Abstract
Designer-receptors exclusively activated by designer drugs (DREADDs) are extensively used to modulate neuronal activity in rodents, but their use in primates remains limited. An essential need that remains is the demonstration that DREADDs are efficiently expressed on the plasma membrane of primate neurons. To address this issue, electron microscopy immunogold was used to determine the subcellular localization of the AAV vector-induced DREADDs hM4Di and hM3Dq fused to different tags in various brain areas of rhesus monkeys and mice. When hM4Di was fused to mCherry, the immunogold labeling was mostly confined to the intracellular space, and poorly expressed at the plasma membrane in monkey dendrites. In contrast, the hM4Di-mCherry labeling was mostly localized to the dendritic plasma membrane in mouse neurons, suggesting species differences in the plasma membrane expression of these exogenous proteins. The lack of hM4Di plasma membrane expression may limit the functional effects of systemic administration of DREADD-actuators in monkey neurons. Removing the mCherry and fusing of hM4Di with the hemagglutinin (HA) tag resulted in strong neuronal plasma membrane immunogold labeling in both monkeys and mice neurons. Finally, hM3Dq-mCherry was expressed mostly at the plasma membrane in monkey neurons, indicating that the fusion of mCherry with hM3Dq does not hamper membrane incorporation of this specific DREADD. Our results suggest that the pattern of ultrastructural expression of DREADDs in monkey neurons depends on the DREADD/tag combination. Therefore, a preliminary characterization of plasma membrane expression of specific DREADD/tag combinations is recommended when using chemogenetic approaches in primates.
Keywords: immunogold, hM4Di, hM3Dq, mCherry, HA-tag
Graphical Abstract
We examined the ultrastructural localization of the DREADDs hM4Di and hM3Dq fused to different tags in monkey and mouse neurons. Poor plasma membrane expression was observed with hM4Di-mCherry in monkeys. In contrast, strong plasma membrane localization was found with HA-hM4Di in mice and monkeys, hM4Di-mCherry in mice and hM3Dq-mCherry in monkeys. Our results suggest that ultrastructural expression of DREADDs depends on the species, and the DREADD/tag combination.
Introduction
Chemogenetic techniques based on Designer Receptors Exclusively Activated by Designer Drugs (DREADDs) have been extensively used to study brain circuits related to sleep, feeding, anxiety, and movement, among many others (Roth, 2016). Due to its minimal invasiveness, adjustability and reversibility, the chemogenetic approach is attractive to basic and translational neuroscience research, as it allows precise modulation of selected neuronal subpopulations, through systemic injections of pharmacological compounds that activate the DREADDs (Rogan & Roth, 2011; Sternson & Roth, 2014). The DREADD-based methods, however, have been applied mostly in rodent studies, while only a handful of reports using DREADDs in non-human primates (NHPs) have been published (Eldridge et al., 2016; Grayson et al., 2016; Nagai et al., 2016; Upright et al., 2018).
Because of the translational significance of NHP studies, based on the close evolutionary relationship between NHPs and humans (Capitanio & Emborg, 2008; Phillips et al., 2014; Roelfsema & Treue, 2014), it is essential to optimize the use of DREADDs (and other chemogenetic approaches) for NHP studies, so as to confirm and expand the impressive advances already achieved in rodent models, and to take a step closer to using these promising techniques as therapeutic approaches for humans.
To further enable the effective use of DREADDs in NHPs and its potential utility for human interventions, it is important to characterize DREADDs expression at cellular and subcellular levels in NHPs. In published studies in which DREADDs have been used in NHPs, the receptor expression has been confirmed post-mortem by light-microscopy visualization of the tag proteins fused to the DREADDs (Eldridge et al., 2016; Grayson et al., 2016; Nagai et al., 2016; Upright et al., 2018). To help interpret the functional capacity of DREADDs, it is also important to demonstrate that DREADDs are inserted into the plasma membrane, where the receptors are functionally linked to their intracellular signaling pathways and can engage with their exogenous ligands (clozapine-n-oxide or clozapine, Armbruster et al., 2007; Gomez et al., 2017). High-resolution electron microscopic (EM) methods can be used to study the ultrastructural localization of endogenous or transduced receptors, including the spatial relationship of receptors and the cytoplasmic plasma membrane. In particular, the use of non-diffusible colloidal gold particles as markers of antigens in the EM-immunogold technique permits reliable localization of membrane proteins with nanometer resolution (Galvan et al., 2006). Previously, we successfully used this approach in monkey tissue to demonstrate that the viral vector-induced expression of channelrhodopsins (ChR2) occurs primarily in the plasma membrane of striatal and thalamic neurons, where they can be effectively activated by light stimulation (Galvan et al., 2012).
In the current study, we use the immunogold method to demonstrate that DREADDs can also be expressed in primate neuronal plasma membranes. The findings suggest that the specificity of the combination of the DREADD and the tag protein plays an important role in the ability of the DREADD to be inserted in the plasma membrane in monkey neurons. The results of this work will help to refine strategies aimed at achieving the effective use of DREADDs in NHPs.
Methods
Animals
These studies were done in accordance with federal guidelines (NRC, 2011), and the United States Public Health Service Policy on the Humane Care and Use of Laboratory Animals (revised 2015), and were approved by the Animal Care and Use Committees at Emory University, University of Wisconsin-Madison and the National Institute on Drug Abuse (NIDA).
Five rhesus macaques (Macaca mulatto, 3 females and 2 males, ages 4 to 14 years old at the time of virus solution injection) were obtained from the colonies at the Yerkes National Primate Research Center (YNPRC, Atlanta, GA), or the Wisconsin National Primate Research Center (WNPRC, Madison, WI). The animals were housed in pairs (YNPRC), or single housed (WNPRC, due to a previous unrelated immunization study), maintained in a controlled environment with a 12-hour light/dark cycle, had free access to water, and were fed twice daily. Five mice (male, C57/Bl6J, 25–30g at the time of the injection) were ordered from Jackson Laboratory, and housed 4 per cage until surgery, and individually housed afterwards. They were fed regular chow and water ad libitum and maintained at a 12-hour light/dark cycle.
Viral vectors and intracerebral injections
Animals received adeno-associated virus encoding hM4Di or hM3Dq fused to mCherry at the C-terminus (AAV-hSyn-hM4Di-mCherry and AAV-hSyn-hM3Dq-mCherry plasmids provided by Dr. Bryan Roth, UNC) or hM4Di fused to HA tag at the N-terminus (AAV-hSyn-HA-hM4Di). To construct the AAV-hSyn-HA-hM4Di plasmid (Addgene plasmid # 121538 ; http://n2t.net/addgene:121538; RRID:Addgene_121538), the sequence encoding HA-tagged hM4D(Gi) was amplified from pAAV-hSyn-HA-hM4D(Gi)-IRES-mCitrine and used to replace the FLAG-tagged iRFP coding region in pOTTC1479 (pAAV SYN1 iRFP-FLAG, unpublished) using ligation-independent cloning. pAAV-hSyn-HA-hM4D(Gi)-IRES-mCitrine was a gift from Bryan Roth (Addgene plasmid # 50464; http://n2t.net/addgene:50464; RRID:Addgene_50464). Table 1 describes sources for the viral vectors, details of the injection sites, and volume of virus solution injected in each animal.
Table 1:
Details of viral injections and systemic injections
| Animal ID |
Species | Injection location |
Vector | Titer (vg/ml) |
Amount injected, (μl) |
Number of systemic CNO or CLZ injections |
Survival days after viral injection |
Survival days after last CNO or CLZ injection |
Days from perfusion to immunohisto- chemistry |
|---|---|---|---|---|---|---|---|---|---|
| MR264 | Rhesus macaque | BLA, left# | AAV5-hSyn-hM4Di-mCherrya | 1.3×1013 | 40 | 9 | 651 | 73 | 180 |
| MR263 | Rhesus macaque | STN, left | AAV5-hSyn-hM4Di-mCherrya | 4.1×1013 | 10 | 3 | 70 | 15 | 181 |
| MR275 | Rhesus macaque | STN, left | AAV5-hSyn-hM4Di-mCherrya | 4.1×1013 | 12 | 27 | 417 | 143 | 62 |
| STN, right | AAV8-hSyn-hM4Di-mCherrya | 2×1012 | 28 | 279 | |||||
| MR290 | Rhesus macaque | Dorsal amygdala, right | AAV5-hSyn-HA-hM4Dic | 6.9×1013 | 24 | 1* | 36 | 7 | 139 |
| Frontal cortex, right | 15 | 50 | |||||||
| MR293 | Rhesus macaque | Cortex area 25 | hSyn-hM3Dq-mCherrya | 3.4×1012 | 22 | 1* | 41 | 14 | 96 |
| RM8, RM9 | Mice | BLA, bilateral | AAV8-hSyn-hM4Di-mCherryb | 3×1012 | 1 | 0 | 9 | n/a | 42 |
| RM34, RM35, RM36 | Mice | BLA,bilateral | AAV5-hSyn-HA-hM4Dic | 4.25×1012 | 0.3 (per side) | 1 * | 35 | 7 | 36 |
Viral vector sources:
University of North Carolina Vector Core,
Addgene,
Boston Children’s Hospital Viral Core
Abbreviations: CNO, clozapine-N-oxide; CLZ, clozapine
For monkey MR264, only the injection in the left BLA was examined, due to poor tissue preservation in the right hemisphere.
Only one injection of trace dose of [C11]-clozapine
In monkey MR264, the injection of the virus solution followed procedures similar to those described in previous studies of amygdala lesions in monkeys (Raper et al., 2014). Briefly, the monkey was sedated with ketamine hydrochloride (1mg/kg) and maintained with isoflurane (1–2% intratracheal) throughout the T1 weighted MRI scan to determine the stereotaxic coordinates of the amygdala. Under aseptic conditions and isoflurane anesthesia (1–2% to effect), craniotomies were made over the left and right amygdala and the viral vector solution was administered in six to eight injection sites within the center of the basolateral amygdala, using a 10 μl Hamilton syringe. Needles were lowered simultaneously in both hemispheres, and the solution was infused at a rate of 0.4 μl/min. After each injection, a 3 min waiting period was allotted to minimize viral spread during needle retractions. Post-surgical treatments included banamine (1 mg/kg for 3 days), dexamethasone (0.5 mg/kg for 3 days), and rocephin, 25 mg/kg for 7 days) to prevent pain, edema, and infection, respectively.
In monkeys MR263 and MR275, the injections of the virus solution were done through chronically implanted recording chambers directed at the subthalamic nucleus (STN). The placement of chambers was done during a surgical procedure performed under anesthesia (1– 3% intratracheal isoflurane) and using aseptic techniques, as previously described (Galvan et al., 2014). Post-surgical treatments were the same as those described above for monkey MR264. The injections of virus solutions were done under electrophysiological guidance, using a custom-made injection system combined with a tungsten electrode (Kliem & Wichmann, 2004). The injection-recording probes were lowered in the STN and the solution was infused at a rate of 0.1–0.2 μl /min, followed by a 10 min wait time before withdrawing the injection system.
In monkeys MR290 and MR293, targeting of the injection site and subsequent viral vector delivery were performed using intraoperative MRI (iMRI) methods (for details, see Kalin et al., 2016). Briefly, before the procedure, the animals were anesthetized with ketamine (up to 20 mg/kg), prepared for surgery, and then placed in a magnetic resonance imaging (MRI)-compatible stereotaxic frame. The animals were intubated and received isoflurane anesthesia (1–3%, intratracheal). Injections of the viral vector solutions were done into the arcuate sulcus of the frontal cortex and the dorsal amygdala of the right hemisphere (MR290), or into cortical area 25 of the left hemisphere (MR293). The viral vector solution was infused along with 0.66 mM Gadobenate dimeglumine to visualize dispersion of the infusate on the MRI. After the surgery, cefazolin (20–25 mg/kg, twice daily for 5 days) and buprenorphine (up to 0.03 mg/kg, twice daily for one day) were given to prevent infection and minimize discomfort.
All monkey surgeries conducted at the YNPRC or WNPRC were done while monitoring vital signs, (heart rate, respiration, oxygen saturation, and CO2), and post-surgical treatments were administered in coordination with the respective veterinary staff.
For intracerebral injections in mice, the animals were anesthetized with a mixture of ketamine (60 mg/kg) and xylazine (12 mg/kg), placed on a stereotaxic apparatus, and received bilateral injections of virus solution (Table 1) in the basolateral amygdala (BLA). Coordinates of injections to target the BLA were: 1.3 mm posterior, ±2.8 mm lateral and 5.0 mm ventral from Bregma (according to the Paxinos mouse brain atlas, (Paxinos & Franklin, 2012). Virus solutions were injected using a 2 μl Hamilton syringe over 10 min, at a rate of 50 nl/min, and the syringe was left in place for an additional 10 minutes to allow diffusion of the suspension. During surgery, mice body temperature was monitored and controlled using a heat pad during surgical procedures and recovery from anesthesia. After surgery, the animals were treated with a saline injection (20 ml/kg) for hydration, and carprofen (5 mg/kg) for analgesia.
Systemic injections of clozapine-n-oxide or clozapine
In the context of other studies that examined functional effects of DREADDs (not reported here), some of the monkeys received systemic injections of clozapine-n-oxide (10 mg/kg each time), while some of the mice and two monkeys received one injection of a trace dose of clozapine. Table 1 provides details of the number of injections done in each animal.
Perfusion and initial preparation of tissue
The animals received an overdose of pentobarbital, and were transcardially perfused with Ringer’s solution, followed by 4% paraformaldehyde and 0.1% glutaraldehyde in phosphate buffer (T, 0.2 M, pH 7.4). After removing the brain from the skull, we collected vibratome coronal sections (60 μm) in cold phosphate-buffered saline (PBS, 0.01M, pH 7.4), that were stored at −20° C in an anti-freeze solution (30% ethylene glycol/30% glycerol in PB), until further processing (Table 1). The period of storage time in the freezer did not affect the quality of immunoreactivity.
Immunohistochemistry and processing of tissue for light and electron microscope
We used antibodies against mCherry or HA-tag and the pre-embedding immunogold method to examine the subcellular localization of hM4Di or hM3Dq. We first used light microscopy immunoperoxidase to localize the viral vector injection sites, and identify regions appropriate for the ultrastructural analysis. For this, we selected brain sections containing or adjacent to the virus solution injection tracks. Sections were pretreated with 1% normal goat serum, 1% bovine serum albumin and 0.3% Triton X-100, and then incubated in the respective antibody solution (mCherry or HA-Tag, Table 2) for 24 hours. This was followed by incubation in secondary biotinylated antibodies, then in Avidin-biotin-peroxidase complex (ABC) solution (1:200; Vectastain standard kit, Vector) for 90 min. The sections were then placed in 0.025% 3–3’-diaminobenzidine tetrahydrochloride (DAB, Sigma-Aldrich, St. Louis, MO), 0.01M Imidazole (Fisher Scientific, Pittsburgh, PA) and 0.006% H2O2 for 10 min. The sections were mounted on slides, cover-slipped, and digitized with an Aperio Scanscope CS system (Aperio Technologies, Vista, CA).
Table 2:
Sources and concentrations of antibodies
| Antibody | Vendor | Catalog number |
Antibody registry number |
Host | Concentration used |
|---|---|---|---|---|---|
| Anti-mCherry | Abcam, Cambridge, MA | AB167453 | AB_2571870 | Rabbit | 1:1,000 |
| Anti-HA Tag (clone C29F4) | Cell Signaling Technology, Danvers, MA | 3724 | AB_1549585 | Rabbit | 1:400 |
| Gold-conjugated anti-rabbit, (1. 4 nm gold particle size) | Nanoprobes, Stony Brook, NY | 2004 | AB_2631182 | Goat | 1:100 |
| Biotinylated anti-rabbit | Vector Laboratories, Burlingame, CA | BA-1000 | AB_2313606 | Goat | 1:200 |
To conduct the ultrastructural analysis of the localization of DREADDs, we selected sections adjacent to those showing strong immunoperoxidase labeling. These sections were treated with 1% sodium borohydride (20 min), placed in a cryoprotectant solution (PB, 0.05 M, pH 7.4, containing 25% sucrose /10% glycerol) for 20 min, frozen at −80° C for 20 min, thawed and washed in PBS. Then, to block non-specific sites, the sections were immersed in PBS containing 5% non-fat dry milk (20 min), and subsequently washed three times in Tris-buffered saline (TBS)-gelatin (Tris 0.02 M, NaCl 0.15 M and 0.1% cold water fish gelatin, 10 min each wash). Control sections were incubated without the primary antibodies. The sections were incubated with the primary antibodies against mCherry or HA-Tag (Table 2) for 24 h. This was followed by rinses in TBS-gelatin and incubation in gold-conjugated secondary antibodies (Table 2) for 2 h. Primary and secondary antibodies were diluted in TBS-gelatin buffer containing 1% dry milk, and antibody incubations were done at room temperature.
The sections were then washed with TBS-gelatin and 2% acetate buffer (pH 7.0) and the gold labeling was silver-intensified (HQ silver; Nanoprobes) for 9–13 min in the dark. The intensification process was stopped by washing several times with acetate buffer. The tissue was then post-fixed in osmium tetroxide (0.5% in 0.1 M PB, pH 7.4), rinsed in PB, and dehydrated by incubating the sections sequentially in 50%, 70% (plus uranyl acetate 1%), 90% and 100% ethanol (10–15 min in each solution, 100% ethanol incubation was repeated twice). Then, the sections were incubated in propylene oxide for 10 min. After post-fixation, the sections were embedded in resin (Durcupan ACM; Fluka, Ft. Washington, PA) on microscope slides, and placed in a 60°C oven for 48 h to polymerize the resin. The sections were examined under the light microscope, and blocks of tissue in the regions of interest were cut and glued onto resin blocks. Ultrathin (60 nm) sections were obtained with an ultramicrotome (Ultracut T2; Leica, Nussloch, Germany) and collected on pioloform-coated copper grids. Sections were stained with lead citrate for 5 min to increase their contrast during EM visualization. The sections were examined with an electron microscope (JEOL model 1011, Peabody, MA) at 20,000–25,000X, and areas containing immunogold particles were photographed with a digital camera (DualView 300W; Gatan, Inc., Pleasanton, CA) controlled by Digital Micrograph software (Gatan).
Analysis of material
Only micrographs obtained from areas with optimal ultrastructural preservation of brain tissue were used for the analysis. We collected a minimum of 25 immunogold-labeled elements (dendrites or terminals) per case. The number of elements collected varied per case depending on the areas of tissue with optimal ultrastructural preservation. The localization and quantification of labeling in the micrographs were assessed using ImageJ (Rasband, 1997; Schneider et al., 2012). Structures containing gold particles were categorized into dendrites or axonal terminals, based on ultrastructural features (Peters et al., 1991). Silver enhancement confers variable sizes to immunogold particles, which sometimes results in particle accumulations or clusters, in which individual gold particles are not easily defined. To quantify gold particles in clusters, discernable circular contours in the cluster were counted as individual particles. In cases where the cluster was amorphous, it was counted as one particle.
For the analysis, only dendrites or axon terminals of which the full length of the plasma membrane was clearly visible in the micrograph were selected. Based on the size of primary and secondary antibodies (~17 nm, Blackstad et al., 1990) plus the radius of the gold particle (0.7 nm), a particle was categorized as “plasma membrane bound” if it was located within ~18 nm from the membrane (measurements were done using ImageJ). All other gold particles were categorized as “intracellular”. The proportion of plasma membrane-bound or intracellular particles was expressed as a fraction of the total number of gold particles within specific neuronal elements (dendrite or terminal). To analyze the relationship between the proportion of plasma membrane-bound gold particles and species (monkey or mouse), tag protein (mCherry or HA) or DREADD type (hM4Di or hM3Dq), we used a linear mixed effects model (SPSS version 24, IBM), that defined species, tag protein and DREADD type as fixed factors, and individual virus injections as random factor.
Finally, we examined the localization of plasma-membrane bound gold particles in relation to synapses, in the cases where synaptic densities were observed. Plasma membrane-bound gold particles were classified into extrasynaptic, if they were attached to parts of the plasma membrane not involved in synapses; or synaptic, if they were located in the main body of postsynaptic specializations. Synapses were classified as symmetric or asymmetric, or remained not classified when a clear identification was not possible.
Results
Localization of injection sites, selection of regions of interest and overall quantification
To identify the areas with strong DREADDs transduction, we used antibodies against mCherry or HA-tag, and revealed these antibodies using biotinylated antibodies and immunoperoxidase. Figure 1 shows representative images of brain sections close to the viral vector injection sites in some of the animals used in this study. In these sections, the core of the injection sites and the related spread of labeling could be assessed by the diffusion of the amorphous brown immunoperoxidase staining generated by DAB staining. Sections adjacent to those depicted in this figure were used for the ultrastructural analyses of DREADD localization in the electron microscope, using immunogold methods. Table 3 describes the total number or elements (dendrites or terminals) sampled and gold particles counted in each case.
Figure. 1: Representative images showing expression of DREADDs in monkey and mouse brain tissue after viral vector injections.
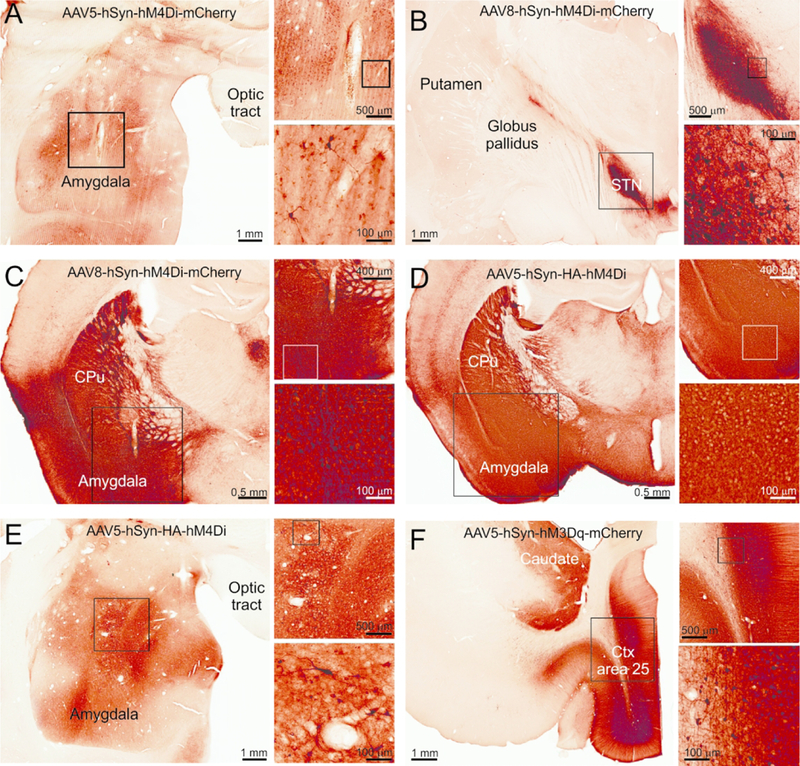
The immunoperoxidase method was used to reveal the tag proteins fused to the DREADDs. All the sections are adjacent or at the site of the injection track. A) Monkey MR264, B) Monkey MR275, C) Mouse RM9, D) Mouse RM34, E) Monkey MR290, F) Monkey MR293. In each panel, the rectangles indicate areas that are shown at higher magnification in the subsequent images.
Table 3:
Number of elements and particles quantified for each injection site
| Animal ID |
Species | Injection location |
Vector | No. elements counted |
Intra- cymiddlelasmic particles |
Membrane- bound particles |
|---|---|---|---|---|---|---|
| MR264 | Rhesus macaque | BLA, center | AAV5-hSyn-hM4Di-mCherrya | 25 | 429 | 63 |
| MR263 | Rhesus macaque | STN, left | AAV5-hSyn-hM4Di-mCherrya | 93 | 1291 | 271 |
| MR275 | Rhesus macaque | STN, left | AAV5-hSyn-hM4Di-mCherrya | 26 | 752 | 106 |
| STN, right | AAV8-hSyn-hM4Di-mCherrya | 33 | 2587 | 489 | ||
| MR290 | Rhesus macaque | Dorsal amygdala, right | AAV5-hSyn-HA-hM4Dic | 83 | 102 | 947 |
| Frontal cortex, right | 48 | 62 | 1564 | |||
| MR293 | Rhesus macaque | Cortex area 25 | hSyn-hM3Dq-mCherrya | 150 | 838 | 4130 |
| RM8 | Mouse | BLA, bilateral | AAV8-hSyn-hM4Di-mCherryb | 52 | 1260 | 1772 |
| RM9 | Mouse | BLA, bilateral | AAV8-hSyn-hM4Di-mCherryb | 39 | 388 | 866 |
| RM34 | Mouse | BLA, bilateral | AAV5-hSyn-HA-hM4Dic | 32 | 92 | 1299 |
| RM35 | Mouse | BLA, bilateral | AAV5-hSyn-HA-hM4Dic | 55 | 134 | 1903 |
| RM36 | Mouse | BLA, bilateral | AAV5-hSyn-HA-hM4Dic | 38 | 68 | 1566 |
Ultrastructural localization of hM4Di-mCherry
Three monkeys received injections of AAV-hSyn-hM4Di-mCherry (one of them in BLA, and the other two animals in the STN). Representative low-magnification images of the injection sites are shown in Fig. 1A and 1B.
The ultrastructural location of hM4Di-mCherry was analyzed at the EM level using immunogold. The majority of immunogold particles (85% of total number of particles, average for the four injections), were found in the intracellular space. The cellular organelles to which the gold particles were attached were putatively identified as microtubules or agranular reticulum. Only a small proportion of gold particles were localized on the plasma membrane (Fig. 2A-C, see summary of data in Fig. 5). This pattern of localization of hM4Di-mCherry was similar in the three monkeys, regardless of the injected target nuclei (basolateral amygdala or the subthalamic nucleus) or the AAV serotype (AAV5 or AAV8).
Figure 2. Ultrastructural localization of hM4Di-mCherry in monkeys and mice.
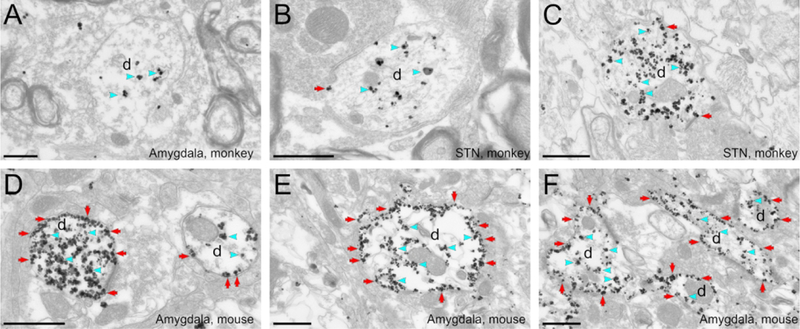
The pre-embedding immunogold method was used to reveal the hM4Di-mCherry. A to C: Electron micrographs showing examples of labeled dendrites in monkey amygdala (A) or STN (B, C). Immunogold particles labeling for hM4Di-mCherry is found mostly in the intracellular compartment (blue arrowheads). D to F: Electron micrographs of labeled dendrites in mice amygdala. The bulk of hM4Di-mCherry gold particles labeling is bound to the plasma membrane (red arrows). All scale bars= 0.6 μm. d, dendrite.
Figure 5: Summary of ultrastructural localization of DREADDs, as revealed by immunogold localization.
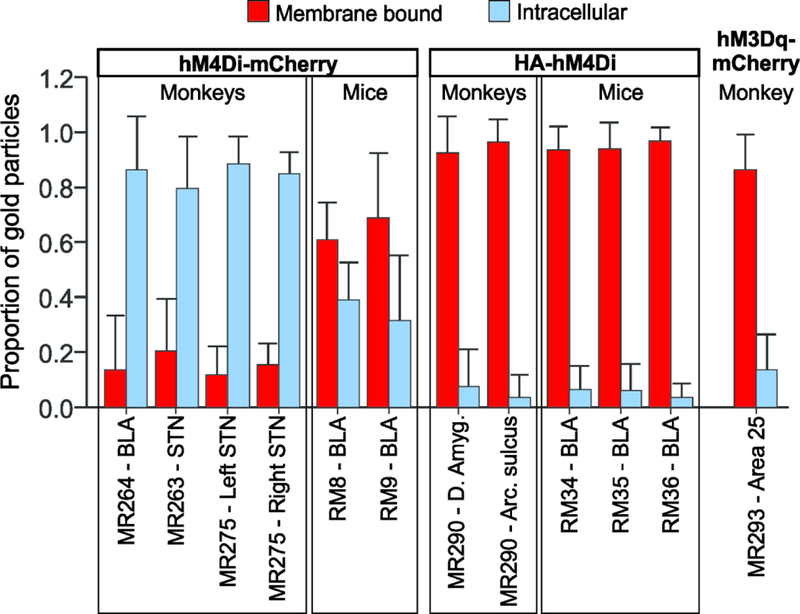
Proportion of gold particles bound to the plasma membrane (red) and in the intracellular compartment (blue) of labeled elements. Data are means + SD (the number of elements quantified for each case is shown in Table 3).
To investigate if this pattern of hM4Di-mCherry subcellular expression was specific to the monkey brain tissue, two mice were injected with AAV-hSyn-hM4Di-mCherry in BLA (Fig. 1C). The expression of hM4Di-mCherry in mice was strikingly different from that observed in monkeys. A large proportion of hM4Di-mCherry in mouse tissue was bound to the plasma membrane (64% of all particles, average for the 2 mice, Fig. 5), while a smaller fraction was observed in the intracellular compartment (Fig. 2D-E). These results suggest that the hM4Di-mCherry fusion has limited access to the dendritic plasma membrane of neurons in monkeys, but not in mice.
Ultrastructural localization of HA-hM4Di
Given the limited expression of hM4Di-mCherry at the plasma membrane in monkey neurons, we hypothesized that the use of a different tag fused to the hM4Di might improve the transport of this DREADD to the plasma membrane. The hemagglutinin (HA) tag is an epitope of the hemagglutinin of the influenza virus. Since its introduction thirty years ago (Field et al., 1988), the HA-tag has been extensively used to label proteins. Thus, we tested if the use of HA-tag fused to hM4Di could improve the transport of this receptor to the plasma membrane.
One monkey received two injections of AAV-hSyn-HA-hM4Di, one in the amygdala (Fig. 1E) and the second in the arcuate sulcus of the frontal cortex (not shown, light microscopy pattern of expression was similar to that observed in the amygdala). We conducted an ultrastructural analysis using immunogold particles to reveal antibodies against HA-tag. In striking contrast to the results obtained with hM4Di-mCherry, we found that the majority of HA-hM4Di immunogold labeling was bound to the plasma membrane in the monkey brain tissue (Fig. 3A-C). Only 7% of gold particles were localized in the intracellular space (Fig. 5). Similarly, in mice that received AAV-hSyn-HA-hM4Di in the amygdala (Fig. 1D), 94% of gold particles on average were bound to the plasma membrane (Fig. 3D-F, and Fig. 5). These results indicate that the fusion of HA-tag greatly improves the trafficking of hM4Di to the membrane, relative to fusion with mCherry. A linear mixed effects model confirmed that species, tag protein and the interaction of species and tag protein, were significant predictors of plasma membrane-bound particles (species: F(1, 7.1)=116.3, p<0.0001; tag protein: F(1, 7.1)=567.4, p<0.0001; interaction of species and tag protein: F(1, 7.1)=112.6, p<0.001).
Figure 3: HA-hM4Di localization in monkeys and mice.
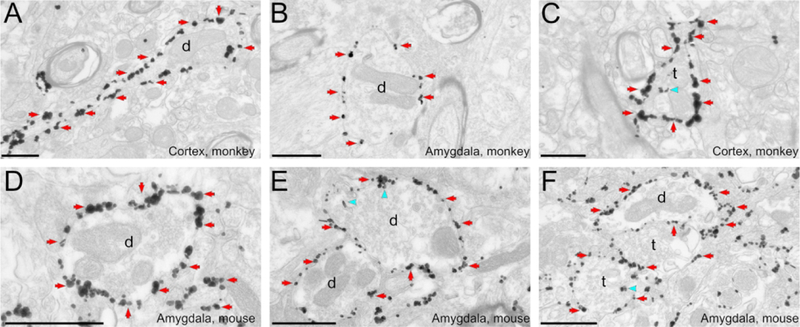
The immunogold method was used to reveal HA-hM4Di. A to C: Electron micrographs of labeled dendrites in the monkey arcuate sulcus (A,C) or amygdala (B). D to F: Electron micrographs of immunoreactive dendrites in the mouse amygdala. Most gold particles indicating HA-hM4Di are bound to the plasma membrane in both monkey and mice tissue. Blue arrowheads and red arrows point to examples of intracellular and plasma membrane-bound gold particles, respectively. All scale bars= 0.6 μm. d, dendrite; t, axon terminal
Ultrastructural localization of hM3Dq-mCherry
To investigate if the limited transport to the plasma membrane of hM4Di-mCherry was a general feature of mCherry-fused DREADDs in monkeys, we studied the ultrastructural localization of hM3Dq-mCherry, after viral injections were performed in area 25 of cortex of one monkey (Fig. 1F). In contrast to what we observed with the hM4Di-mCherry, we found that 83% of gold particles labeling hM3Dq-mCherry were bound to the plasma membrane (Fig. 4, and Fig. 5). In agreement, a linear mixed effect model showed that, when the DREADD is fused to mCherry, the type of DREADD can predict the proportion of plasma membrane-bound particles in monkey neurons (F(1, 2.2)=291.6, p=0.002). This observation suggests that fusion to mCherry does not hamper the transport of all DREADDS to the dendritic plasma membrane in monkey neurons, and that specific DREADD/Tag combinations exhibit different neuronal plasma membrane expression patterns.
Figure 4: hM3Dq-mCherry localization in monkey, as revealed with immunogold.
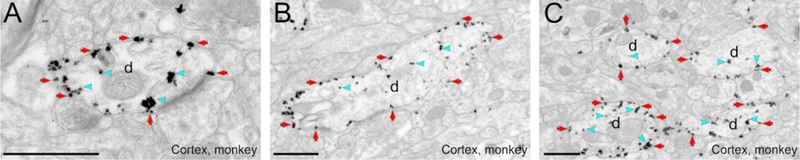
Electron micrographs of labeled dendrites in the monkey cortex, area 25. hM3Dq dendritic labeling is mostly bound to the plasma membrane. Blue arrowheads and red arrows point to examples of intracellular and plasma membrane bound gold particles, respectively. All scale bars= 0.6 μm. d, dendrite
Finally, we examined if plasma membrane-bound particles revealing hM4Di-mCherry, HA-Tag or hM3Dq-mCherry were associated with synapses. In all cases, and for both species, the immunogold particles were very rarely found at synapses; with only 0.1 to 1.4% of total plasma membrane-bound particles located at the main body of synaptic specializations. However, as we and others have previously reported, the pre-embedding immungold method may lead to false negative synaptic labeling for plasma-membrane bound receptors, perhaps due to the antibodies being hindered from accessing the antigenic sites in the synaptic cleft (Baude et al., 1993; Nusser et al., 1994; Galvan et al., 2006).
Discussion
Using the immunogold method and electron microscopic analysis, we have described the ultrastructural localization of hM4Di and hM3Dq in monkeys and mice.
We found that, in monkeys, hM4Di is poorly transported to the plasma membrane when fused to the mCherry tag protein. Conversely, a large proportion of immunogold particles revealing hM4Di-mCherry were observed at the plasma membrane in mouse neurons. The different expression patterns of hM4Di-mCherry between monkeys and mice suggests an important species difference in the intracellular trafficking and/or membrane insertion of these foreign proteins, although it is unclear why this is the case.
The pattern of hM4Di-mCherry expression in mice, with most of the gold-particles bound to the plasma membrane, is consistent with the robust behavioral effects observed during hM4Di-mediated neuronal silencing in numerous rodent studies (reviewed in Sternson & Roth, 2014; Roth, 2016). On the other hand, our findings suggest that the effects of systemically applied DREADD-activating compounds may be compromised by the low proportion of hM4Di at the plasma membrane in monkey neurons. However, it is possible that when activated by the exogenous compound, only a small number of plasma-membrane hM4Di receptors are necessary to decrease neuronal firing. Indeed, two studies have reported functional effects of hM4Di-mCherry activation in monkeys. In one study, hM4Di-mCherry receptors were expressed in the amygdala, and CNO injections altered amygdalo-cortical functional connectivity (Grayson et al., 2016), while in the second study CNO-mediated activation of hM4Di-mCherry in prefrontal cortex disrupted spatial working memory (Upright et al., 2018). Other studies, using a lentiviral vector to express hM4Di fused to cyan fluorescent protein (Eldridge et al., 2016) or hM4Di without a tag protein (Nagai et al., 2016) also showed behavioral changes with hM4Di activation in monkeys. Overall, these positive results strongly support use of DREADDs to modulate neuronal activity in monkeys. Furthermore, we suggest that increased trafficking of the receptors to the plasma membrane has the potential to facilitate more robust and consistent effects of chemogenetic silencing in monkeys.
The monkeys that were injected with AAV5-hSyn-hM4Di-mCherry received several systemic injections of CNO, while the mice were not treated with this drug (Table 2). Receptor internalization is known to occur for G-protein coupled receptors after agonist exposure (Gainetdinov et al., 2004), so we cannot completely rule out the possibility that the large proportion of hM4Di-mCherry gold particles found in the intracellular compartment in monkey neurons was a result of internalization. However, this is unlikely, because in our study there was an interval of at least 15 days (and up to 143 days; see Table 1) between the last CNO injection and euthanasia, allowing washing out of the drug and restoration of receptors to basal conditions (Brancaccio et al., 2013; Anaclet et al., 2015).
In contrast to the findings with hM4Di-mCherry, the same receptor showed robust plasma membrane expression when fused to the HA-tag, in both monkey and mouse neurons. Compared to mCherry, which is 256 amino acids, the HA tag is a small 9 amino acid fragment of hemagglutinin (Field et al., 1988). Given its small size, the fact that it can be readily detected using antibodies (Green et al., 1982), and the fact that it does not appear to interfere with the function of most recombinant proteins, this tag has been extensively used as a general epitope tag in viral vectors. Our results suggest that the HA tag is advantageous over mCherry when fused to hM4Di, because it does not hamper the positioning of hM4Di in the plasma membrane in primate neurons. The HA-tag is fused to the hM4Di at the N-terminus, leaving the C-terminus unhindered to incorporate with scaffolding proteins that might anchor the receptor to the membrane (Ritter & Hall, 2009).
One potential confound of our study is that the monkeys that received AAV-hM4Di-mCherry and those that received AAV-HA-hM4Di were obtained from different colonies, and the injections were done using slightly different methods. However, the improved trafficking of the hM4Di to the plasma membrane in the presence of the HA-tag was also observed in mice, further supporting the notion that this construct does not interfere with membrane trafficking of DREADDs. Our results suggest that hM4Di fused to HA could be a better and more reliable tool than hM4Di-mCherry to be used in monkey DREADD-related experiments. An additional consideration that favors HA over mCherry is that the expression of fluorescent proteins can induce immune responses and neuroinflammation in NHPs (Samaranch et al., 2014).
We also studied the localization of hM3Dq-mCherry in monkey neurons, and found that the fusion of this excitatory DREADD with the mCherry protein did not prevent transport to the plasma membrane. It is possible that structural differences between hM4Di and hM3Dq could account for the discrepant patterns of localization when fused to mCherry. For instance, the C-terminus is shorter in the M4 receptor than in the M3 receptor (Kruse et al., 2014). It is possible that a large tag protein (such as mCherry) fused to the C-terminus may be spaced far enough away from the receptor’s transmembrane core and cytoplasmic loops so it does not interfere with efficient membrane incorporation. In contrast, fusion of the same tag to the M4 receptor’s shorter C-terminus may interfere with the transmembrane core and either globally perturb receptor folding or hinder interactions with chaperone proteins that are necessary for proper plasma membrane transport, anchoring and/or recycling. Thus, the inclusion of mCherry fused to a DREADD does not always result in poor plasma membrane incorporation in primate neurons, but rather, the fusion of mCherry to specific exogenous proteins (i. e., hM4Di) can interfere with expression at the plasma membrane. Consistent with this idea, fusion of mCherry with the opsin ChR2 results in a lower expression level of the opsin than were observed upon ChR2 fusion to yellow fluorescent protein (Yazdan-Shahmorad et al., 2018).
A limitation of our study is that the observations of hM3Dq-mCherry localization were based on a single injection in one monkey. However, these data provide the first description of the ultrastructural localization of hM3Dq (in monkeys or other species), and suggests that the fusion of mCherry to hM3Dq does not hamper the incorporation of the receptor to the membrane, supporting the use of this construct in DREADD-related studies in monkeys.
In summary, this study reveals that the choice of tags to visualize the expression of DREADDs can significantly affect their subcellular localization and, presumably, their function in the primate brain. In addition, our observations suggest that the expression patterns of some exogenous proteins may not be the same in primate and rodent neurons, therefore highlighting the need to understand potential species-specific differences in viral vector-transduced protein expression. This consideration is of utmost importance in non-human primate experiments, where the number of subjects is typically low and the possibility of repeated experiments is limited. Thus, strategies to optimize the expression of DREADDs in NHPs, such as careful selection of tags to identify the receptor, can help further advance both the research and therapeutic use of chemogenetic approaches in monkeys and humans.
Acknowledgements
We thank Susan Jenkins for technical assistance, the staffs of the Yerkes National Primate Research Center (YNPRC), the Harlow Center for Biological Psychology, the HealthEmotions Research Institute at the University of Wisconsin-Madison, and the Wisconsin National Primate Research Center (WNPRC). We also thank Drs. Mark Baxter, Michael Caiola and Thomas Wichmann for constructive comments. We acknowledge the UNC Gene Therapy Vector Core and Dr. R. Jude Samulski, Addgene, and Boston Children’s Hospital Viral Core for manufacturing viral vectors used in this study. This work was funded by National Institutes of Health Office of Research Infrastructure Programs OD P51-OD011132 to the YNPRC, P51-OD011106 to the WNPRC, grant R01-MH046729 to NHK and National Institute on Drug Abuse Intramural Research Program support DA00069 to MM.
Abbreviations
- AAV
adeno-associated virus
- BLA
basolateral amygdala
- ChR2
channelrhodopsin
- CLZ
clozapine
- CNO
clozapine-N-oxide
- d
dendrite
- DREADDs
designer-receptors exclusively activated by designer drugs
- EM
electron microscope
- HA
hemagglutinin
- NHP
non-human primate
- PB
phosphate buffer
- PBS
phosphate-buffered saline
- STN
subthalamic nucleus
- t
axon terminal
- TBS
tris-buffered saline
Footnotes
Conflict of interest
The authors report no conflict of interests
Data accessibility
Data are available upon request to the corresponding author.
References
- Anaclet C, Pedersen NP, Ferrari LL, Venner A, Bass CE, Arrigoni E & Fuller PM (2015) Basal forebrain control of wakefulness and cortical rhythms. Nat Commun, 6, 8744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armbruster BN, Li X, Pausch MH, Herlitze S & Roth BL (2007) Evolving the lock to fit the key to create a family of G protein-coupled receptors potently activated by an inert ligand. Proc Natl Acad Sci U S A, 104, 5163–5168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baude A, Nusser Z, Roberts JD, Mulvihill E, McIlhinney RA & Somogyi P (1993) The metabotropic glutamate receptor (mGluR1 alpha) is concentrated at perisynaptic membrane of neuronal subpopulations as detected by immunogold reaction. Neuron, 11, 771–787. [DOI] [PubMed] [Google Scholar]
- Blackstad TW, Karagulle T & Ottersen OP (1990) MORFOREL, a computer program for two-dimensional analysis of micrographs of biological specimens, with emphasis on immunogold preparations. Comput Biol Med, 20, 15–34. [DOI] [PubMed] [Google Scholar]
- Brancaccio M, Maywood ES, Chesham JE, Loudon AS & Hastings MH (2013) A Gq-Ca2+ axis controls circuit-level encoding of circadian time in the suprachiasmatic nucleus. Neuron, 78, 714–728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Capitanio JP & Emborg ME (2008) Contributions of non-human primates to neuroscience research. Lancet, 371, 1126–1135. [DOI] [PubMed] [Google Scholar]
- Eldridge MA, Lerchner W, Saunders RC, Kaneko H, Krausz KW, Gonzalez FJ, Ji B, Higuchi M, Minamimoto T & Richmond BJ (2016) Chemogenetic disconnection of monkey orbitofrontal and rhinal cortex reversibly disrupts reward value. Nat Neurosci, 19, 37–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Field J, Nikawa J, Broek D, MacDonald B, Rodgers L, Wilson IA, Lerner RA & Wigler M (1988) Purification of a RAS-responsive adenylyl cyclase complex from Saccharomyces cerevisiae by use of an epitope addition method. Mol Cell Biol, 8, 2159–2165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gainetdinov RR, Premont RT, Bohn LM, Lefkowitz RJ & Caron MG (2004) Desensitization of G protein-coupled receptors and neuronal functions. Annu Rev Neurosci, 27, 107–144. [DOI] [PubMed] [Google Scholar]
- Galvan A, Hu X, Rommelfanger KS, Pare JF, Khan ZU, Smith Y & Wichmann T (2014) Localization and function of dopamine receptors in the subthalamic nucleus of normal and parkinsonian monkeys. J Neurophysiol, 112, 467–479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galvan A, Hu X, Smith Y & Wichmann T (2012) In vivo optogenetic control of striatal and thalamic neurons in non-human primates. PLoS One, 7, e50808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galvan A, Kuwajima M & Smith Y (2006) Glutamate and GABA receptors and transporters in the basal ganglia: what does their subsynaptic localization reveal about their function? Neuroscience, 143, 351–375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomez JL, Bonaventura J, Lesniak W, Mathews WB, Sysa-Shah P, Rodriguez LA, Ellis RJ, Richie CT, Harvey BK, Dannals RF, Pomper MG, Bonci A & Michaelides M (2017) Chemogenetics revealed: DREADD occupancy and activation via converted clozapine. Science, 357, 503–507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grayson DS, Bliss-Moreau E, Machado CJ, Bennett J, Shen K, Grant KA, Fair DA & Amaral DG (2016) The Rhesus Monkey Connectome Predicts Disrupted Functional Networks Resulting from Pharmacogenetic Inactivation of the Amygdala. Neuron, 91, 453–466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green N, Alexander H, Olson A, Alexander S, Shinnick TM, Sutcliffe JG & Lerner RA (1982) Immunogenic structure of the influenza virus hemagglutinin. Cell, 28, 477–487. [DOI] [PubMed] [Google Scholar]
- Kalin NH, Fox AS, Kovner R, Riedel MK, Fekete EM, Roseboom PH, Tromp do PM, Grabow BP, Olsen ME, Brodsky EK, McFarlin DR, Alexander AL, Emborg ME, Block WF, Fudge JL & Oler JA (2016) Overexpressing Corticotropin-Releasing Factor in the Primate Amygdala Increases Anxious Temperament and Alters Its Neural Circuit. Biol Psychiatry, 80, 345–355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kliem MA & Wichmann T (2004) A method to record changes in local neuronal discharge in response to infusion of small drug quantities in awake monkeys. J Neurosci Methods, 138, 45–49. [DOI] [PubMed] [Google Scholar]
- Kruse AC, Kobilka BK, Gautam D, Sexton PM, Christopoulos A & Wess J (2014) Muscarinic acetylcholine receptors: novel opportunities for drug development. Nat Rev Drug Discov, 13, 549–560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagai Y, Kikuchi E, Lerchner W, Inoue KI, Ji B, Eldridge MA, Kaneko H, Kimura Y, Oh-Nishi A, Hori Y, Kato Y, Hirabayashi T, Fujimoto A, Kumata K, Zhang MR, Aoki I, Suhara T, Higuchi M, Takada M, Richmond BJ & Minamimoto T (2016) PET imaging-guided chemogenetic silencing reveals a critical role of primate rostromedial caudate in reward evaluation. Nat Commun, 7, 13605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NRC (2011) Guide for the Care and Use of Laboratory Animals: Eighth Edition. The National Academies Press, Washington, DC. [Google Scholar]
- Nusser Z, Mulvihill E, Streit P & Somogyi P (1994) Subsynaptic segregation of metabotropic and ionotropic glutamate receptors as revealed by immunogold localization. Neuroscience, 61, 421–427. [DOI] [PubMed] [Google Scholar]
- Paxinos G & Franklin KBJ (2012) The Mouse Brain in Stereotaxic Coordinates. Academic Press, Sidney, Australia. [Google Scholar]
- Peters A, Palay S & Webster HD (1991) The Fine Structure of the Nervous System. Oxford University Press, New York. [Google Scholar]
- Phillips KA, Bales KL, Capitanio JP, Conley A, Czoty PW, t Hart BA, Hopkins WD, Hu SL, Miller LA, Nader MA, Nathanielsz PW, Rogers J, Shively CA & Voytko ML (2014) Why primate models matter. Am J Primatol, 76, 801–827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raper J, Stephens SB, Henry A, Villarreal T, Bachevalier J, Wallen K & Sanchez MM (2014) Neonatal amygdala lesions lead to increased activity of brain CRF systems and hypothalamic-pituitary-adrenal axis of juvenile rhesus monkeys. J Neurosci, 34, 11452–11460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rasband W (1997) ImageJ US National Institutes of Health, Bethesda, MD. [Google Scholar]
- Ritter SL & Hall RA (2009) Fine-tuning of GPCR activity by receptor-interacting proteins. Nat Rev Mol Cell Biol, 10, 819–830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roelfsema PR & Treue S (2014) Basic neuroscience research with nonhuman primates: a small but indispensable component of biomedical research. Neuron, 82, 1200–1204. [DOI] [PubMed] [Google Scholar]
- Rogan SC & Roth BL (2011) Remote control of neuronal signaling. Pharmacol Rev, 63, 291–315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roth Bryan L. (2016) DREADDs for Neuroscientists. Neuron, 89, 683–694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samaranch L, San Sebastian W, Kells AP, Salegio EA, Heller G, Bringas JR, Pivirotto P, DeArmond S, Forsayeth J & Bankiewicz KS (2014) AAV9-mediated expression of a non-self protein in nonhuman primate central nervous system triggers widespread neuroinflammation driven by antigen-presenting cell transduction. Mol Ther, 22, 329–337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider CA, Rasband WS & Eliceiri KW (2012) NIH Image to ImageJ: 25 years of image analysis. Nat Meth, 9, 671–675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sternson SM & Roth BL (2014) Chemogenetic tools to interrogate brain functions. Annu Rev Neurosci, 37, 387–407. [DOI] [PubMed] [Google Scholar]
- Upright NA, Brookshire SW, Schnebelen W, Damatac CG, Hof PR, Browning PGF, Croxson PL, Rudebeck PH & Baxter MG (2018) Behavioral effect of chemogenetic inhibition is directly related to receptor transduction levels in rhesus monkeys. J Neurosci. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yazdan-Shahmorad A, Tian N, Kharazia V, Samaranch L, Kells A, Bringas J, He J, Bankiewicz K & Sabes PN (2018) Widespread optogenetic expression in macaque cortex obtained with MR-guided, convection enhanced delivery (CED) of AAV vector to the thalamus. J Neurosci Methods, 293, 347–358. [DOI] [PubMed] [Google Scholar]


