Abstract
The process of deciding whether to pursue preimplantation genetic testing (PGT) of an embryo is highly stressful for individuals and couples and has adverse emotional consequences (e.g. distress and uncertainty). PGT influences patients’ lives in both positive and negative ways and is experienced at an individual level, as a dyadic unit, as a family member and as part of the society. Here, we argue that providing a conceptual framework with which to understand the `experience of decision making’ about PGT for monogenic disease (PGT-M) testing specifically, as well as the factors contributing to `decisional distress’ and `uncertainty’ that patients endure as a result—apart from what decision they make—is crucial to optimizing patient counseling, satisfaction and outcomes in the field of ART. Derived from psychological theory, the framework proposed here identifies three categories of contributing factors to decisional distress and uncertainty in considering PGT-M; namely, ‘intraindividual’, ‘interpersonal’ and ‘situational’ factors. We reviewed evidence from the PGT literature to inform our framework. Well-accepted theories of stress and health decision making were also reviewed for their relevance to PGT-M decision making, focusing on potential distress and uncertainty. Our novel conceptual framework can be used to inform clinical practice, to advance research and to aid the development of interventions for individuals and couples who are deciding whether or not to use PGT-M. Alleviating emotional distress and uncertainty can improve patients’ well-being during their reproductive journey.
Keywords: health decision making, preimplantation genetic testing, genetic counseling, couples, reproductive endocrinology and infertility, distress, psychology, decision uncertainty
Introduction
Advances in genetics are changing the landscape of reproductive options and decisions for an increasing number of individuals. Choosing parenthood and the challenges of pregnancy and childbearing are among the most consequential experiences of modern life. Preimplantation genetic testing (PGT) is a specialized technique used to identify genetic conditions in embryos created through IVF. PGT involves testing for a particular monogenic disease (PGT for monogenic diseases [PGT-M]), for structural rearrangements in chromosomes (PGT-SR) or for an abnormal number of chromosomes i.e. aneuploidy (PGT-A) (Practice Committees of the American Society for Reproductive Medicine and the Society for Assisted Reproductive Technology, 2018). Previously, PGT-M was referred to as PGD, and PGT-A (PGT for aneuploidy) was termed PGS (reviewed in Harper et al., 2017).
Indications for PGT-M are recessive single-gene disorders (e.g. cystic fibrosis), dominant single-gene disorders (e.g. Huntington’s Disease), sex-linked disorders (e.g. Duchenne’s muscular dystrophy), chromosomal disorders (e.g. translocation) or HLA matching (e.g. savior siblings; Dayal and Lucidi, 2013). PGT-M provides patients who are at risk of passing heritable diseases to their offspring with the ability to have healthy, unaffected children, while avoiding the ethically and emotionally challenging alternative of deciding whether or not to terminate a pregnancy found `intrapartum’ to be affected by that genetic disease (reviewed in Harper et al., 2017). However, the decision-making process surrounding the use of PGT-M can raise profound, complex issues related to reproduction. Individually, and often as a couple, patients are forced to consider moral and ethical questions regarding the value of a potential life affected by genetic disease; to evaluate their ability to tolerate the physical and emotional risks and financials costs associated with IVF treatment; to contemplate the risks associated with inaccurate or indeterminate testing results—such as those issues posed by embryo mosaicism (Harper et al., 2017)—and the myriad other limitations of genetic testing; and, following failed treatments, to decide whether or not to use PGT-M again in the future (e.g. Derks-Smeets et al., 2014). These factors are likely to affect the extent to which couples experience emotional distress and decisional uncertainty when considering testing.
Prior survey studies of couples who have previously elected to proceed with IVF and PGT-M do demonstrate that the psychological effects of the process remain present for up to 3 years (Järvholm et al., 2017). Thus, it seems reasonable to suspect that the experience of deciding whether or not to use PGT-M—especially for those already coping with infertility or concerned about serious heritable conditions—is highly stressful, with consequences for emotional well-being. Although a few prior studies have assessed factors that affect the ultimate decision that patients make in considering PGT-M (Hershberger and Pierce, 2010), little is known regarding the distress and uncertainty that patients have during the process of contemplating testing, regardless of what decision they ultimately make. These early experiences may affect not only patients’ ultimate reproductive decisions and thus long-term reproductive outcomes but also the health of their future offspring.
Developing a conceptual framework grounded in psychological theory
Currently, to the authors’ knowledge, there are two previously published models from one group regarding the PGT decision-making process (Hershberger and Pierce, 2010; Hershberger et al., 2012); however, their models consider the steps made in deciding whether or not to pursue PGT (i.e. the `process of getting to the uptake decision’), but not the `psychological experience’ of the decision-making process itself, and the factors that contribute to the extent of patients’ experiences of decisional uncertainty and distress. Specifically, Hershberger and colleagues used their systematic review findings to suggest a framework in which couples’ PGT decision making is composed of three iterative and dynamic dimensions: cognitive appraisal tasks (e.g. evaluating risks and success rates), emotional responses and moral judgments (e.g. status of the embryo). More recently, among couples who were considering or recently used PGT-M (Hershberger et al., 2012), Hershberger and colleagues identified four phases associated with the test uptake decision. In the `Identify’ phase, couples determined that there was a reason to consider repro-genetic testing. In the `Contemplate’ phase, couples decided whether or not to pursue parenthood and, if so, they explored various options relevant to their genetic family history such as PGT-M. When couples decided whether or not to use PGT-M, they moved into the `Resolve’ phase. At this point, Hershberger et al. (2012) classified their participants into three sub-categories according to whether they decided to test, declined testing or were `oscillating’ (e.g. considering PGT-M if another reproductive strategy was not successful). Couples who subsequently initiated an IVF/PGT-M cycle entered the `Engage’ phase. Study results indicated that progression through these phases was not linear, but instead dynamic as couples revisited their decisions and obtained new information. Furthermore, this study observed considerable variability across couples in the degree of decisional difficulty they experienced.
Although this model is useful in characterizing the stage of decision making at which a couple may be during any given time period, it does not address the factors affecting this process and the psychological outcomes that patients experience as a result. In the authors’ opinion, a conceptual framework with which to understand the `process’ of decision making that these patients experience, as well as the factors contributing to decisional `distress’ that they endure as a result, is crucial to optimize patient counseling, satisfaction and outcomes in the field of ART. In our opinion, this type of framework, focusing upon psychological experiences and outcomes, is critical to the identification of intervention opportunities that hopefully will improve upon patient experiences with this decision-making process—regardless of what they ultimately choose to do. Given that the process of PGT-M decision making is first and foremost cognitive/emotional, we suggest that the best way to understand and study patient experiences with PGT-M decision making is through psychological theory.
It is valuable to conceptualize the PGT-M decision-making process through the lens of Lazarus’s widely accepted theoretical approach to coping with stressful life events (Lazarus and Folkman, 1984). This approach holds that stressful life events are dynamic `person-environment transactions’, influenced both by characteristics and resources of individuals and also by characteristics of the event itself. These individual and contextual factors influence the way a person evaluates an event and the extent to which they believe that the event threatens their welfare (`primary appraisal’). According to this approach, events that are perceived to pose harm, threat or challenge to one’s well-being because they tax or exceed one’s resources are experienced as stressful. From this perspective, based on the few but informative reports of the experience of individuals referred for PGT-M (as reviewed in Genoff Garzon et al., 2018), the decision about whether or not to undergo testing is a stressful process. The decision-making process is emotionally and cognitively taxing, adds strain to one’s relationships and creates logistical and financial burdens as well (e.g. Lavery et al., 2002; Roberts and Franklin, 2004; Valdrez et al., 2014). Furthermore, as the Lazarus approach suggests and numerous studies document, the ways that people respond to or cope with a stressful event (`secondary appraisal’) affect their emotional states and other outcomes. Thus, both individual and contextual factors contribute to decisional distress and uncertainty in a patient considering PGT-M. In the context of this decision-making process, our framework identifies two factors that contribute to decisional distress and uncertainty and are consistent with Lazarus’ theoretical approach, namely `intraindividual’ and `situational factors’ (Fig. 1).
Figure 1.
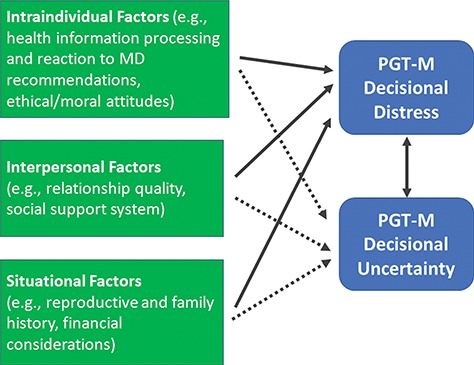
Framework of PGT-M decision-making influences on decisional distress and uncertainty. PGT-M: preimplantation genetic testing for monogenic diseases, MD: medical doctor.
Relevant to our conceptual framework for understanding the outcomes of distress and uncertainty in the context of critical decision making is the work of Luce (2005) in the cancer control domain. Luce elaborates on Lazarus’s approach as it relates to making decisions with strong emotional valence and difficult trade-offs with uncertain outcomes. Luce describes decision-generated stress as a unique component of a health-related stressor, which can be tempered by intraindividual and interpersonal coping resources such as ethical attitudes and relationship quality. Based on Luce’s work, we derived a third contributing factor to distress in our framework, `interpersonal factors’ (Fig. 1).
It is of interest to note that the aforementioned framework developed by Hershberger and Pierce (2010) offers support for our psychological framework describing the decision-making process and its psychological outcomes in the realm of PGT-M. Hershberger and colleagues’ model provides an important synthesis of the decisional tasks involved in PGT-M decision making and offers a basis for identifying factors that influence whether individuals get `stuck’ in these tasks and what adds to their decisional distress and uncertainty as they navigate these tasks individually and dyadically. Our framework moves a step beyond to focus upon these latter aspects of the process and to formulate an understanding of contributing factors and the subsequent results of those stressors.
Applying current knowledge to support and build upon our conceptual framework
Thus, as described in Fig. 1, we propose three factors contributing to patient decisional distress and uncertainty during the contemplation of PGT-M: `intraindividual’, `interpersonal’ and `situational’ factors. These factors combine to create a complex emotional and cognitive environment, which influences patient decision making in this area. The resultant decisional distress and uncertainty that occur can have a variety of adverse outcomes for patients.
To further develop our framework, we next moved to better characterize the specific components, which make up these groups of distress-inducing factors via a literature review of currently published literature on decision making and PGT-M. (Due to the scarcity of research on PGT-A and PGT-SR, we restrict our framework to PGT-M. This restriction also makes intuitive sense given the differences in motivation for single-gene testing for a specific disorder as opposed to chromosomal screening for other purposes, e.g. to help/potentially avoid a miscarriage.) As alluded to earlier, the majority of existing literature on the topic of decision making regarding PGT-M assesses factors that influence the decision that patients ultimately make regarding PGT-M, rather than assessing the components contributing to decisional distress and uncertainty for all patients while considering whether or not to opt for PGT-M. We therefore considered findings from these studies, as well as from well-accepted psychological theories of stress and health decision making, to inform our framework.
Intraindividual factors
Two primary intraindividual factors likely to influence PGT decisional distress and uncertainty include health information processing and ethical/moral attitudes. How people collect, interpret and revisit health information influences their decision making and their later satisfaction with it. There is evidence that individual differences exist in preferences for health information: some people prefer to avoid it or feel frightened and overwhelmed by it (`blunters’), while others seek out information and may be reassured by it (`monitors’) (Miller, 1987, 1995). Monitors focus on and evaluate threatening or stressful information, whereas blunters tend to distract themselves in order to blunt the information’s emotional impact. People experience better mental and physical health when they receive information that matches their informational coping style. Gender differences in informational coping styles may also exist. In one recent study, husbands of subfertile women sought online information more than their wives to supplement what they learned in the clinic (Pastore et al., 2014).
Although research has not yet examined the role of individual characteristics, such as informational coping style, in PGT-M decisional distress and uncertainty specifically, information itself does play a central role (Genoff Garzon et al., 2018). For example, women with a family history of breast or ovarian cancer report needing education and unbiased information about PGT-M, and they prefer receiving it from someone not invested in the testing decision (e.g. genetic counselor) (Quinn et al., 2009; Hurley et al., 2012). Couples may have difficulty understanding the educational materials that are provided and may turn to the Internet and social media for their information (Rubin et al., 2018). Information from one’s healthcare provider may be particularly important. Provider influence and information was one of the top three factors associated with uptake of PGT-A in a 2016 study (Gebhart et al., 2016), and this may also be true for PGT-M decision making. Provision of pre-test information about medical procedures has also been shown to decrease infertility-specific anxiety and stress (Pook and Krause, 2005).
Additionally, the ability that patients have to process their health information and their reactions to it are affected by provider education and recommendations. Using a sample of 27 infertility providers (4 of whom were also patients) and 10 patients, Klitzman (2018) described how the providers’ experience and knowledge of PGT and genetics influence their referral/recommendations about PGT to patients. Therefore, in addition to the patient’s own attitude and knowledge about PGT, providers’ attitudes and knowledge also have an impact on patients, in terms of whether they are referred for PGT counseling or if testing is recommended. This decision-making influence was novel among the reviewed reports. Other influences on the decision-making process that echo sentiments expressed elsewhere (Genoff Garzon et al., 2018) include the severity of the genetic disorder, childhood versus adult onset of the genetic condition, potential testing for multiple genetic conditions, the complexity of the PGT process and related gaps in understanding the procedures, unanticipated test results and unsuccessful prior fertility treatment, all of which can increase emotional distress. Patients also noted that social acceptance of PGT has increased over time, which altered their perception in a more favorable direction.
Another relevant intraindividual factor is a person’s ethical or moral perceptions of PGT-M. The selection and disposition of embryos (affected and unaffected and used and unused) involves ethical considerations among individuals contemplating PGT (Katz et al., 2002; Karatas et al., 2010; van Rij et al., 2013). Across studies, 30% of PGT-M considerers expressed ethical concerns (Quinn et al., 2012); other research confirms that in deciding about PGT testing, many patients reported ethical conflicts (Roberts and Franklin, 2004; Olesen et al., 2016). Because PGT-M is more controversial for adult-onset (versus childhood-onset) hereditary diseases, such as the breast cancer BRCA1/2 gene that increases lifetime cancer risk (Ethics Committee of the American Society for Reproductive Medicine, 2010), this may also contribute to decisional distress or uncertainty.
Interpersonal factors
The quality of a couple’s relationship, how they typically make decisions and their joint decision-making approach are also primary factors likely to affect distress and uncertainty in PGT-M decision making. Because PGT decision making usually involves a couple, it is important to consider dyadic factors. BRCA1/2 carriers (Werner-Lin et al., 2012), for example, reported negotiating whether one partner had greater decision-making power in terms of both PGT-M and reproductive decision making in general. Greater decision-making power may rest with the partner whose body is most affected by the IVF process or the person whose genetic history is driving the consideration of PGT-M (Werner-Lin et al., 2012). Individuals at risk for Huntington’s disease (Klitzman et al., 2007) also reported negotiating their (sometimes unequal) balance in making joint reproductive decisions (unrestricted to PGT-M). In the case of PGT-M, where there is a positive family history, siblings and other family members are also likely to influence decision making (Rubin et al., 2014).
Considerable research underscores the value of interpersonal resources, such as supportive relationships, in alleviating the impact of stressful life events on emotional and psychological outcomes (Uchino and Birmingham, 2011). In the context of infertility and PGT decision making, there is some relevant evidence. Individuals who perceive their partner to be available and responsive have been shown to experience lower stress related to their infertility than individuals who perceive their partner to be avoidant and non-responsive (Van den Broeck et al., 2010; Donarelli et al., 2012). In one study of patients contemplating the use of PGT-A, social support or acceptance from partner, family and/or friends was a strong influence on test uptake (Gebhart et al., 2016). In contrast, in a separate study (van Rij et al., 2011), social support was not predictive of intended or actual use among women, although `insufficient’ social support had some influence on the male partner’s uptake decision. Neither of these two studies assessed the extent to which social support may have alleviated decisional distress or uncertainty.
The interpersonal relationship between the patient and the provider may also influence the level of distress and uncertainty that accompanies this decision making. As discussed above, the provider influences the health information received and can influence the perception of the medical choices and their relative merits.
Situational factors
Finally, a wide array of situational factors is likely to affect decisional distress and uncertainty, including financial aspects of testing and reasons for PGT referral (e.g. genetic history). The psychological milieu is very likely to differ for people with an offspring affected with a known inherited genetic condition (Dallaire et al., 1995; Quittner et al., 2016). A study of 246 couples found that living with an affected child had a negative association with PGT-M intention (van Rij et al., 2011), although the authors did not report whether these parents had a different emotional or psychological response to testing. Having an affected child or just a positive family history was also a factor influencing reproductive decision making (defined broadly) in cystic fibrosis carrier couples (Myring et al., 2011).
The ability to pay for IVF and PGT-M is influenced by insurance coverage and access to financial assistance from personal funds, family, bank or credit card loans etc. In addition, the perceived value of this expenditure relative to other ways to spend resources, whether in the form of money, time or emotional energy, is also likely to elevate uncertainty and distress. Couples from the UK reported that the myriad risks and choices involved with PGT-M testing affected their financial security, relationships, career and health (Roberts and Franklin, 2004). Two studies (Drazba et al., 2014; Gebhart et al., 2016) have also found financial cost to be a critical factor in deciding whether to use PGT-M. Having an affected child influences the financial situation of the family (Stettner, 2018), so those considering PGT-M to avoid a second affected child may already be financially challenged. As reported (https://www.fertilityiq.com/cost), while IVF alone costs approximately US $12 000, the required medications cost an additional $5000, and PGT-M adds another $5000–6000. In the UK, 40% of couples that use IVF undergo more than one cycle (Smith et al., 2015), and across the ESHRE consortium (Calhaz-Jorge et al., 2017) and in the USA (using fresh non-donor eggs or embryos; Centers for Disease Control and Prevention, 2016), ~29–30% of IVF cycles result in a clinical pregnancy. Thus, for many couples, the cost of PGT-M is multiplied by each IVF cycle and insurance coverage for these procedures varies widely. That multiple cycles are often required adds to the uncertainty of the costs, and this uncertainty regarding cost can increase decision-related distress.
Testing the framework: hypotheses and prediction
As shown in Fig. 1, we hypothesize that the three sets of factors—intraindividual, interpersonal and situational—contribute to decisional distress and uncertainty in patients considering PGT-M. That is, we predict that knowledge about the characteristics of patients and about the testing context itself will help to identify those who are experiencing greatest distress and uncertainty. The framework also incorporates several subsidiary, specific hypotheses. For example, as articulated above, we expect that patients with moral quandaries about the testing, and those with poorer relationship quality, insufficient social support, prior experience of subfertility or financial strain, will experience greater distress and uncertainty.
Research applications
The framework presented here offers a foundation for empirical research to further our understanding of the PGT-M decision-making process. For some of the predictor variables identified in our framework, particularly intraindividual and interpersonal factors, well-validated instruments exist that can be administered easily in clinical settings or made available to patients online. Instruments designed to assess variables, such as informational preferences, marital/relationship quality, dyadic coping and social support from friends, family and health care providers, can be selected from published literature, using versions appropriate to the characteristics of a sample, such as their cultural background and level of education. Researchers also have the potential to examine a wide range of situational factors as predictors of decisional distress and uncertainty. Presumably, the situational factors of interest will reflect the particular testing and patient care context, such as reasons for testing referral and financial issues that are pertinent.
In comparison to the availability of appropriate instruments for many of the variables represented by the three sets of predictor factors in our conceptual framework, there is a lack of valid measurement tools to assess the outcomes identified by this framework: that is, the decisional distress and uncertainty faced by patients who are deciding whether to use PGT-M. Our team has therefore undertaken a research program to develop, pilot and standardize instruments to assess decisional distress and uncertainty associated with PGT-M (e.g. Pastore et al., 2017; Rubin et al., 2018). We are now collecting data to establish the feasibility, reliability, validity and acceptability to patients of these instruments and will soon make them available to others for research and clinical use.
Clinical applications
Specifically relevant to this framework, the decisional distress and decisional uncertainty tools could be used in a predictive manner in the clinic. That is, patients could complete the questionnaires and then the delivery of the services (education, genetic counseling, test result communication etc.) would be modified accordingly in response to the questionnaire responses and overall scores. As a hypothetical specific example, a person with high decisional distress or uncertainty may indicate through a health information processing instrument (such as the Health Information Orientation Scale; DuBenske et al., 2009) that they are information seekers as opposed to reporting `information apprehension’. In this instance, the patient will likely benefit from extended education/details and provision of resources for subsequent self-education before, during or after the PGT-M testing. As an example of this approach in pediatrics, a patient-facing enhanced genomic report (designed by patients for patients) was found by a small group of information seeker parents to improve communication with their providers and led to increased engagement and high satisfaction (Williams et al., 2018).
By considering the major contributors to decisional distress in patient decision making about PGT-M, providers can tailor their interactions with patients to minimize decisional distress and uncertainty on an individualized basis. We suggest that patient counseling about PGT-M should begin, universally, with an acknowledgement that the decision to undergo PGT-M can be difficult and affected by the couple’s relationship, reproductive history, belief systems, finances and interactions with the healthcare system (i.e. the intraindividual, interpersonal and situational factors outlined in our framework). After introducing the option for PGT-M, including the relevant clinical and financial information, providers should encourage an open dialog both between the partners and with the clinic regarding the factors that may influence their decision making about PGT-M. This will highlight any outstanding factual/educational gaps that the providers can fill in. Equally important, this facilitation of dialog in a non-judgmental manner will improve providers’ ability to identify whether there is decisional distress for each patient. With that knowledge, the provider can help to address concerns specifically during subsequent counseling sessions and/or to make an appropriate referral (e.g. genetic counselor or repro-psychologist). Open patient/provider communication in the context of cancer patients and fertility concerns has been shown to be associated with lower patient distress, greater knowledge, greater involvement in the decision-making process about fertility preservation and greater satisfaction with health care (Ussher et al., 2018). Detailed guidelines for psychosocial care in fertility clinics have been outlined by ESHRE (Gameiro et al., 2015), and our suggestions dovetail well with these guidelines.
This communication approach, armed with knowledge of the conceptual framework described here, can also be of direct use to health care providers (e.g. physicians, genetic counselors etc.) working within the shared decision-making model (SDM; Barry and Edgman-Levitan, 2012). SDM guides attention to specific situational, intraindividual and interindividual factors that can impinge on the decision-making process, particularly for those struggling with the decision. Genetic counselors are in a unique position to address gaps in patient knowledge, the genetic implications and decisional considerations relevant to PGT-M. Thus, it can be particularly valuable for health care providers in the fertility clinic to use decision aids in the context of SDM to help alleviate potential PGT decision-making-related distress and uncertainty through patient/couple-centered interactions.
Couples considering PGT-M can be empowered to make informed decisions that they are less likely to regret in the future—thereby, hopefully, improving their retrospective beliefs about the process postnatally as well. Finally, in general, such improved communication and quality of interpersonal interactions during the PGT-M decision-making process could increase patient compliance with provider recommendations and improve patient perceptions of the quality of care they receive during reproductive treatments (Aarts et al., 2012). As reviewed elsewhere (Gameiro et al., 2015), patients value how staff relate to them and want staff to acknowledge the emotional impact of infertility, and we presume these observations also pertain to the experience of PGT-M decision making.
Limitations of our proposed framework
Limitations of this framework include the fact that there is scant prior research on this topic. Additionally, although Hershberger and colleagues did apply their `cognitive dimensions’ of the PGT decision-making process (Hershberger and Pierce, 2010) to interpret findings from a study exploring the reasons that young women with cancer choose to undergo fertility preservation (Hershberger et al., 2016), to the authors’ knowledge, their conceptual model of the PGT-M decision-making `process’ (Hershberger et al., 2012) has not been empirically tested with patients considering PGT. Further, because of the lack of any conceptual framework to describe the `emotional’ aspects of decisional distress experienced by patients considering PGT-M (prior to the one proposed here), there is not any empirical research published on PGT decisional distress or uncertainty.
Conclusion
Here, we propose a novel way of conceptualizing patient decision making regarding the use of PGT-M. Our framework emphasizes the experiences of patients considering this option and the factors that can result in decisional distress and uncertainty for them—rather than focusing on stages of contemplation and whether or not they decide to pursue PGT-M.
Clinical implementation and next steps for research
Our psychological framework for interpreting the patient experience in deciding about PGT-M identifies several factors that contribute to decisional distress and uncertainty. These factors will form the basis for future research to better characterize the nature of these stressors, the risk factors that patients have for adverse psychological outcomes when exposed to those stressors and ultimately for quality improvement projects to avoid adverse patient experiences. For example, it appears clear that improved quality and timing of provider-to-patient communication and patient education could be highly beneficial (Pook and Krause, 2005; Quinn et al., 2009; Gebhart et al., 2016; Genoff Garzon et al., 2018). A patient-centered approach, however, is critical, as our framework highlights individual differences in informational processing and reactions to recommendations, as well as differing ethical and moral atittudes that inform patient decision making and their personal interpretations of their experiences with healthcare. Patient-centered care is associated with better patient well-being (Aarts et al., 2012; Gameiro et al., 2013). Generating a method to identify the individual differences in preferences about receiving health information (Miller, 1987, 1995), while considering the broad range of situational factors that are unique to each patient and couple, is critical to providing sensitive and optimal patient counseling and care. Recent research has found that a multimedia educational platform was successful in increasing patient understanding of risks and informed consent regarding various fertility interventions (Madeira et al., 2018), and this same approach could be applied to PGT services.
We propose that the first steps for researchers and clinicians are to determine the magnitude of distress and uncertainty that individuals experience in this context, to identify which patients may need directed counseling and support, to identify which factors contribute most significantly to their distress and uncertainty and to assess whether and how these contributors are modifiable. We believe that improved communication between providers and patients (open dialog through the SDM model), specifically regarding the couple’s decision-making considerations, will facilitate greater PGT-M decisional certainty and decreased decisional distress among patients. Although alleviating emotional distress is important in its own right, it is also likely that distress and uncertainty affect the quality of decisions that people make about PGT-M and their perceptions of the quality of fertility care they received (Aarts et al., 2012). Empowering couples to make more informed PGT decisions may reduce future decision regret and may improve postnatal well-being, both of which are areas for future researchers to explore.
Authors’ roles
The framework was designed by M.L. with significant input from L.P. and L.R. The literature review on related frameworks and theoretical models was led by L.P. and L.R., with input from M.C.G.G. and C.N.C.M. The review of the relevant psychological literature was led by M.L. and L.R., with input from L.P. and J.N.S.B. Substantial contributions to this opinion were added by J.N.S.B. and C.N.C.M., while the overall structure of the final article was created by C.N.C.M. All authors were involved in drafting and revising the final manuscript and have approved this version for publication.
Funding
None.
Conflict of interest
The authors declare that there is no conflict of interest.
References
- Aarts JW, Huppelschoten AG, van Empel IW, Boivin J, Verhaak CM, Kremer JA, Nelen WL. How patient-centred care relates to patients' quality of life and distress: a study in 427 women experiencing infertility. Hum Reprod 2012;27:488–495. [DOI] [PubMed] [Google Scholar]
- Barry MJ, Edgman-Levitan S. Shared decision making—pinnacle of patient-centered care. N Engl J Med 2012;366:780–781. [DOI] [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention 2014 Assisted Reproductive Technology National Summary Report, 2016. https://www.cdc.gov/art/reports/2014/national-summary.html (15 January 2018, date last accessed).
- Dallaire L, Lortie G, Des Rochers M, Clermont R, Vachon C. Parental reaction and adaptability to the prenatal diagnosis of fetal defect or genetic disease leading to pregnancy interruption. Prenat Diagn 1995;15:249–259. [DOI] [PubMed] [Google Scholar]
- Dayal M, Lucidi R. Preimplantation Genetic Diagnosis, 2013. http://emedicine.medscape.com/article/273415-overview#a2 (23 September 2015, date last accessed).
- Derks-Smeets IA, Gietel-Habets JJ, Tibben A, Tjan-Heijnen VC, Meijer-Hoogeveen M, Geraedts JP, van Golde R, Gomez-Garcia E, van den Bogaart E, van Hooijdonk M et al. Decision-making on preimplantation genetic diagnosis and prenatal diagnosis: a challenge for couples with hereditary breast and ovarian cancer. Hum Reprod 2014;29:1103–1112. [DOI] [PubMed] [Google Scholar]
- Donarelli Z, Lo Coco G, Gullo S, Marino A, Volpes A, Allegra A. Are attachment dimensions associated with infertility-related stress in couples undergoing their first IVF treatment? A study on the individual and cross-partner effect. Hum Reprod 2012;27:3215–3225. [DOI] [PubMed] [Google Scholar]
- Drazba KT, Kelley MA, Hershberger P. A qualitative inquiry of the financial concerns of couples opting to use preimplantation genetic diagnosis to prevent the transmission of known genetic disorders. J Genet Couns 2014;23:202–211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DuBenske LL, Burke Beckjord E, Hawkins RP, Gustafson DH. Psychometric evaluation of the Health Information Orientation Scale: a brief measure for assessing health information engagement and apprehension. J Health Psychol 2009;14:721–730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ethics Committee of the American Society for Reproductive Medicine Use of preimplantation genetic diagnosis for serious adult onset conditions: a committee opinion. Fertil Steril 2010;100:54–57. [DOI] [PubMed] [Google Scholar]
- European IVF-monitoring Consortium (EIM), European Society of Human Reproduction and Embryology (ESHRE),Calhaz-Jorge C, De Geyter C, Kupka MS, de Mouzon J, Erb K, Mocanu E, Motrenko T, Scaravelli G, Wyns C, Goossens V. Assisted reproductive technology in Europe, 2013: results generated from European registers by ESHRE. Hum Reprod 2017;32:1957–1973. [DOI] [PubMed] [Google Scholar]
- Gameiro S, Boivin J, Dancet E, de Klerk C, Emery M, Lewis-Jones C, Thorn P, Van den Broeck U, Venetis C, Verhaak CM et al. ESHRE guideline: routine psychosocial care in infertility and medically assisted reproduction—a guide for fertility staff. Hum Reprod 2015;30:2476–2485. [DOI] [PubMed] [Google Scholar]
- Gameiro S, Canavarro MC, Boivin J. Patient centred care in infertility health care: direct and indirect associations with wellbeing during treatment. Patient Educ Couns 2013;93:646–654. [DOI] [PubMed] [Google Scholar]
- Gebhart MB, Hines RS, Penman A, Holland AC. How do patient perceived determinants influence the decision-making process to accept or decline preimplantation genetic screening? Fertil Steril 2016;105:188–193. [DOI] [PubMed] [Google Scholar]
- Genoff Garzon MC, Rubin LR, Lobel M, Stelling J, Pastore LM. Review of patient decision-making factors and attitudes regarding preimplantation genetic diagnosis. Clin Genet 2018;94:22–42. [DOI] [PubMed] [Google Scholar]
- Harper JC, Aittomäki K, Borry P, Cornel MC, de Wert G, Dondorp W, Geraedts J, Gianaroli L, Ketterson K, Liebaers I et al. Recent developments in genetics and medically-assisted reproduction: from research to clinical applications. Hum Reprod Open 2017;107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hershberger PE, Gallo AM, Kavanaugh K, Olshansky E, Schwartz A, Tur-Kaspa I. The decision-making process of genetically at-risk couples considering preimplantation genetic diagnosis: initial findings from a grounded theory study. Soc Sci Med 2012;74:1536–1543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hershberger PE, Pierce P. Conceptualizing couples’ decision making in PGD: emerging cognitive, emotional, and moral dimensions. Patient Educ Couns 2010;81:53–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hershberger PE, Sipsma H, Finnegan L, Hirshfeld-Cytron J. Reasons why young women accept or decline fertility preservation after cancer diagnosis. J Obstet Gynecol Neonatal Nurs 2016;45:123–134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hurley K, Rubin LR, Werner-Lin A, Sagi M, Kemel Y, Stern R, Phillips A, Cholst I, Kauff N, Offit K. Incorporating information regarding preimplantation genetic diagnosis into discussions concerning testing and risk management for BRCA1/2 mutations: a qualitative study of patient preferences. Cancer 2012;118:6270–6277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Järvholm S, Thurin-Kjellberg A, Broberg M. Experiences of pre-implantation genetic diagnosis (PGD) in Sweden: a three-year follow-up of men and women. J Genet Couns 2017;26:1008–1016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karatas JC, Barlow-Stewart K, Meiser B, McMahon C, Strong KA, Hill W, Roberts C, Kelly P. Psychological adjustment, knowledge and unmet information needs in women undergoing PGD. Hum Reprod 2010;25:1481–1489. [DOI] [PubMed] [Google Scholar]
- Katz MG, Fitzgerald L, Bankier A, Savulescu J, Cram DS. Issues and concerns of couples presenting for preimplantation genetic diagnosis (PGD). Prenat Diagn 2002;22:1117–1122. [DOI] [PubMed] [Google Scholar]
- Klitzman R. Challenges, dilemmas and factors involved in PGD decision-making: providers' and patients' views, experiences and decisions. J Genet Couns 2018;27:909–919. [DOI] [PubMed] [Google Scholar]
- Klitzman R, Thorne D, Williamson J, Chung W, Marder K. Decision-making about reproductive choices among individuals at-risk for Huntington's disease. J Genet Couns 2007;16:347–362. [DOI] [PubMed] [Google Scholar]
- Lavery SA, Aurell R, Turner C, Castello C, Veiga A, Barri PN, Winston RM. Preimplantation genetic diagnosis: patients’ experiences and attitudes. Hum Reprod 2002;17:2464–2467. [DOI] [PubMed] [Google Scholar]
- Lazarus R, Folkman S. Stress, Appraisal, and Coping. New York: Springer Publishing Company, 1984 [Google Scholar]
- Luce MF. Decision making as coping. Health Psychol 2005;24:S23–S28. [DOI] [PubMed] [Google Scholar]
- Madeira JL, Rehbein J, Christianson MS, Lee M, Parry JP, Pennings G, Lindheim SR. Using the EngagedMD multimedia platform to improve informed consent for ovulation induction, intrauterine insemination, and in vitro fertilization. Fertil Steril 2018;110:1338–1346. [DOI] [PubMed] [Google Scholar]
- Miller SM. Monitoring and blunting: validation of a questionnaire to assess styles of information seeking under threat. J Pers Soc Psychol 1987;52:345–353. [DOI] [PubMed] [Google Scholar]
- Miller SM. Monitoring versus blunting styles of coping with cancer influence the information patients want and need about their disease. Implications for cancer screening and management. Cancer 1995;76:167–177. [DOI] [PubMed] [Google Scholar]
- Myring J, Beckett W, Jassi R, Roberts T, Sayers R, Scotcher D, McAllister M. Shock, adjust, decide: reproductive decision making in cystic fibrosis (CF) carrier couples—a qualitative study. J Genet Couns 2011;20:404–417. [DOI] [PubMed] [Google Scholar]
- Olesen AP, Nor SN, Amin L. Attitudes toward pre-implantation genetic diagnosis (PGD) for genetic disorders among potential users in Malaysia. Sci Eng Ethics 2016;22:133–146. [DOI] [PubMed] [Google Scholar]
- Pastore LM, Karns LB, Ventura K, Clark ML, Steeves RH, Callanan NP. Longitudinal interviews of couples diagnosed with diminished ovarian reserve undergoing fragile X premutation testing. J Genet Couns 2014;23:97–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pastore LM, Lobel M, Stelling J, Rubin LR Preimplantation Genetic Testing Patient Decision-Making: New Tools to Assess Decisional Distress and Decisional Uncertainty, June 2017. Paper presented at the Genomics and Society: Expanding the ELSI Universe Congress, CT, 2017.
- Pook M, Krause W. Stress reduction in male infertility patients: a randomized, controlled trial. Fertil Steril 2005;83:68–73. [DOI] [PubMed] [Google Scholar]
- Practice Committees of the American Society for Reproductive Medicine and the Society for Assisted Reproductive Technology The use of preimplantation genetic testing for aneuploidy (PGT-A): a committee opinion. Fertil Steril 2018;109:429–436. [DOI] [PubMed] [Google Scholar]
- Quinn GP, Pal T, Murphy D, Vadaparampil ST, Kumar A. High-risk consumers' perceptions of preimplantation genetic diagnosis for hereditary cancers: a systematic review and meta-analysis. Genet Med 2012;14:191–200. [DOI] [PubMed] [Google Scholar]
- Quinn GP, Vadaparampil ST, King LM, Miree CA, Friedman S. Conflict between values and technology: perceptions of preimplantation genetic diagnosis among women at increased risk for hereditary breast and ovarian cancer. Fam Cancer 2009;8:441–449. [DOI] [PubMed] [Google Scholar]
- Quittner AL, Abbott J, Georgiopoulos AM, Goldbeck L, Smith B, Hempstead SE, Marshall B, Sabadosa KA, Elborn S. International Committee on Mental Health in Cystic Fibrosis: Cystic Fibrosis Foundation and European Cystic Fibrosis Society consensus statements for screening and treating depression and anxiety. Thorax 2016;71:26–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts C, Franklin S. Experiencing new forms of genetic choice: findings from an ethnographic study of preimplantation genetic diagnosis. Hum Fertil (Camb) 2004;7:285–293. [DOI] [PubMed] [Google Scholar]
- Rubin LR, Pastore LM, Subramony S, Lobel M, Stelling J. Navigating preimplantation genetic testing decisions in the age of social media: a qualitative study. Fertil Steril 2018;110:e146. [Google Scholar]
- Rubin LR, Werner-Lin A, Sagi M, Cholst I, Stern R, Lilienthal D, Hurley K. The BRCA clock is ticking!': negotiating medical concerns and reproductive goals in preimplantation genetic diagnosis. Hum Fertil (Camb) 2014;17:159–164. [DOI] [PubMed] [Google Scholar]
- Smith ADAC, Tilling K, Nelson SM, Lawlor DA. Live-birth rate associated with repeat in vitro fertilisation treatment cycles. JAMA 2015;314:2654–2662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stettner M. How one father meets the financial needs of sons with cystic fibrosis, 2018. https://www.marketwatch.com/story/how-one-father-meets-the-financial-needs-of-sons-with-cystic-fibrosis-2018-03-30(9 April 2018, date last accessed).
- Uchino BN, Birmingham W. Stress and support processes In: Contrada RJ, Baum A (eds). The Handbook of Stress Science: Biology, Psychology, and Health. New York: Springer, 2011,111–121 [Google Scholar]
- Ussher JM, Parton C, Perz J. Need for information, honesty and respect: patient perspectives on health care professionals communication about cancer and fertility. Reprod Health 2018;15:2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valdrez K, Silva S, Coelho T, Alves E. Awareness and motives for use and non-use of preimplantation genetic diagnosis in familial amyloid polyneuropathy mutation carriers. Prenat Diagn 2014;34:886–892. [DOI] [PubMed] [Google Scholar]
- Van den U, D'Hooghe T, Enzlin P, Demyttenaere K. Predictors of psychological distress in patients starting IVF treatment: infertility-specific versus general psychological characteristics. Hum Reprod 2010;25:1471–1480. [DOI] [PubMed] [Google Scholar]
- van MC, de Die-Smulders CE, Bijlsma EK, de Wert GM, Geraedts JP, Roos RA, Tibben A. Evaluation of exclusion prenatal and exclusion preimplantation genetic diagnosis for Huntington's disease in the Netherlands. Clin Genet 2013;83:118–124. [DOI] [PubMed] [Google Scholar]
- van Rij MC, Gielen M, Lulofs R, Evers JL, van Osch L, Muntjewerff N, Geraedts JP, de Die-Smulders CE. Profiles and motives for PGD: a prospective cohort study of couples referred for PGD in the Netherlands. Hum Reprod 2011;26:1826–1835. [DOI] [PubMed] [Google Scholar]
- Werner-Lin A, Rubin LR, Doyle M, Stern R, Savin K, Hurley K, Sagi M. "My funky genetics": BRCA1/2 mutation carriers' understanding of genetic inheritance and reproductive merger in the context of new reprogenetic technologies. Fam Syst Health 2012;30:166–180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams JL, Rahm AK, Zallen DT, Stuckey H, Fultz K, Fan AL, Bonhag M, Feldman L, Segal MM, Williams MS. Impact of a patient-facing enhanced genomic results report to improve understanding, engagement, and communication. J Genet Couns 2018;27:358–369. [DOI] [PMC free article] [PubMed] [Google Scholar]


