Abstract
Background:
Basal cell carcinoma (BCC) treatment modalities can be stratified based on tumor subtype and recurrence risk. The main limitation of non-surgical treatment modalities is the lack of histopathological confirmation. Reflectance confocal microscopy (RCM) is a non-invasive imaging device that provides quasi-histological images.
Objective:
To evaluate the feasibility and efficacy of RCM-guided carbon dioxide (CO2) laser ablation of low-risk BCCs.
Methods:
Prospective study with biopsy-proven low-risk BCCs imaged with RCM. RCM was performed on these sites and ablated; if residual tumor was found, a new series of laser passes were performed. The patients were then followed for recurrence clinically and with RCM.
Results:
Twenty-two tumor sites in nine patients were imaged and treated. Median age was 59±12.9 years (range 30 – 74). Male-to-female ratio was 5:4. Mean tumor size was 7.7 mm (range 5 – 10 mm). Residual tumor was identified in 5/22 cases (22.7%) under RCM on immediate first pass post-ablation sites, prompting additional laser passes. Median follow-up was 28.5 month (22 – 32 months) with no recurrences found.
Conclusions:
Addition of RCM to laser ablation workflow can detect subclinical persistent tumor after initial ablation and may serve as an aid to increase the efficacy of laser ablation.
Keywords: reflectance confocal microscopy, laser, basal cell carcinoma, diagnosis, treatment, follow-up, ablation, carbon dioxide laser
Capsule summary
– In this prospective case-series including 22 BCCs, RCM found 22.7% of residual BCC immediately after first pass of carbon-dioxide laser ablation. No recurrences have been found after median of 28.5 months follow-up.
– RCM can better guide non-surgical BCC treatment modalities ultimately improving treatment efficacy.
Introduction:
According to the National Comprehensive Cancer Network (NCCN), BCC treatment modality can be selected depending on tumor subtype and recurrence risk: low-risk BCCs are amenable to non-surgical management.1 Laser ablation is a localized treatment modality that can be used for managing low-risk BCC;2–4 however, as with all non-surgical treatments, it lacks histopathological confirmation of clearance.
Reflectance confocal microscopy (RCM) can diagnose5 and monitor treatment response of BCC.6, 7 Preliminary studies have evaluated the feasibility of RCM for monitoring low-risk BCCs with ablative lasers.8–10 Our objective was to evaluate the feasibility and efficacy of RCMguided carbon dioxide (CO2) laser-ablation of low-risk BCCs.8
Patients and methods:
We prospectively included adult patients, with history of multiple BCCs (≥3 tumors), located on NCCN low-risk areas,1 presenting with biopsy-proven BCCs between November 2014, and April 2018. This study was IRB approved and all patients signed informed consent. Patient demographics and tumor data were recorded in a deidentified database.
Reflectance confocal microscopy and laser ablation protocol:
- Baseline examination:
Lesion site was delineated with specially-designed paper rings11 with a 4-mm normal skin margin. Pre-ablation RCM was performed with a handheld device (Vivascope 3000, Caliber ID, Rochester, NY) scouting the entire area and margins to evaluate for presence of BCC. We used previously described RCM criteria12–14 to define ‘positive’ or ‘negative’ sites. All patients underwent laser ablation, irrespective of RCM residual status. RCM modified the lateral extension and/or number of laser passes.
- Laser ablation:
After RCM mapping, and under local anesthesia, laser ablation was performed in the entirety of the paper ring-demarcated lesional area with CO2 laser (Lumenis Ultrapulse 5000C; Lumenis Inc., San Jose, CA). The fluence was 300 – 350 mJ/cm2, density of 100%, and a uniform spot size of 2.25 mm diameter. The number of “passes” was determined according our previous hsitological study8 and the RCM estimated tumor depth (‘RCM-guided’). Each laser pass removes approximately 20–30 µm.15
- Immediate post-laser examination:
Immediately after laser ablation, a new RCM evaluation was performed to scout for deepseating residual BCC. We used topical aluminum chloride (35%) for 30 seconds as contrast agent to enhance possible residual tumor, via a mechanism of chromatin condensation (Figure 1).16 Post ablation RCM was performed with sterilized plastic caps and sterile gel applied directly to the wound. If residual BCC was identified on RCM, an additional series of passes based on RCM-estimated depth were performed.
Figure 1.
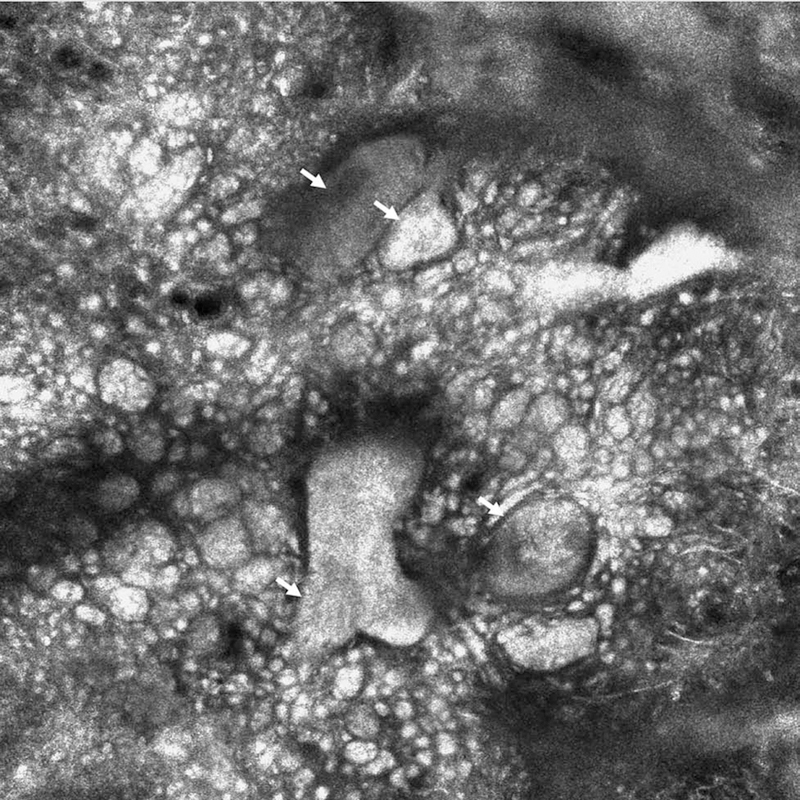
Reflectance confocal microscopy appearance of aluminium chloride-highlighted basal cell carcinoma tumor nodules (white arrows), immediately after a first pass of laser ablation (no epidermis present). This patient had a second pass of laser ablation showing no residual tumor under RCM (750 × 750 µm).
- RCM follow-up
Patients were followed at 3-, 6-, and 12-months and every 6 months thereafter. If BCC was suspected, biopsy was to be performed. After 12 months, patients continued their regular clinical and RCM follow-up as determined by the physician.
Results:
Twenty-two BCCs were included (mean size 7.7 mm [5 – 10 mm]) in 9 patients (median age 59±12.9 years, range 30 – 74; 5 males, 4 females). Two patients had history of radiation during childhood. Twenty-one cases were superficial BCCs; one case had a mixed type of superficial, nodular, and infiltrative.
Baseline, pre-ablation, RCM examination of biopsy-proven BCC sites identified residual tumor in 81.8% (18/22 lesions) (Figure 2A, Table 1). After imaging, the first laser ablation pass was performed.
Figure 2.
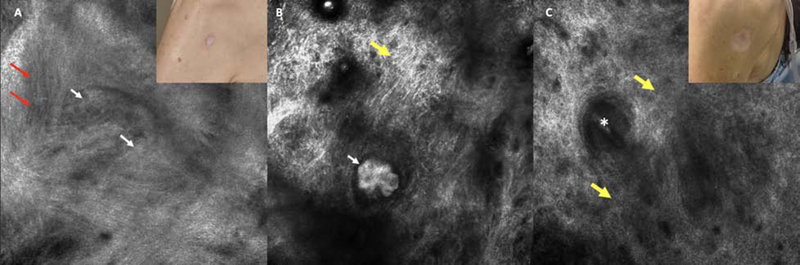
Superficial basal cell carcinoma on the posterior shoulder of a female in her 50s with metastatic breast cancer. A. Pre-ablation reflectance confocal microscopy (RCM) image showing a suspicious cord-like structures/early tumor nodule with clefting (white arrows) and horizontal vessels (red arrows). Inlet displaying pre-ablation clinical appearance (750 × 750 µm). B. Immediate post ablation RCM showed clear-cut tumor nodules after ablation of the epidermis. These tumor nodules were highlighted by aluminium chloride (white arrow). Reticulated collagen is also seen (yellow arrow). This patient underwent 3 subsequent passes. C. A 12-month RCM follow-up showed scar with dense collagen (yellow arrows); the asterisk corresponds to a hair follicle. Insert showing the appearance of the scar (750 × 750 µm).
Table 1:
Reflectance confocal microscopy characteristics of basal cell carcinomas before (baseline) laser ablation, immediate after (post-ablation), and 3-, 6-, and 12-months of follow-up.
| Confocal features | Baseline % (n) | Immediate post-ablation % (n) | 3-month follow-up % (n)* | 6-month follow-up % (n)* | 12-month follow-up % (n)* |
|---|---|---|---|---|---|
| Atypical honeycomb | 13.6% (3) | No epidermis | 0 | 0 | 0 |
| Ulceration | 13.6% (3) | 100% | 0 | 0 | 0 |
| Streaming | 63.6% (14) | No epidermis | 0 | 0 | 0 |
| Cobblestone pattern | 13.6% (3) | No epidermis | 0 | 0 | 0 |
| Tumor nests | 45.5% (10) | 13.6% (3) | 0 | 0 | 0 |
| Palisading | 50% (11) | 18.2% (4) | 0 | 0 | 0 |
| Clefting | 54.5% (12) | 18.2% (4) | 0 | 0 | 0 |
| Cord-like structures | 72.7% (16) | 4.5% (1) | 0 | 0 | 0 |
| Dark Silhouettes | 4.5% (1) | 0 | 0 | 0 | 0 |
| Horizontal vessels | 81.8% (18) | 22.7% (5) | 0 | 0 | 0 |
| Bundled collagen | 63.6% (14) | 68.2% (15) | 0 | 0 | 0 |
| Plump cells | 13.6% (3) | 4.5% (1) | 0 | 0 | 0 |
| Inflammation | 18.2% (4) | 22.7% (5) | 0 | 0 | 0 |
| Reticulated collagen | 0 | 81.8% (18) | 0 | 0 | 0 |
| Debris | 0 | 81.8% (18) | 0 | 0 | 0 |
| Scar tissue | 0 | 0 | 100% (17/17) | 100% (17/17) | 100% (17/17) |
Initial N=25;
N=17.
Immediate, post-ablation RCM examination:
Residual BCC was identified in 5/22 (22.7%) of sites upon immediate, post-ablation, RCM examination (Figure 1 and 2B, Table 1). A second set of passes of CO2 laser was performed, using the same parameters. In the repeated, post-laser, RCM group (n=5), no BCC features were identified. Mean number of passes was 3.6 (range 2 – 8).
RCM follow-up:
At the 12-month follow-up, 3 patients (n=5 lesions; 22%) were lost and excluded from the recurrence analysis. Six patients (17 lesions) completed at least 12-months of follow-up and no clinical or RCM evidence of recurrence was identified (0/17) (Figure 2C). Patients remained under clinical and RCM monitoring every 6 months with no recurrences (median follow-up 28.5 months [22 – 32 months]). Sites have healed well with good cosmetic outcomes.
Discussion:
The main limitation of non-surgical treatments of BCC is lack of histological clearance verification. Clinicians rely on visual appearance to assess whether a tumor was completely removed or not.8 The complementary use of RCM in different stages: pre-ablation, immediately post-ablation, and during follow-up can guide BCC ablative treatments.8–10, 15
Immediate post-ablation RCM examination identified non-clinically evident residual tumor in 22.7% of lesions. This guided further laser treatment, achieving probable complete removal of tumors. As a result, after 28.5 months of follow-up, no recurrences have been identified. However, BCC recurrences may occur later, and longer follow-up is needed.17
Detection of residual BCC with immediate, post-ablation RCM herein (22.7%), is comparable to a study showing 21.2% of residual BCC on histopathology, 3 months post laserablation.3 Therefore, RCM-guided laser-ablation could achieve a higher cure rate. Since RCM is limited to a depth of 200–250µm, deep-seating residual tumor could have been missed on initial RCM evaluation. Given that epidermis was ablated with initial laser treatment, we were able to evaluate deeper skin levels, allowing detection of deeper residual tumor that would be otherwise missed.
RCM can also be used to determine residual status of BCC after biopsy.18 Herein, we found residual tumor in 82% of biopsy-proven BCCs included. Potentially, use of RCM as a screening tool, can potentially spare some unnecessary treatment in selected, low-risk patients. A recent study showed that RCM is a useful tool to assess residual status in clinically-negative BCC biopsy sites.19
Limitations:
A relatively small sample size and short follow-up time. An important inherent limitation of RCM is the maximum image depth of 200 – 250 µm: The use of multimodal imaging such as RCM-optical coherence tomography can potentially help overcome this, by enabling evaluation of deeper tumoral components.20–22 Additionally, there was no histopathologic proof of clearance; nevertheless, correlation of RCM with final histopathological status was demonstrated previously.8 Finally, this study was performed in a Tertiary Cancer Center with expertise in use of RCM.
Conclusion:
RCM-guided laser ablation can detect subclinical BCC after initial laser ablation and may aid to increased efficacy of laser treatments. RCM may emerge as a noninvasive tool to monitor different treatment modalities.23
Acknowledgments
Founding source: This research is funded in part by a grant from the National Cancer Institute / National Institutes of Health (P30-CA008748) made to the Memorial Sloan Kettering Cancer Center. Parts of this research were also funded by grants from the Skin Cancer Foundation and the A. Ward Ford Memorial Grant.
Anthony Rossi: Dr. Rossi has no relevant conflicts of interest related to this manuscript but has received grant funding from The Skin Cancer Foundation and the A.Ward Ford Memorial Grant for research related to this work. He also served on advisory board, as a consultant, or given educational presentations: for Allergan, Inc; Galderma Inc; Evolus Inc; Elekta; Biofrontera, Quantia; Merz Inc; Dynamed; Skinuvia, Perf-Action, and LAM therapeutics.
Footnotes
Conflict of interest:
Milind Rajadhyaksha: he is a former employee of and owns equity in Caliber I.D. (formerly, Lucid Inc.), the company that manufactures and sells a reflectance confocal microscope (VivaScope). The VivaScope, for reflectance confocal microscopy (RCM) imaging, is the commercial version of an original laboratory prototype that was developed by Dr. Rajadhyaksha when he was at Massachusetts General Hospital, Harvard Medical School.
Consent for publication: The authors consent the publication of this submission (manuscript and figures).
Prior presentation: none.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References:
- 1.National Comprehensive Cancer Network N. Basal Cell Skin Cancer (Version 1.2018) 2018.
- 2.Mirza FN, Khatri KA. The use of lasers in the treatment of skin cancer: A review. J Cosmet Laser Ther 2017;19:451–8. [DOI] [PubMed] [Google Scholar]
- 3.Zane C, Facchinetti E, Arisi M, Ortel B, Calzavara-Pinton P. Pulsed CO2 Laser Ablation of Superficial Basal Cell of Limbs and Trunk: A Comparative Randomized Clinical Trial With Cryotherapy and Surgical Ablation. Dermatol Surg 2017;43:920–7. [DOI] [PubMed] [Google Scholar]
- 4.Telfer NR, Colver GB, Morton CA, British Association of D. Guidelines for the management of basal cell carcinoma. Br J Dermatol 2008;159:35–48. [DOI] [PubMed] [Google Scholar]
- 5.Kadouch DJ, Schram ME, Leeflang MM, Limpens J, Spuls PI, de Rie MA. In vivo confocal microscopy of basal cell carcinoma: a systematic review of diagnostic accuracy. J Eur Acad Dermatol Venereol 2015;29:1890–7. [DOI] [PubMed] [Google Scholar]
- 6.Torres A, Niemeyer A, Berkes B, Marra D, Schanbacher C, Gonzalez S et al. 5% imiquimod cream and reflectance-mode confocal microscopy as adjunct modalities to Mohs micrographic surgery for treatment of basal cell carcinoma. Dermatol Surg 2004;30:1462–9. [DOI] [PubMed] [Google Scholar]
- 7.Pasquali P, Segurado-Miravalles G, Freites-Martinez A, Gonzalez-Rodriguez S. Cryosurgical management of basal cell carcinoma: in vivo follow-up using reflectance confocal microscopy. Int J Dermatol 2018. [DOI] [PubMed]
- 8.Hibler BP, Sierra H, Cordova M, Phillips W, Rajadhyaksha M, Nehal KS et al. Carbon dioxide laser ablation of basal cell carcinoma with visual guidance by reflectance confocal microscopy: a proof-ofprinciple pilot study. Br J Dermatol 2016;174:1359–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sierra H, Yelamos O, Cordova M, Chen CJ, Rajadhyaksha M. Reflectance confocal microscopy-guided laser ablation of basal cell carcinomas: initial clinical experience. J Biomed Opt 2017;22:1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sierra H, Cordova M, Chen CJ, Rajadhyaksha M. Confocal imaging-guided laser ablation of basal cell carcinomas: an ex vivo study. J Invest Dermatol 2015;135:612–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Marino ML, Rogers T, Sierra Gil H, Rajadhyaksha M, Cordova MA, Marghoob AA. Improving lesion localization when imaging with handheld reflectance confocal microscope. Skin Res Technol 2016;22:519–20. [DOI] [PubMed] [Google Scholar]
- 12.Navarrete-Dechent C, DeRosa AP, Longo C, Liopyris K, Oliviero M, Rabinovitz H et al. Reflectance confocal microscopy terminology glossary for non-melanocytic skin lesions: A systematic review. J Am Acad Dermatol 2018. [DOI] [PMC free article] [PubMed]
- 13.Longo C, Lallas A, Kyrgidis A, Rabinovitz H, Moscarella E, Ciardo S et al. Classifying distinct basal cell carcinoma subtype by means of dermatoscopy and reflectance confocal microscopy. J Am Acad Dermatol 2014;71:716–24 e1. [DOI] [PubMed] [Google Scholar]
- 14.Peppelman M, Wolberink EA, Blokx WA, van de Kerkhof PC, van Erp PE, Gerritsen MJ. In vivo diagnosis of basal cell carcinoma subtype by reflectance confocal microscopy. Dermatology 2013;227:255–62. [DOI] [PubMed] [Google Scholar]
- 15.Sierra H, Damanpour S, Hibler B, Nehal K, Rossi A, Rajadhyaksha M. Confocal imaging of carbon dioxide laser-ablated basal cell carcinomas: An ex-vivo study on the uptake of contrast agent and ablation parameters. Lasers Surg Med 2016;48:133–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Scope A, Mahmood U, Gareau DS, Kenkre M, Lieb JA, Nehal KS et al. In vivo reflectance confocal microscopy of shave biopsy wounds: feasibility of intraoperative mapping of cancer margins. Br J Dermatol 2010;163:1218–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.van Loo E, Mosterd K, Krekels GA, Roozeboom MH, Ostertag JU, Dirksen CD et al. Surgical excision versus Mohs’ micrographic surgery for basal cell carcinoma of the face: A randomised clinical trial with 10 year follow-up. Eur J Cancer 2014;50:3011–20. [DOI] [PubMed] [Google Scholar]
- 18.Navarrete-Dechent C, Mori S, Cordova M, Nehal KS. Reflectance confocal microscopy as a novel tool for presurgical identification of basal cell carcinoma biopsy site. J Am Acad Dermatol 2019;80:e7–e8. [DOI] [PubMed] [Google Scholar]
- 19.Navarrete-Dechent C, Cordova M, Aleissa S, Liopyris K, Dusza SW, Phillips W et al. Reflectance confocal microscopy confirms residual basal cell carcinoma on clinically negative biopsy sites before Mohs surgery: a prospective study. J Am Acad Dermatol 2019;In Press. [DOI] [PMC free article] [PubMed]
- 20.Rossi AM, Navarrete-Dechent C, Nehal KS. Beyond skin deep: taking bedside dermatology to the next level with noninvasive technologies. Br J Dermatol 2018;178:994–6. [DOI] [PubMed] [Google Scholar]
- 21.Sahu A, Yelamos O, Iftimia N, Cordova M, Alessi-Fox C, Gill M et al. Evaluation of a Combined Reflectance Confocal Microscopy-Optical Coherence Tomography Device for Detection and Depth Assessment of Basal Cell Carcinoma. JAMA Dermatol 2018. [DOI] [PMC free article] [PubMed]
- 22.Navarrete-Dechent C, Rajadhyaksha M, Nehal KS. Can optical coherence tomography improve the management of basal cell carcinoma? Br J Dermatol 2018;Epub ahead of print. [DOI] [PubMed]
- 23.Navarrete-Dechent C, Cordova M, Postow MA, Pulitzer M, Lezcano C, Halpern AC et al. Evaluation of the Response of Unresectable Primary Cutaneous Melanoma to Immunotherapy Visualized With Reflectance Confocal Microscopy: A Report of 2 Cases. JAMA Dermatol 2019. [DOI] [PMC free article] [PubMed]


