Abstract
The incidences of heart failure with preserved ejection fraction (HFpEF) are increased in the aged populations as well as diabetes and hypertension. Coronary microvascular dysfunction has been contributed to the development of HFpEF. Endothelial cells (ECs) depend on glycolysis rather than oxidative phosphorylation for generating ATP to maintain vascular homeostasis. Glycolytic metabolism has a critical role in the process of angiogenesis since endothelial cells rely on the energy produced predominantly from glycolysis for migration and proliferation. Sirtuin 3 (SIRT3) is found predominantly in mitochondria and its expression declines progressively with aging, diabetes, obesity, and hypertension. Emerging evidence indicates that endothelial SIRT33 regulates a metabolic switch between glycolysis and mitochondrial respiration. SIRT3 deficiency in EC resulted in a significant decrease in glycolysis, whereas, it exhibited higher mitochondrial respiration and more prominent production of reactive oxygen species (ROS). SIRT3 deficiency also displayed strikingly increases in acetylation of p53, EC apoptosis, and senescence. Impairment of SIRT3-mediated EC metabolism may lead to a disruption of EC/pericyte/cardiomyocyte communications and coronary microvascular rarefaction which promotes cardiomyocyte hypoxia, Titin-based cardiomyocyte stiffness, and myocardial fibrosis thus leading to a diastolic dysfunction and HFpEF. This review summarizes current knowledge of SIRT3 in EC metabolic reprograming, EC/pericyte interactions, coronary microvascular dysfunction, and HFpEF.
Keywords: SIRTUIN3 (SIRT3), endothelial cell, glycolysis, mitochondrial respiration, angiogenesis, diastolic function, Heart failure with preserve ejection fraction (HFpEF)
Introduction
Heart failure (HF) is a progressive disease with high incidence that develops with advanced age, hypertension, and diabetes [1–3], 4, 5]. Each year about 600,000 patients are newly diagnosed with HF in the United States; costs of care are estimated at $34.8 billion per year. More than half of these patients have heart failure with preserved ejection fraction (HFpEF) [6, 7]. Heart failure with reduced ejection fraction (HFrEF) is another phenotype of HF that is defined as reduced left ventricle ejection fraction. So far, standard-of-care of HFrEF medications have failed to show efficacy in large clinical trials in patients with HFpEF [8]. HFpEF is commonly seen in older patients who usually have cardiovascular, metabolic, and inflammatory comorbidities [4]. Paulus and colleagues suggest that these comorbidities lead to systematic inflammation that results in coronary endothelial dysfunction and thus decreased bioavailability of NO which ultimately causes diastolic dysfunction [9]. Despite its importance, our understanding of HFpEF in regards to either pathophysiology or molecular mechanism is very limited.
Compelling evidence indicates that epigenetic modification is involved in the regulation of cellular function and stresses as well as progression of cardiovascular disease [5, 10]. Recently, Sirtuins have been investigated intensively regarding to the ability to regulate energy metabolism, reactive oxygen species (ROS) production, and cell survival [5]. In particular, SIRT3, a NAD+-dependent lysine residue deacetylase which was first identified in the heart mitochondria, has emerged as a novel regulator of mitochondrial function and cellular metabolism [5, 11–13]. As compared to younger individuals, SIRT3 levels were found to be decreased in old adults with sedentary lifestyles [14]. SIRT3 levels are also decreased in the heart of diabetic db/db mice which are associated with microvascular rarefaction and cardiac dysfunction in diabetes [15]. Our recent studies indicate that reduction of SIRT3 leads to a metabolic reprogramming in EC and diastolic dysfunction in mice that might be relevant to the HFpEF phenotypes such as aging, diabetes, obesity, and hypertension [16–19].
Endothelial glycolysis has a critical role in the regulation of angiogenesis since ECs rely on glycolysis-derived ATP for migration and proliferation [20, 21]. Among the many enzymes in the glycolytic metabolism of glucose, 6-phosphofructo-2-kinase/fructose-2, 6-bisphosphatase, isoform 3 (PFKFB3), is a key regulator of glycolysis in endothelial cells. PFKFB3 has been shown to promote endothelial proliferation and angiogenic sprouting [21–23]. Patients with HFpEF have coronary microvascular rarefaction and more cardiac hypertrophy [4]. At physiological conditions, myocardial growth and angiogenesis are well coordinated. However, with the presence of hypertension, coronary vascular disease, and myocardial infarction, cardiac hypertrophy and new vessel formation are imbalanced, known as pathological hypertrophy, leading to progression of eventual heart failure [24]. Coronary microvascular rarefaction with reduced coronary flow reserve (CFR) results in poor perfusion to the myocardium thus creating a hypoxic environment which would exaggerate ischemic injury and apoptosis in cardiomyocytes. Therefore, therapeutic myocardial angiogenesis and improvement of CFR are promising approaches for the prevention and treatment of heart failure [24, 25]. Previous study demonstrated that overexpression of angiogenic growth factor apelin increased myocardial vascular density and attenuated ischemia-induced heart failure (HF) in STZ-induced hyperglycemic mice, but these protective effects were abolished in STZ-SIRT3KO mice [26]. Although accumulating evidence reveals a regulatory role of SIRT3 in angiogenesis and HF [15, 26], there is still much to uncover about the precise mechanisms in the pathogenesis of HFpEF and HFrEF. This review discusses the emerging role of SIRT3 in reprograming cell metabolism, EC/pericyte interactions, and HFpEF.
Cell specific metabolic phenotypes of SIRT3
SIRT3 is a mitochondrial deacetylase and its expression is in the highest metabolically active organs including brain, heart, kidney, liver, and skeletal muscle [27]. SIRT3 has been shown to regulate almost every major aspect of mitochondrial function, including oxygen consumption, ATP generation, and ROS formation. Previous study revealed non-tissue-specific roles for SIRT3 in global metabolic homeostasis [28]. However, a recent study also indicated that SIRT3 regulates the acetyl-proteome in core mitochondrial processes common to brain, heart, kidney, liver, and skeletal muscle but regulates metabolic pathways differentially in fuel-producing and fuel-utilizing tissues [27]; this suggests SIRT3 regulates mitochondrial acetylation in a tissue-specific manner. Thus, functional role of SIRT3 is likely much more complex than initially appreciated, particularly involving tissue and cell specific. Some studies have been reported that deficiency of SIRT3 in myoblast and cancer cells decreases mitochondrial respiration and increases ROS formation [29, 30]. Moreover, respiratory capacity and ATP synthesis were decreased in cardiac mitochondria of SIRT3 KO mice [31]. Although the function of mitochondrial SIRT3 has been well investigated, the metabolic modifications of SIRT3 in EC has not been highlighted. We are particularly interested in the functional role of endothelial SIRT3 deficiency on the glucose metabolic switch and cardiac diastolic function. Our study demonstrated that loss of SIRT3 in EC reprogrammed cell metabolism by reduced glycolysis and increased oxidative phosphorylation [17]. Moreover, specific deletion of endothelial SIRT3 caused an impairment of coronary microvascular function and led to a diastolic dysfunction in mice. Our study indicated a cell specific role of SIRT3 on cell metabolism and diastolic function [17].
SIRT3, endothelial metabolic flexibility, and angiogenesis
ECs have high glycolytic activity that is comparable with that of tumor cells and much higher than that of cardiomyocytes and other oxidative cell types [23]. Recent study indicated that glycolytic flux in endothelial cells was over 200-fold higher than glucose oxidation, fatty acid oxidation, and glutamine oxidation; in addition, mitochondrial respiration was lower in ECs than in cardiomyocytes [21]. In the process of glycolysis, the conversion of fructose-6-phosphate (F6P) to fructose-1, 6-bisphosphate (F1,6P2) is catalyzed by 6-Phosphofructo-1 kinase (PFK-1). PFK-1, a bottleneck enzyme in the glycolytic pathway, is activated by its allosteric activator, fructose-2,6-bisphosphate (F2,6P2) [21, 23]. In endothelial cells, F2,6P2 is mainly synthesized and regulated by PFKFB3 and TP53-induced glycolysis and apoptosis regulator (TIGAR). Despite immediate access to oxygen in the blood, healthy ECs depend on glycolysis rather than oxidative phosphorylation to maintain vascular barrier function, tissue homeostasis, and capacity to quickly respond to stress such as hypoxia, nutrient deprivation, or tissue damage [21, 32]. ECs possess the ability to switch from oxidative phosphorylation to glycolysis as the primary energy source, known as metabolic flexibility, to protect cells with increased energy demand from elevated oxidative stress [4]. Upon stimulation in pathological conditions, ECs increase glycolytic flux mediated by, at least in part, upregulation of PFKFB3 [21, 33]. Previous study showed that expression of PFKFB3 was significantly reduced in the culture ECs isolated from SIRT3 KO mice. Moreover, the glycolytic function of SIRT3 KO-ECs was impaired, as evidenced by the decreased glycolysis, glycolytic capacity, and glycolytic reserve [17]. Intriguingly, loss of SIRT3 resulted in elevated basal oxygen consumption rate in ECs. These findings indicated SIRT3 deficiency in ECs resulted in a metabolic reprogramming that ECs were more depended on oxygen-dependent oxidative phosphorylation thus impaired metabolic flexibility. Moreover, the production of ROS was significantly increased in SIRT3 KO ECs [17]. Recent studies have shown that PFKFB3-mediated glycolysis is required for angiogenesis, and pharmacological inhibition of PFKFB3 blocks pathological angiogenesis [21–23]. Study also showed that inhibition of PFKFB3 suppressed endothelial glycolysis. This led to a significant reduction of angiogenic factors and increase in pro-inflammatory factors. These results support the possible mechanism of which SIRT3 deletion or inhibition of PFKFB3 triggers endothelial dysfunction via promoting pro-inflammatory state [17]. Impaired angiogenesis has been described in many cardiac disease associated with aging, diabetes, and obesity. Loss of SIRT3 impaired hypoxic signaling which altered glycolytic metabolism and oxygen consumption and, in turn, contributed to reduced angiogenic capabilities of ECs [16, 17, 19]. ECs are quiescent and rarely sprout, migrate, or proliferate under normal conditions. Under ischemic conditions, EC senses and responds to signals released from hypoxic tissue resulting in a metabolic shift to favoring glycolytic metabolism and leading to a highly migratory and proliferative phenotype to stimulate angiogenesis [12, 34]. This phenotype change is mainly mediated by the mechanisms involving HIFs activation, VEGF/VEGFR2, or FGF stimulation-induced glycolysis via PFKFB3 [21, 23, 35, 36]. Increasing the levels of HIFs via the inhibition of PHDs by a PHD inhibitor DMOG rescued the glycolysis in the SIRT3 KO ECs suggesting that HIFs are involved in the regulation of glycolytic metabolism [18]. The HIFs are the transcriptional factors that regulate oxygen-related energy metabolism and angiogenesis [37]. The level of HIFs has been shown to decrease with aging that is correlated to an increased expression of PHDs [37]. Treatment with PHD inhibitor DMOG dose-dependently upregulated the expression of HIF-2α and PFKFB3 in WT and SIRT3 ECs suggesting that deficiency of SIRT3 may damage HIF-2α signaling in endothelial cells during hypoxia; this may lead to impaired hypoxia-induced angiogenesis [16, 18].
Microvascular rarefaction, CFR, and HFpEF
Coronary flow reserve (CFR) is defined as the ratio of maximal flow velocity in hyperemia to baseline flow velocity and usually represents functional change of coronary microvasculature. In clinic, coronary microvascular function is mainly evaluated by determination of CFR. Reduction of CFR is strongly associated with cardiac mortality in patients with heart failure, especially in HFpEF. Diabetic patients have higher prevalence of reduced CFR [38]. Preexisting reduction of CFR in diabetic patients may contribute to the microvascular dysfunction and obstruction thus leading to no-reflow after percutaneous coronary intervention. Myocardial capillary density is the main determinant of CFR [39]. Impairment of angiogenesis and reduced myocardial capillary density (microvascular rarefaction) are considered a major feature of HFpEF [40]. Microvascular rarefaction decreased eNOS and NO bioavailability, impaired cGS/cGMP signaling pathway, and resulted in cardiomyocyte stiffness, cardiac hypertrophy, and HFpEF [40–43]. Microvascular rarefaction also led to an impairment of CFR which sensitized the heart to hypoxia that resulted in an increased ROS formation, cardiomyocyte death, and heart failure with reduced ejection fraction (HFrEF).
SIRT3 ECKO mice have been reported to develop a diastolic dysfunction without having abnormalities in systolic function [17]. HFpEF patients are usually presented with abnormalities in coronary microcirculation due to endothelial dysfunction and coronary microvascular rarefaction [4]. Endothelial dysfunction and decreased microvascular density may limit coronary blood flow during reactive hyperemia [4]. CFR was decreased in SIRT3 ECKO mice [17, 18] indicating impaired myocardial perfusion and possible LV remodeling such as dilated cardiomyopathy [44]. Indeed, LV end-diastolic dimension and end-diastolic volume were found to be significantly increased in SIRT3 ECKO mice [17, 18]. Increasing ROS formation has been shown to diminish the nitric oxide bioavailability in cardiomyocytes [45]. Deficiency of SIRT3 KO in ECs resulted in a dramatic increase in ROS formation suggesting a possible impaired NO-cGMP-dependent mechanism that contributes to decreased CFR and impaired cardiomyocytes relaxation leading to a subsequent prolonged IVRT [16–19]. The heart is one of the most oxygen- and energy-demanding organs which mainly depends on oxidative phosphorylation in mitochondria [46]. In addition, decreased glycolysis and higher oxygen demand in ECs mean less oxygen is delivered to the cardiomyocytes. Therefore, advanced microvascular dysfunction may contribute to chronic hypoxia in the heart. Normal tissue counters hypoxia by upregulation of expression of HIFα system and its downstream signaling angiogenic factors such as VEGF and Ang-1. It also promotes angiogenesis to preserve oxygen supply and alleviates hypoxia-induced injury. In contrast, hypoxia-induced expression of HIF-1α, HIF-2α, Ang-1, and VEGF was impaired in SIRT3 KO-ECs as well as endothelial angiogenic capabilities indicating a defective hypoxia response in SIRT3 ECKO mice [17]. Study has shown that knockout of HIF-2α has been shown to impair homeostasis of ROS and cause cardiac hypertrophy which is one of the key risk factors for diastolic dysfunction [47]. In addition, microRNA profile analysis of the isolated ECs projected a putative highly interconnected subnetwork that was linked to fibrosis involving Smad2/3, TGF-β1, and Notch 2 (Figure 1A and B). Taken together, all these changes could result in diastolic dysfunction and HFpEF phenotype.
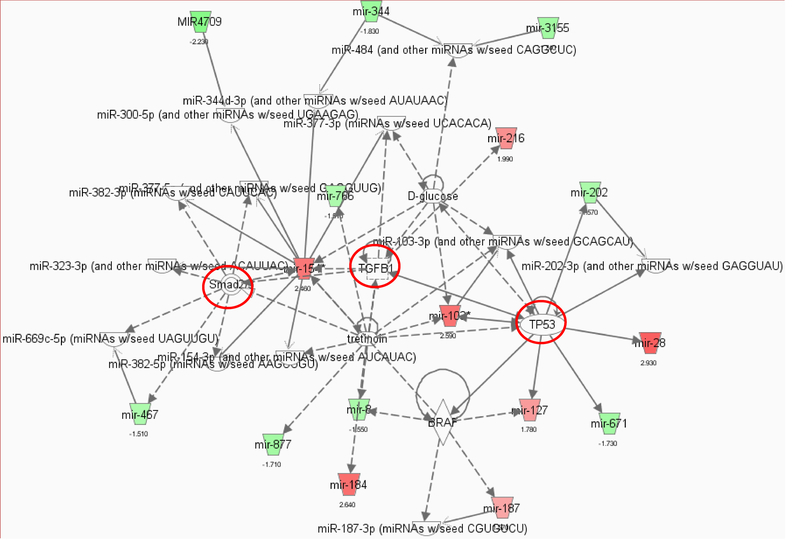
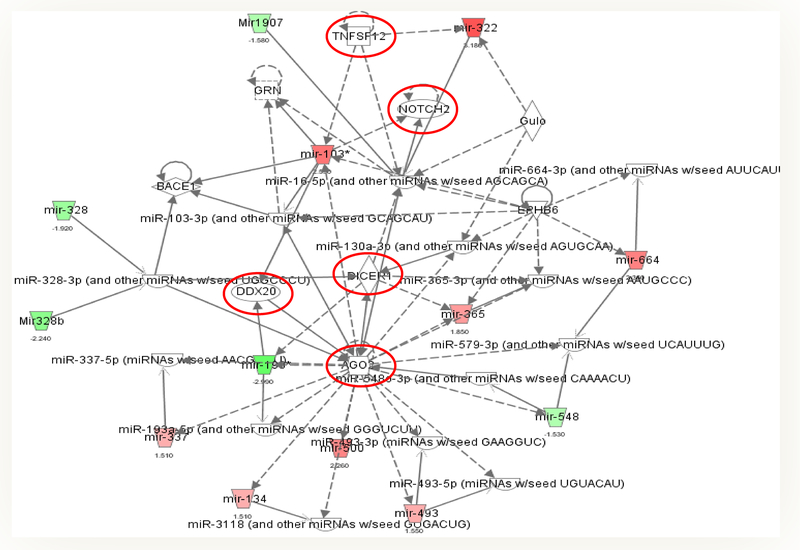
Figure 1.
miRNA-gene-network associated with SIRT3 ablation from the microarray data. Pathway analysis was performed with a statistical cutoff of p <0.02. Green trapezoid represents downregulated miRNA, and red trapezoid represents upregulated miRNA. There were two highly interconnected networks associated with endothelial SIRT3 deletion.
A, Network 1 is involved in the regulation Smad2/3, TGF-β1, and TP53. The red circle represents upregulated genes.
B, Network 2 is associated with DICER1, AGO2, TNFSF12, and Notch signaling. The red circle represents upregulated genes.
EC/pericyte interaction and coronary microvascular function
Pericytes are vascular mural cells of mesenchymal origin, embedded in the basement membrane of microvasculature, where they make specific local contacts with endothelium [50, 51]. The microvasculature of human hearts contains an abundance of pericytes. Each pericyte is associated with two or three ECs in the heart [52]. Loss of pericytes, known to lead to diabetic retinopathy [53–56], has been shown to also cause microvascular dysfunction in other vascular beds [57]. For example, recent studies have highlighted the critical role of capillary pericytes in the regulation of cerebral blood flow in ischemia/reperfusion (I/R)-injury [58, 59]. Pericytes are the second most frequent cell type in the coronary vasculature after endothelial cells (ECs) [52, 57, 58, 60–62]; surprisingly, almost nothing is known about cardiac pericyte in the regulation of coronary microvascular function and heart failure. A study showed that treatment of mice with sunitinib malate (FDA approved for the treatment of metastatic renal cell carcinoma) disrupted EC/pericyte interactions and led to impaired coronary blood flow (CBF) and cardiac dysfunction [57]. Notch3, a receptor expressed in pericytes, has a critical role in the regulation of pericyte differentiation [63, 64]. Notch3 has been reported to regulate pericyte number and maintain vascular integrity [65, 66]. Studies showed that Notch3KO mice challenged with Angiotensin II caused coronary microvascular dysfunction and heart failure [67, 68]. Deficiency of Notch3 also led to a significant reduction of pericytes in the mouse heart which resulted in an impairment of pericyte/capillary coverage and reduced CFR in mice. Furthermore, deficiency of Notch3 sensitized the heart to ischemic injury with larger infarcted size and higher rates of mortality [69]. In addition, deficiency of Notch3 decreased the numbers of NG2+ (pericyte)/Sca1+/c-kit+ progenitor cells and impaired microvascular stabilization thus promoting ischemia-induced microvascular leakage and inflammation [69]. This study strongly suggests that cardiac pericyte is necessary to maintain both the maturation and the integrity of coronary microvasculature in response to myocardial ischemia. Therefore, cardiac pericyte is a promising therapeutic target for coronary no-reflow and progression of heart failure after myocardial infarction or I/R injury [69, 70].
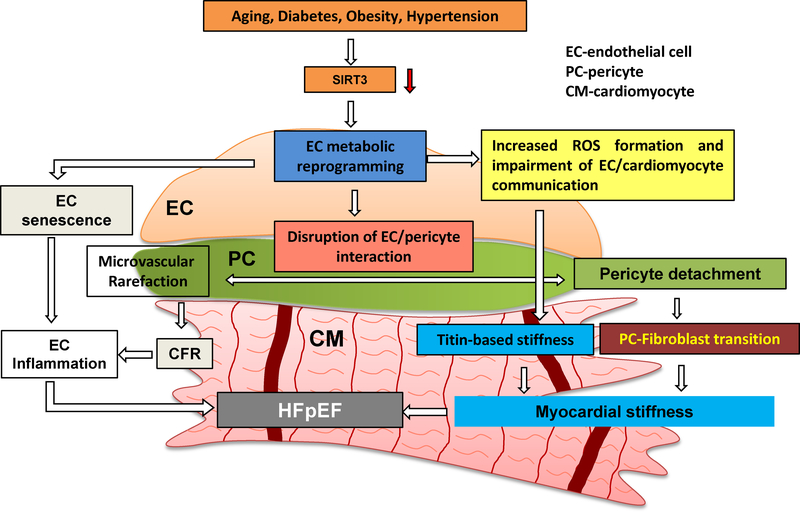
In our previous study, we have demonstrated that pericyte/EC coverage was severely impaired in the heart of obese mice [71]. SIRT3 KO mice had a reduced pericyte/EC coverage in the heart together with a significant reduction of CFR [16]. Although the mechanism of decreased pericyte/EC coverage was not clear, our study indicated that impaired angiopoietins/Tie-2 and HIF-2α/Notch3 signaling pathways may be involved in the pericyte loss in SIRT3 KO mice [72]. Although the exact mechanism of loss of pericyte was not investigated, recent studies have shown that loss of pericyte or detachment of pericyte from capillary has the potential to differentiate into myofibroblasts. This may contribute to deposition of excessive fibrosis and myocardial stiffness which may contribute to HFpEF [48, 49]. Therefore, we reason that disruption of SIRT3 signaling may promote endothelial dysfunction and lead to cardiac diastolic dysfunction through blocking endothelial metabolism and disrupting endothelial cell/pericyte communications and pericyte detachment which leads to pericyte-fibroblast transition and results in myocardial and vascular stiffness (Figure 3).
Figure 3.
Hypothesis regarding the role of endothelial metabolic reprogramming in the pathophysiology of HFpEF.
Risk factors, such as aging, diabetes, obesity, and hypertension, have been shown to reduce the expression of SIRT3 in EC. Myocardial stiffness (cardiomyocyte stiffness and myocardial fibrosis) is the center feature of HFpEF. In HFpEF, diastolic dysfunction and myocardial stiffness are driven by endothelial metabolic reprogramming. Loss of SIRT3 alters EC glycolytic metabolism and shifts ECs from oxygen-independent metabolism to highly oxygen-consuming metabolism. This metabolic reprogramming in EC increases oxygen demand and induces production of ROS, which diminishes EC/cardiomyocyte interaction, thus resulting in an increase in Titin-based cardiomyocyte stiffness. Disruption of endothelial glycolytic metabolism also leads to EC senescence and increases EC inflammation. Moreover, impairment of EC glycolysis decreases angiogenic capacity as well as promotes microvascular rarefaction and inflammatory response by disruption of EC/pericyte interaction. In addition, pericyte detachment promotes pericyte differentiation into myofibroblast and increase interstitial fibrosis deposition. All these critical steps may lead to microvascular dysfunction and diastolic dysfunction and HFpEF. SIRT3, Sirtuin 3, CFR, coronary flow reserve, EC, endothelial cell, PC, pericyte, CM, cardiomyocyte, HFpEF, heart failure with preserved ejection fraction.
SIRT3 and Titin-based cardiomyocyte stiffness
Cardiac stiffness (cardiomyocyte stiffness and fibrosis) is the center feature of HFpEF. The giant cytoskeletal protein Titin is the largest protein known and spans the entire half-sarcomere from the Z-disk to the M-band. It functions as a molecular spring that develops passive tension during diastole when sarcomeres elongate. The Titin-based cardiomyocyte stiffness is the key determinant for HFpEF [73–76]. Titin-based cardiomyocyte stiffness is mediated mainly through isoform shifts (N2BA/N2B) or alterations of phosphorylation of its elastic regions N2-Bus (decreases passive tension, PT) and PEVK (increases PT) [77, 78]. ECs have been reported to support cardiomyocyte function by increasing angiogenesis and releasing regulatory proteins. Surprisingly, little is known about EC/cardiomyocyte interactions on cardiac stiffness and HFpEF. Our recent study revealed that infusion of SIRT3KO mice with angiotensin II (Ang-II) significantly increased myocardial stiffness and accentuated Ang II-induced diastolic dysfunction (unpublished data). Although knockout of SIRT3 or specific knockout of endothelial SIRT3 (SIRT3ECKO) in mice displayed an HFpEF phenotype with impaired CFR and cardiomyocyte stiffness, the regulatory role of SIRT3 on Titin-based cardiomyocyte stiffness has not been explored. Similar as phosphorylation, SIRT3 may also alter acetylation of Titin elastic regions N2-Bus and PEVK which subsequently regulates Titin-based cardiomyocyte stiffness. This notion leads us to further investigate in the future. We, therefore, propose that disruption of SIRT3 in EC reprograms endothelial glycolytic metabolism that leads to derangement of EC/cardiomyocyte/pericyte interactions and microvascular rarefaction. These alterations may promote cardiomyocyte hypoxia and Titin-based cardiomyocyte stiffness and fibrosis thus leading to a diastolic dysfunction and HFpEF (Figure 3).
SIRT3, cardiomyocytes apoptosis, and HFrEF
SIRT3 is a mitochondrial lysine residue deacetylase that regulates energy metabolism and cellular functions in various tissues; the level of SIRT3 declines progressively with aging [79]. Cardiomyocytes are intense energy-utilizing cells that are highly dependent on the energy produced from mitochondrial oxidative phosphorylation [27]. Therefore, it is not surprising that SIRT3 has an important role in regulating cardiomyocyte function. Recent studies have shown that mice lacking SIRT3 have impaired mitochondrial fatty acid oxidation, reduced oxidative phosphorylation complex activity, and ATP production in the heart along with increased ROS production [79–83]. Thus, impaired mitochondrial dynamics seen in SIRT3 KO mice may be one of the most important factors that lead to cardiac hypertrophy and eventually heart failure. Oxygen conformance refers to the ability of cells to reduce energy expenditure which allows them to lower oxygen consumption in advance of an energetic crisis under insufficient oxygen such as ischemia. Such energy conservation induces a state of hypoxia tolerance and enhances survival at low oxygen tension. Recent study implicates PHD1 is the key regulator of oxygen conformance and induces hypoxia tolerance under hypoxic conditions. Deficiency of PHD1 lowers oxygen consumption and reduces mitochondrial oxidative stress and protects muscle against ischemic injury in a HIF-2α-dependent fashion [84]. This endogenous protection is mediated, at least in part, through a reprogramming of the basal glycolytic metabolism, in particular, by a reduction of oxidative glucose metabolism due to an up-regulation of pyruvate dehydrogenase kinase1 (PDK1) and PDK4 expression which correlates with a lower pyruvate dehydrogenase complex activity and restricts the entry of glycolytic intermediates in the tricarboxylic acid (TCA) cycle. Intriguingly, this protection is not attributable to an increase in oxygen supply through enhancing angiogenesis or vasodilatation but to reduction of reactive oxygen species (ROS) formation and enhancement of cells against deleterious effects of oxidative damage under hypoxic conditions. Intriguingly, even transient knockdown of PHD1 induces hypoxia tolerance and protects muscle against ischemic injury [84]. These findings suggest a novel role of PHD1 in the protection of ischemic muscle against oxidative damage by induction of hypoxia tolerance through a mechanism involving induction of a hibernating state and persevering mitochondrial integrity. Global SIRT3 KO mice had an increased PHD1 and decreased HIF-2 levels as well as developed systolic dysfunction at 12 months of age [18]. Intriguingly, PHD inhibition by DMOG treatment improved systolic function further suggesting HIFαs in cardiomyocytes may be involved in the regulation of cardiac function [18]. These findings implicate that SIRT3 deficiency-induced upregulation of PHD1 may attenuate endogenous protection and exacerbate cardiac dysfunction via reducing myocardial tolerance to hypoxia.
To identify miRNA-mRNA regulatory network, we systematically evaluated miRNA-mRNA associations using expression profiles of ECs from WT and SIRT3 KO mice. The inferred putative target interactions formed two highly interconnected subnetworks which were linked to fibrosis, inflammation, cell senescence, and death. We used microRNA processing with a statistical cutoff of p<0.02 (Figure 1 A&B). Interestingly, network 1 is clustered around Smad2/3, TGF-β1, and TP53 (Figure 1A), while network 2 is associated with DICER1, AGO2, TNFSF12, and Notch2 signaling (Figure 1B). Among them, p53 is a pro-apoptotic protein which requires acetylation to be active for recruitment of transcription co-factors, such as PUMA and Bax [5, 85]. SIRT3 has been reported to interact with and directly deacetylate p53 in bladder carcinoma cells [5, 86]. Although the role of SIRT3 in regulation of p53-mediated apoptosis in cardiomyocyte remains unclear, our microRNA profile analysis indicated that loss of SIRT3 affected miRNAs expression that were associated with TNFSF12 and TP53 signaling which may provide a possible mechanism of SIRT3-mediated regulation of cell death and inflammation (Figure 1A and B). This was further confirmed by the data that the acetylation of p53 was significantly increased in the heart of SIRT3 KO mice (Figure 2). Cardiomyocyte death induced by increased oxidative stress and decreased survival signaling and fibrosis that replaces dead cardiomyocytes might count for another very important factor that contributes to heart failure.
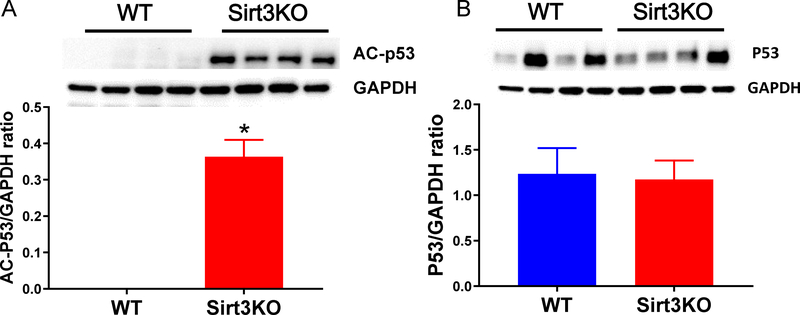
Figure 2.
Western blots analysis indicated that SIRT3 deletion altered cardiac expression of p53.
A, Expression of p53 acetylation was significantly increased in SIRT3 KO mice (n=4) vs. WT mice (n=4).
B, Expression of p53 was not significantly increased in SIRT3 KO mice. (n=4) vs. WT mice (n=4). *p < 0.05. p values were determined by Student’s t test. Data are shown as the means ± s.e.m.
P53, endothelial senescence, and HFpEF
HFpEF, which is one of the leading causes of mortality in aging, can be viewed as a systemic syndrome of aging-related phenotype. P53 is the master regulator of aging and has been shown to have a critical role in the pathogenesis of heart failure. The expression of p53 is elevated in the human failing heart [87, 88]. P53 and its transcriptional target gene TP53-induced glycolysis and apoptosis regulator (TIGAR) are activated in the cardiomyocyte under hypoxia [89, 90]. So far, two roles of p53 accumulation in the failing heart have been demonstrated: (1) induction of cell cycle arrest that causes proliferative cells arrest in the G1 stage of the cell cycle to induce senescence and (2) suppression of angiogenesis [91, 92]. Endothelial senescence has been reported to contribute to the development of HFpEF by promoting myocardial endothelial inflammation and peripheral vascular endothelial inflammation via the senescence-associated secretory mechanism. Endothelial senescence led to an impairment of systemic vasodilator reserve and coronary microvascular dysfunction in mice [93]. Elevation of p53 reduced capillary formation by inhibition of HIF-1α in the hypertrophic hearts [94]. In contrast, global knockout of p53 in mice attenuates doxorubicin-induced cardiac dysfunction [95, 96]. Specific knockout of p53 in EC reduces EC apoptosis and increases capillary density which attenuates pressure-overload induced heart failure [97]. Our data also showed that p53 acetylation was significantly increased whereas total p53 remains unaltered in the hearts of SIRT3 KO mice (Figure 2). This indicates p53 acetylation may cause endothelial senescence and microvascular dysfunction that promotes a diastolic dysfunction.
In summary, recent studies uncover a molecular mechanism of SIRT3 in the regulation of angiogenesis by reprogramming EC glycolytic metabolism. Based upon these studies, we proposed two cardiac phenotypes of SIRT3: 1) endothelial deletion of SIRT3 developed HFpEF phenotype due to endothelial metabolic dysfunction which may lead to EC senescence, microvascular rarefaction, and myocardial stiffness (Figure 3); whereas, 2) global knockout of SIRT3 resulted in HFrEF phenotype that is contributed by mitochondrial dysfunction and oxidative stress induced cardiomyocyte death [18, 19]. In addition, the current available therapeutic interventions, such as ACE-inhibitors, beta-blockers, and mineralocorticoid receptor antagonists, benefit HFrEF patients but have not been proven to improve the outcomes in HFpEF patients [1, 98]. Pharmacological trials are not promising in HFpEF patients, either [99–102]. As humans age, cardiac function declines especially diastolic function. Although it is clear that SIRT3 deficiency causes cardiac dysfunction, unfortunately, there is no clinical trial to explore the therapeutic role of SIRT3. This is due to lack of SIRT3 specific activators so far. Due to its unique location, it is urgent to develop new agents which are specific to target SIRT3 in the mitochondria. Honokiol, a Chinese medicine, has been shown to bind and activate SIRT3 as well as provide protective action against cardiac injury [103, 104]. Our recent studies also revealed a critical role of endothelial SIRT3 in regulation of cardiac diastolic function during aging as well as hypertension. These results indicate not only a link between aging and HFpEF, but also a valuable target and mechanism for therapeutic intervention in cardiac pathologies and aging process. Further studies are warranted to address the intracellular molecular mechanisms mediated by endothelial SIRT3 as well as discover new SIRT3 specific agonists and potential therapeutic approaches for targeting EC metabolism such as glycolytic metabolism in the treatment of HFpEF.
Acknowledgments
This study was supported by National Institutes of Health 2RO1 HL102042-7 (Chen).
Abbreviations
- HFpEF
Heart failure with preserved ejection fraction
- HFrEF
Heart failure with reduced ejection fraction
- NO
nitric oxide
- ROS
reactive oxygen species
- PFKFB3
phosphofructose kinase/fructose 2, 6 bisphosphatase 3
- CFR
coronary flow reserve
- TIGAR
TP53-induced glycolysis and apoptosis regulator
- F-6P
fructose 6 phosphate
- PFK-1
Phosphofructose kinase 1
- VEGF
vascular endothelia growth factor
- FGF
fibroblast growth factor
- LV
left ventricular
- HIF
hypoxia-inducible factor
- PDK1
pyruvated dehydrogenase kinase-1
- TCA
tricarboxylic acid cycle
Footnotes
Conflicts of interest: None.
Reference List
- [1].Brouwers FP, de Boer RA, van der Harst P, Voors AA, Gansevoort RT, Bakker SJ et al. Incidence and epidemiology of new onset heart failure with preserved vs. reduced ejection fraction in a community-based cohort: 11-year follow-up of PREVEND. Eur Heart J 2013;34:1424–31. [DOI] [PubMed] [Google Scholar]
- [2].Cheng S, Vasan RS. Advances in the epidemiology of heart failure and left ventricular remodeling. Circulation 2011;124:e516–e519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Lam CS, Donal E, Kraigher-Krainer E, Vasan RS. Epidemiology and clinical course of heart failure with preserved ejection fraction. Eur J Heart Fail 2011;13:18–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Mohammed SF, Hussain S, Mirzoyev SA, Edwards WD, Maleszewski JJ, Redfield MM. Coronary microvascular rarefaction and myocardial fibrosis in heart failure with preserved ejection fraction. Circulation 2015;131:550–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Tanno M, Kuno A, Horio Y, Miura T. Emerging beneficial roles of sirtuins in heart failure. Basic Res Cardiol 2012;107:273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Upadhya B, Taffet GE, Cheng CP, Kitzman DW. Heart failure with preserved ejection fraction in the elderly: scope of the problem. J Mol Cell Cardiol 2015;83:73–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Dhingra A, Garg A, Kaur S, Chopra S, Batra JS, Pandey A et al. Epidemiology of heart failure with preserved ejection fraction. Curr Heart Fail Rep 2014;11:354–65. [DOI] [PubMed] [Google Scholar]
- [8].Andersen MJ, Borlaug BA. Heart failure with preserved ejection fraction: current understandings and challenges. Curr Cardiol Rep 2014;16:501. [DOI] [PubMed] [Google Scholar]
- [9].Paulus WJ, Tschope C. A novel paradigm for heart failure with preserved ejection fraction: comorbidities drive myocardial dysfunction and remodeling through coronary microvascular endothelial inflammation. J Am Coll Cardiol 2013;62:263–71. [DOI] [PubMed] [Google Scholar]
- [10].Lorenzen JM, Martino F, Thum T. Epigenetic modifications in cardiovascular disease. Basic Res Cardiol 2012;107:245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Nogueiras R, Habegger KM, Chaudhary N, Finan B, Banks AS, Dietrich MO et al. Sirtuin 1 and sirtuin 3: physiological modulators of metabolism. Physiol Rev 2012;92:1479–514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Guarani V, Potente M. SIRT1 - a metabolic sensor that controls blood vessel growth. Curr Opin Pharmacol 2010;10:139–45. [DOI] [PubMed] [Google Scholar]
- [13].Tseng AH, Wu LH, Shieh SS, Wang DL. SIRT3 interactions with FOXO3 acetylation, phosphorylation and ubiquitinylation mediate endothelial cell responses to hypoxia. Biochem J 2014;464:157–68. [DOI] [PubMed] [Google Scholar]
- [14].Lanza IR, Short DK, Short KR, Raghavakaimal S, Basu R, Joyner MJ et al. Endurance exercise as a countermeasure for aging. Diabetes 2008;57:2933–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Zeng H, He X, Hou X, Li L, Chen JX. Apelin gene therapy increases myocardial vascular density and ameliorates diabetic cardiomyopathy via upregulation of sirtuin 3. Am J Physiol Heart Circ Physiol 2014;306:H585–H597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].He X, Zeng H, Chen JX. Ablation of SIRT3 causes coronary microvascular dysfunction and impairs cardiac recovery post myocardial ischemia. Int J Cardiol 2016;215:349–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].He X, Zeng H, Chen ST, Roman RJ, Aschner JL, Didion S et al. Endothelial specific SIRT3 deletion impairs glycolysis and angiogenesis and causes diastolic dysfunction. J Mol Cell Cardiol 2017;112:104–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].He X, Zeng H, Roman RJ, Chen JX. Inhibition of prolyl hydroxylases alters cell metabolism and reverses pre-existing diastolic dysfunction in mice. Int J Cardiol 2018;272:281–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].He X, Zeng H, Chen JX. Emerging role of SIRT3 in endothelial metabolism, angiogenesis, and cardiovascular disease. J Cell Physiol 2019;234:2252–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Goveia J, Stapor P, Carmeliet P. Principles of targeting endothelial cell metabolism to treat angiogenesis and endothelial cell dysfunction in disease. EMBO Mol Med 2014;6:1105–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].De BK, Georgiadou M, Schoors S, Kuchnio A, Wong BW, Cantelmo AR et al. Role of PFKFB3-driven glycolysis in vessel sprouting. Cell 2013;154:651–63. [DOI] [PubMed] [Google Scholar]
- [22].Schoors S, De BK, Cantelmo AR, Georgiadou M, Ghesquiere B, Cauwenberghs S et al. Partial and transient reduction of glycolysis by PFKFB3 blockade reduces pathological angiogenesis. Cell Metab 2014;19:37–48. [DOI] [PubMed] [Google Scholar]
- [23].Xu Y, An X, Guo X, Habtetsion TG, Wang Y, Xu X et al. Endothelial PFKFB3 plays a critical role in angiogenesis. Arterioscler Thromb Vasc Biol 2014;34:1231–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Oka T, Akazawa H, Naito AT, Komuro I. Angiogenesis and cardiac hypertrophy: maintenance of cardiac function and causative roles in heart failure. Circ Res 2014;114:565–71. [DOI] [PubMed] [Google Scholar]
- [25].Hou J, Kang YJ. Regression of pathological cardiac hypertrophy: signaling pathways and therapeutic targets. Pharmacol Ther 2012;135:337–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Hou X, Zeng H, He X, Chen JX. Sirt3 is essential for apelin-induced angiogenesis in post-myocardial infarction of diabetes. J Cell Mol Med 2015;19:53–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Dittenhafer-Reed KE, Richards AL, Fan J, Smallegan MJ, Fotuhi SA, Kemmerer ZA et al. SIRT3 mediates multi-tissue coupling for metabolic fuel switching. Cell Metab 2015;21:637–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Fernandez-Marcos PJ, Jeninga EH, Canto C, Harach T, de Boer VC, Andreux P et al. Muscle or liver-specific Sirt3 deficiency induces hyperacetylation of mitochondrial proteins without affecting global metabolic homeostasis. Sci Rep 2012;2:425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Jing E, Emanuelli B, Hirschey MD, Boucher J, Lee KY, Lombard D et al. Sirtuin-3 (Sirt3) regulates skeletal muscle metabolism and insulin signaling via altered mitochondrial oxidation and reactive oxygen species production. Proc Natl Acad Sci U S A 2011;108:14608–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Li H, Feng Z, Wu W, Li J, Zhang J, Xia T. SIRT3 regulates cell proliferation and apoptosis related to energy metabolism in non-small cell lung cancer cells through deacetylation of NMNAT2. Int J Oncol 2013;43:1420–30. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- [31].Koentges C, Pfeil K, Schnick T, Wiese S, Dahlbock R, Cimolai MC et al. SIRT3 deficiency impairs mitochondrial and contractile function in the heart. Basic Res Cardiol 2015;110:36. [DOI] [PubMed] [Google Scholar]
- [32].Vallerie SN, Bornfeldt KE. Metabolic Flexibility and Dysfunction in Cardiovascular Cells. Arterioscler Thromb Vasc Biol 2015;35:e37–e42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Eelen G, de ZP, Simons M, Carmeliet P. Endothelial cell metabolism in normal and diseased vasculature. Circ Res 2015;116:1231–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Fraisl P, Mazzone M, Schmidt T, Carmeliet P. Regulation of angiogenesis by oxygen and metabolism. Dev Cell 2009;16:167–79. [DOI] [PubMed] [Google Scholar]
- [35].Obach M, Navarro-Sabate A, Caro J, Kong X, Duran J, Gomez M et al. 6-Phosphofructo-2-kinase (pfkfb3) gene promoter contains hypoxia-inducible factor-1 binding sites necessary for transactivation in response to hypoxia. J Biol Chem 2004;279:53562–70. [DOI] [PubMed] [Google Scholar]
- [36].Minchenko O, Opentanova I, Caro J. Hypoxic regulation of the 6-phosphofructo-2-kinase/fructose-2,6-bisphosphatase gene family (PFKFB-1–4) expression in vivo. FEBS Lett 2003;554:264–70. [DOI] [PubMed] [Google Scholar]
- [37].Rohrbach S, Simm A, Pregla R, Franke C, Katschinski DM. Age-dependent increase of prolyl-4-hydroxylase domain (PHD) 3 expression in human and mouse heart. Biogerontology 2005;6:165–71. [DOI] [PubMed] [Google Scholar]
- [38].Garg R, Rao AD, Baimas-George M, Hurwitz S, Foster C, Shah RV et al. Mineralocorticoid receptor blockade improves coronary microvascular function in individuals with type 2 diabetes. Diabetes 2015;64:236–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Kaul S, Jayaweera AR. Myocardial capillaries and coronary flow reserve. J Am Coll Cardiol 2008;52:1399–401. [DOI] [PubMed] [Google Scholar]
- [40].Mohammed SF, Hussain S, Mirzoyev SA, Edwards WD, Maleszewski JJ, Redfield MM. Coronary microvascular rarefaction and myocardial fibrosis in heart failure with preserved ejection fraction. Circulation 2015;131:550–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Stratmann B, Gawlowski T, Tschoepe D. Diabetic cardiomyopathy--to take a long story serious. Herz 2010;35:161–8. [DOI] [PubMed] [Google Scholar]
- [42].Boudina S, Abel ED. Diabetic cardiomyopathy revisited. Circulation 2007;115:3213–23. [DOI] [PubMed] [Google Scholar]
- [43].Seferovic PM, Paulus WJ. Clinical diabetic cardiomyopathy: a two-faced disease with restrictive and dilated phenotypes. Eur Heart J 2015;36:1718–27. [DOI] [PubMed] [Google Scholar]
- [44].Chang WT, Chen JS, Hung YK, Tsai WC, Juang JN, Liu PY. Characterization of aging-associated cardiac diastolic dysfunction. PLoS One 2014;9:e97455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Liu H, Chen T, Li N, Wang S, Bu P. Role of SIRT3 in Angiotensin II-induced human umbilical vein endothelial cells dysfunction. BMC Cardiovasc Disord 2015;15:81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Porter GA, Urciuoli WR, Brookes PS, Nadtochiy SM. SIRT3 deficiency exacerbates ischemia-reperfusion injury: implication for aged hearts. Am J Physiol Heart Circ Physiol 2014;306:H1602–H1609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Scortegagna M, Ding K, Oktay Y, Gaur A, Thurmond F, Yan LJ et al. Multiple organ pathology, metabolic abnormalities and impaired homeostasis of reactive oxygen species in Epas1−/− mice. Nat Genet 2003;35:331–40. [DOI] [PubMed] [Google Scholar]
- [48].Souders CA, Bowers SL, Baudino TA. Cardiac fibroblast: the renaissance cell. Circ Res 2009;105:1164–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Lin SL, Kisseleva T, Brenner DA, Duffield JS. Pericytes and perivascular fibroblasts are the primary source of collagen-producing cells in obstructive fibrosis of the kidney. Am J Pathol 2008;173:1617–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Gaengel K, Genove G, Armulik A, Betsholtz C. Endothelial-mural cell signaling in vascular development and angiogenesis. Arterioscler Thromb Vasc Biol 2009;29:630–8. [DOI] [PubMed] [Google Scholar]
- [51].Armulik A, Abramsson A, Betsholtz C. Endothelial/pericyte interactions. Circ Res 2005;97:512–23. [DOI] [PubMed] [Google Scholar]
- [52].Nees S, Weiss DR, Senftl A, Knott M, Forch S, Schnurr M et al. Isolation, bulk cultivation, and characterization of coronary microvascular pericytes: the second most frequent myocardial cell type in vitro. Am J Physiol Heart Circ Physiol 2012;302:H69–H84. [DOI] [PubMed] [Google Scholar]
- [53].Ejaz S, Chekarova I, Ejaz A, Sohail A, Lim CW. Importance of pericytes and mechanisms of pericyte loss during diabetes retinopathy. Diabetes Obes Metab 2008;10:53–63. [DOI] [PubMed] [Google Scholar]
- [54].Hammes HP, Lin J, Renner O, Shani M, Lundqvist A, Betsholtz C et al. Pericytes and the pathogenesis of diabetic retinopathy. Diabetes 2002;51:3107–12. [DOI] [PubMed] [Google Scholar]
- [55].Pfister F, Feng Y, vom HF, Hoffmann S, Molema G, Hillebrands JL et al. Pericyte migration: a novel mechanism of pericyte loss in experimental diabetic retinopathy. Diabetes 2008;57:2495–502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Yamagishi S, Imaizumi T. Pericyte biology and diseases. Int J Tissue React 2005;27:125–35. [PubMed] [Google Scholar]
- [57].Chintalgattu V, Rees ML, Culver JC, Goel A, Jiffar T, Zhang J et al. Coronary microvascular pericytes are the cellular target of sunitinib malate-induced cardiotoxicity. Sci Transl Med 2013;5:187ra69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Hall CN, Reynell C, Gesslein B, Hamilton NB, Mishra A, Sutherland BA et al. Capillary pericytes regulate cerebral blood flow in health and disease. Nature 2014;508:55–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Fernandez-Klett F, Priller J. Diverse functions of pericytes in cerebral blood flow regulation and ischemia. J Cereb Blood Flow Metab 2015;35:883–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].O’Farrell FM, Attwell D. A role for pericytes in coronary no-reflow. Nat Rev Cardiol 2014;11:427–32. [DOI] [PubMed] [Google Scholar]
- [61].Nykanen AI, Tuuminen R, Lemstrom KB. Donor simvastatin treatment and cardiac allograft ischemia/reperfusion injury. Trends Cardiovasc Med 2013;23:85–90. [DOI] [PubMed] [Google Scholar]
- [62].Tuuminen R, Syrjala S, Krebs R, Keranen MA, Koli K, Abo-Ramadan U et al. Donor simvastatin treatment abolishes rat cardiac allograft ischemia/reperfusion injury and chronic rejection through microvascular protection. Circulation 2011;124:1138–50. [DOI] [PubMed] [Google Scholar]
- [63].Jin S, Hansson EM, Tikka S, Lanner F, Sahlgren C, Farnebo F et al. Notch signaling regulates platelet-derived growth factor receptor-beta expression in vascular smooth muscle cells. Circ Res 2008;102:1483–91. [DOI] [PubMed] [Google Scholar]
- [64].Weber DS. A novel mechanism of vascular smooth muscle cell regulation by Notch: platelet-derived growth factor receptor-beta expression? Circ Res 2008;102:1448–50. [DOI] [PubMed] [Google Scholar]
- [65].Gu X, Liu XY, Fagan A, Gonzalez-Toledo ME, Zhao LR. Ultrastructural changes in cerebral capillary pericytes in aged Notch3 mutant transgenic mice. Ultrastruct Pathol 2012;36:48–55. [DOI] [PubMed] [Google Scholar]
- [66].Wang Y, Pan L, Moens CB, Appel B. Notch3 establishes brain vascular integrity by regulating pericyte number. Development 2014;141:307–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [67].Boulos N, Helle F, Dussaule JC, Placier S, Milliez P, Djudjaj S et al. Notch3 is essential for regulation of the renal vascular tone. Hypertension 2011;57:1176–82. [DOI] [PubMed] [Google Scholar]
- [68].Ragot H, Merval R, Baudet M, Fazal L, Polidano E, Delcayre C et al. P720Notch3 is an important mediator of cardiac adaptation to pressure overload. Cardiovasc Res 2014;103 Suppl 1:S131–S132. [Google Scholar]
- [69].Tao YK, Zeng H, Zhang GQ, Chen ST, Xie XJ, He X et al. Notch3 deficiency impairs coronary microvascular maturation and reduces cardiac recovery after myocardial ischemia. Int J Cardiol 2017;236:413–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [70].Chen JX, Chen ST, Tao YK. Cardiac pericyte is promising target for ischemic heart diseases: Role of Notch3. Int J Cardiol 2017;246:57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [71].Zeng H, Vaka VR, He X, Booz GW, Chen JX. High-fat diet induces cardiac remodelling and dysfunction: assessment of the role played by SIRT3 loss. J Cell Mol Med 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [72].Zeng H, He X, Tuo QH, Liao DF, Zhang GQ, Chen JX. LPS causes pericyte loss and microvascular dysfunction via disruption of Sirt3/angiopoietins/Tie-2 and HIF-2alpha/Notch3 pathways. Sci Rep 2016;6:20931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [73].Methawasin M, Strom JG, Slater RE, Fernandez V, Saripalli C, Granzier H. Experimentally Increasing the Compliance of Titin Through RNA Binding Motif-20 (RBM20) Inhibition Improves Diastolic Function In a Mouse Model of Heart Failure With Preserved Ejection Fraction. Circulation 2016;134:1085–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [74].Franssen C, Gonzalez MA. The role of titin and extracellular matrix remodelling in heart failure with preserved ejection fraction. Neth Heart J 2016;24:259–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [75].Zile MR, Baicu CF, Ikonomidis JS, Stroud RE, Nietert PJ, Bradshaw AD et al. Myocardial stiffness in patients with heart failure and a preserved ejection fraction: contributions of collagen and titin. Circulation 2015;131:1247–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [76].van HL, Franssen CP, Hamdani N, Verheugt FW, Somsen GA, Paulus WJ. Molecular and cellular basis for diastolic dysfunction. Curr Heart Fail Rep 2012;9:293–302. [DOI] [PubMed] [Google Scholar]
- [77].Hidalgo C, Granzier H. Tuning the molecular giant titin through phosphorylation: role in health and disease. Trends Cardiovasc Med 2013;23:165–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [78].Slater RE, Strom JG, Granzier H. Effect of exercise on passive myocardial stiffness in mice with diastolic dysfunction. J Mol Cell Cardiol 2017. [DOI] [PubMed] [Google Scholar]
- [79].Koentges C, Pfeil K, Schnick T, Wiese S, Dahlbock R, Cimolai MC et al. SIRT3 deficiency impairs mitochondrial and contractile function in the heart. Basic Res Cardiol 2015;110:36. [DOI] [PubMed] [Google Scholar]
- [80].McDonnell E, Peterson BS, Bomze HM, Hirschey MD. SIRT3 regulates progression and development of diseases of aging. Trends Endocrinol Metab 2015;26:486–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [81].Ahn BH, Kim HS, Song S, Lee IH, Liu J, Vassilopoulos A et al. A role for the mitochondrial deacetylase Sirt3 in regulating energy homeostasis. Proc Natl Acad Sci U S A 2008;105:14447–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [82].Hirschey MD, Shimazu T, Goetzman E, Jing E, Schwer B, Lombard DB et al. SIRT3 regulates mitochondrial fatty-acid oxidation by reversible enzyme deacetylation. Nature 2010;464:121–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [83].Alrob OA, Sankaralingam S, Ma C, Wagg CS, Fillmore N, Jaswal JS et al. Obesity-induced lysine acetylation increases cardiac fatty acid oxidation and impairs insulin signalling. Cardiovasc Res 2014;103:485–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [84].Aragones J, Schneider M, Van GK, Fraisl P, Dresselaers T, Mazzone M et al. Deficiency or inhibition of oxygen sensor Phd1 induces hypoxia tolerance by reprogramming basal metabolism. Nat Genet 2008;40:170–80. [DOI] [PubMed] [Google Scholar]
- [85].Brooks CL, Gu W. The impact of acetylation and deacetylation on the p53 pathway. Protein Cell 2011;2:456–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [86].Li S, Banck M, Mujtaba S, Zhou MM, Sugrue MM, Walsh MJ. p53-induced growth arrest is regulated by the mitochondrial SirT3 deacetylase. PLoS One 2010;5:e10486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [87].Birks EJ, Latif N, Enesa K, Folkvang T, Luong lA, Sarathchandra P et al. Elevated p53 expression is associated with dysregulation of the ubiquitin-proteasome system in dilated cardiomyopathy. Cardiovasc Res 2008;79:472–80. [DOI] [PubMed] [Google Scholar]
- [88].Song H, Conte JV, Jr., Foster AH, McLaughlin JS, Wei C. Increased p53 protein expression in human failing myocardium. J Heart Lung Transplant 1999;18:744–9. [DOI] [PubMed] [Google Scholar]
- [89].Hoshino A, Matoba S, Iwai-Kanai E, Nakamura H, Kimata M, Nakaoka M et al. p53-TIGAR axis attenuates mitophagy to exacerbate cardiac damage after ischemia. J Mol Cell Cardiol 2012;52:175–84. [DOI] [PubMed] [Google Scholar]
- [90].Kimata M, Matoba S, Iwai-Kanai E, Nakamura H, Hoshino A, Nakaoka M et al. p53 and TIGAR regulate cardiac myocyte energy homeostasis under hypoxic stress. Am J Physiol Heart Circ Physiol 2010;299:H1908–H1916. [DOI] [PubMed] [Google Scholar]
- [91].Oka T, Morita H, Komuro I. Novel molecular mechanisms and regeneration therapy for heart failure. J Mol Cell Cardiol 2016;92:46–51. [DOI] [PubMed] [Google Scholar]
- [92].Oka T, Akazawa H, Naito AT, Komuro I. Angiogenesis and cardiac hypertrophy: maintenance of cardiac function and causative roles in heart failure. Circ Res 2014;114:565–71. [DOI] [PubMed] [Google Scholar]
- [93].Gevaert AB, Shakeri H, Leloup AJ, Van Hove CE, De Meyer GRY, Vrints CJ et al. Endothelial Senescence Contributes to Heart Failure With Preserved Ejection Fraction in an Aging Mouse Model. Circ Heart Fail 2017;10. [DOI] [PubMed] [Google Scholar]
- [94].Sano M, Minamino T, Toko H, Miyauchi H, Orimo M, Qin Y et al. p53-induced inhibition of Hif-1 causes cardiac dysfunction during pressure overload. Nature 2007;446:444–8. [DOI] [PubMed] [Google Scholar]
- [95].Nithipongvanitch R, Ittarat W, Velez JM, Zhao R, St Clair DK, Oberley TD. Evidence for p53 as guardian of the cardiomyocyte mitochondrial genome following acute adriamycin treatment. J Histochem Cytochem 2007;55:629–39. [DOI] [PubMed] [Google Scholar]
- [96].Shizukuda Y, Matoba S, Mian OY, Nguyen T, Hwang PM. Targeted disruption of p53 attenuates doxorubicin-induced cardiac toxicity in mice. Mol Cell Biochem 2005;273:25–32. [DOI] [PubMed] [Google Scholar]
- [97].Gogiraju R, Xu X, Bochenek ML, Steinbrecher JH, Lehnart SE, Wenzel P et al. Endothelial p53 deletion improves angiogenesis and prevents cardiac fibrosis and heart failure induced by pressure overload in mice. J Am Heart Assoc 2015;4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [98].Holland DJ, Kumbhani DJ, Ahmed SH, Marwick TH. Effects of treatment on exercise tolerance, cardiac function, and mortality in heart failure with preserved ejection fraction. A meta-analysis. J Am Coll Cardiol 2011;57:1676–86. [DOI] [PubMed] [Google Scholar]
- [99].Massie BM, Carson PE, McMurray JJ, Komajda M, McKelvie R, Zile MR et al. Irbesartan in patients with heart failure and preserved ejection fraction. N Engl J Med 2008;359:2456–67. [DOI] [PubMed] [Google Scholar]
- [100].Kitzman DW, Hundley WG, Brubaker PH, Morgan TM, Moore JB, Stewart KP et al. A randomized double-blind trial of enalapril in older patients with heart failure and preserved ejection fraction: effects on exercise tolerance and arterial distensibility. Circ Heart Fail 2010;3:477–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [101].Edelmann F, Wachter R, Schmidt AG, Kraigher-Krainer E, Colantonio C, Kamke W et al. Effect of spironolactone on diastolic function and exercise capacity in patients with heart failure with preserved ejection fraction: the Aldo-DHF randomized controlled trial. JAMA 2013;309:781–91. [DOI] [PubMed] [Google Scholar]
- [102].Pitt B, Pfeffer MA, Assmann SF, Boineau R, Anand IS, Claggett B et al. Spironolactone for heart failure with preserved ejection fraction. N Engl J Med 2014;370:1383–92. [DOI] [PubMed] [Google Scholar]
- [103].Pillai VB, Kanwal A, Fang YH, Sharp WW, Samant S, Arbiser J et al. Honokiol, an activator of Sirtuin-3 (SIRT3) preserves mitochondria and protects the heart from doxorubicin-induced cardiomyopathy in mice. Oncotarget 2017;8:34082–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [104].Pillai VB, Samant S, Sundaresan NR, Raghuraman H, Kim G, Bonner MY et al. Honokiol blocks and reverses cardiac hypertrophy in mice by activating mitochondrial Sirt3. Nat Commun 2015;6:6656. [DOI] [PMC free article] [PubMed] [Google Scholar]