI] [PubMed] [Google Scholar]
- 72.Drummond MB, Upson D. Electronic cigarettes. Potential harms and benefits. Ann Am Thorac Soc 2014; 11:236–242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Christensen CH, Rostron B, Cosgrove C, Altekruse SF, Hartman AM, Gibson JT, et al. Association of Cigarette, Cigar, and Pipe Use With Mortality Risk in the US Population. JAMA Intern Med 2018; 178:469–476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Lee PN, Forey BA, Coombs KJ. Systematic review with meta-analysis of the epidemiological evidence in the 1900s relating smoking to lung cancer. BMC Cancer 2012; 12:385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Malhotra J, Borron C, Freedman ND, Abnet CC, van den Brandt PA, White E, et al. Association between Cigar or Pipe Smoking and Cancer Risk in Men: A Pooled Analysis of Five Cohort Studies. Cancer Prev Res (Phila) 2017; 10:704–709. [DOI] [PubMed] [Google Scholar]
- 76.Waziry R, Jawad M, Ballout RA, Al Akel M, Akl EA. The effects of waterpipe tobacco smoking on health outcomes: an updated systematic review and meta-analysis. Int J Epidemiol 2017; 46:32–43. [DOI] [PubMed] [Google Scholar]
- 77.Jett J, Stone E, Warren G, Cummings KM. Cannabis Use, Lung Cancer, and Related Issues. J Thorac Oncol 2018; 13:480–487. [DOI] [PubMed] [Google Scholar]
- 78.Azofeifa A, Mattson ME, Schauer G, McAfee T, Grant A, Lyerla R. National Estimates of Marijuana Use and Related Indicators - National Survey on Drug Use and Health, United States, 2002-2014. Mmwr Surveill Summ 2016; 65:1-Cover3. [DOI] [PubMed] [Google Scholar]
- 79.National Conference of State Legislatures: Marijuna Laws. URL at http://www.ncsl.org/bookstore/state-legislatures-magazine/marijuana-deep-dive.aspx webpage accessed 5/21/2019
- 80.Moir D, Rickert WS, Levasseur G, Larose Y, Maertens R, White P, et al. A comparison of mainstream and sidestream marijuana and tobacco cigarette smoke produced under two machine smoking conditions. Chem Res Toxicol 2008; 21:494–502. [DOI] [PubMed] [Google Scholar]
- 81.Mohite PN, Zeriouh M, Saez DG, Popov AF, Sabashnikov A, Zych B, et al. Influence of history of cannabis smoking in selected donors on the outcomes of lung transplantation. Eur J Cardiothorac Surg 2017; 51:142–147. [DOI] [PubMed] [Google Scholar]
- 82.Martinasek MP, McGrogan JB, Maysonet A. A Systematic Review of the Respiratory Effects of Inhalational Marijuana. Respir Care 2016; 61:1543–1551. [DOI] [PubMed] [Google Scholar]
- 83.Zhang LR, Morgenstern H, Greenland S, Chang SC, Lazarus P, Teare MD, et al. Cannabis smoking and lung cancer risk: Pooled analysis in the International Lung Cancer Consortium. Int J Cancer 2015; 136:894–903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Substance Abuse and Mental Health Services Administration. (2017). Key substance use and mental health indicators in the United States: Results from the 2016 National Survey on Drug Use and Health (HHS Publication No. SMA 17-5044, NSDUH Series H-52). Rockville, MD: Center for Behavioral Health Statistics and Quality, Substance Abuse and Mental Health Services Administration; Retrieved from https://www.samhsa.gov/data/. [Google Scholar]
- 85.Miech RA, Johnston L, O’Malley PM, Bachman JG, Schulenberg J, Patrick ME. Trends in use of marijuana and attitudes toward marijuana among youth before and after decriminalization: the case of California 2007-2013. Int J Drug Policy 2015; 26:336–344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Miech R, Johnston L, O’Malley PM. Prevalence and Attitudes Regarding Marijuana Use Among Adolescents Over the Past Decade. Pediatrics 2017; 140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.World Health Organization.: WHO handbook on indoor radon : a public health perspective. Geneva, Switzerland: World Health Organization; 2009. [PubMed] [Google Scholar]
- 88.Driscoll T, Nelson DI, Steenland K, Leigh J, Concha-Barrientos M, Fingerhut M, et al. The global burden of disease due to occupational carcinogens. Am J Ind Med 2005; 48:419–431. [DOI] [PubMed] [Google Scholar]
- 89.Garzillo C, Pugliese M, Loffredo F, Quarto M. Indoor radon exposure and lung cancer risk: a meta-analysis of case-control studies. Transl Cancer Res 2017; 6:S934–S943. [Google Scholar]
- 90.Lubin JH, Boice JD Jr. Lung cancer risk from residential radon: meta-analysis of eight epidemiologic studies. J Natl Cancer Inst 1997; 89:49–57. [DOI] [PubMed] [Google Scholar]
- 91.Zhang ZL, Sun J, Dong JY, Tian HL, Xue L, Qin LQ, et al. Residential radon and lung cancer risk: an updated meta- analysis of case-control studies. Asian Pac J Cancer Prev 2012; 13:2459–2465. [DOI] [PubMed] [Google Scholar]
- 92.Dela Cruz CS, Tanoue LT, Matthay RA. Lung cancer: epidemiology, etiology, and prevention. Clin Chest Med 2011; 32:605–644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Alberg AJ, Brock MV, Ford JG, Samet JM, Spivack SD. Epidemiology of lung cancer: Diagnosis and management of lung cancer, 3rd ed: American College of Chest Physicians evidence-based clinical practice guidelines; Chest 2013; 143:e1S–29S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Albin M, Magnani C, Krstev S, Rapiti E, Shefer I. Asbestos and cancer: An overview of current trends in Europe. Environ Health Perspect 1999; 107 Suppl 2:289–298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Huang SX, Jaurand MC, Kamp DW, Whysner J, Hei TK. Role of mutagenicity in asbestos fiber-induced carcinogenicity and other diseases. J Toxicol Environ Health B Crit Rev 2011; 14:179–245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Olsson AC, Vermeulen R, Schuz J, Kromhout H, Pesch B, Peters S, et al. Exposure-Response Analyses of Asbestos and Lung Cancer Subtypes in a Pooled Analysis of Case-Control Studies. Epidemiology 2017; 28:288–299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Case BW. Asbestos, smoking, and lung cancer: interaction and attribution. Occup Environ Med 2006; 63:507–508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Hammond EC, Selikoff IJ, Seidman H. Asbestos exposure, cigarette smoking and death rates. Ann N Y Acad Sci 1979; 330:473–490. [DOI] [PubMed] [Google Scholar]
- 99.Reid A, de Klerk NH, Ambrosini GL, Berry G, Musk AW. The risk of lung cancer with increasing time since ceasing exposure to asbestos and quitting smoking. Occupational and Environmental Medicine 2006; 63:509–512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.IARC. Agents classified by the IARC Monographs volumes 1-123. 2019. https://monographs.iarc.fr/agents-classified-by-the-iarc/. (accessed May 9, 2019).
- 101.Field RW, Withers BL. Occupational and environmental causes of lung cancer. Clin Chest Med 2012; 33:681–703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Croft JB, Wheaton AG, Liu Y, Xu F, Lu H, Matthews KA, et al. Urban-Rural County and State Differences in Chronic Obstructive Pulmonary Disease - United States, 2015. MMWR Morb Mortal Wkly Rep 2018; 67:205–211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.MacNee W Pathogenesis of chronic obstructive pulmonary disease. Proc Am Thorac Soc 2005; 2:258–266; discussion 290-251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Zhang X, Jiang N, Wang L, Liu H, He R. Chronic obstructive pulmonary disease and risk of lung cancer: a meta-analysis of prospective cohort studies. Oncotarget 2017; 8:78044–78056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Brenner DR, McLaughlin JR, Hung RJ. Previous lung diseases and lung cancer risk: a systematic review and meta-analysis. PLoS One 2011; 6:e17479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Wang H, Yang L, Zou L, Huang D, Guo Y, Pan M, et al. Association between chronic obstructive pulmonary disease and lung cancer: a case-control study in Southern Chinese and a meta-analysis. PLoS One 2012; 7:e46144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Brenner DR, Boffetta P, Duell EJ, Bickeboller H, Rosenberger A, McCormack V, et al. Previous lung diseases and lung cancer risk: a pooled analysis from the International Lung Cancer Consortium. Am J Epidemiol 2012; 176:573–585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Masoli M, Fabian D, Holt S, Beasley R, Global Initiative for Asthma P. The global burden of asthma: executive summary of the GINA Dissemination Committee report. Allergy 2004; 59:469–478. [DOI] [PubMed] [Google Scholar]
- 109.Rosenberger A, Bickeboller H, McCormack V, Brenner DR, Duell EJ, Tjonneland A, et al. Asthma and lung cancer risk: a systematic investigation by the International Lung Cancer Consortium. Carcinogenesis 2012; 33:587–597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Qu YL, Liu J, Zhang LX, Wu CM, Chu AJ, Wen BL, et al. Asthma and the risk of lung cancer: a meta-analysis. Oncotarget 2017; 8:11614–11620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Jain V, Bhardwaj A: Pneumonia, Pathology In: StatPearls. Treasure Island (FL); 2018. [Google Scholar]
- 112.Mackenzie G The definition and classification of pneumonia. Pneumonia (Nathan) 2016; 8:14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Mantovani A, Allavena P, Sica A, Balkwill F. Cancer-related inflammation. Nature 2008; 454:436–444. [DOI] [PubMed] [Google Scholar]
- 114.Sogaard KK, Farkas DK, Pedersen L, Weiss NS, Thomsen RW, Sorensen HT. Pneumonia and the incidence of cancer: a Danish nationwide cohort study. J Intern Med 2015; 277:429–438. [DOI] [PubMed] [Google Scholar]
- 115.Schabath MB, Delclos GL, Martynowicz MM, Greisinger AJ, Lu C, Wu X, et al. Opposing effects of emphysema, hay fever, and select genetic variants on lung cancer risk. Am J Epidemiol 2005; 161:412–422. [DOI] [PubMed] [Google Scholar]
- 116.Blasi F, Tarsia P, Arosio C, Fagetti L, Allegra L. Epidemiology of Chlamydia pneumoniae. Clin Microbiol Infect 1998; 4 Suppl 4:S1–S6. [PubMed] [Google Scholar]
- 117.Littman AJ, Jackson LA, Vaughan TL. Chlamydia pneumoniae and lung cancer: epidemiologic evidence. Cancer Epidemiol Biomarkers Prev 2005; 14:773–778. [DOI] [PubMed] [Google Scholar]
- 118.Zhan P, Suo LJ, Qian Q, Shen XK, Qiu LX, Yu LK, et al. Chlamydia pneumoniae infection and lung cancer risk: a meta-analysis. Eur J Cancer 2011; 47:742–747. [DOI] [PubMed] [Google Scholar]
- 119.Dheda K, Barry CE 3rd, Maartens G. Tuberculosis. Lancet 2016; 387:1211–1226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.LoBue PA, Mermin JH. Latent tuberculosis infection: the final frontier of tuberculosis elimination in the USA. Lancet Infect Dis 2017; 17:e327–e333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Keikha M, Esfahani BN. The Relationship between Tuberculosis and Lung Cancer. Adv Biomed Res 2018; 7:58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Shiels MS, Engels EA. Evolving epidemiology of HIV-associated malignancies. Curr Opin HIV AIDS 2017; 12:6–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Dubrow R, Silverberg MJ, Park LS, Crothers K, Justice AC. HIV infection, aging, and immune function: implications for cancer risk and prevention. Curr Opin Oncol 2012; 24:506–516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Franzetti M, Ricci E, Bonfanti P. The pattern of non-AIDS-defining cancers in the HIV population: epidemiology, risk factors and prognosis. A review. Curr HIV Res 2019. [DOI] [PubMed] [Google Scholar]
- 125.Sigel K, Makinson A, Thaler J. Lung cancer in persons with HIV. Curr Opin HIV AIDS 2017; 12:31–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Mdodo R, Frazier EL, Dube SR, Mattson CL, Sutton MY, Brooks JT, et al. Cigarette smoking prevalence among adults with HIV compared with the general adult population in the United States: cross-sectional surveys. Ann Intern Med 2015; 162:335–344. [DOI] [PubMed] [Google Scholar]
- 127.Engels EA, Brock MV, Chen J, Hooker CM, Gillison M, Moore RD. Elevated incidence of lung cancer among HIV-infected individuals. J Clin Oncol 2006; 24:1383–1388. [DOI] [PubMed] [Google Scholar]
- 128.Hernandez-Ramirez RU, Shiels MS, Dubrow R, Engels EA. Cancer risk in HIV-infected people in the USA from 1996 to 2012: a population-based, registry-linkage study. Lancet HIV 2017; 4:e495–e504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Yang Y, Dong J, Sun K, Zhao L, Zhao F, Wang L, et al. Obesity and incidence of lung cancer: a meta-analysis. Int J Cancer 2013; 132:1162–1169. [DOI] [PubMed] [Google Scholar]
- 130.Smith L, Brinton LA, Spitz MR, Lam TK, Park Y, Hollenbeck AR, et al. Body mass index and risk of lung cancer among never, former, and current smokers. J Natl Cancer Inst 2012; 104:778–789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Wu SH, Liu Z. Soy food consumption and lung cancer risk: a meta-analysis using a common measure across studies. Nutr Cancer 2013; 65:625–632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Wu QJ, Xie L, Zheng W, Vogtmann E, Li HL, Yang G, et al. Cruciferous vegetables consumption and the risk of female lung cancer: a prospective study and a meta-analysis. Ann Oncol 2013; 24:1918–1924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Song J, Su H, Wang BL, Zhou YY, Guo LL. Fish consumption and lung cancer risk: systematic review and meta-analysis. Nutr Cancer 2014; 66:539–549. [DOI] [PubMed] [Google Scholar]
- 134.Zhu H, Zhang S. Body mass index and lung cancer risk in never smokers: a meta-analysis. BMC Cancer 2018; 18:635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Duan P, Hu C, Quan C, Yi X, Zhou W, Yuan M, et al. Body mass index and risk of lung cancer: Systematic review and dose-response meta-analysis. Sci Rep 2015; 5:16938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Yang JJ, Yu D, Takata Y, Smith-Warner SA, Blot W, White E, et al. Dietary Fat Intake and Lung Cancer Risk: A Pooled Analysis. J Clin Oncol 2017; 35:3055–3064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Sun Y, Li Z, Li J, Li Z, Han J. A Healthy Dietary Pattern Reduces Lung Cancer Risk: A Systematic Review and Meta-Analysis. Nutrients 2016; 8:134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Bailey-Wilson JE, Amos CI, Pinney SM, Petersen GM, de Andrade M, Wiest JS, et al. A major lung cancer susceptibility locus maps to chromosome 6q23-25. Am J Hum Genet 2004; 75:460–474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Bosse Y, Amos CI. A Decade of GWAS Results in Lung Cancer. Cancer Epidemiol Biomarkers Prev 2018; 27:363–379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.McKay JD, Hung RJ, Han Y, Zong X, Carreras-Torres R, Christiani DC, et al. Large-scale association analysis identifies new lung cancer susceptibility loci and heterogeneity in genetic susceptibility across histological subtypes. Nat Genet 2017; 49:1126–1132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Chatterjee N, Shi J, Garcia-Closas M. Developing and evaluating polygenic risk prediction models for stratified disease prevention. Nat Rev Genet 2016; 17:392–406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Parkin DM, Bray F, Ferlay J, Pisani P. Global cancer statistics, 2002. CA Cancer J Clin 2005; 55:74–108. [DOI] [PubMed] [Google Scholar]
- 143.de Groot PM, Wu CC, Carter BW, Munden RF. The epidemiology of lung cancer. Transl Lung Cancer Res 2018; 7:220–233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.McCarthy WJ, Meza R, Jeon J, Moolgavkar SH. Chapter 6: Lung cancer in never smokers: epidemiology and risk prediction models. Risk Anal 2012; 32 Suppl 1:S69–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Rudin CM, Avila-Tang E, Harris CC, Herman JG, Hirsch FR, Pao W, et al. Lung cancer in never smokers: molecular profiles and therapeutic implications. Clin Cancer Res 2009; 15:5646–5661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.National Cancer Institute. Sherlock-lung: A Genomic Epidemiologic Study of Lung Cancer in Never Smokers. URL at: https://dceg.cancer.gov/research/cancer-types/lung/sherlock-lung-study Webpage accessed May 21, 2019.
- 147.Jamal A, Phillips E, Gentzke AS, Homa DM, Babb SD, King BA, et al. Current Cigarette Smoking Among Adults - United States, 2016. MMWR Morb Mortal Wkly Rep 2018; 67:53–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Halpern MT, Gillespie BW, Warner KE. Patterns of absolute risk of lung cancer mortality in former smokers. J Natl Cancer Inst 1993; 85:457–464. [DOI] [PubMed] [Google Scholar]
- 149.Schabath MB. Risk models to select high risk candidates for lung cancer screening. Ann Transl Med 2018; 6:65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Aberle DR, Adams AM, Berg CD, Black WC, Clapp JD, Fagerstrom RM, et al. Reduced lung-cancer mortality with low-dose computed tomographic screening. The New England journal of medicine 2011; 365:395–409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Schabath MB, Massion PP, Thompson ZJ, Eschrich SA, Balagurunathan Y, Goldof D, et al. Differences in Patient Outcomes of Prevalence, Interval, and Screen-Detected Lung Cancers in the CT Arm of the National Lung Screening Trial. PLoS One 2016; 11:e0159880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.U.S. Preventive Services Task Force. Final Update Summary: Lung Cancer: Screening. July 2015. URL at: https://www.uspreventiveservicestaskforce.org/Page/Document/UpdateSummaryFinal/lung-cancer-screening.
- 153.Centers for Medicare & Medicard Services. Decision Memo for Screening for Lung Cancer with Low Dose Computed Tomography (LDCT) (CAG-00439N). URL at: https://www.cms.gov/medicare-coverage-database/details/nca-decision-memo.aspx?NCAId=274.
- 154.Minnix JA, Karam-Hage M, Blalock JA, Cinciripini PM. The importance of incorporating smoking cessation into lung cancer screening. Transl Lung Cancer Res 2018; 7:272–280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Silva M, Pastorino U, Sverzellati N. Lung cancer screening with low-dose CT in Europe: strength and weakness of diverse independent screening trials. Clin Radiol 2017; 72:389–400. [DOI] [PubMed] [Google Scholar]
- 156.De Koning H, Van der Aalst C, Ten Haaf K, Oudkerk M. Effects of Volume CT Lung Cancer Screening: Mortality Results of the NELSON Randomised-Controlled Population Based Trial. Journal of Thoracic Oncology 2018; 13:S185–S185. [Google Scholar]
- 157.Pastorino U, Silva M, Sestini S, Sabia F, Boeri M, Cantarutti A, et al. Prolonged lung cancer screening reduced 10-year mortality in the MILD trial: new confirmation of lung cancer screening efficacy. Ann Oncol 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Becker N, Motsch E, Trotter A, Heussel CP, Dienemann H, Schnabel PA, et al. Lung cancer mortality reduction by LDCT screening-Results from the randomized German LUSI trial. Int J Cancer 2019. [DOI] [PubMed] [Google Scholar]