Introduction
Aneurysmal subarachnoid hemorrhage (aSAH) affects approximately 30,000 Americans annually,1,2 with estimated mortality rates in the 45% to 50% range.2,3 Current surgical and endovascular treatments aim to control re-bleeding, ischemia, hydrocephalus, seizures, and other neurological sequelae.3 However, despite advances in surgical and endovascular treatment, neurological impairment has been noted in up to 20% of aSAH survivors.4 Fewer than 60% of individuals return to functional baseline.2 Following treatment, patients remain at risk for both neurological (vasospasm, seizure, hydrocephalus, etc.)5,6 and medical (natremia and glycemia abnormalities, fever, anemia)5,7–9 complications. Prevention and treatment of these sequelae require multidisciplinary management and continuity of care. These patients are at high risk for readmission, with short-term estimates ranging from 7.5% to 26%.10–14 Readmission to a hospital other than the original site of treatment (nonindex hospital) causes a discontinuity in care that may be particularly impactful for aSAH patients during perioperative and subacute follow-up periods. Detailed patient history and baseline neurological status are integral to the assessment of vasospasm risk factors, neurological status change, hydrocephalus, and management of resulting systemic medical complications. Therefore, it is important to identify factors associated with nonindex readmission and the impact of nonindex readmissions on outcomes for these patients.
The Medicare Hospital Readmission Reduction Program was instituted in 2012 as part of the Affordable Care Act in an effort to reduce short term readmissions and associated costs.15 Subsequent research on patients that underwent surgical treatment of aortic aneurysms or general cancer reported that 20.0%–46.7% of short-term readmissions were to nonindex hospitals.16–24 These studies cited worse outcomes and greater costs for these patients. The purpose of this study is to characterize nonindex readmission following treatment for ruptured aneurysms. Using a large national database, our aims were to: [1] determine the rate of readmission to nonindex hospitals, [2] evaluate patient and hospital factors associated with nonindex readmission, and [3] evaluate the association of readmission to nonindex (versus index) facility with patient outcomes following aSAH treatment. We hypothesized that illness severity and disadvantaged socioeconomic indicators are associated with nonindex hospital readmissions and that patients readmitted to nonindex hospitals have increased likelihood of patient morbidity and subsequent readmissions.
Materials and Methods
Study Design
A retrospective analysis of the Nationwide Readmissions Database (NRD) was performed from 2010–2014. The NRD is the Health Care Utilization Project’s (HCUP) National Readmission Database that allows for analysis of patient admission and readmissions over the course of a given calendar year. The dataset includes patient and hospital demographic characteristics, disease diagnosis and procedure codes, and clinical outcome variables. Data tabulations with n-value of ten or fewer was suppressed in accordance with HCUP privacy guidelines; the present study was not subject to Institutional Review Board review. This article adhered to the Enhancing the QUAlity and Transparency Of health Research (EQUATOR) reporting guideline of Strengthening the Reporting of OBservational studies in Epidemiology (STROBE).25
Study Population
International Classification of Disease, Ninth Edition (ICD-9) codes were used to identify patients with aneurysmal subarachnoid hemorrhage (430.xx) that were treated with either endovascular embolization (39.79, 39.72, 39.52) or clip ligation via craniotomy (39.51). ICD-9 diagnosis methodology has been previously validated for sensitivity, specificity, and positive predictive value.26 Patients without 90 days follow-up after admission within the calendar year were excluded. Patients who were under 18 years of age, experienced inpatient hospital death, were admitted electively, or were missing mortality or length of stay records were also excluded from analysis. A nonindex readmission was defined as a readmission to a hospital other than the initial site of procedure. To evaluate the effect of non-index readmission on treatment outcomes, patients were stratified into either the index or non-index readmission groups.
Variables
Patient and hospital variables derived from the NRD were analyzed. Initial admission demographic variables included age, gender, primary insurance (Medicare, Medicaid, private, self-pay, no charge, other), residency status within the state of admission (yes/no), and median household income quartile (based on ZIP code). Patient age is included in the NRD as a continuous variable but was redefined as a categorical variable for analysis (18–44, 45–59, 60–74, 75+).19
The following clinical covariates were also included from patient admission records: initial procedure received (clip ligation, endovascular coiling, or multiple), Elixhauser comorbidity score (0, 1, 2, 3+), All Patient Refined – Diagnosis Related Group (APR-DRG) severity score (minor, moderate, major, extreme), discharge disposition (home versus another facility), discharge quarter (January-March, April-June, July-September), length of stay, major complication, neurological complication, treated hydrocephalus, and ventriculostomy placement. The Elixhauser comorbidity index is the aggregate of Agency for Healthcare Research and Quality’s (AHRQ) defined comorbidities present in the NRD. APR-DRG is a classification system that includes sub-scores to stratify the risk of severity of illness.27 Hospital factors, including hospital ownership (government nonfederal, private not-profit, private investor-owned), teaching status, bedsize (small, medium, large), and urban-rural designation (large metropolitan ≥ 1 million residents, small metropolitan < 1 million residents) were also determined from admission patient records. The presence of a major complication (by ICD-9 code) was defined as pneumonia (481–482), pulmonary embolism (415.1–415.9), renal failure (584.5–584.9), cerebrovascular accident (CVA, 433.01, 433.11, 433.21, 433.31, 433.81, 433.91), myocardial infarction (410.00–410.90), cardiac arrest (427.5), sepsis (995.91), or septic shock (995.92) at admission. The presence of a neurological complication at admission was defined by ICD-9 codes for intracerebral hemorrhage (431, 998.11–12), seizures (345), or other neurological complications after procedure (997.01–997.09).
Readmission Outcomes
The outcomes of interest were major complication at readmission and incidence of a second readmission within the 90-day period, which were analyzed and compared between nonindex and index readmission groups. The presence of a major complication at readmission was defined using the identical set of ICD-9 code criteria as major complication at admission.
Statistical Analysis
90-day readmission rates for both index and nonindex hospital readmission groups were determined. Analysis of readmission at 90 days post-discharge (versus 30-days) was utilized to capture delayed complications following neurosurgical or endovascular treatment of ruptured aneurysms.28 Descriptive analyses were performed for all variables. Patient and hospital variables associated with index versus nonindex readmissions were determined using multivariable logistic regression incorporating NRD clustering. A p-value of 0.05 was used to assess for variables to include in the model. Patient and hospital variables included age, insurance status, APR-DRG severity at readmission, presence of major complication during hospital stay, length of stay, hospital ownership, hospital procedure volume, Elixhauser comorbidity score, residency of same state as procedure, hospital bed size, discharge disposition, and treated hydrocephalus. Sensitivity analysis was done using same variables, but using hospital level variables at readmission, and we got similar results.
We used multivariable logistic regression to evaluate association of patient readmission to nonindex versus index hospitals with categorical outcomes of major complication and second readmission. For each logistic model, adjusted covariates included all patient and hospital characteristics listed above. Missing data was insignificant (<5%) and excluded from analysis in accordance with complete case analysis methodology. Analysis was conducted using SAS 9.4 (Cary, NC) with a significance level set at 0.05.
Results
Study Group
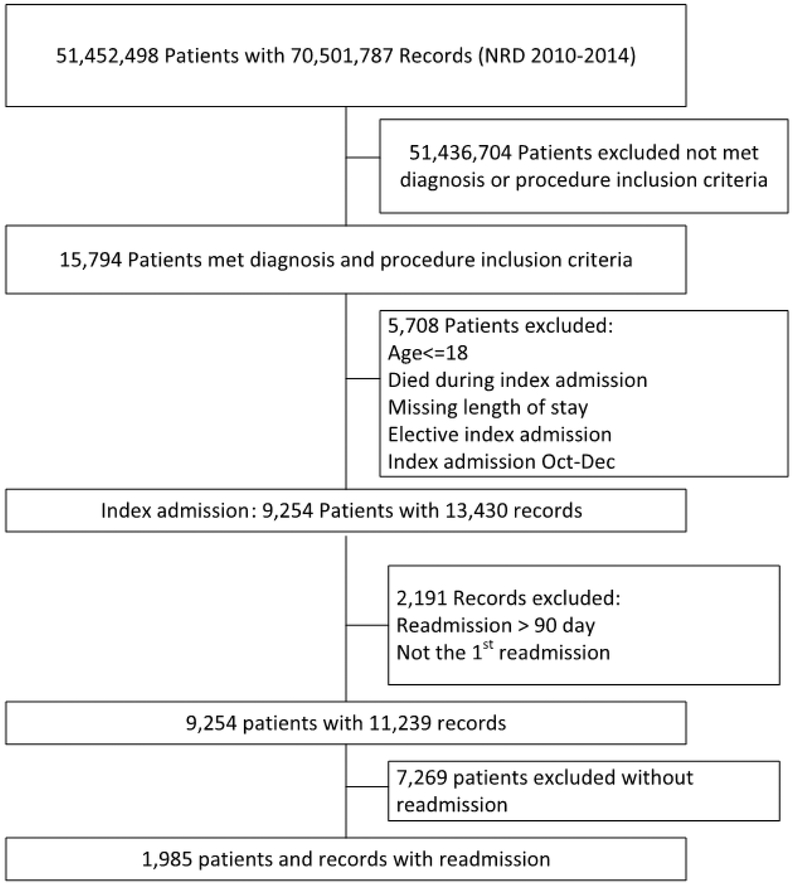
Out of 9,254 patients admitted for treated ruptured aneurysms recorded in the NRD, 1,985 (21.5%) were readmitted within 90 days (Figure 1). 355 of readmissions were to a hospital other than that of original admission (17.9% nonindex readmission rate). The mean admission length of stay was 27.2 days (standard deviation=19.2). The rates of aneurysmal clip ligation (n=958, 48.3%) and endovascular embolization (n=961, 48.4%) for treatment were similar amongst readmissions Most readmissions were female (n=1,336, 67.3%), privately (n=759, 38.2%) or Medicare insured (n=638, 32.1%), and received neither ventriculostomy (n=1,405, 70.8%) nor treatment for hydrocephalus (n=1,389, 70.0%). Of the hospitals represented, most were private non-profit (n=1,419, 71.5%) teaching hospitals (n=1,732, 87.3%), located in a large metropolitan area with at least 1 million residents (n=1,345, 67.8%), and had a large patient bed capacity (n=1,676, 84.4%). Most readmissions lacked major (n=1,384, 69.7%) or neurological complications (n=1,549, 78.0%), and were discharged to another facility (1,298, 65.4%). A summary of patient and hospital variables found in index and nonindex readmission groups is included in Tables 1 and 2.
Figure 1.
Patient inclusion/exclusion flow chart from NRD 2010–2014. NRD, Nationwide Readmissions Database.
Table 1.
Patient Readmission Demographics
| Variable | All Readmissions | Index | Nonindex | p-value |
|---|---|---|---|---|
| Total | 1985 | 1630 (82.1%) | 355 (17.9%) | |
| Procedure | ||||
| Clipping | 958 (48.3%) | 795 (48.8%) | 163 (45.9%) | 0.459 |
| Coiling | 961 (48.4%) | 779 (47.8%) | 182 (51.2%) | |
| Multiple | 66 (3.3%) | 56 (3.4%) | DS* | |
| Age | ||||
| 18–44 | 395 (19.9%) | 306 (18.8%) | 54 (15.2%) | 0.008* |
| 45–59 | 791 (39.8%) | 665 (40.8%) | 126 (35.5%) | |
| 60–74 | 639 (32.2%) | 512 (31.4%) | 127 (35.8%) | |
| >=75 | 195 (9.8%) | 147 (9.0%) | 48 (13.5%) | |
| Gender | ||||
| Male | 649 (32.7%) | 519 (31.8%) | 130 (36.6%) | 0.082 |
| Female | 1336 (67.3%) | 1111 (68.1%) | 225 (63.4%) | |
| Primary insurance | ||||
| Medicare | 638 (32.1%) | 492 (30.2%) | 146 (41.1%) | 0.0003* |
| Medicaid | 375 (18.9%) | 301 (18.5%) | 74 (20.9%) | |
| Private insurance | 759 (38.2%) | 659 (40.4%) | 100 (28.1%) | |
| Self-pay | 123 (6.2%) | 104 (6.4%) | 19 (5.4%) | |
| No charge | 11 (0.6%) | DS | DS | |
| Other | 77 (3.9%) | 63 (3.8%) | 14 (3.9%) | |
| Missing | DS | DS | DS | |
| Elixhauser comorbidity score | ||||
| 0 | 102 (5.1%) | 87 (5.3%) | 15 (4.2%) | 0.0001* |
| 1 | 289 (14.6%) | 255 (15.6%) | 34 (9.6%) | |
| 2 | 395 (19.9%) | 341 (20.9%) | 54 (15.2%) | |
| >= 3 | 1199 (60.4%) | 947 (58.1%) | 252 (71.0%) | |
| Median household income for patient’s ZIP code | ||||
| 0–25 percentile | 542 (27.3%) | 441 (27.1%) | 101 (28.5%) | 0.262 |
| 26–50 percentile | 505 (25.4%) | 410 (25.2%) | 95 (26.8%) | |
| 51–75 percentile | 467 (23.5%) | 379 (23.3%) | 88 (24.8%) | |
| 76–100 percentile | 438 (22.1%) | 374 (22.9%) | 64 (18.0%) | |
| Missing | 33 (1.7%) | 26 (1.6%) | DS | |
| All Patient Refined DRG: Severity of Illness Subclass | ||||
| Minor | 51 (2.6%) | 43 (2.6%) | DS | 0.076 |
| Moderate | 174 (8.8%) | 144 (8.8%) | 30 (8.5%) | |
| Major | 701 (35.3%) | 595 (36.5%) | 106 (29.9%) | |
| Extreme | 1059 (53.4%) | 848 (52.0%) | 211 (59.4%) | |
| Resident of state where procedure was performed | ||||
| Nonresident | 142 (7.2%) | 135 (8.3%) | DS | <0.0001* |
| Resident | 1843 (92.8%) | 1495 (91.7%) | 348 (98.0%) | |
| Discharged to another facility | ||||
| Yes | 1298 (65.4%) | 1025 (62.9%) | 273 (76.9%) | <0.0001* |
| No | 684 (34.5%) | 603 (37.0%) | 81 (22.8%) | |
| Missing | DS | DS | DS | |
| Discharge quarter | ||||
| Jan-March | 703 (35.4%) | 567 (34.8%) | 136 (38.3%) | 0.395 |
| April-June | 658 (33.1%) | 542 (33.3%) | 116 (32.7%) | |
| July-Sep | 624 (31.4%) | 521 (32.0%) | 103 (29.0%) | |
| Admission major complication | ||||
| Yes | 601 (30.3%) | 465 (28.3%) | 136 (38.3%) | 0.0003* |
| No | 1384 (69.7%) | 1165 (71.5%) | 219 (61.7%) | |
| Admission neurological complication | ||||
| Yes | 436 (22.0%) | 357 (21.9%) | 79 (22.25%) | 0.885 |
| No | 1549 (78.0%) | 1273 (78.1%) | 276 (77.75%) | |
| Treated for hydrocephalus | ||||
| Yes | 596 (30.0%) | 471 (28.9%) | 125 (35.2%) | 0.019* |
| No | 1389 (70.0%) | 1159 (71.1%) | 230 (64.8%) | |
| Ventriculostomy | ||||
| Yes | 580 (29.2%) | 471 (28.9%) | 109 (30.7%) | 0.497 |
| No | 1405 (70.8%) | 1159 (71.1%) | 246 (69.3%) | |
Data suppressed (DS) for patient privacy considerations, in accordance with the Healthcare Cost and Utilization Project NRD guidelines for publishing privacy protections.
Table 2.
Hospital Demographics
| Variable | All Readmissions | Index | Nonindex | p-value |
|---|---|---|---|---|
| Control/ownership of hospital | ||||
| Government, nonfederal | 418 (21.1%) | 342 (20.1%) | 76 (21.4%) | 0.002* |
| Private, not-profit | 1419 (71.5%) | 1182 (72.5%) | 237 (66.7%) | |
| Private, investor-owned | 148 (7.5%) | 106 (6.5%) | 42 (11.8%) | |
| Teaching status | ||||
| Teaching | 1732 (87.3%) | 1429 (87.7%) | 303 (85.4%) | 0.236 |
| Non-teaching | 253 (12.7%) | 201 (12.3%) | 52 (14.7%) | |
| Hospital bedsize | ||||
| Small | 71 (3.6%) | 55 (3.4%) | 16 (4.5%) | 0.006* |
| Medium | 238 (12.0%) | 179 (11.0%) | 59 (16.6%) | |
| Large | 1676 (84.4%) | 1396 (85.6%) | 280 (78.9%) | |
| Hospital urban-rural designation | ||||
| Large metropolitan area > 1 million residents | 1345 (67.8%) | 1102 (67.6%) | 243 (68.5%) | 0.758 |
| Small metropolitan area < 1 million residents, or micropolitan | 640 (32.2%) | 528 (32.4%) | 112 (31.6%) | |
Associations with Nonindex Readmission
Multivariable regression analysis demonstrated covariates associated with readmission to a nonindex hospital. Patients initially treated at hospitals considered to be private and investor-owned (OR=1.70 [95% CI: 1.09 – 2.67], p =0.020) had greater odds of readmission to a nonindex hospital. Patients discharged to a skilled nursing or other facility after their primary admission (OR=1.70 [95% CI: 1.27 – 2.28], p=0.0004) were also more likely to be readmitted to a nonindex facility. Having private insurance was associated with decreased odds of nonindex readmission (OR=0.65 [95% CI: 0.46 – 0.92], p=0.014). Compared to patients with an Elixhauser comorbidity score of 3 or greater, patients with Elixhauser comorbidity of 2 had lower odds of nonindex readmission (OR=0.66 [95% CI: 0.47 – 0.92], p=0.014). The results for all variables assessed for associations with 90-day nonindex readmission are summarized in Table 3.
Table 3.
Predictors of 90-Day Nonindex Readmission
| Variable | Nonindex N (%) | Odds Ratio (95% Confidence Interval) | p-value |
|---|---|---|---|
| Primary insurance | |||
| Medicare | 145 (41.0%) | 1.09 (0.78 – 1.52) | 0.609 |
| Medicaid | 74 (20.9%) | Reference | |
| Private insurance | 100 (28.3%) | 0.65 (0.46 – 0.92) | 0.014* |
| Self-pay | 19 (5.4%) | 0.93 (0.53 – 1.63) | 0.791 |
| No charge | DS* | 1.18 (0.24 – 5.73) | 0.834 |
| Other | 14 (4.0%) | 1.11 (0.58–2.12) | 0.940 |
| Control/ownership of hospital | |||
| Government, nonfederal | 76 (21.5%) | Reference | |
| Private, not-profit | 236 (66.7%) | 0.86 (0.64 – 1.15) | 0.300 |
| Private, investor-owned | 42 (11.9%) | 1.70 (1.09 – 2.67) | 0.020* |
| Resident of state where procedure was performed | |||
| Nonresident | DS | 0.25 (0.12– 0.54) | 0.0004* |
| Resident | 347 (98.0%) | Reference | |
| Discharged to another facility | |||
| Yes | 347 (98%) | 1.70 (1.27 – 2.28) | 0.0004* |
| No | 81 (22.9%) | Reference | |
| Comorbidity score | |||
| 0 | 15 (4.2%) | 0.97 (0.54 – 1.75) | 0.921 |
| 1 | 34 (9.6%) | 0.68 (0.46 – 1.02) | 0.062 |
| 2 | 53 (15.0%) | 0.66 (0.47 – 0.92) | 0.014* |
| >= 3 | 252 (71.2%) | Reference | |
Data suppressed (DS) for patient privacy considerations, in accordance with the Healthcare Cost and Utilization Project NRD guidelines for publishing privacy protections.
Outcome comparisons between Index vs. Nonindex Readmission
When compared to those readmitted to index facilities, patients readmitted to nonindex facilities were associated with increased likelihood of major complication (OR=1.71 [95% CI: 1.18 – 2.48], p=0.005) and second readmission (OR=1.51 [95% CI: 1.17 – 1.96], p=0.002). Odds ratios for outcomes analyzed between nonindex and index readmissions are summarized in Table 4.
Table 4.
Multivariable Regression for Clinical Outcomes: Nonindex Readmission versus Index Readmission
| Outcome | Nonindex N(%) | OR (95% Confidence Interval) | p-value |
|---|---|---|---|
| Major Complication | 76 (23.5) | 1.71 (1.18 – 2.48) | <.0001* |
| Second Readmission | 108 (24.0) | 1.51 (1.17 – 1.96) | 0.002* |
Discussion
This study characterized readmission after treatment for aneurysmal subarachnoid hemorrhage (aSAH) by identifying factors associated with readmission to a different hospital (nonindex), and the impact on clinical outcomes. Approximately 18% of readmissions occurred at non-index hospitals. Patients that were discharged from their primary admission to a nursing home or other facility were more likely to be readmitted to a nonindex hospital. Nonindex readmission was associated with a greater risk for major complications and second readmission when compared to index readmissions.
Nonindex readmissions following surgical or endovascular aSAH treatment creates interruptions in the follow-up care of complex and often critically ill patients. Discontinuity can place healthcare providers at a substantial disadvantage, lacking complete integration and understanding of the patients’ prior clinical history and management plan. Increased continuity of care is generally known to be associated with fewer complications, better medication adherence, and better disclosure of clinically relevant medical history.29–31 Systems of care that share health information have shown promise in improving efficiency and outcomes for nonindex readmissions.29,32 Care fragmentation may contribute to secondary readmissions and complications associated with nonindex aSAH readmissions.
Studies of other surgical cohorts have noted nonindex readmission rates between 22.1% and 28.4%.16–19 These investigations observed higher morbidity rates associated with nonindex readmissions. Zafar et al. investigated NRD patients undergoing major cancer surgery, and found an approximate 30% increase in major complication risk for patients readmitted to a nonindex hopsital.19 A similar cohort of major cancer surgery patients from the State Inpatient Database of California was found to have 16% increased odds of having a second readmission following a nonindex readmission (when compared to index readmissions).20
While our findings parallel the trends of these general surgery investigations, the associations with poor outcomes noted with nonindex readmission following aSAH were much greater in magnitude. The complexity of treating aneurysmal hemorrhage may exacerbate the impact of care discontinuity as aneurysmal SAH patients require comprehensive follow-up by a multidisciplinary team.3,6,9 Aneurysm rupture is often associated with a hypercoagulable state and multi-system complications.33,34 Intracranial blood may persist for weeks.35,36 Fever, hyperglycemia, anemia, hyponatremia, hypernatremia, and DVT often occur at delayed time points.3,9 Management of complex medical issues by a care team with access to the complete clinical history has previously been shown to improve outcomes during readmissions.29,30,37. If a previously treated aSAH patient is readmitted for new onset weakness, it is important to know whether they had suffered from cerebral vasospasm or seizures during the index admission, to guide diagnosis and treatment. Likewise, whether a patient presents with confusion, history of electrolyte abnormalities, or a CSF diversion procedure is important in guiding further management. Oftentimes, aSAH patients cannot recount their own medical histories and are not accompanied by their primary care provider. Post-treatment aSAH patients also take multiple medications that have complex interactions and specific indications for use. These issues can be difficult for an admitting physician/care team to resolve without prior knowledge of the patient, or at least familiarity with the protocols and management paradigms of the treating physician or hospital center.
In our dataset, discharge to a secondary care facility was identified as a risk factor for nonindex readmission. This finding highlights the heightened vulnerability of post-treatment aSAH patients at outside care facilities. Although these patients are generally sicker, surgical cancer studies have demonstrated that patients discharged to skilled nursing facilities are at increased risk for nonindex readmission, even after adjusting for disease severity.18–20 By contrast, having private insurance was associated with a lower likelihood of readmission to a non-index hospital, which support prior studies that have demonstrated that private insurance payer status is associated with better patient outcomes following neurosurgical procedures.38 The decreased readmission rate to non-index hospitals may impact morbidity or subsequent patient readmissions.
During initial model building for nonindex readmission outcomes, we found procedure volume at the readmission hospital to be an important confounder for nonindex hospitalization morbidity. Prior to the current model, omitting adjustment for readmission hospital procedure volume artificially inflated the impact of nonindex readmission on patient morbidity (previously OR = 2.12). This finding underscores the importance of hospital volume as a potential confounder in evaluating clinical readmission outcomes for ruptured aneurysm patients. Indeed, prior ischemic stroke studies suggest that transfer to a high-volume facility can improve outcomes, despite the potential detriments associated with the transfer.3,39,40 The impact of procedure volume on nonindex readmission and subsequent outcomes warrants further investigation.
Retrospective cohort studies are inherently limited by their design. While large administrative datasets such as the NRD provide significant numbers of data points, they are restricted by coding accuracy,41 and lack of disease specificity.42,43 However, previous efforts at validation for use of ICD-9 coding in other administrative databases have shown high sensitivity and specificity, as well as positive predictive value estimates ranging from 80% to 94%.26,44 The NRD does not contain information on patient neurological status and aneurysm size/location at presentation, which have been shown to correlate with surgical and endovascular outcomes.11,45 The NRD lacks patient readmission data between calendar years and across state lines. Data on travel distance, which is generally associated with greater nonindex readmission rates,20,22,24 is also not available. Finally, NRD data is a projection of the unique American healthcare environment. Further investigation in other international settings may elucidate important driving forces for nonindex readmission unique to the variety of healthcare systems.
Conclusion
The current study demonstrates high nonindex readmission rates following initial hospitalizations for aneurysmal subarachnoid hemorrhage treatment. Approximately 18% of readmissions occurred to nonindex hospitals. Nonindex readmissions were associated with increased patient morbidity and risks of secondary readmission. These adverse outcomes may relate to disruption of care continuity. Future efforts targeting reduction in nonindex readmissions could potentially improve patient outcomes following treatment of ruptured aneurysms.
Sources of Funding:
This work was supported by the National Institutes of Health Southern Clinical and Translational Science Institute KL2 Research Scholar Award to Frank J. Attenello, MD [grant number 5KL2TR001854-03].
Glossary
- aSAH
aneurysmal subarachnoid hemorrhage
- EQUATOR
Enhancing the QUAlity and Transparency Of health Research
- STROBE
Strengthening the Reporting of OBservational studies in Epidemiology
- ICD-9
International Classification of Disease, Ninth Edition
- APR-DRG
All Patient Refined – Diagnosis Related Group
- AHRQ
Agency for Healthcare Research and Quality
- OR
Odds ratio
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosures:
WJM reports the following: consultant: Rebound Therapeutics, Viseon TSP, Medtronic, Penumbra, Stream Biomedical; investor: Cerebrotech, Endostream, Viseon, Rebound
All other authors: No competing financial disclosures exist.
Portions of this work were presented in poster form at the American Association of Neurological Surgeons Annual Meeting, San Diego, California, United States of America, April 13, 2019.
References
- 1.Bederson JB, Connolly ES Jr., Batjer HH, Dacey RG, Dion JE, Diringer MN, et al. Guidelines for the management of aneurysmal subarachnoid hemorrhage: a statement for healthcare professionals from a special writing group of the Stroke Council, American Heart Association. Stroke. 2009;40(3):994–1025. [DOI] [PubMed] [Google Scholar]
- 2.Zacharia BE, Hickman ZL, Grobelny BT, DeRosa P, Kotchetkov I, Ducruet AF, et al. Epidemiology of aneurysmal subarachnoid hemorrhage. Neurosurgery clinics of North America. 2010;21(2):221–233. [DOI] [PubMed] [Google Scholar]
- 3.Connolly ES Jr., Rabinstein AA, Carhuapoma JR, Derdeyn CP, Dion J, Higashida RT, et al. Guidelines for the management of aneurysmal subarachnoid hemorrhage: a guideline for healthcare professionals from the American Heart Association/american Stroke Association. Stroke. 2012;43(6):1711–1737. [DOI] [PubMed] [Google Scholar]
- 4.Anderson SW, Todd MM, Hindman BJ, Clarke WR, Torner JC, Tranel D, et al. Effects of intraoperative hypothermia on neuropsychological outcomes after intracranial aneurysm surgery. Annals of neurology. 2006;60(5):518–527. [DOI] [PubMed] [Google Scholar]
- 5.Connolly ES, Rabinstein AA, Carhuapoma JR, Derdeyn CP, Dion J, Higashida RT, et al. Guidelines for the Management of Aneurysmal Subarachnoid Hemorrhage. Stroke. 2012;43(6):1711–1737. [DOI] [PubMed] [Google Scholar]
- 6.Mascia L, Del Sorbo L. Diagnosis and management of vasospasm. F1000 Med Rep. 2009;1:33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hasan D, Wijdicks EF, Vermeulen M. Hyponatremia is associated with cerebral ischemia in patients with aneurysmal subarachnoid hemorrhage. Annals of neurology. 1990;27(1):106–108. [DOI] [PubMed] [Google Scholar]
- 8.Hoffman H, Ziechmann R, Gould G, Chin LS. The Impact of Aneurysm Location on Incidence and Etiology of Hyponatremia Following Subarachnoid Hemorrhage. World neurosurgery. 2018;110:e621–e626. [DOI] [PubMed] [Google Scholar]
- 9.Wartenberg KE, Schmidt JM, Claassen J, Temes RE, Frontera JA, Ostapkovich N, et al. Impact of medical complications on outcome after subarachnoid hemorrhage. Critical care medicine. 2006;34(3):617–623; quiz 624. [DOI] [PubMed] [Google Scholar]
- 10.Alaraj A, Hussein AE, Esfahani DR, Amin-Hanjani S, Aletich VA, Charbel FT. Reducing length of stay in aneurysmal subarachnoid hemorrhage: A three year institutional experience. Journal of Clinical Neuroscience. 2017;42:66–70. [DOI] [PubMed] [Google Scholar]
- 11.Greenberg JK, Washington CW, Guniganti R, Dacey RG Jr., Derdeyn CP, Zipfel GJ. Causes of 30-day readmission after aneurysmal subarachnoid hemorrhage. Journal of Neurosurgery. 2016;124(3):743–749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lichtman JH, Jones SB, Leifheit-Limson EC, Wang Y, Goldstein LB. 30-day mortality and readmission after hemorrhagic stroke among Medicare beneficiaries in Joint Commission primary stroke center-certified and noncertified hospitals. Stroke. 2011;42(12):3387–3391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Liang JW, Cifrese L, Ostojic LV, Shah SO, Dhamoon MS. Preventable Readmissions and Predictors of Readmission After Subarachnoid Hemorrhage. Neurocritical Care. 2018;08:08. [DOI] [PubMed] [Google Scholar]
- 14.Rumalla K, Smith KA, Arnold PM, Mittal MK. Subarachnoid Hemorrhage and Readmissions: National Rates, Causes, Risk Factors, and Outcomes in 16,001 Hospitalized Patients. World neurosurgery. 2018;110:e100–e111. [DOI] [PubMed] [Google Scholar]
- 15.Weber SM, Greenberg CC. Medicare Hospital Readmission Reduction Program: what is the effect on surgery? Surgery. 2014;156(5):1066–1068. [DOI] [PubMed] [Google Scholar]
- 16.Burke RE, Jones CD, Hosokawa P, Glorioso TJ, Coleman EA, Ginde AA. Influence of Nonindex Hospital Readmission on Length of Stay and Mortality. Medical Care. 2018;56(1):85–90. [DOI] [PubMed] [Google Scholar]
- 17.Chappidi MR, Kates M, Stimson CJ, Bivalacqua TJ, Pierorazio PM. Quantifying Nonindex Hospital Readmissions and Care Fragmentation after Major Urological Oncology Surgeries in a Nationally Representative Sample. Journal of Urology. 2017;197(1):235–240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chappidi MR, Kates M, Stimson CJ, Johnson MH, Pierorazio PM, Bivalacqua TJ. Causes, Timing, Hospital Costs and Perioperative Outcomes of Index vs Nonindex Hospital Readmissions after Radical Cystectomy: Implications for Regionalization of Care. Journal of Urology. 2017;197(2):296–301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zafar SN, Shah AA, Channa H, Raoof M, Wilson L, Wasif N. Comparison of Rates and Outcomes of Readmission to Index vs Nonindex Hospitals After Major Cancer Surgery. JAMA Surgery. 2018;11:11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zheng C, Habermann EB, Shara NM, Langan RC, Hong Y, Johnson LB, et al. Fragmentation of Care after Surgical Discharge: Non-Index Readmission after Major Cancer Surgery. Journal of the American College of Surgeons. 2016;222(5):780–789 e782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Glebova NO, Hicks CW, Taylor R, Tosoian JJ, Orion KC, Arnaoutakis KD, et al. Readmissions after complex aneurysm repair are frequent, costly, and primarily at nonindex hospitals. Journal of Vascular Surgery. 2014;60(6):1429–1437. [DOI] [PubMed] [Google Scholar]
- 22.Kelley KA, Young JI, Bassale S, Herzig DO, Martindale RG, Sheppard BC, et al. Travel distance influences readmissions in colorectal cancer patients-what the primary operative team needs to know. J Surg Res. 2018;227:220–227. [DOI] [PubMed] [Google Scholar]
- 23.Patel MS, Fong ZV, Wojcik BM, Noorbakhsh A, Wilson SE, Chang DC. Hospital Teaching Status and Readmission after Open Abdominal Aortic Aneurysm Repair. Ann Vasc Surg. 2018;50:186–194. [DOI] [PubMed] [Google Scholar]
- 24.Smith AB, Meyer AM, Meng K, Nielsen ME, Pruthi R, Wallen E, et al. The relationship of travel distance with cystectomy access and outcomes. Urol. 2018;36(6):308.e301–308.e309. [DOI] [PubMed] [Google Scholar]
- 25.von Elm E, Altman DG, Egger M, Pocock SJ, Gotzsche PC, Vandenbroucke JP, et al. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. Epidemiology. 2007;18(6):800–804. [DOI] [PubMed] [Google Scholar]
- 26.Tirschwell DL, Longstreth WT Jr. Validating administrative data in stroke research. Stroke. 2002;33(10):2465–2470. [DOI] [PubMed] [Google Scholar]
- 27.Averill R, Goldfield N, Hughes J, Bonazelli J, McCullough E, Steinbeck B, et al. All Patient Refined Diagnosis Related Groups (APR-DRGs), version 20.0 Methodology Overview. 3M Health Information Systems; 2012. [Google Scholar]
- 28.Hillis AE, Anderson N, Sampath P, Rigamonti D. Cognitive impairments after surgical repair of ruptured and unruptured aneurysms. Journal of Neurology, Neurosurgery & Psychiatry. 2000; 69(5):608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pereira Gray DJ, Sidaway-Lee K, White E, Thorne A, Evans PH. Continuity of care with doctors—a matter of life and death? A systematic review of continuity of care and mortality. BMJ Open. 2018;8(6). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chen CC, Tseng CH, Cheng SH. Continuity of care, medication adherence, and health care outcomes among patients with newly diagnosed type 2 diabetes: a longitudinal analysis. Med Care. 2013;51(3):231–237. [DOI] [PubMed] [Google Scholar]
- 31.Chen CC, Cheng SH. Continuity of care and changes in medication adherence among patients with newly diagnosed diabetes. The American journal of managed care. 2016;22(2):136–142. [PubMed] [Google Scholar]
- 32.Lammers EJ, Adler-Milstein J, Kocher KE. Does Health Information Exchange Reduce Redundant Imaging? Evidence From Emergency Departments. Medical Care. 2014;52(3):227–234. [DOI] [PubMed] [Google Scholar]
- 33.Peltonen S, Juvela S, Kaste M, Lassila R. Hemostasis and fibrinolysis activation after subarachnoid hemorrhage. J Neurosurg. 1997;87(2):207–214. [DOI] [PubMed] [Google Scholar]
- 34.Nina P, Schisano G, Chiappetta F, Luisa Papa M, Maddaloni E, Brunori A, et al. A study of blood coagulation and fibrinolytic system in spontaneous subarachnoid hemorrhage. Correlation with hunt-hess grade and outcome. Surgical neurology. 2001;55(4):197–203. [DOI] [PubMed] [Google Scholar]
- 35.Dolinskas CA, Bilaniuk LT, Zimmerman RA, Kuhl DE. Computed tomography of intracerebral hematomas. I. Transmission CT observations on hematoma resolution. AJR American journal of roentgenology. 1977;129(4):681–688. [DOI] [PubMed] [Google Scholar]
- 36.Broderick J, Connolly S, Feldmann E, Hanley D, Kase C, Krieger D, et al. Guidelines for the management of spontaneous intracerebral hemorrhage in adults: 2007 update: a guideline from the American Heart Association/American Stroke Association Stroke Council, High Blood Pressure Research Council, and the Quality of Care and Outcomes in Research Interdisciplinary Working Group. Stroke. 2007;38(6):2001–2023. [DOI] [PubMed] [Google Scholar]
- 37.Cabana MD, Jee SH. Does continuity of care improve patient outcomes? The Journal of family practice. 2004;53(12):974–980. [PubMed] [Google Scholar]
- 38.McClelland S 3rd, Guo H, Okuyemi KS. Population-based analysis of morbidity and mortality following surgery for intractable temporal lobe epilepsy in the United States. Archives of neurology. 2011;68(6):725–729. [DOI] [PubMed] [Google Scholar]
- 39.Nuno M, Patil CG, Lyden P, Drazin D. The effect of transfer and hospital volume in subarachnoid hemorrhage patients. Neurocrit Care. 2012;17(3):312–323. [DOI] [PubMed] [Google Scholar]
- 40.Parikh NS, Chatterjee A, Diaz I, Pandya A, Merkler AE, Gialdini G, et al. Modeling the Impact of Interhospital Transfer Network Design on Stroke Outcomes in a Large City. Stroke. 2018;49(2):370–376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Southern DA, Roberts B, Edwards A, Dean S, Norton P, Svenson LW, et al. Validity of administrative data claim-based methods for identifying individuals with diabetes at a population level. Canadian journal of public health = Revue canadienne de sante publique. 2010;101(1):61–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Johnson EK, Nelson CP. Values and pitfalls of the use of administrative databases for outcomes assessment. J Urol. 2013;190(1):17–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.McClelland S 3rd, Jalai CM, Ryu S, Passias PG. Limitations of using population-based databases to assess trends in spinal stereotactic radiosurgery. Journal of radiosurgery and SBRT. 2016;4(3):177–180. [PMC free article] [PubMed] [Google Scholar]
- 44.Roumie CL, Mitchel E, Gideon PS, Varas-Lorenzo C, Castellsague J, Griffin MR. Validation of ICD-9 codes with a high positive predictive value for incident strokes resulting in hospitalization using Medicaid health data. Pharmacoepidemiology and drug safety. 2008;17(1):20–26. [DOI] [PubMed] [Google Scholar]
- 45.Wiebers DO, Whisnant JP, Huston J 3rd, Meissner I, Brown RD Jr., Piepgras DG, et al. Unruptured intracranial aneurysms: natural history, clinical outcome, and risks of surgical and endovascular treatment. Lancet (London, England). 2003;362(9378):103–110. [DOI] [PubMed] [Google Scholar]