INTRODUCTION
The reality of inter-individual variability in drug disposition or response when viewed from the perspective of practitioners treating patients one-at-a-time is quite different from the population-oriented perspective of pharmaceutical companies and regulatory agencies, challenging the field to think differently when applying population data to guide therapeutic decisions for individual, unique patients. Paradoxically, novel approaches for collecting and analyzing population data offer opportunities to ensure that no patient ever feels they are “just average”.
PATIENTS AS UNIQUE INDIVIDUALS
It is implicitly understood that populations consist of large numbers of individuals, and yet it has proved challenging to use population data to inform therapeutic decisions for individual patients. For example, the questions that most concern the pharmaceutical industry and regulatory agencies, such as the US Food and Drug Administration (FDA), relate to the doses of medications that, on average, are safe and effective -- achieve the desired clinical outcome while minimizing the risk of toxicity -- in the patient populations to which those medications will be prescribed. Dosing recommendations in the product label, therefore, are most appropriate for patients who exhibit characteristics of the “population average”. Unfortunately, many individuals possess characteristics that make them unique. A study of 1,013 patients conducted at the Mayo Clinic1 revealed that 99% of patients carried an actionable variant in at least one of five major pharmacogenomic genes (“normal metabolizers” for CYP2C9, CYP2C19, CYP2D6, SLCO1B1 and VKORC1; only 1% of the patients had no actionable variants). Given that currentclinical pharmacogenomic implementation strategies designed to individualize drug treatment tend to focus on single genes that influence the dose-exposure relationship (e.g., CYP2C9, CYP2C19, CYP2D6 and SLCO1B1), it is reasonable to consider the following questions:
Will each patient prescribed a particular dose, 10 mg for example, be expected to have the same exposure?
If not, how much variability in exposure is present in the population?
What fold-range -- maximum value to minimum value for systemic exposure following a fixed dose -- is acceptable? 2-fold? 3-fold? 5-fold? 10-fold? (for context, an average ± 50% range equates to 1.5/0.5 = a 3-fold range).
What factors contribute to the observed variability in systemic exposure? How much of the observed variability in systemic exposure is contributed by pharmacogenetic variation?
What systemic exposure is required to maximize the probability of achieving the desired therapeutic response and minimize the risk of toxicity? In other words, what is the target exposure required to optimize clinical response, assuming an exposure-response relationship has been established?
Do all patients require the same systemic exposure?
EXPLORING VARIABILITY FROM AN INDIVIDUAL PERSPECTIVE
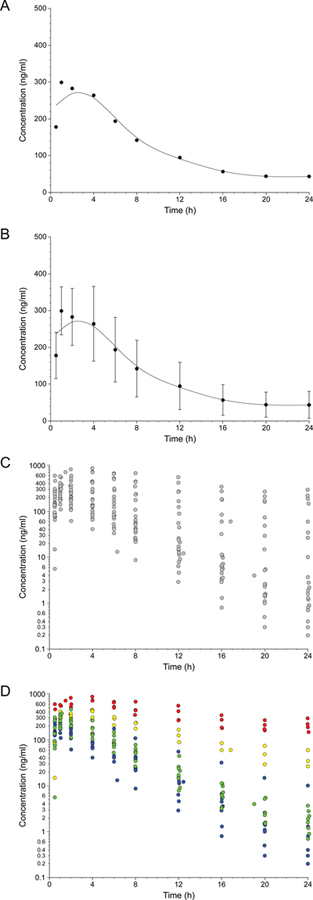
It is helpful to explore these questions from the perspective of an individual patient, parent or prescriber. Assume a situation where a single dose of a medication, 0.5 mg/kg, was administered to 23 pediatric patients aged 6 to 17 years of age. If we were to ask the question, “what is the average plasma concentration-time profile for a 0.5 mg/kg dose of this medication in 23 patients 6–17 years of age?”, the answer would be the profile presented in Fig. 1A. However, we know that there will be some variability in the sample, and a common way to represent this variability is to plot the mean ± one standard deviation of the plasma concentration at each time point, assuming the population data are normally distributed (Fig. 1B). In reality, prescribers treat individual patients, and each patient will have his or her individual plasma concentration profile for the 0.5 mg/kg dose that they were administered (Fig. 1C); when the dataset is viewed from the perspective of an individual patient, knowledge of the mean ± SD becomes irrelevant because a prescriber (and parent or patient) may want to know where within the range of possibilities (25-fold range of concentrations at 4 hours; 2,000-fold range at 24 hours; 50-fold range of systemic exposure) an individual patient may fall prior to making subsequent dosing decisions. What factors, then, contribute to the observed variability?
FIGURE 1.
Perceptual framework for the transition from a population perspective of inter-individual variability to one that focuses on individual patients: average plasma concentration-time profile for a group (panel A), representing inter-individual variability as the mean ± one standard deviation of the plasma concentration at each time point (panel B), individual plasma concentration profiles contributing to the population mean (panel C), and identification of factors, such as pharmacogenetic data, that allow the range of possibilities for an individual patient to be reduced (panel D). Raw data used to construct the figure were derived from Brown et al2, and symbol colors in paned D refer to CYP2D6 genotypes corresponding to the following phenotypes: poor metabolizers (no functional alleles; red), intermediate metabolizers (one null allele and one partial function allele; yellow), extensive metabolizers with the equivalent of one functional allele (green), and extensive metabolizers with two functional alleles (blue).
The data presented in Fig. 1 were derived from a previously published study of atomoxetine in patients with attention-deficit/hyperactivity disorder (ADHD)2 in which study participants were administered the recommended starting dose of the drug. Consideration of a sample mean ± SD may not allow the extent of individual variability in systemic exposure present in a patient population to be readily appreciated, especially when polymorphic clearance pathways are involved. For atomoxetine CYP2D6 is the primary clearance pathway, and knowledge of individual CYP2D6 genotype helps to explain some of the observed variability (Fig. 1D); however, inspection of the green (one functional CYP2D6 allele) and blue points (two functional alleles) in Fig. 1D, reveals considerable inter-individual variability in plasma concentrations at a given time point after dosing. In the absence of CYP2D6 genotype information for an individual patient about to be prescribed the medication, it is difficult to know which of the profiles in Fig. 1C that patient is likely to have. Furthermore, if the patient does not experience the desired therapeutic response, what is the next step? Increase the dose? Try a different medication? In the absence of additional individual information, such as plasma concentration data to guide the decision-making process (e.g., low concentrations/systemic exposure may benefit from an increase in dose, whereas lack of response in the presence of high systemic exposures may reflect a patient who will not respond to this medication regardless of exposure and require a change in medication), precision therapeutics is a mirage.
When one considers that in the Mayo study1 31% and 37% of patients had actionable variants in two or three of the genes, respectively, and three times as many patients (3%) had actionable variants in all five genes as the number of patients with no actionable variants it becomes apparent that on a population basis, individuals with a “normal metabolizer” status across the board represent a relatively small segment of the population, whereas an “average patient” is, in fact, likely to harbor actionable variants in 2–3 genes. It is also important to recognize that non-genetic sources of variability (not to mention day-to-day variability) contribute to observed variability within a given genotype group as we discovered in an investigation of pravastatin pharmacogenetics in children and adolescents published recently in CP&T. In other words, the more unique we are as individuals, the more we will deviate from the population average. If this is the case, how many parents will be satisfied knowing that their child will fall somewhere within the range of values presented in Fig. 1C after receiving the FDA-recommended starting dose of atomoxetine? I wouldn’t be … for my child, or for myself.
MOVING BEYOND DOSE TO EXPOSURE AND RESPONSE
Given the range of exposures present in the population, what exposure should be targeted to optimize drug response? “Somewhere in the middle” would not be an uncommon response, and in a recent commentary my colleagues and I rather simplistically suggest that the optimum exposure may be a function of drug target expression, itself subject to influence by genetic variation. Extending this argument further we illustrate the challenges of translating drug target genotype information from “population” statistical association studies to actionable information for individual patients3. Why is that our traditional approaches to collect more and more data on a population basis (covariates for population pharmacokinetic models; genetic variants associated with drug response) have not translated into a readily demonstrative impact on the treatment of individual patients?
POPULATION DATA AS A SOLUTION
I propose that the hope for precision therapeutic strategies that are truly individualized lies with … population data -- not bludgeoning problems into submission simply by collecting more, and more, and more data, but being more intelligent about what data will be collected and how they will be used. Recently, Eric Topol described his journey with big data and artificial intelligence to shatter the naïveté of an optimal diet for all people4. Analogously, testing for a limited number of common variants in one or two genes to predict drug response seems rather naïve considering the number of genes involved the processes of absorption, distribution, metabolism, excretion, interaction with cellular targets, and the accompanying downstream signal transduction pathways that occur between drug ingestion and therapeutic response. Furthermore, the impact of rare variants with large effects as predictors of individual drug responses cannot be ignored. The challenge is to collect data reflecting the uniqueness of an individual’s genome, metabolome, microbiome, current state of health (including pre-existing medical conditions and concurrent medications), lifestyle (diet, stress level, physical activity, sleep) on many people – a population – and allow machine learning algorithms to select the features most relevant and informative for a particular situation. Examples of artificial intelligence successfully applied to predict drug response are encouraging5, but successful clinical implementation on a population basis will require further evidence from prospective clinical trials that the method performs as intended in a broader range of patients than originally studied.
CONCLUSION
Asking “What dose (and implicitly implied exposure at the site of action), on average, is safe and effective for the patient population receiving a medication” is very different from “What dose (exposure) is right for this patient”?; data traditionally collected to answer the first question may not be sufficient to answer the second. The challenge is to think beyond patient characteristics as covariates for models, and expand the data acquisition process to include big data sources that facilitate development of tools to recognize the uniqueness of not-so-average individual patients.
Acknowledgments
FUNDING
Work by the author related to precision therapeutics and individualization of drug treatment is funded by grant 1 U54 HD090258, Genomic- and Ontogeny-Linked Dose Individualization and cLinical Optimization for Kids, from the Eunice Kennedy Shriver National Institute of Child Health and Human Development, and support from the Sarli Family Foundation.
Footnotes
CONFLICT OF INTEREST
The authors declared no competing interests for this work.
References
- 1.Ji Y, Skierka JM, Blommel JH, Moore BE, VanCuyk DL, Bruflat JK, Peterson LM, Veldhuizen TL, Fadra N, Peterson SE, Lagerstedt SA, Train LJ, Baudhuin LM, Klee EW, Ferber MJ, Bielinski SJ, Caraballo PJ, Weinshilboum RM, Black JL. Preemptive pharmacogenomic testing for precision medicine: A comprehensive analysis of five actionable pharmacogenomic genes using next-generation DNA sequencing and a customized CYP2D6 genotyping cascade. J. Mol. Diagn 18, 438–445 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Brown JT, Abdel-Rahman SM, van Haandel L, Gaedigk A, Lin YS, Leeder JS. Single dose, CYP2D6 genotype-stratified pharmacokinetic study of atomoxetine in children with ADHD. Clin Pharmacol Ther 99:642–50 (2016) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.McLaughlin MJ, Wagner JB, Carleton B, Shakhnovich V, Leeder JS. Considerations for implementing precision therapeutics in children. Clin Transl Sci 12:140–150 (2019) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Topol E. https://www.nytimes.com/2019/03/02/opinion/sunday/diet-artificial-intelligence-diabetes.html (accessed June 17, 2019).
- 5.Athreya AP, Neavin D, Carillo-Roa T, Skime M, Biernacka J, Frye MA, Rush AJ, Wang L, Binder EB, Iyer RK, Weinshilboum RM, Bobo WV. Pharmacogenomics-driven prediction of antidepressant treatment outcomes: A machine learning approach with multi-trial replication. Clin Pharmacol Ther doi: 10.1002/cpt.1482 (2019) [DOI] [PMC free article] [PubMed] [Google Scholar]