Abstract
Research shows that rats and humans on a high-fat diet (HFD) are less sensitive to satiety signals known to act via vagal afferent pathways. We hypothesize that HFD causes an upregulation of 2-pore domain potassium channels, resulting in hyperpolarization of nodose ganglia (NG) and decreased vagal response to satiety signals, which contribute to hyperphagia. We show that a 2-week HFD caused an upregulation of 2-pore domain TWIK-related spinal cord K+ (TRESK) and TWIK-related acid-sensitive K+ 1 (TASK1) channels by 330% ± 50% and 60% ± 20%, respectively, in NG. Patch-clamp studies of isolated NG neurons demonstrated a decrease in excitability. In vivo single-unit NG recordings showed that a 2-week HFD led to a 55% reduction in firing frequency in response to CCK-8 or leptin stimulation. NG electroporation with TRESK siRNA restored NG responsiveness to CCK-8 and leptin. Rats fed a 2-week HFD consumed ~40% more calories compared with controls. Silencing NG TRESK but not TASK1 channel expression in HFD-fed rats restored normal calorie consumption. In conclusion, HFD caused upregulation of TRESK channels, resulting in NG hyperpolarization and decreased vagal responsiveness to satiety signals. This finding provides a pharmacological target to prevent or treat HFD-induced hyperphagia.
Keywords: Endocrinology, Gastroenterology
Keywords: Homeostasis, Ion channels, Obesity
High fat diet upregulates the TRESK channel, resulting in nodose ganglia hyperpolarization and decreased vagal responsiveness to satiety signals that contributed to hyperphagia.
Introduction
Diet-induced obesity (DIO) and hyperphagia are common in Western countries; however, our understanding of the mechanisms underlying the development of these conditions remains unclear. Vagal afferent signaling is an important link between the gastrointestinal (GI) tract and centers in the central nervous system that control food intake and energy expenditure (1). Prior studies show that obese animals and humans exhibit abnormal satiety signaling (2). Vagal afferent sensitivity to GI hormones (cholecystokinin [CCK], bombesin, and serotonin) and mechanical stimulation is significantly reduced in high-fat diet–induced (HFD-induced) obese rodents in comparison with control diet–fed animals (3–9), suggesting abnormal vagal sensory functioning.
It has been demonstrated that 1 to 2 weeks of exposure to HFD was sufficient to impair the ability of CCK to inhibit gastric emptying and suppress food intake (6, 10), indicating that HFD feeding dysregulates vagal sensory functioning before the onset of obesity. Impaired vagal afferent responsiveness to both gastric satiety hormones (CCK and leptin) and mechanical stimulation raises the possibility that changes in electrophysiological properties may be the underlying mechanism responsible for impaired vagal responsiveness to a wide variety of satiety signals. Daly and colleagues (6) performed electrophysiological recordings from isolated nodose ganglion neurons and demonstrated that electrical excitability is significantly reduced in neurons from HFD-induced obese mice. This change in excitability is accompanied by a reduction in input resistance of the cell membrane resting potential.
Potassium channels are crucial determinants of neuronal excitability throughout the nervous system. We recently demonstrated that upregulation of 2-pore domain TWIK-related spinal cord K+ (TRESK or K2P18.1) channels is responsible for the impaired vagal sensory functioning in diabetic conditions (11). It is conceivable that similar abnormalities may explain reduced vagal responsiveness to satiety signals after chronic high-fat feeding. In this study, we hypothesized that upregulation of the 2-pore domain K+ (2PK) channel TRESK occurs following high-fat feeding and is responsible for abnormal vagal responsiveness to satiety signals. Using in vivo and in vitro electrophysiological and gene silencing techniques, we investigated the role of the TRESK and TWIK-related acid-sensitive K+ 1 (TASK1) channels in the malfunctioning of vagal nodose ganglion neurons’ responsiveness to satiety signals and the development of hyperphagia.
Results
TRESK is upregulated in vagal sensory ganglia.
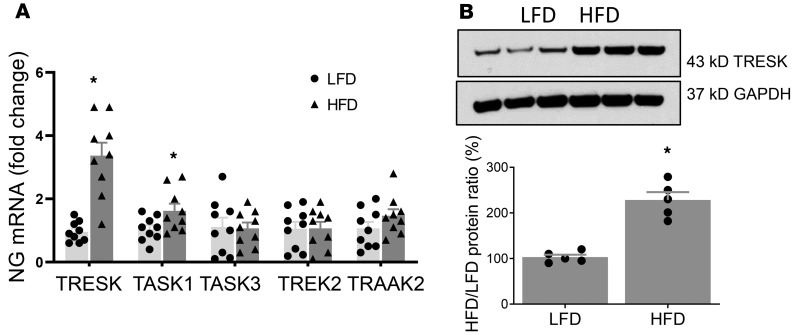
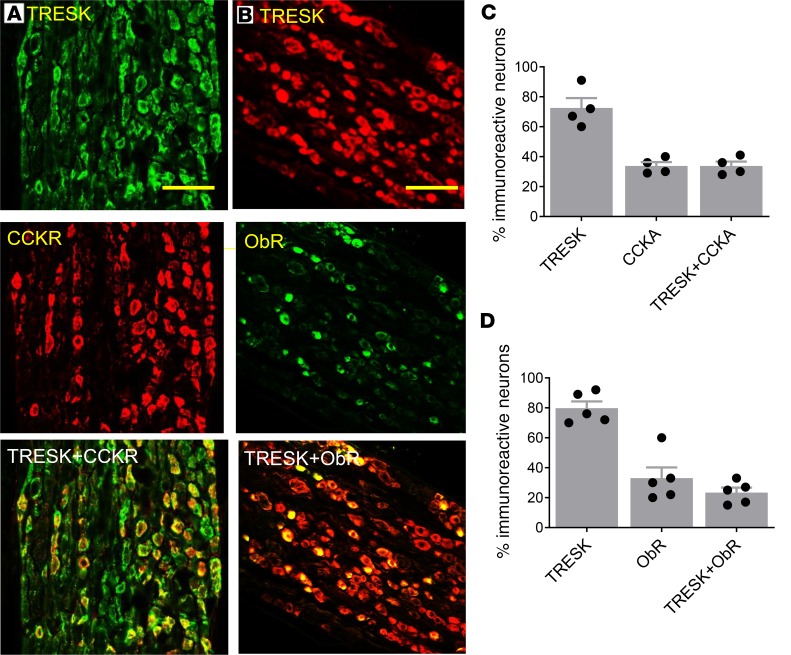
Our quantitative PCR studies showed that nodose ganglia (NG) neurons expressed TRESK, TWIK-related K+ channel type 2 (TREK2), TASK1 and -3, and TRAAK2 mRNA (Figure 1). Quantitation of mRNA of various 2PK channels, normalized to the reference HPRT mRNA ratio, revealed a significant increase in the expression of TRESK and TASK1 mRNA by 330% ± 50% and 60% ± 20% (P < 0.05, and n = 9 each group), respectively, while the levels of TASK3, TREK2, and TRAAK mRNA remained unchanged (Figure 1A, P < 0.05) in the vagal ganglia from HFD-fed rats when compared with low-fat diet–fed (LFD-fed) rats. Western blot analysis results showed that HFD feeding caused an increase in the level of TRESK protein by 227% ± 17% (Figure 1B; n = 5, and P < 0.05). We performed immunohistochemical staining of the vagal ganglia to demonstrate expression of the TRESK channel and to determine whether it colocalizes with the CCK-A receptor (CCK-AR). Our studies showed intense staining of TRESK and CCK-AR immunoreactivities in 72% ± 6% and 35% ± 6% of vagal neurons, respectively (Figure 2). Among the TRESK-positive neurons, 48% expressed CCK-AR and 31% expressed ObR. Conversely, all CCK-AR– and ObR–positive neurons expressed TRESK immunoreactivities. In separate studies, we showed that 24% of NG neurons expressed ObR and all NG neurons containing ObR expressed TRESK immunoreactivities. These immunocytochemistry data support the premise that TRESK channels may modulate the satiety actions of CCK and leptin.
Figure 1. Demonstration of increased levels of 2PK channels in vagal sensory ganglia following 2-week HFD.
(A) RT-PCR data showing TRESK, TASK1 and -3, TREK2, and TRAAK2 mRNA in vagal sensory ganglia after 2 weeks of HFD feeding when compared with LFD-fed rats. HPRT was used as a loading control. n = 9 in each group. *P < 0.05 vs. LFD. Data are represented as mean ± SEM. One-way ANOVA with Bonferroni’s test. (B) A representative immunoblot from the vagal sensory ganglia shows TRESK protein expression after 2 weeks of HFD feeding (n = 5, and P < 0.05 compared with 2 weeks of LFD); GAPDH was used as a loading control. Summary histogram shows significant upregulation of TRESK protein expression in vagal sensory ganglia as early as 2 weeks after HFD feeding when compared with LFD-fed group. Student’s t test. *P < 0.05 vs. LFD. HFD, high-fat diet; HPRT, hypoxanthine-guanine phosphoribosyltransferase; LFD, low-fat diet; RT-PCR, reverse transcription PCR; TASK, TWIK-related acid-sensitive K+ channel; TREK2, TWIK-related K+ channel type 2; TRESK, TWIK-related spinal cord K+ channel.
Figure 2. Localization of TRESK and CCK-AR and ObR immunoreactivities in the rat vagal sensory ganglia.
(A) TRESK (green, upper), CCK-AR (red, middle), and superimposed pictures show colocalization of TRESK and CCK-AR (yellow, lower) and in the vagal sensory ganglia. Scale bar: 100 µm. (B) TRESK (red, upper), ObR (green, middle), and superimposed pictures show colocalization of TRESK and ObR (yellow, lower) in the vagal sensory ganglia. Scale bar: 100 µm. (C) Summary bar graph showing distribution and colocalization of TRESK and CCK-AR immunoreactivities in rat vagal sensory ganglia. Data are represented as mean ± SEM. (D) Summary bar graph showing distribution and colocalization of TRESK and ObR immunoreactivities in rat vagal sensory ganglia. Data are represented as mean ± SEM. Student’s t test. CCKR, CCK-A receptor; ObR, leptin receptor.
TRESK siRNA treatment restores abnormal responses to CCK and leptin in HFD-fed rats.
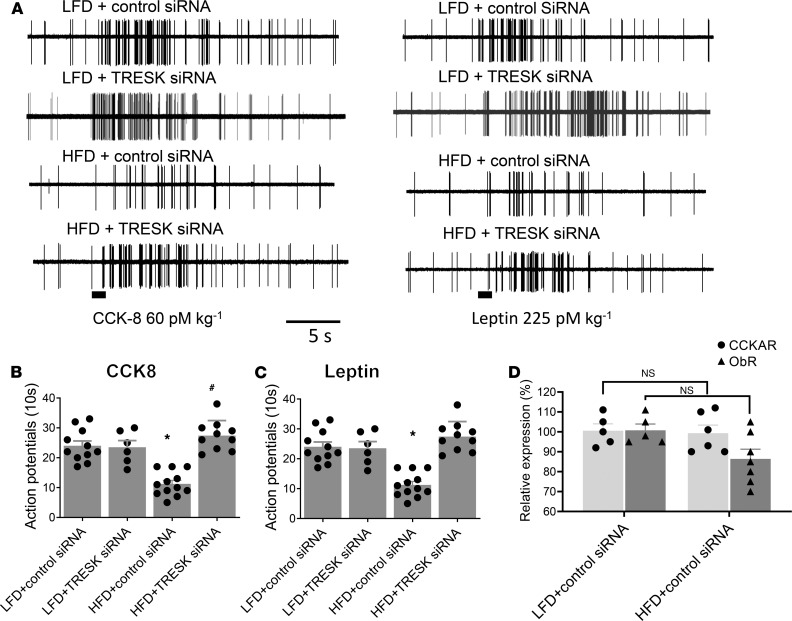
In vivo single-unit discharges of vagal primary afferent neurons innervating the GI tract were recorded from rat vagal sensory ganglia neurons as described previously (12). Only units that were activated by electrical stimulation of the subdiaphragmatic vagus nerve were included in the data analyses. Under basal conditions, all units recorded in rats given LFD + control siRNA or TRESK siRNA, HFD + control siRNA, or HFD + TRESK siRNA were either silent or displayed very low spontaneous activities (0–5 spikes/10 s). I.v. administration of CCK-8 (60 pmol/kg) caused the neuronal firing rates to increase from a basal rate of 1.1 ± 1 spike per 10 s to 24 ± 5 spikes per 10 s (n = 11 responders of 30 recorded, Figure 3, A and B) in LFD-fed rats transfected with control siRNA (data pooled). In contrast, after i.v. administration of CCK-8 (60 μg/kg) in HFD-fed rats whose vagal sensory ganglia were pretreated with control siRNA, the nodose neuron response rates were significantly dampened to 11.5 ± 3.5 spikes per 10 s (n = 12 responders of 32 recorded, and P < 0.05 compared with LFD group) (Figure 3, A and B). Silencing of the TRESK channel by electroporation of the right vagal sensory ganglia with TRESK siRNA restored the effect of CCK-8 in HFD-fed rats (27.5 ± 5 spikes per 10 s) (n = 10 responders of 30 recorded, and P < 0.05 compared with HFD) (Figure 3, A and B). Our quantitative PCR study data demonstrated that the levels of CCK-AR and ObR were not significantly different between the 2 groups of rats (LFD + control siRNA vs. HFD + control siRNA) (Figure 3D), indicating that the differences in CCK-8–generated neuronal firing rates were not due to changes in expression of CCK-ARs.
Figure 3. HFD feeding for 2 weeks reduces the frequency of neuronal firing in response to CCK-8 and leptin in vivo.
(A) Representative recordings of NG neuron responses to intra–superior pancreaticoduodenal artery infusions of CCK-8 (60 pmol/kg) and leptin (60 pmol/kg) in LFD-fed or HFD-fed rats and transfected with control siRNA or TRESK siRNA. Note that CCK-8 generated significantly fewer action potentials in HFD-fed rats compared with those fed an LFD. (B) Summary histograms showing single-unit discharges in response to CCK-8 in rats given an LFD and transfected with control siRNA (n = 11) or TRESK siRNA (n = 6), HFD + control siRNA (n = 12), and HFD treated with TRESK siRNA (n = 10). Data are represented as mean ± SEM. One-way ANOVA with Bonferroni’s test, *P < 0.05 vs. LFD + control siRNA; #P < 0.05 vs. HFD + control siRNA. (C) Summary histogram showing single-unit discharges in response to leptin in rats given an LFD and transfected with control siRNA (n = 11) and TRESK siRNA (n = 5), HFD (n = 12), and HFD treated with TRESK siRNA (n = 10). Data are represented as mean ± SEM. One-way ANOVA with Bonferroni’s test, *P < 0.05 vs. LFD + control siRNA; #P < 0.05 vs. HFD + control siRNA. (D) Summary histogram showing CCK-AR and ObR expression in vagal sensory ganglia from LFD- and HFD-fed rats were not significantly different. HPRT was used as a loading control. Data are represented as mean ± SEM. CCK-8, cholecystokinin-8.
Similar observations were made with leptin stimulation. In LFD + control siRNA rats, i.v. infusion of leptin (225 pmol/kg) stimulated neuronal firing from a basal rate of 1.5 ± 0.8 spikes per 10 s to 19.4 ± 3.1 spikes per 10 s (n = 6 of 30 recorded). In rats fed with 2 weeks of HFD + control siRNA, the response rates to leptin stimulation were reduced to 8.8 ± 2.6 spikes per 10 s (n = 6 of 30 recorded, and P < 0.05 compared to LFD + control siRNA group; Figure 3, A and C). Silencing of the TRESK channel with specific siRNA restored the responsiveness of the nodose neurons to stimulation to a level similar to that observed in the control LFD group (21 ± 4.3 spikes per 10 s; n = 6 of 32 recorded, and P < 0.05 compared with HFD + control siRNA) (Figure 3, A and C). Our quantitative PCR demonstrated that the levels of ObR were not significantly different between the 2 groups of rats (LFD and HFD), indicating that the differences in leptin-generated neuronal firing rates were not due to changes of expression of ObR (Figure 3D). These observations suggest that irrespective of the stimulants used, silencing TRESK in NG normalized the response to stimulation by CCK-8 or leptin in rats given an HFD. As controls, we showed that silencing TRESK channels in the NG of rats given LFD did not affect the NG responsiveness to CCK-8 or leptin stimulation (Figure 3, A–C).
Increased fat, not calories, in the diet is responsible for abnormal vagal sensory functioning.
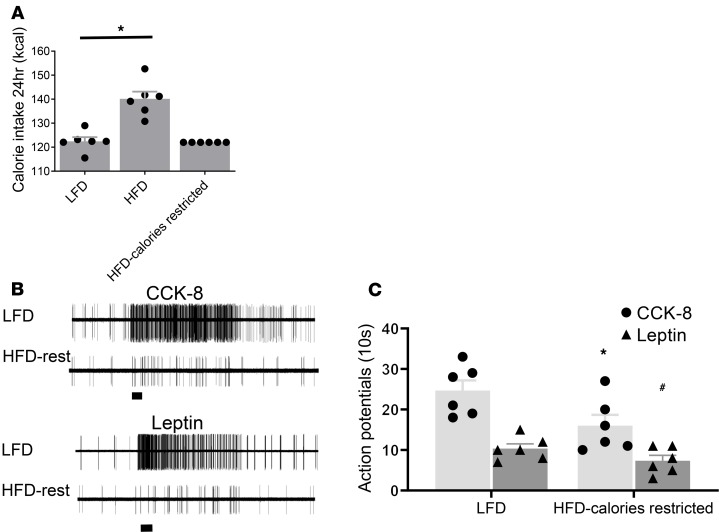
Our feeding studies showed that the HFD group consumed significantly more calories compared with the LFD group (144 ± 2.4 kcal vs. 122 ± 1.3 kcal, and n = 6) (P < 0.05) (Figure 4A). To demonstrate that it was increased fat and not calories that altered the vagal excitability, we conducted single-unit recordings on the nodose neurons from rats that received an HFD but within calories restricted to amounts similar to that consumed by the LFD group (122 kcal daily). Our electrophysiological recordings showed reduced vagal firings in response to CCK-8 and leptin stimulation (Figure 4, B and C) similar to the recordings obtained from NG of the HFD group without calorie restriction (Figure 3).
Figure 4. HFD calorie-restricted feeding for 2 weeks reduces the frequency of neuronal firing in response to CCK-8 and leptin in vivo.
(A) Summary bar graph showing 24 hours of cumulative calorie intake in LFD, HFD, and HFD calorie-restricted groups (n = 6 in each group). Data are represented as mean ± SEM. *P < 0.05 vs. LFD. (B) Representative recordings of NG neuron responses to intra–superior pancreaticoduodenal artery infusions of CCK-8 (60 pmol/kg) and leptin (60 pmol/kg) in LFD-fed or HFD calorie-restricted–fed rats. (C) Summary histogram showing single-unit discharges in response to CCK-8 and leptin in rats given an LFD (n = 6) and HFD calorie-restricted diet, respectively (n = 6). Data are represented as mean ± SEM. Student’s t test. *P < 0.05 vs. LFD stimulated with CCK-8; #P < 0.05 vs. LFD stimulated with leptin.
Patch-clamp studies show reduced excitability in vagal sensory neurons from HFD-fed rats is reversed by silencing TRESK but not TASK channels in vagal sensory ganglia.
We performed whole-cell patch-clamp recordings in isolated vagal ganglion neurons to examine the active and passive membrane properties of randomly selected or retrograde traced duodenum-projecting nodose neurons from LFD- or HFD-fed rats. Our data showed that duodenum-projecting neurons were not significantly different from randomly selected neurons. Compared to LFD + control siRNA (n = 14), HFD + control siRNA (n = 13) induced a decrease in neuronal input resistance (295 ± 7 MΩ vs. 355 ± 10 MΩ, and P < 0.05), a significant increase in rheobase (107.4 ± 20 pA vs. 51.6 ± 12 pA, and P < 0.05), and a reduction in the number of action potentials at twice rheobase (3 ± 1.2 vs. 10 ± 3, and P < 0.05) (Figure 5). These data indicate a global decrease in the excitability of vagal sensory neurons of rats given an HFD. Our electrophysiological data also demonstrated that treatment of vagal sensory ganglia with TRESK siRNA (n = 12) but not TASK1 siRNA (n = 11) reversed the HFD-induced changes on the membrane properties of vagal sensory ganglia neurons (Figure 5).
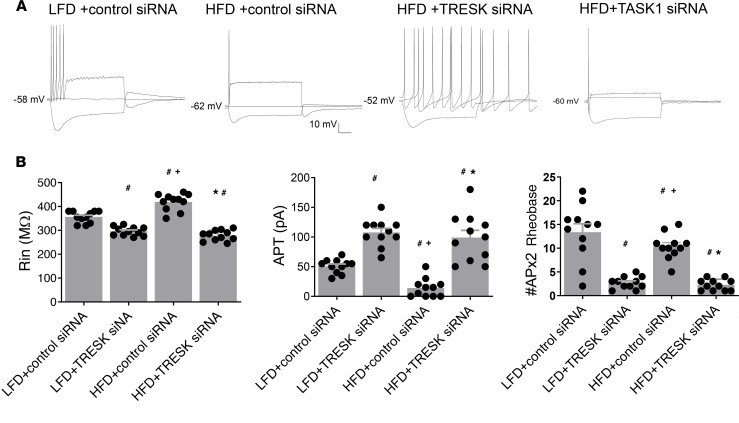
Figure 5. Effects of TRESK and TASK1 siRNA transfection on basic membrane properties of vagal sensory neurons from HFD-fed rats.
(A) Representative current-clamp recordings from vagal sensory neurons obtained from LFD or HFD + control siRNA and HFD + TRESK siRNA or HFD + TASK1 siRNA groups of rats. Membrane voltage responses were generated in response to –100 pA, 0 pA, and double rheobase amplitude current pulse of 500-ms duration. (B) Summary histograms illustrate significantly reduced neuronal input resistance (Rin), increased amplitude of the rheobase (pA), and reduced number of APs discharged by double amplitude current pulse in nodose neurons from 2-week HFD + control siRNA (n = 13) rats compared with those given an LFD + control siRNA (n = 14). These data show that pretreatment of vagal sensory ganglia with TRESK siRNA (n = 12) but not TASK1 siRNA (n = 11) reversed the effect of HFD on the excitability of sensory neurons. Data are represented as mean ± SEM. #P < 0.05 vs. LFD + control siRNA; +P < 0.05 vs. HFD + control siRNA; *P < 0.05 vs. HFD + TRESK siRNA, 1-way ANOVA with Bonferroni’s test. AP, action potential.
Silencing TRESK channels in the vagal sensory ganglia abolishes HFD orexigenic actions on feeding.
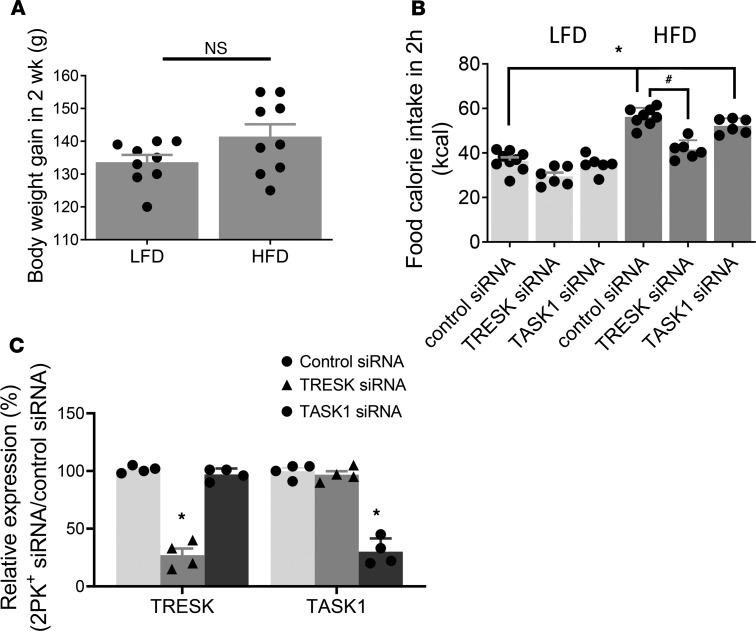
Rats fed a 2-week HFD (n = 18) did not gain more weight than those fed an LFD (n = 15) (34% ± 5.3% vs. 32% ± 3.2%, and P > 0.05), when compared to the respective initial body weights (Figure 6A). But these rats were not obese. To examine whether upregulation of TRESK and TASK1 channels in the vagal NG contribute to hyperphagia observed in rats given an HFD, we performed feeding studies in the following groups of rats after a 12-hour fast: (a) LFD rats injected with control siRNA (n = 6) or TRESK siRNA (n = 6) or TASK1 siRNA (n = 6) and (b) HFD rats injected with control siRNA (n = 6), TRESK siRNA (n = 6), or TASK1 siRNA (n = 6). Our data showed that, compared with LFD-fed (treated with control siRNA), HFD-fed (treated with control siRNA) rats consumed significantly more calories within 2 hours after 12 hours of fasting (56.1 ± 1.9 kcal/2 hours vs. 36.7 ± 2.3 kcal/2 hours, and P < 0.05 vs. LFD; Figure 6B). Silencing TRESK expression (25%–45% of control siRNA, Figure 6C) but not TASK1 channel expression in vagal sensory ganglia of HFD-fed rats reduced calorie intake to levels similar to those observed in LFD-fed rats (P < 0.05 vs. HFD) (Figure 6B). These data provide direct evidence that upregulation of TRESK channels in vagal sensory ganglia contributes to hyperphagia following 2-week HFD feeding.
Figure 6. Silencing the TRESK channel but not TASK1 channel in vagal sensory ganglia abolishes the orexigenic effect of HFD on food intake.
(A) The effect of 2-week LFD and HFD feeding on body weight. Data are represented as mean ± SEM. Student’s t test. (B) The effect of control (n = 9) and TRESK (n = 6) and TASK1 (n = 6) channel silencing in LFD- and HFD-fed rats on cumulative food intake. Data are represented as mean ± SEM. *P < 0.05 vs. LFD treated with control siRNA; #P < 0.05 vs. HFD whose vagal ganglia were treated with control and TRESK and TASK1 siRNA, 1-way ANOVA with Bonferroni’s test. (C) PCR data showed decreased TRESK and TASK1 mRNA on day 5 after siRNA injection in vagal ganglia of HFD-fed rats. HPRT gene was used as a loading control. Data are represented as mean ± SEM. *P < 0.05 compared with control siRNA. One-way ANOVA with Bonferroni’s test.
Discussion
We have shown that enhanced expression of TRESK and to a lesser extent TASK1 channels occurred in the NG of rats given an HFD. These were accompanied by reduced vagal excitability and may contribute to hyperphagia in animals ingesting HFDs. These changes occurred as early as 2 weeks of HFD feeding. Silencing the expression of TRESK but not TASK1 gene expression in the NG reverted the electrophysiological changes on the NG evoked by the HFD and normalized the feeding behavior of rats given an HFD. For the first time to our knowledge, we demonstrated the following: (a) upregulation of TRESK and TASK1 channels occurred in NG of rats after 2 weeks of HFD; (b) in vivo single-unit recordings of nodose neurons showed that HFD decreased responsiveness to satiety peptides, such as CCK-8 and leptin; (c) in vitro patch-clamp studies of nodose neurons innervating the GI tract showed a global decrease in excitability; (d) silencing of TRESK but not TASK1 channels normalized the electrophysiological properties of nodose neurons of rats given an HFD; and (e) in vivo silencing of the TRESK channel restored NG responsiveness to CCK and leptin stimulation and prevented hyperphagia induced by HFD.
Prior studies in obese rodents after prolonged high-fat feeding showed reduced sensitivity to satiety signals (13–15). However, it is unclear whether this is a cause or consequence of obesity. Hyperphagia in obese rats is partially related to defective central satiety signaling, including permanently disrupted hypothalamic leptin projections (16) and enhanced orexigenic peptide expression that overrides or impinges on the gut and orexigenic signaling to influence food intake. Other studies focused on adipose tissue as a source of obesity-related inflammation leading to insulin resistance. More recent studies showed impaired satiety control occurred after prolonged HFD feeding, leading to hyperphagia and obesity (13–16). In this study, we showed rats given HFD exhibited reduced sensitivity to satiety signals acting via vagal afferents within 2 weeks. These rats showed no significant increase in weight gain versus control rats given LFD. Thus, it is an ideal model to investigate HFD-induced hyperphagia because it does not have confounding factors related to obesity. Our studies showed a blunted response to satiety signals after 2 weeks of HFD feeding, indicating that HFD-induced hyperphagia is not a consequence of obesity. Furthermore, we observed an impaired NG response to different satiety peptides CCK and leptin, suggesting vagal dysfunction may be secondary to altered electrophysiological properties of the NG.
2PK channels play an important role in setting the resting membrane potential and excitability of neurons (17, 18). Regulation of 2PK channels by neurotransmitters and second messengers is essential for neuronal functioning (19). The mammalian 2PK channel family, which consists of 15 subunits, is divided into 6 subfamilies: TWIK, TASK, TREK, TALK, THIK, and TRESK (17, 19). They exhibit diverse electrophysiological and pharmacological properties and may mediate different physiological functions. Recently, Park et al. (20) showed that the reduction in vagal afferent excitability in obesity is due in part to an increase of resting (leak) K+ conductance. Quantitative PCR demonstrated upregulation of TASK. Voltage-clamp experiments showed that the increased leak K+ channel current was reduced by chemical inhibitors of TASK1 and TASK3, leading the authors to conclude that TASK channels may account for the impairment of satiety signaling in DIO (20). However, this claim was not confirmed by feeding studies. Furthermore, the inhibitors for TASK1 and -3 are not specific. Our current study demonstrated that rat NG expressed TRESK, TREK2, TRAAK, and TASK1 and -3 mRNA, similar to the studies by Cadaveira et al. (21) and Zhao et al. (22). We further showed that high-fat feeding for 2 weeks caused a modest increase in TASK1 and a marked upregulation of TRESK in the NG. Cadaveira and colleagues reported that TRESK and TREK1 are the main functional 2PK channel subunits contributing to “leak” conductance in vagal sensory neurons (21).
To date, no specific 2PK channel inhibitors are available. To provide direct evidence that the enhanced TRESK channel expression in the NG is mainly responsible for the impaired response of the NG to CCK and leptin stimulation, we silenced TRESK and TASK1 gene expression by injecting NG with TRESK and TASK1 siRNA. We administered specific siRNAs with a cell membrane–permeabilizing agent, i-Fect, to silence the membrane channel expression in the NG. By silencing TRESK and TASK1 channel expression in vivo without affecting gene expression in other systems, we could specifically show that the TRESK channel and not the TASK1 channel in the NG is mainly responsible for reduced NG neuron excitability following a 2-week HFD. We validated successful (60%–80%) knockdown of TRESK and TASK1 gene expression with gene expression studies. Our studies showed that rats given an HFD with TRESK siRNA exhibited normal response to CCK and leptin stimulation. On the other hand, silencing TRESK expression in the NG in the control rats did not affect the vagal response to CCK-8 or leptin. Furthermore, silencing the TRESK but not TASK1 channel in the NG of rats given an HFD restored normal feeding behavior. To ensure that the defective vagal firing in response to CCK-8 and leptin in the HFD group was due to increased fat and not increased calories in the diet, we showed markedly reduced vagal firings in response to CCK-8 and leptin stimulation in rats given an HFD with restricted calories (Figure 4, B and C) similar to recordings obtained from NG of the HFD group without calorie restriction.
In conclusion, our studies provide a molecular explanation for impaired vagally mediated satiety signaling in rats given an HFD. Following 2 weeks of high-fat feeding, there was a significant upregulation of TRESK and a modest increase in TASK1 channels in the NG. Silencing studies indicate that the upregulation by TRESK channels is mainly responsible for a global decrease in excitability of vagal sensory neurons, which in turn dampens the response to satiety signals, such as CCK and leptin. These plastic changes in vagal sensory signaling occur as early as 2 weeks of high-fat feeding, preceding the development of obesity, and result in hyperphagia. Understanding the abnormalities in NG signaling may provide therapeutic targets to prevent or treat hyperphagia in patients on HFDs.
Methods
Animals.
Male Sprague-Dawley rats (170–190 g) were purchased from Harlan Laboratories and allowed to acclimatize to the local animal facility for 1 week before experimentation. Animals were separated into 2 groups and were fed either an HFD (60% kcal from fat; 5.21 kcal/g; obtained from Research Diets Inc., D12492) or a standard laboratory rat diet/LFD (control, 10% kcal from fat; 3.85 kcal/g; Lab Diet, catalog 5LOD) for 2 weeks. To ensure that the defective vagal firing was due to increased fat, not increased calories, in the diet, we performed additional experiments in which we restricted the calorie intake to match the calories in the LFD group while maintaining amounts of fat similar to the HFD group. Rats were housed at 22°C under a 12-hour light/12-hour dark cycle, with lights on at 0600 hours and off at 1800 hours, with free access to food and water.
Retrograde tracing of NG.
Rats were deeply anesthetized with a 4% mixture of isoflurane in air, as described previously (11). After laparotomy, crystals of the retrograde tracer 1,1′-dioctadecyl-3,3,3′,3′-tetramethylindocarbocyanine (DiI; Molecular Probes, Invitrogen) were applied to the duodenum. To confine the dye to the site of application, crystals of DiI were embedded in a fast-hardening epoxy resin (Tra-Con Inc.) that was allowed to harden for approximately 5 minutes. The surgical area was then washed with warm sterile saline, and the wound was closed with 4-0 nylon sutures. The animal was allowed to recover for 10 to 15 days before being killed for NG dissection.
Isolation and culture of vagal sensory neurons.
Rats were killed by CO2 asphyxiation, and vagal sensory ganglia were dissected, sliced into small fragments, and placed in a 1.5-ml centrifuge tube containing digestion buffer (dispase II; Life Science, Roche Diagnostics) and collagenase I (Invitrogen, Invitrogen) (1 mg/ml). After incubation at 37°C for 60 minutes, cells were dispersed by gentle trituration through Pasteur pipettes and washed in DMEM (Invitrogen). The cells were resuspended in DMEM containing 10% fetal bovine serum (FBS, Invitrogen) and plated onto poly-l-lysine–coated (100 μg/ml) coverslips for 30 minutes and cultured in DMEM with 10% FBS, with penicillin and streptomycin, at 37°C. Primary neuronal cultures were maintained in culture for 16 to 30 hours at 37°C before recording.
Whole-cell patch-clamp electrophysiology.
Coverslips containing vagal sensory ganglion neurons were placed in a recording chamber and mounted on an inverted microscope (Nikon Ti, Mager Scientific). Our pilot studies and data from the literature (20) suggested that DIO generates similar changes in vagal sensory neurons regardless of their anatomical projection. Hence, NG neurons were randomly selected for recording. All measurements were made in a physiological saline solution composed of (in mM) 140 NaCl, 5 KCl, 2 CaCl2, 1 MgCl2, 5 glucose, and 10 HEPES, with pH adjusted to 7.3 with NaOH (13). Whole-cell recordings were made using borosilicate glass electrodes with resistance between 3 and 6 MΩ (A-M Systems) backfilled with a saline solution composed of (in mM) 130 potassium gluconate, 10 HEPES, 10 EGTA, 1.0 MgCl2, 2.5 CaCl2, 1.0 ATP, and 0.3 GTP, with pH adjusted to 7.3 with KOH. Current and voltage recordings were acquired from discrete, isolated vagal sensory neurons using an Axopatch 200B Amplifier (Molecular Devices) filtered at 2 kHz using a 4-pole, low-pass Bessel filter and digitized with a Digidata 1440A A/D converter (Molecular Devices), then stored and analyzed on a personal computer running pCLAMP 9 software (Molecular Devices). After obtaining a whole-cell formation, membrane access series resistance was compensated 75%–80%. The input resistance was calculated by measuring the neuron membrane potential displacement from –60 mV in response to a –10-pA current pulse. The rheobase was determined as the lowest current leading to an action potential.
Western blotting.
Vagal ganglia were harvested in accordance with a technique described previously (12). After washing, lysis buffer (30 μl) with protease inhibitor (Life Science, Roche Diagnostics) was added for 15 minutes at 4°C. The lysate was centrifuged at 14,000 g for 10 minutes. Protein samples were then run on 10% Ready Gel Tris-HCl (Bio-Rad Laboratories) for 1.5 hours at 80 V. Proteins were then transferred to PVDF membranes for 1 hour at 80 V. The membranes were blocked with StartingBlockT20 blocking buffer (Invitrogen) at room temperature, probed with primary antibodies against TRESK (APC-122, Alomone Labs) at a dilution of 1:1000 at 4°C overnight, and then washed in Tris-buffered saline for 1 hour. The membranes were probed with corresponding horseradish peroxidase–conjugated secondary antibodies (Invitrogen, catalog 31464 )at a dilution of 1:2000. The resulting bands were scanned with an Epson Stylus Photo R2400 (Epson Corp.) and analyzed using ImageJ (NIH).
NG quantitative RT-PCR.
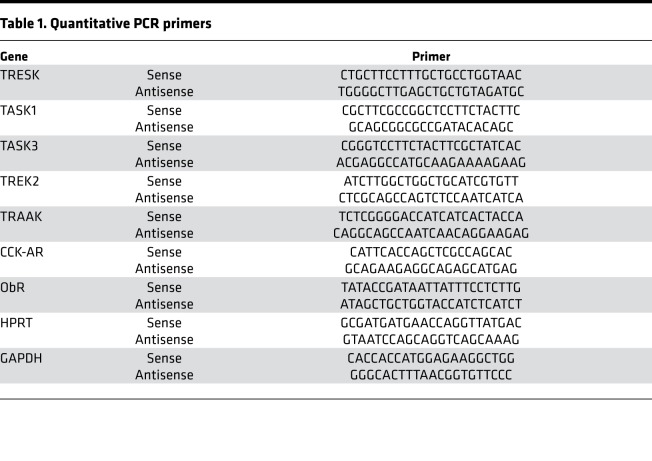
NG were removed bilaterally from rats from all experimental groups. Total RNA was extracted using an RNeasy Micro Kit (Qiagen) according to the manufacturer’s instructions. RNA was quantified by measuring the absorbance at 260 nm (A260) using a NanoDrop 2000c spectrophotometer (Thermo Fisher Scientific), and RNA purity was estimated via the 260/280 absorbance ratio. Total RNA (2–4 μg) was transcribed into cDNA using oligo-dT primers and superscript II (Invitrogen). Quantitative RT-PCR was performed using Bio-Rad CFX-Connect Real Time System and SYBR Green Supermix. Primers targeting ObR, TRESK, TASK1 and -3, TREK2, TRAAK, CCK-AR, and HPRT are listed in Table 1. All primer pairs span 1 or more introns. The quantitative PCR conditions were 95°C for 5 minutes, 1 cycle; 95°C for 10 seconds, 60°C for 45 seconds, 40 cycles. A melt curve was obtained to confirm the specificity of the products.
Table 1. Quantitative PCR primers.
RT-PCR was carried out using the GoTaq DNA polymerase (Promega) with the following conditions: 95°C for 3 minutes, 1 cycle; 95°C for 30 seconds, 55°C for 30 seconds, 72°C for 1 minute, 38 cycles; 72°C for 10 minutes, 1 cycle. Products were visualized using 3% agarose gel electrophoresis with ethidium bromide staining. Relative RNA levels were calculated using comparative computed tomography. Quantitative data were expressed as mean ± SEM.
Immunocytochemistry.
Immunocytochemistry studies to localize TRESK (APC-122, Alomone Labs) and CCK-AR (sc-270029, Cell Signaling Technology) were performed on vagal sensory ganglia as described previously (12). Following anesthesia with urethane, a transcardial perfusion was performed with ice-cold PBS and subsequently with fixative 4% paraformaldehyde, 0.2% picric acid, and 0.35% glutaraldehyde in phosphate buffer (0.1 mol/L, pH 7.4). The vagal sensory ganglia were removed and placed in the same fixative for 2 hours at room temperature and then in 25% sucrose in PBS (0.1 mol/L) overnight at 4°C. The ganglia were cut into 5-μm longitudinal sections using a precision cryostat (Leica Microsystems). Sections of the vagal ganglia were incubated in 5% normal donkey serum in PBS for 1 hour at room temperature. Staining was performed using the primary antibodies against TRESK and CCK-AR or ObR (sc-8325, Santa Cruz Biotechnology) at a dilution of 1:200 in PBS containing 2% normal donkey serum, 0.3% Triton X-100, and 0.1% sodium azide overnight at room temperature; washed in PBS; and then exposed for 1 hour to species-specific Alexa Fluor 488–conjugated (Molecular Probes, Invitrogen) and Cy3-conjugated secondary antibodies (Jackson ImmunoResearch Laboratories) and AMCA streptavidin (Vector Laboratories) diluted in PBS containing 0.3% Triton X-100 (dilution 1:200). The slides were examined using the ×20 objective with a BX51 epifluorescence microscope (Olympus) equipped with a digital camera. The images were analyzed in bright-field light and in fluorescent light using filter combinations that enabled separate visualizations of multiple fluorophores.
In duplex-labeled ganglia, we counted the neuronal profiles exhibiting neuronal profiles that could be clearly identified, with the visible nucleus profile obtained in bright-field illumination and fluorescent light. Five sections per ganglion were counted, the sections being separated by a minimum distance of 80 μm. Data were included in the bar graph (Figure 2) representing the percentage of cell profiles expressing green, red, or both green and red staining.
In vivo silencing.
TRESK (AM16708) and TASK1 (AM16708) siRNA were purchased from Invitrogen, and random siRNA (sc-37007) was purchased from Santa Cruz Biotechnology. The pEGFP-N1 vector (BD 6085-1), which encodes the Aequorea victoria GFP, was purchased from BD Biosciences. The in vivo electroporation was performed as described previously (23). Each rat was anesthetized and placed in a custom-made surgical plate. Body temperature was maintained at 37 ± 1°C with a homeothermic blanket system. The vagal NG were exposed by way of a ventral approach. The incision was made from the midline of the neck. With the use of a surgical operating microscope, the caudal end of the ganglion with the attached vagus nerve was separated from the adjacent cervical sympathetic trunk and carotid artery. The vagus ganglion was carefully isolated, and the area was moistened with saline. A piece of filter paper soaked with protease (type XIV, 0.3 mg/ml) was applied to the ganglion for 15 minutes. A beveled glass micropipette with tip diameter 35–45 μm (Clunbury Scientific) was filled with a 5:1 mixture of TRESK siRNA, TASK siRNA, or control siRNA (14 µl of 100 µM concentration) with i-Fect (Neuromics Edina). The micropipette, connected to a nanopump (PV830 Pneumatic PicoPump; World Precision Instruments), was guided by a micromanipulator through a small incision on the surface of the vagal sensory ganglion. siRNAs and i-Fect mixture were then injected into the left and right vagal ganglia (20 nl each). The micropipette was left in the ganglion for 10 minutes and then slowly withdrawn. A pair of stainless steel electrodes was placed on the ganglion 15 minutes after the injection. The gap between the electrodes was fixed at 2 mm. Square-wave electric pulses were delivered by an isolated pulse stimulator (Model 2100; A-M Systems). A train of square-wave pulses with a pulse duration of 20 ms was delivered at 50 V/cm at a frequency of 1 Hz, followed by the same stimulation with the opposite polarity.
Transfection efficiency was assessed by measuring GFP expression, specific protein immunoreactivity, mRNA expression, and protein expression. GFP expression, which is a novel genetic reporter system, was measured using fluorescence microscopy with excitation at 488 nm. Because the specific target siRNA construct was packaged with GFP reporter gene, the distribution of siRNA expression and GFP expression overlap.
Our previous study demonstrated that transfection of siRNA into neurons has a maximal effect 3– 6 days after transfection, with silencing lasting up to 2 weeks (11, 12). Feeding studies and single-unit recordings were performed 5– 6 days after electroporation. Only fully recovered animals were included in the study. Our preliminary studies showed that rats that do have surgery and those with control siRNA injected into the NG consumed similar amounts of food, indicating that surgery alone does not affect the feeding behavior.
Recording of single-unit vagal sensory neuronal activity.
Rats were anesthetized with a mixture of xylazine and ketamine (13 mg/kg and 87 mg/kg body weight, respectively). Recording of single-unit vagal sensory activity was performed as described previously by Li et al. (24). Supplemental doses of the anesthetic agents were administered as needed to maintain a deep level of anesthesia and muscle relaxation. The rats were ventilated with a respirator, and a tracheal tube permitted artificial ventilation with room air (75–85 strokes/min; tidal volume 3.5–4.0 cm3). Rats were placed in a small-animal stereotaxic frame (David Kopf Instruments). Body temperature was maintained with a special heating pad. The right vagal sensory ganglion was exposed by a short dorsal approach. The beveled glass micropipette filled with 1.0 M KCl was lowered into the vagal ganglion. A reference electrode was placed on a skin incision near the recording electrode. Only GI C-fibers were recorded. These fibers were identified according to the following parameters measured in response to electrical stimulation of the subdiaphragmatic branch of the vagus nerve: latency (60–80 ms), conduction distance between stimulating electrode and NG (0.06 m), and conduction velocity (<1.0 m/s). Neuronal discharges recorded were amplified by a high-input impedance preamplifier (A-M Systems), monitored with an oscilloscope and audio monitor and displayed, and then stored on a computer using Axon tape software (Molecular Devices).
Food intake studies and effects of electroporation with TRESK siRNA.
Before starting the food intake studies, rats were randomly divided into 3 groups of 5 rats each and the right and left vagal ganglions were transfected with random siRNA (sc-37007, Santa Cruz Biotechnology) and TASK1 (AM16708) or TRESK siRNA (AM16708) (both from Invitrogen). Rats were housed individually and allowed to acclimate in the metabolic cages for at least 5 days before feeding experiments. Feeding studies were initiated 5 days after transfection with siRNA. Before food intake experiments, rats were 12 hours fasted but with free access to water. The feeding study was initiated at 0900 hours. Rats were given weighed food and their cumulative food intake was recorded at 1-hour intervals over 2 hours, as described previously (12).
Statistics.
All values are expressed as mean ± SEM. One-way ANOVA with Bonferroni’s correction for multiple comparisons was used to compare more than 2 groups, and unpaired 2-tailed Student’s t test was used to compare 2 groups. P < 0.05 was considered significant. Data management and statistical analyses were performed using Origin 9 (Originlab) and Microsoft Office Excel.
Study approval.
All animal procedures were performed in accordance with the NIH guidelines and with the approval of the University Committee on Use and Care of Animals at the University of Michigan.
Author contributions
CO supervised the project and obtained grant support. GG performed patch-clamp studies and relevant analysis. XW performed single-unit recording and relevant analysis. JG performed Western blotting studies and relevant analysis. JL performed PCR studies and relevant analysis. SZ performed genetic silencing and feeding studies and relevant analysis.
Acknowledgments
This work was supported by the National Institute of Diabetes and Digestive and Kidney Diseases grants R01-DK048419 (to CO) and P30-DK34933 (to CO).
Version 1. 09/05/2019
Electronic publication
Footnotes
Conflict of interest: The authors have declared that no conflict of interest exists.
Copyright: © 2019, American Society for Clinical Investigation.
Reference information: JCI Insight. 2019;4(17):e130402.https://doi.org/10.1172/jci.insight.130402.
Contributor Information
Gintautas Grabauskas, Email: gintas@med.umich.edu.
Xiaoyin Wu, Email: wxy@med.umich.edu.
Jun Gao, Email: gaojun@med.umich.edu.
Chung Owyang, Email: Cowyang@umich.edu.
References
- 1.Grabauskas G, Owyang C. Plasticity of vagal afferent signaling in the gut. Medicina (Kaunas) 2017;53(2):73–84. doi: 10.1016/j.medici.2017.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Geliebter A. Gastric distension and gastric capacity in relation to food intake in humans. Physiol Behav. 1988;44(4-5):665–668. doi: 10.1016/0031-9384(88)90333-2. [DOI] [PubMed] [Google Scholar]
- 3.Covasa M, Ritter RC. Rats maintained on high-fat diets exhibit reduced satiety in response to CCK and bombesin. Peptides. 1998;19(8):1407–1415. doi: 10.1016/S0196-9781(98)00096-5. [DOI] [PubMed] [Google Scholar]
- 4.Covasa M, Ritter RC. Reduced sensitivity to the satiation effect of intestinal oleate in rats adapted to high-fat diet. Am J Physiol. 1999;277(1):R279–R285. doi: 10.1152/ajpregu.1999.277.1.R279. [DOI] [PubMed] [Google Scholar]
- 5.Covasa M, Grahn J, Ritter RC. High fat maintenance diet attenuates hindbrain neuronal response to CCK. Regul Pept. 2000;86(1-3):83–88. doi: 10.1016/S0167-0115(99)00084-1. [DOI] [PubMed] [Google Scholar]
- 6.Daly DM, Park SJ, Valinsky WC, Beyak MJ. Impaired intestinal afferent nerve satiety signalling and vagal afferent excitability in diet induced obesity in the mouse. J Physiol (Lond) 2011;589(pt 11):2857–2870. doi: 10.1113/jphysiol.2010.204594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.de Lartigue G, Barbier de la Serre C, Espero E, Lee J, Raybould HE. Diet-induced obesity leads to the development of leptin resistance in vagal afferent neurons. Am J Physiol Endocrinol Metab. 2011;301(1):E187–E195. doi: 10.1152/ajpendo.00056.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.de Lartigue G, Barbier de la Serre C, Espero E, Lee J, Raybould HE. Leptin resistance in vagal afferent neurons inhibits cholecystokinin signaling and satiation in diet induced obese rats. PLoS One. 2012;7(3):e32967. doi: 10.1371/journal.pone.0032967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kentish S, et al. Diet-induced adaptation of vagal afferent function. J Physiol (Lond) 2012;590(1):209–221. doi: 10.1113/jphysiol.2011.222158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Swartz TD, Duca FA, Covasa M. Differential feeding behavior and neuronal responses to CCK in obesity-prone and -resistant rats. Brain Res. 2010;1308:79–86. doi: 10.1016/j.brainres.2009.10.045. [DOI] [PubMed] [Google Scholar]
- 11.Grabauskas G, Wu X, Song I, Zhou SY, Lanigan T, Owyang C. Increased activation of the TRESK K+ mediates vago-vagal reflex malfunction in diabetic rats. Gastroenterology. 2016;151(5):910–922.e7. doi: 10.1053/j.gastro.2016.07.029. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 12.Grabauskas G, et al. KATP channels in the nodose ganglia mediate the orexigenic actions of ghrelin. J Physiol (Lond) 2015;593(17):3973–3989. doi: 10.1113/JP270788. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 13.Savastano DM, Covasa M. Adaptation to a high-fat diet leads to hyperphagia and diminished sensitivity to cholecystokinin in rats. J Nutr. 2005;135(8):1953–1959. doi: 10.1093/jn/135.8.1953. [DOI] [PubMed] [Google Scholar]
- 14.Covasa M. Deficits in gastrointestinal responses controlling food intake and body weight. Am J Physiol Regul Integr Comp Physiol. 2010;299(6):R1423–R1439. doi: 10.1152/ajpregu.00126.2010. [DOI] [PubMed] [Google Scholar]
- 15.Woods SC, et al. Consumption of a high-fat diet alters the homeostatic regulation of energy balance. Physiol Behav. 2004;83(4):573–578. doi: 10.1016/j.physbeh.2004.07.026. [DOI] [PubMed] [Google Scholar]
- 16.Bouret SG, Gorski JN, Patterson CM, Chen S, Levin BE, Simerly RB. Hypothalamic neural projections are permanently disrupted in diet-induced obese rats. Cell Metab. 2008;7(2):179–185. doi: 10.1016/j.cmet.2007.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Goldstein SA, Bockenhauer D, O’Kelly I, Zilberberg N. Potassium leak channels and the KCNK family of two-P-domain subunits. Nat Rev Neurosci. 2001;2(3):175–184. doi: 10.1038/35058574. [DOI] [PubMed] [Google Scholar]
- 18.Kang D, Kim D. TREK-2 (K2P10.1) and TRESK (K2P18.1) are major background K+ channels in dorsal root ganglion neurons. Am J Physiol, Cell Physiol. 2006;291(1):C138–C146. doi: 10.1152/ajpcell.00629.2005. [DOI] [PubMed] [Google Scholar]
- 19.Enyedi P, Czirják G. Molecular background of leak K+ currents: two-pore domain potassium channels. Physiol Rev. 2010;90(2):559–605. doi: 10.1152/physrev.00029.2009. [DOI] [PubMed] [Google Scholar]
- 20.Park SJ, Yu Y, Wagner B, Valinsky WC, Lomax AE, Beyak MJ. Increased TASK channel-mediated currents underlie high-fat diet induced vagal afferent dysfunction. Am J Physiol Gastrointest Liver Physiol. 2018;315(4):G592–G601. doi: 10.1152/ajpgi.00335.2017. [DOI] [PubMed] [Google Scholar]
- 21.Cadaveira-Mosquera A, Pérez M, Reboreda A, Rivas-Ramírez P, Fernández-Fernández D, Lamas JA. Expression of K2P channels in sensory and motor neurons of the autonomic nervous system. J Mol Neurosci. 2012;48(1):86–96. doi: 10.1007/s12031-012-9780-y. [DOI] [PubMed] [Google Scholar]
- 22.Zhao H, Sprunger LK, Simasko SM. Expression of transient receptor potential channels and two-pore potassium channels in subtypes of vagal afferent neurons in rat. Am J Physiol Gastrointest Liver Physiol. 2010;298(2):G212–G221. doi: 10.1152/ajpgi.00396.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhou SY, Lu Y, Song I, Owyang C. Inhibition of gastric motility by hyperglycemia is mediated by nodose ganglia KATP channels. Am J Physiol Gastrointest Liver Physiol. 2011;300(3):G394–G400. doi: 10.1152/ajpgi.00493.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Li Y, Zhu J, Owyang C. Electrical physiological evidence for high and low-affinity vagal CCK-A receptors. Am J Physiol. 1999;277(2):G469–G477. doi: 10.1152/ajpgi.1999.277.2.G469. [DOI] [PubMed] [Google Scholar]