Abstract
Objective:
To assess the pharmacokinetics of combined oral contraceptive (COC) components and prevalence of ovulation in HIV-positive women using ritonavir-containing antiretroviral regimens compared to those using regimens previously found not to interact with COCs or not using any antiretrovirals.
Study Design:
We conducted a prospective cohort pharmacokinetic pilot study comparing the pharmacokinetics of levonorgestrel (LNG) and ethinyl estradiol (EE) in HIV-positive women taking ritonavir-containing antiretroviral regimens to those in women using non-ritonavir-containing regimens or no antiretrovirals. Participants received COCs containing LNG/EE 150/30 mcg for 21 days. Beginning day 21, we collected serial blood samples over 72 hours. The primary outcome was area-under-the-curve (AUC) of LNG, with secondary outcomes including other LNG pharmacokinetic measures, EE pharmacokinetics, and ovulation as measured by serum progesterone.
Results:
Pharmacokinetic parameters of LNG showed a trend towards increased exposure in women on ritonavir. LNG AUClast increased by 32.6% (312 ±60.9 vs 243 ±82.6 ng/mL*h, p=0.033, n=5) in women taking ritonavir compared to the control group (n=10). The Cmax (9.68 ±1.81 vs 7.62 ±2.29 ng/mL) and Cmin (4.97 ±1.15 vs 3.70 ±1.29 ng/mL) were also higher in the ritonavir arm. After excluding the inconsistent users (n=2), CL of LNG was reduced in the ritonavir arm (p=0.032). EE pharmacokinetic profiles were not different between groups. The progesterone concentrations were similar in women of both groups and none were consistent with ovulation during the treatment cycle.
Conclusion:
Women on ritonavir showed an approximately 30% increase in LNG exposure but no difference in EE exposure.
Implications:
The current data suggest that ritonavir does not have a clinically significant impact on oral contraceptive pharmacokinetics.
Keywords: Combined oral contraceptives, HIV-positive, Pharmacokinetics, Clearance, Volume of distribution
1. Introduction
Human Immunodeficiency Virus (HIV) currently occurs in approximately 18.2 million women worldwide [1], including an estimated 256,500 women in the United States [2]. Effective contraception is a key component of the care of women infected with HIV, both for optimization of maternal health and prevention of perinatal transmission [1]. However, data to guide contraceptive use among HIV-positive women remain markedly limited. Although Centers for Disease Control Medical Eligibility Criteria classify ritonavir-boosted protease inhibitors as Category 2 (ie, benefits outweigh risks), theoretical concerns remain regarding the efficacy of combined oral contraceptives (COCs) in the large number of women using antiretroviral (ARV) regimens containing the protease inhibitor ritonavir [3].
Protease inhibitors including ritonavir, saquinavir, nelvirapine, and atazanavir are considered strong inhibitors of the CYP3A4 enzymes [3]. Ritonavir specifically acts via competitive reversible binding to CYP3A4 enzymes [4]. In practice, it is given in conjunction with other ARV medications, particularly other protease inhibitors, to enhance their activity through inhibition of drug metabolism. As the CYP3A4 system also metabolizes contraceptive steroids [5, 6], ritonavir would be expected to increase levonorgestrel (LNG) and ethinyl estradiol (EE) concentrations in women using COCs. Surprisingly, prior pharmacokinetic studies of COC users paradoxically demonstrated decreased EE exposure in women taking protease inhibitors [7–17], including ritonavir [9–13]. Conversely, the same studies showed great variation in the effects of protease inhibitors on the progestin components of COCs [7–18], ranging from a 18% decrease in norethindrone area-under-the-curve (AUC) [15] to a 110% increase in AUC of norethindrone [14]. In addition, the majority of existing studies of pill use in conjunction with protease inhibitors evaluated norethindrone-containing COCs [7–9, 12, 14, 18]. Although LNG-containing COCs are among the most commonly-prescribed COCs in the United States [19], only one small study investigated the pharmacokinetics of a pill containing norgestrel [17]. In that study LNG AUC appeared to increase in women taking nevirapine.
Moreover, few studies correlated observed pharmacokinetic effects of protease inhibitors with pharmacodynamic effects such as ovulation; those that did evaluate ovulation generally relied on a single progesterone level [4, 7, 14, 17].
We hypothesized that LNG AUC would increase in women taking ritonavir while EE AUC would decrease. We further hypothesized that suppression of ovulation would be unaffected.
2. Materials and Methods
We recruited HIV-positive women (Age 18–45, BMI ≤40 kg/m2, not currently using hormonal contraception) taking antiretroviral regimens that include ritonavir from the Los Angeles County-University of Southern California (USC) Maternal, Child, and Adolescent Clinic for Infectious Disease and Virology (MCA clinic) between October 2015 and October 2016. The control group comprised HIV-positive women using regimens previously shown not to affect COC metabolism or taking no antiretroviral medication [20]. We excluded potential participants if they took other CYP3A4 inhibitors or inducers. Details of the exclusion criteria can be found at ClinicalTrials.gov (Identifier: ). The USC Institutional Review Board approved the study.
Eligible women attended a screening visit during the luteal phase of their menstrual cycle (approximately day 21); we ascertained ovulatory status using serum progesterone values at this visit. We considered a value of 3 ng/mL or greater indicative of ovulation. After confirmation of ovulatory status, women took a COC containing LNG/EE 150/30 mcg (Marlissa, Glenmark Generics Inc, Mahwah, NJ) for one 21-day cycle beginning the first day of their next menses. During this cycle, participants underwent twice-weekly blood draws for measurement of serum progesterone in order to detect ovulation, with a value greater than or equal to 3 ng/mL considered consistent with ovulation. On day 21, participants completed a 12-hour visit for pharmacokinetic assessment of LNG and EE with concentrations measured at 0, 1, 2, 3, 4, 6, 8, and 12 hours. Participants returned at 24, 48, and 72 hours for additional LNG and EE samples. Participants were not required to fast on presentation nor fed a standard diet but could eat as desired throughout the visit. We separated whole blood specimens into serum and stored them at 4°C before being transferring them to a -80°C freezer. To assess compliance, participants returned their study pill package to investigators at the time of their pharmacokinetic visit. In addition, we measured LNG levels weekly. To assess compliance with ARV medication in the ritonavir arm, we evaluated ritonavir serum levels at the screening visit, during the second week of COC use, and on the day of the pharmacokinetic visit of participants using ritonavir. Participants were not randomized to ritonavir versus other ARV therapy given the complexity of choosing an ARV regimen and concerns about development of drug resistance. The ARV formulations and doses were at the discretion of the prescribing physicians.
The primary outcome was difference in LNG area under the concentration versus time curve over 72 hours (denoted AUClast) measured beginning on day 21. We further assessed additional LNG PK parameters, including time to maximum concentration (Tmax), maximum concentration (Cmax), minimum concentration (Cmin), and clearance (CL) and compared these between groups. Secondary outcomes included EE PK measures and ovulation as assessed by serial progesterone measurements.
2.1. Laboratory assays
The USC Reproductive Endocrine Research Laboratory performed all hormonal assays under the direction of Dr. Frank Z Stanczyk. We used specific and sensitive radioimmunoassays (RIAs) to quantify LNG and EE serum levels as described [21–23] previously. We measured progesterone using a competitive chemiluminescent immunoassay on the Immulite analyzer (Siemens Healthcare Diagnostics, Deerfield, IL). The sensitivity of this assay was 0.1 ng/mL.
The Drug Research Unit at the University of California, San Francisco performed ritonavir assays using liquid chromatography/tandem mass spectrometry in accordance with previously described methods [11].
2.2. Statistical Analysis
We examined Cmax (maximum concentration), Cmin (concentration at 24 h), AUCτ (area under the concentration versus time curve in 24 h), AUClast (area under the concentration versus time curve over 72 h), AUCinf (area under the concentration versus time curve to infinity), Tmax (time to maximum drug concentration), MRT (mean residence time), T1/2 (half-life), CL (clearance), and Vss (volume at steady-state) for both LNG and EE in all participants. We used Phoenix WinNonlin version 7.0 (Certarar, St. Louis, MO) with linear up-log down method to perform the non-compartmental analysis (NCA) and R (version 3.4.3) for the Shapiro-Wilk Normality test. Comparison of non-normally distributed PK parameters, including Tmax, T1/2, Vss and CL, utilized nonparametric tests (Mann-Whitney), while comparison of normally distributed PK parameters (AUClast, AUCτ, Cmax, and Cmin) utilized the unpaired t test with Welch’s correction. We used GraphPad Prism 7.04 to perform all statistical analyses with significance defined as p<0.05.
A prior study compared norgestrel pharmacokinetics in women taking ARV therapy to women not taking ARV medication and found an increase of approximately 30% in norgestrel AUC in women taking ARV[17]. In order to have 80% power to detect the same change in LNG AUC with an alpha of 0.05, we needed 10 women per group to complete the study.
3. Results
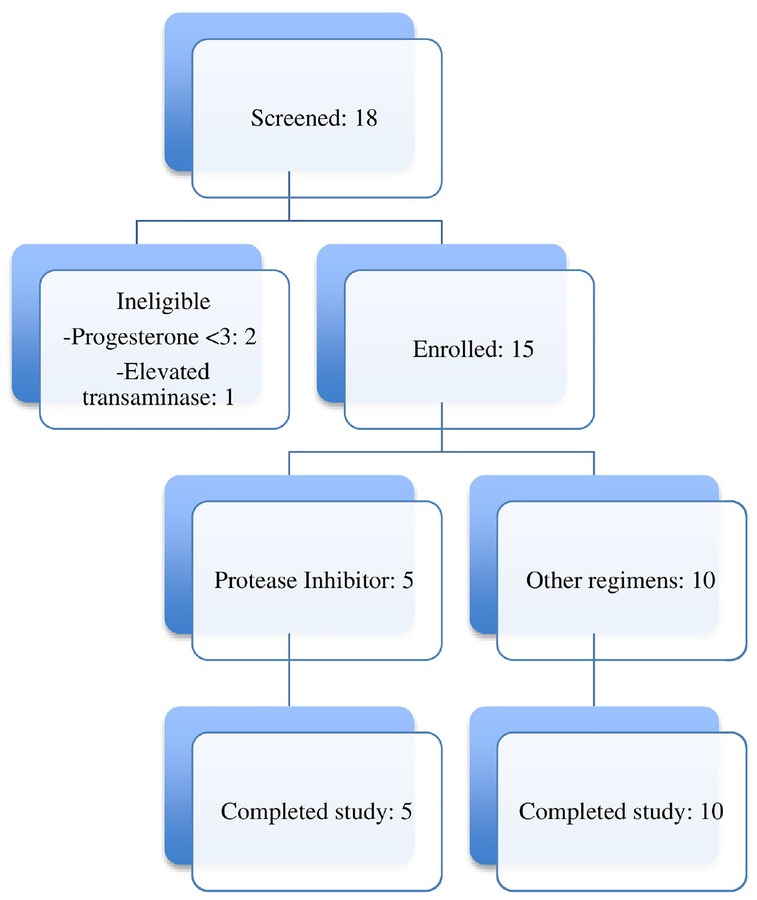
Twenty participants completed the screening visit. We excluded three women after screening: two for anovulatory status and one with elevated transaminases. The remaining 17 candidates enrolled in and completed the study, including seven in the ritonavir arm and 10 in the control arm. One participant in the ritonavir arm has unavailable EE data. We excluded two participants in the ritonavir arm from analysis due to non-compliance with ritonavir. The final analysis included five participants (including two inconsistent users with some levels below detectable limits) for the ritonavir arm and 10 for the control arm (Figure 1).
Figure 1.
Flow of screening and enrollment of women taking ritonavir-boosted protease inhibitors or antiretroviral regimens found not to interact with COCs
Both groups had similar baseline characteristics with respect to age, BMI, parity, CD4 numbers, smoking status, history of opportunistic infections, baseline progesterone, and serum creatinine (Table 1).
Table 1.
Baseline Characteristics of Subjects in the Control arm (COCs) or Ritonavir Arm (COCs+Ritonavir)
| COCs | COCs+Ritonavir | |
|---|---|---|
| 10 | 5 | |
| Hispanic N (%) | 8 (80) | 5 (100) |
| Age (years)a | 34.5 (30,42) | 38 (29,41) |
| Weight (kg)a | 72.3 (58.5,86.6) | 58.5 (57.6,70.8) |
| BMI (kg/m2)a | 28.6 (24.8,36.1) | 26.4 (24.0,29.5) |
| Paritya | 2 (1,4) | 3 (3,4) |
| Smoking N (%) | 1 (10) | 0 (0) |
| History of opportunistic infection | none | none |
| CD4 (cells/mm3)a | 830 (571,1082) | 811 (229,1047) |
| Baseline progesterone (ng/mL)a | 8.6 (4.5,11.6) | 6.4 (5.9,12.2) |
| Creatinine (mg/dL)a | 0.68 (0.55,0.90) | 0.71 (0.51,0.99) |
Data are presented as median (min, max).
3.1. COC Pharmacokinetics
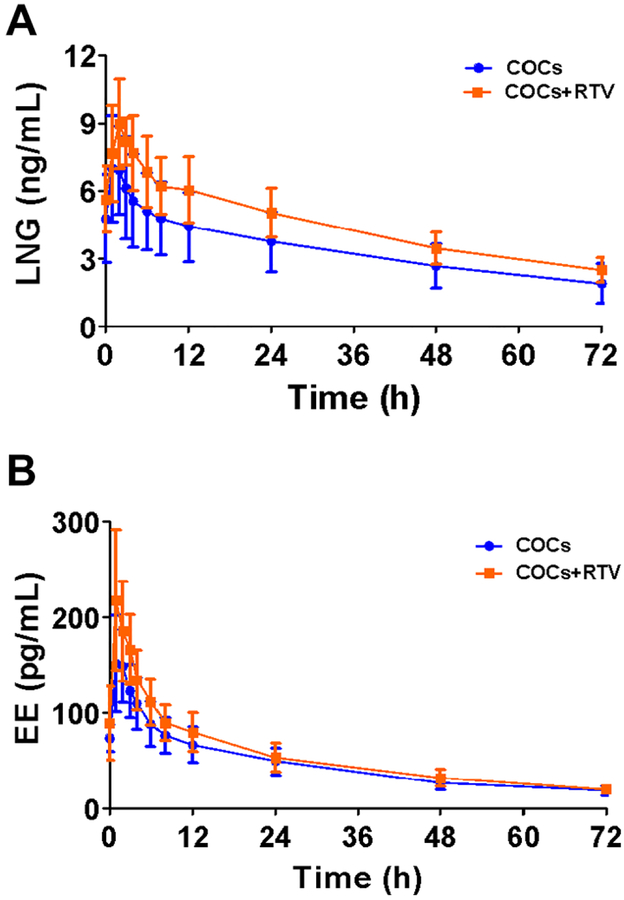
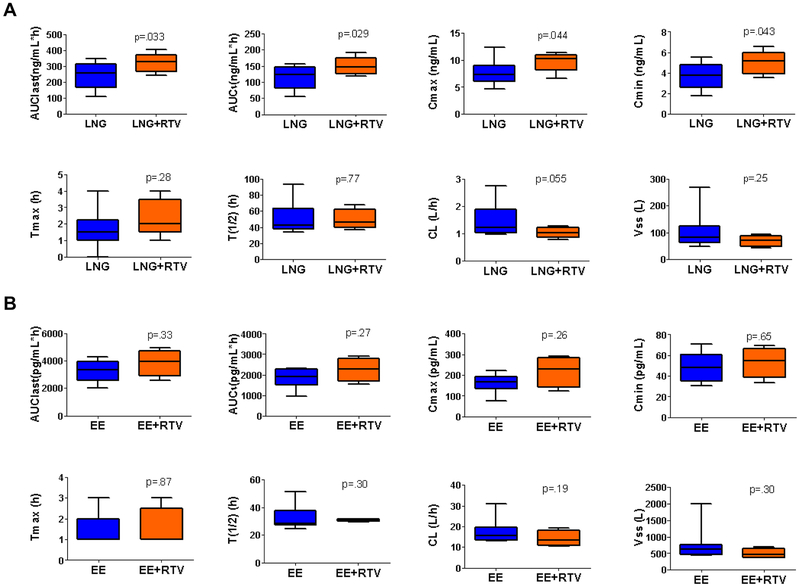
The LNG AUClast (321 ±61 ng/mL*h) was 32.0% higher (p=0.033) in the ritonavir group than in the control group (243 ±83 ng/mL*h). Overall participants on ritonavir had higher LNG exposure, with differences in Cmin, Cmax, and AUCτ higher than the control group (Figure 2A, Table 2). Table 2 includes details of all other LNG and EE pharmacokinetic parameters.
Figure 2.
Steady-state and washout plasma concentration vs time profiles for LNG (A) and EE (B) in women taking ritonavir-boosted ARV regimens (COCs+ritonavir (RTV), n=5) compared to women taking ARV regimens not interacting with COCs or no ARV medication (COCs, n=10). Data are presented as mean ±SD.
Table 2.
LNG and EE PK Parameters in Women taking COCs (Control, n=10) or COCs + Ritonavir (Ritonavir, n=5)
| LNG | EE | |||||||
|---|---|---|---|---|---|---|---|---|
| Parameter | Control arm | Ritonavir arm | Change. (%) | p value | Control Arm | Ritonavir arm | Change (%) | p value |
| AUClasta | 243 ±83 | 321 ±61 | 32.0 | 0.033 | 3270 ±744 | 3867 ±976 | 18.2 | 0.33 |
| AUCTa | 113 ±37 | 150 ±28 | 32.6 | 0.029 | 1848 ±433 | 2251 ±567 | 21.8 | 0.27 |
| Cmaxb | 7.62 ±2.29 | 9.68 ±1.81 | 27.0 | 0.044 | 163.3 ±43.8 | 218.3 ±73.4 | 33.7 | 0.26 |
| Cminb | 3.70 ±1.29 | 4.97 ±1.15 | 34.4 | 0.043 | 49.2 ±14.1 | 53.3 ±14.7 | 8.5 | 0.65 |
| Tmax (h) c | 1.5 (0.2,3.8) 42.6 |
2.0 (1.1,3.9) 46.2 |
33.3 | 0.28 | 1.0 (1.0,2.8) 28.4 |
1.0 (1.0,2.9) 31.3 |
0.0 | 0.87 |
| T1/2 (h) c | (34.5,89.7) 1.23 |
(37.6,66.8) 1.02 |
8.2 | 0.77 | (24.9,49.1) 15.6 |
(29.8,32.0) 13.2 |
10.5 | 0.30 |
| CL(L/h) c | (0.96,2.65) 82.7 |
(0.80,1.27) 71.8 |
−16.7 | 0.055 | (13.0,28.5) 626 |
(10.5,19.0) | −15.1 | 0.19 |
| Vss (L) c | (51.2,238.3) | (44.0,93.4) | −13.2 | 0.25 | (427.1730) | 475 (370,671) | −24.1 | 0.30 |
AUClast: area under the concentration-time curve till the last observation (72h) of the last dose. AUCτ: area under the concentration curve (24h) of the last dose during the 21-day cycle. Units for LNG AUC (includes AUClast, AUCτ) are ng/mL*h, Units for EE AUC (includes AUClast, AUCτ) are pg/mL*h. Data presented as mean±SD as these parameters follow normal distribution by Shapiro-Wilk test. Statistical tests were performed with non-paired, 1-tailed t test with Welch’s correction for LNG with the hypothesis that exposures would be higher in the Ritonavir arm. 2-tailed t tests were used for EE because there was no clear direction of the change for EE.
Cmax: maximum observed concentration for the last dose of the cycle. Cmin: observed concentration 24h after the last dose of the cycle. Units of LNG Cmax, Cmin are ng/mL; Units of EE Cmax, Cmin are pg/mL.
Tmax: Time for maximum concentration observed. T1/2 (h): Half-life calculated by NCA method. CL: drug clearance calculated by NCA method. Vss: volume at steady state calculated by NCA method. Data are presented as median (95% Confidence interval) as Tmax, T1/2, CL and Vss don’t follow normal distribution, non-parametric tests (Mann-Whitney test) were used for statistical tests of these four parameters. 2-tailed tests were used for these parameters except for LNG CL where 1-tailed was used with the hypothesis that the clearance was lower in Ritonavir arm.
After excluding the two inconsistent users, we found LNG AUC values were still higher (p=0.033 for AUClast, p=0.039 for AUCτ) in the ritonavir group and the level of significance for Cmax increased (p=0.003 vs p=0.044). The CL was also lower after this exclusion (p=0.032 vs p=0.11).
Excluding the tobacco user in the control group did not affect the results (data not shown).
3.2. Progesterone concentrations during COC cycle
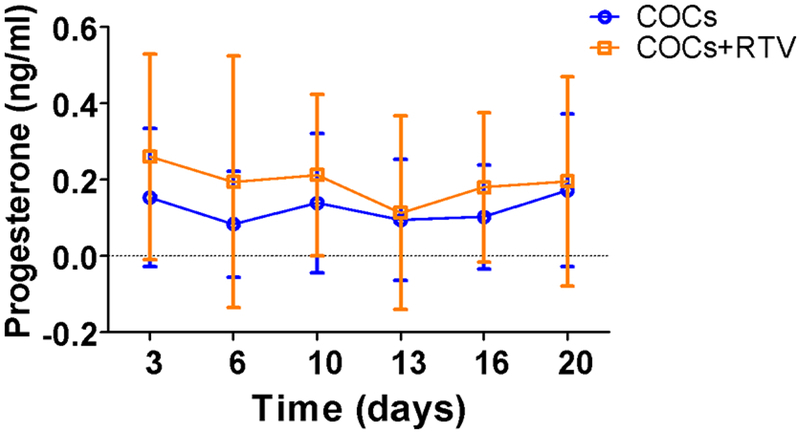
Progesterone concentrations in HIV-positive women using ritonavir-containing regimens and those using other regimens were similar at all times of visits during the treatment cycle (p≥0.5), with the mean progesterone at each visit in all women below 0.3 ng/mL (Figure 4). All progesterone concentrations were above 3 ng/mL at screening and below 1 ng/mL during COC usage, suggesting effective prevention of ovulation.
Figure 4.
Progesterone concentrations during COC cycle 1 in women taking ritonavir (RTV)-boosted ARV regimens (COCs+RTV, n=5) compared to women taking ARV regimens not interacting with COCs or no ARV medication (COCs, n=10). Data are presented as mean ±SD. Sampling times can be off by 1 or 2 days for each visit.
3.3. Compliance
One participant in the control arm had LNG monitoring values suggestive of inconsistent use (one undetectable level and an additional value less than 1 ng/mL). A second participant in the control arm had an initial monitoring LNG less than 1 ng/mL but subsequent concentrations were all greater than 1 ng/mL. The remainder of participants all had LNG values indicative of consistent use. In the ritonavir arm, two participants had undetectable RTV concentrations at all times, suggestive of nonuse. Two additional participants had undetectable values at two of four time points, suggesting inconsistent use.
4. Discussion
Although LNG-containing COCs are among the most commonly prescribed COCs in the United States, no prior studies specifically evaluated the effects of ritonavir on a LNG-containing COC [18]. The majority of existing studies of pill use in conjunction with protease inhibitors used norethindrone-containing COCs. For instance, Atrio et al studied the effects of ritonavir on the pharmacokinetics of a norethindrone progestin-only contraceptive pill, observing a 50% increase in norethindrone AUC among women using ritonavir [18]. Notably, Stuart et al [17] evaluated the effects of a combined ARV including the protease inhibitor nevirapine on the pharmacokinetics of a COC containing EE 30 mcg and norgestrel 300 mcg, finding an increase in LNG AUC from 114 to 147 ng*mL/h in women taking nevirapine, while EE AUC decreased from 1457 to 1384 ng*mL/h. Our data suggest that ritonavir may have a similar impact on LNG metabolism as we observed higher exposures in the ritonavir arm. Such results fit the expected effect of CYP3A4 inhibition on LNG metabolism and are comparable to the slight increases in LNG exposure observed with other moderate and strong CYP3A inhibitors [24]. However, other mechanisms may also contribute. For instance, ritonavir-induced insulin resistance may decrease sex-hormone binding globulin (SHBG) levels, which in turn may increase effective LNG exposure.
In contrast, we did not detect a difference in EE PK between groups. Most prior studies found decreases in EE exposure associated with ritonavir use [9–13]. This difference may be due to our small sample size, with only four participants in the ritonavir arm able to contribute EE data. Alternately, an SHBG-mediated mechanism could explain why LNG and not EE pharmacokinetics were affected; however, this would be inconsistent with other studies demonstrating decreased EE exposure in women taking ritonavir. Finally, variable effects on EE PK may be related to non-CYP3A4-mediated EE metabolism, including oxidation by CYP2C9 and metabolism by other non-CYP-mediated pathways as described by Zhang and colleagues [24].
In addition, prior studies of ritonavir effects on COC use have not included rigorous assessment of effects on ovulation. Stuart et al [17] measured a single progesterone value during the PK visit and found all women had values consistent with anovulation. We obtained samples throughout an entire cycle of COC use and found that progesterone values were uniformly low, suggesting that ritonavir use does not affect the ability of COCs to suppress ovulation. Although our study was small and the ritonavir group did not reach desired recruitment size, we did find an increase in LNG exposure in the ritonavir arm. Given the modest increase in LNG AUC and effective suppression of progesterone, these results suggest that LNG-based COCs are likely to have similar efficacy in women taking ritonavir-based regimens and those taking regimens that do not interact with COCs.
Our study has several notable limitations. We were unable to reach our target sample size for the ritonavir arm, resulting in a lack of power. Logistical considerations precluded extending the study through a second cycle of pill use. A cycle of 21 days -longer than five half-lives- should be sufficient duration to reach steady-state and to assess the effect of ritonavir on pharmacokinetic parameters. Nevertheless, limiting the study to a single cycle means we may have missed effects on time to steady-state or therapeutic concentration during initiation of a second cycle, which could have implications for recommendations for pill-free or placebo duration in women taking ritonavir. The inclusion of two participants who were intermittently compliant with ritonavir may have impacted our estimates; if ritonavir does increase LNG exposure, this intermittent compliance would bias our results toward the null hypothesis. In fact, we found that the difference in LNG CL became greater after excluding the inconsistent users, suggesting their inclusion may have diminished the impact of ritonavir on LNG metabolism. Though the analytical methodology used is a long-established and validated radioimmunoassay (RIA) newer methodologies such as LC-MS or GC-MS may have allowed measurement of LNG with greater specificity. Finally, participants were not randomized to ritonavir versus other therapies
In spite of these limitations, this study aligns with existing data supporting the safety and efficacy of oral contraceptives in women using ritonavir-based antiretroviral regimens and supports the current recommendation that women taking ritonavir are appropriate candidates for COC use [3].
Figure 3.
Boxplot of LNG (A) and EE (B) pharmacokinetic parameters of subjects in participants taking ritonavir-boosted ARV regimens (LNG/EE+Ritonavir (RTV), n=5) compared to women taking ARV regimens not interacting with COCs or no ARV medication (LNG/EE, n=10). Data are presented as boxplot with whiskers from minimum to maximum. Abbreviations of each parameter can be found in Table 2.
Acknowledgments
The authors thank Dr. Alice Stek and the staff of the MCA clinic for their assistance throughout this project.
Funding Source: The Society of Family Planning funded this project, and was not involved in the design, conduct, analysis, interpretation of results, writing of the report or decision to submit the article for publication. This work was also supported by National Institutes of Health Grant GM 24211
Financial Disclosures: Nicole M. Bender receives honoraria from Merck, Inc. William J. Jusko has been a recent consultant for Novartis, Boehringer Ingelheim, Reveragen, and Bayer Healthcare Products. Frank Z. Stanczyk serves as a consultant for Agile Therapeutics, TherapeuticsMD, Pantarhei Bioscience, and Mithra Pharmaceuticals.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
5. References
- [1].The Joint United Nations Programme on HIV/AIDS. wwwunaidsorg.Accessed on September 17, 2018. [PubMed] [Google Scholar]
- [2].Prevention CfDC. Estimated HIV incidence and prevalence in the United States, 2010–2015. HIV Surveillance Supplemental Report 2018; 23 (No. 1). [Google Scholar]
- [3].Centers for Disease Control and Prevention. US Medical Eligibility Criteria for Contraceptive Use. 2016;MMWR:1–108. [Google Scholar]
- [4].Department of Health U.S. and Human Services Food and Drug Administration. Guidance for Industry Drug Interaction Studies-Study Design Data Analysis and Implications for Dosing and Labeling. Clinical Pharmacology. September 2006. [Google Scholar]
- [5].Edelman AB, Cherala G, Stanczyk FZ. Metabolism and pharmacokinetics of contraceptive steroids in obese women: a review. Contraception. 2010;82:314–23. [DOI] [PubMed] [Google Scholar]
- [6].Fotherby K Pharmacokinetics of ethynyloestradiol in humans. Methods Find Exp Clin Pharmacol. 1982;4:133–41. [PubMed] [Google Scholar]
- [7].Lin WH, Feng HP, Shadle CR, O'Reilly T, Wagner JA, Butterton JR. Pharmacokinetic and pharmacodynamic interactions between the hepatitis C virus protease inhibitor, boceprevir, and the oral contraceptive ethinyl estradiol/norethindrone. Eur J Clin Pharmacol. 2014;70:1107–13. [DOI] [PubMed] [Google Scholar]
- [8].Garg V, van Heeswijk R, Yang Y, Kauffman R, Smith F, Adda N. The pharmacokinetic interaction between an oral contraceptive containing ethinyl estradiol and norethindrone and the HCV protease inhibitor telaprevir. J Clin Pharmacol. 2012;52:1574–83. [DOI] [PubMed] [Google Scholar]
- [9].Kasserra C, Li J, March B, O'Mara E. Effect of vicriviroc with or without ritonavir on oral contraceptive pharmacokinetics: a randomized, open-label, parallel-group, fixed-sequence crossover trial in healthy women. Clin Ther. 2011;33:1503–14. [DOI] [PubMed] [Google Scholar]
- [10].Zhang J, Chung E, Yones C, et al. The effect of atazanavir/ritonavir on the pharmacokinetics of an oral contraceptive containing ethinyl estradiol and norgestimate in healthy women. Antivir Ther. 2011;16:157–64. [DOI] [PubMed] [Google Scholar]
- [11].Vogler MA, Patterson K, Kamemoto L, et al. Contraceptive efficacy of oral and transdermal hormones when co-administered with protease inhibitors in HIV-1-infected women: pharmacokinetic results of ACTG trial A5188. J Acquir Immune Defic Syndr. 2010;55:473–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Sekar VJ, Lefebvre E, Guzman SS, et al. Pharmacokinetic interaction between ethinyl estradiol, norethindrone and darunavir with low-dose ritonavir in healthy women. Antivir Ther. 2008;13:563–9. [PubMed] [Google Scholar]
- [13].Ouellet D, Hsu A, Qian J, et al. Effect of ritonavir on the pharmacokinetics of ethinyl oestradiol in healthy female volunteers. Br J Clin Pharmacol. 1998;46:111–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Bristol-Meyers Squibb P NJ. Reyataz (atazanavir): US Prescribing Information 2009. [Google Scholar]
- [15].Mildvan D, Yarrish R, Marshak A, et al. Pharmacokinetic interaction between nevirapine and ethinyl estradiol/norethindrone when administered concurrently to HIV-infected women. J Acquir Immune Defic Syndr. 2002;29:471–7. [DOI] [PubMed] [Google Scholar]
- [16].Merck & Co I, Whitehouse Station, NJ: VICTRELIS™ (boceprivir) Capsules: US Prescribing Information 2011. [Google Scholar]
- [17].Stuart GS, Moses A, Corbett A, et al. Pharmacokinetics and Pharmacodynamics of a Combined Oral Contraceptive and a Generic Combined Formulation Antiretroviral in Malawi. J Acquir Immune Defic Syndr. 2011;58:e40–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Atrio J, Stanczyk FZ, Neely M, Cherala G, Kovacs A, Mishell DR Jr, Effect of protease inhibitors on steady-state pharmacokinetics of oral norethindrone contraception in HIV-infected women. J Acquir Immune Defic Syndr. 2014;65:72–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Creinin MD. Types of combined oral contraceptives used by US women. Contraception. 2013;88:192–3. [DOI] [PubMed] [Google Scholar]
- [20].Tseng A, Hills-Nieminen C. Drug interactions between antiretrovirals and hormonal contraceptives. Expert Opin Drug Metab Toxicol. 2013;9:559–72. [DOI] [PubMed] [Google Scholar]
- [21].Edelman AB, Carlson NE, Cherala G, et al. Impact of obesity on oral contraceptive pharmacokinetics and hypothalamic-pituitary-ovarian activity. Contraception. 2009;80:119–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Edelman AB, Cherala G, Munar MY, et al. Prolonged monitoring of ethinyl estradiol and levonorgestrel levels confirms an altered pharmacokinetic profile in obese oral contraceptives users. Contraception. 2013;87:220–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Westhoff CL, Torgal AH, Mayeda ER, Pike MC, Stanczyk FZ. Pharmacokinetics of a combined oral contraceptive in obese and normal weight women. Contraception. 2010;81:474–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Zhang N, Shon J, Kim M, et al. Role of CYP3A in oral contraceptives clearance. Clin Transl Sci. 2018;11:251–260. [DOI] [PMC free article] [PubMed] [Google Scholar]