Abstract
Recent research in functional genomics shows that social stressors affect the expression of immune response genes. These effects are mediated in part via our adaptive capacity for intracellular molecules to respond to extracellular signals, a process called signal transduction. Under this framework, one way stressors can be transduced into cellular changes is through central nervous system (CNS) modulation of peripheral neural, endocrine, and molecular activity. Mindfulness meditation is a consciousness discipline used to cultivate attention and self-regulation, and may thus be relevant to the signal transduction process outlined in the social genomics literature. In this opinion article, we briefly review results from existing controlled trials that test the effects of mindfulness meditation on gene expression. We then speculate on a mind-body conceptual model, grounded in existing social genomics theory. In the spirit of hypothesis generation, we argue that mindfulness meditation changes brain activity patterns related to attention, self-regulation, and threat evaluation and so may alter the signal transduction process that regulates the expression of immune response genes.
Keywords: Meditation, mindfulness, genomics, gene expression, theory, hypothesis generating
Mindfulness meditation and modern life stressors
Modern life is complicated. Advances in technological speed as well as growing social and political discord create an environment replete with real and imagined stressors. This mass information feed interfaces with our more slowly evolved hominid processing system, the brain, and its associated downstream molecular signals. As an example of modern demands on our brain, we routinely take in an estimated five times more information per day than 40 years ago (Levitin, 2014), and this information is often packaged to arouse fear. In this climate, it is not surprising that over 60% of American adults report feeling significant stress over money, work, or about the future of the nation (American Psychological Association, 2017). It is an ongoing question as to how our brain and biological systems will adapt to these unrelenting challenges. No evidence suggests a lull in this social complexity, and so adaptations, at least those that will be health promoting, will likely involve the use of practices applied by the individual that modulate attention and foster self-regulation in the context of environments replete with stressors.
Self-regulation of attention and awareness is one type of cognitive adaptation to stressors, and mindfulness meditation represents one learning technology for developing such skills. Mindfulness is defined as the state of awareness that emerges by way of paying purposeful and nonjudgmental attention to the present and the unfolding of experience moment by moment. (Kabat-Zinn, 2003) Meditation practiced over time is believed to promote a trait-like characteristic of mindfulness, representing a modification of central nervous system and/or mental functioning (i.e., changes in attention, awareness, perception, evaluation, and self-regulation). Although by no means conclusive, mindfulness meditation is associated with various brain changes, one being in the region of the amygdala, a region that is important for interpreting stressors and their threat valence (Arnsten 2009, Gianaros 2008, Holzel 2010). It has also been argued that mindfulness meditation correlates with increased parasympathetic nervous system activation (Ditto 2006), which might reciprocally down-regulate activity of the sympathetic nervous system-mediated stress response (Thayer & Lane 2000). A recent study reported that a stressed sample practicing mindfulness meditation showed a coupling of established brain regions to be correlated with a downstream molecular marker (i.e., reduced circulating levels of inflammation) (Creswell et al., 2016). Overall, these findings suggest that mindfulness meditation is associated with brain activity patterns suggestive of less stress reactivity, and that pattern may affect downstream peripheral stress response systems in a manner that has been detailed in the field of social genomics.
Genomic impact of mindfulness training: Initial evidence
Within the recent decade, several studies have assessed the effects of mindfulness meditation on gene expression in immune cells, with particular focus on stress-related inflammatory markers and associated biological pathways (see Table 1). Results of several randomized controlled trials share several common features, including a significant reduction in activity of the pro-inflammaory transcription factor nuclear factor (NF)-κB among samples of community adults (Black et al., 2015; Creswell et al., 2012), breast cancer survivors (Bower et al., 2015), and long-term meditators (García-Campayo J, 2017). In addition to NF-κB, investigators have assessed other biomarkers of inflammation and have found increased activity of anti-inflammatory glucocorticoid receptor (GR) and Type I interferon antiviral genes following a mindfulness meditation intervention (Bower etal., 2015). Although the majority of the genomic studies that assess mindfulness training have reported results inflammatory gene expression, investigations that examine alternate pathways involved in depression, stress, and common diseases provide additional insight on how mindfulness training exerts changes at the genomic level.
Table 1.
Controlled studies testing the effects of MT on immune cell genomic markers
| Article | Main study purpose |
Analysis N |
Comparator | Participants | Intervention | Specimen/ Bioinformatics analysis |
Genomic findings |
|---|---|---|---|---|---|---|---|
| Black 2015 | Test effect of MM or sleep hygiene on sleep quality among older adults | 49 | Sleep hygiene education * | Community adults; 67% female; ages 66.3±7.4 years | MAPs; 6 weekly 2 hour group sessions | PBMC/TELi S Illumina HT 12 v4 BeadArrays | <NFkB (both groups) |
| Bower 2015 | Test effect of MM on stress and depressive symptoms amongwomen diagnosed with early stage breast cancer | 71 | Waiting list | Breast cancer patients; 100% female; ages 46.1± years (agerange 28.4-60) | MAPs; 6 weekly 2 hour group sessions | PBMC/Genome-wide TELiS Illumina HT12 v4 BeadArrays | <NFkB (dependent on practice dosage); >GR; >IRF ; <CREB (NS) |
| Creswell 2012 | Test effect of MM on loneliness among older adults | 40 | Waiting list | Lonely older adults; 80% female; ages 64.4±6.0 years | MBSR; 8 weekly 2 hour group sessions + retreat | PBMC/Geno me-wide TELiS Illumina HT 12 BeadChips | <NFkB (MBSR group) |
| Kaliman 2014 | To test the effect of MM on the expression of circadian, chromatic modulatory, and inflammatory genes | 40 | Leisure Time | Long-term meditators and meditation-naïve adults; 58%female; ages 50.38+8.96 years for controls; 49.89±11.18 years for meditators | 1-day mindfulness practice period formeditators (content extracted from MBSR session) | PBMC/Ficol l-Paque-plus method Sigma | <RIPK2 and <COX2 (pro-inflammatorygenes)(med itator group) |
| Lim 2018 | To test effects of MM on cognition and health among elderly, Asian participants | 60 | Health Education Program | Mildly cognitively impaired, elderly community adults; Asian; (age range 60-90) | MAPs; 12 weekly 40 min group sessions followed by 1x month for 45 min for 6 months | PBMC/Affymetrix GeneChip Human Genome U133 Plus 2.0 Arrays | Differentially expressed genes between groups were identified |
| Wang 2018 | To test effects of MM on levels of macrophage migration inhibitory factor (MIF) and its regulating microRNA-451a | 168 | TAU (mainly CBT) | Swedish outpatient adults with depression, anxiety, or stress and adjustment disorders; ages 42.2+11.0 years (age range 21-65) | MBSR/MBC T 8 weekly 2 hour group sessions | PBMC/Bead-based multiplex assay for Luminex platform (R&D Systems) | <MIF levels (both groups); >microRNA-451a levels (NS) |
Data garnered from these controlled trials indicate a link between mindfulness and immune regulation, but the psychological and neural mechanisms involved remain poorly understood. In this article we articulate a hypothesis about how the neural correlates of mindfulness experience may translate into peripheral gene regulatory activity, with the goal of guiding future mechanistic research in this area. This mind-body genomics model describes how cultivating mindfulness can change the way stressors and the social environment are perceived via attentional and self-regulatory shifts, and may thus alter signal transduction into downstream cellular processes such as inflammation. Thus, mindfulness meditation adds important information to existing social genomics models in that it offers a potential intervention approach to test established social genomics models in human subjects.
Current social genomics models propose that the activation of inflammatory genes when individuals feel threatened was likely adaptive under ancestral conditions, that is, when the perceptions of threat often forecasted physical injury (e.g., fleeing from an aggressive animal). However, in the context of more complex and dynamic contemporary social systems and stressors (e.g., unemployment, discrimination, anxiety), activating the pro-inflammatory profile in response to perception of threat is poorly adaptive because chronic activation of inflammatory genes provides little protection in the absence of wounding injury or bacterial infection, but may nevertheless promote chronic diseases (e.g., cardiovascular, metabolic, neurodegenerative, and neoplastic diseases) and compromises responsiveness to viral infections.
Our theory extends the current social genomics model relating perceived threat to peripheral stress response systems that regulate gene expression by hypothesizing that mindfulness meditation can inhibit these effects by recalibrating attention and awareness (i.e., types of perception) and thereby alter evaluation of, and self-regulatory response to threats inherent in modern social life. Given the social genomic pathways outlined above, we theorize that mindfulness capacities serve to reduce activation of threat-responsive social signal transduction pathways and thereby reduce the stimulation of the pro-inflammatory molecular defense programs that can ultimately contribute to chronic diseases.
Mind-body signal transduction process: Speculating on a possible framework
Attention and awareness are essential components of perception. Recent research has shown how our perceptions of the social world can influence our molecular self (Cole, 2014; Slavich and Cole, 2013). Although seemingly intuitive that environment affects biology, it is only recently that we have been able to address how environmental factors interact with our DNA to affect protein production and cellular function. Our DNA encodes within its ~20,000 genes the fundamental information needed to structure life by creating human cells. However, the potential of DNA is realized only if a gene is expressed, that is, if DNA is transcribed into RNA. Absent gene expression in the form of RNA, DNA genes have no effect on the cellular processes that mediate human life. As such, RNA and its downstream protein products perform the fundamental tasks of life, from determining hair color to mediating cellular behaviors and structuring organs.
Intracellular proteins known as transcription factors regulate transcription of DNA into RNA. While transcription factors are diverse and serve multiple functions within the cell, some transcription factors act to alter gene expression in response to extracellular signals, such as hormones, neurotransmitters, or growth factors (Webster et al., 2002). The set of genes that are expressed in a cell varies across cell types and tissues, and the specific subset of genes expressed in a particular cell population at a given time is called its transcriptome. Social environments can influence human gene expression via psychological processes (e.g., experiences of threat or fear) that trigger neural and endocrine responses (e.g., activation of the sympathetic nervous system) that ultimately modulate transcription factor activity (Cole, 2014; Slavich and Cole, 2013). Biochemical mediators such as hormones or neurotransmitters engage cellular receptor systems, which activate intracellular biochemical cascades that culminate in the activation of transcription factors that bind to DNA and prompt its transcription into RNA.
The adaptive capacity for intracellular molecules to respond to extracellular signals has been referred to as signal transduction. Biologists have traditionally defined signal transduction as the biochemical processes that translate extracellular signals, such as hormones or neurotransmitters, into changes in gene expression through the activation of protein transcription factors that bind to DNA and flag it for transcription into RNA. Social signal transduction extends this analysis to include the upstream neural dynamics that translate social conditions into systemically distributed signaling molecules (e.g., release of norepinephrine during fight-or-flight stress responses) and to include the specific downstream gene modules that are activated by a given transcription factor. Figure 1 depicts an example of a signal transduction pathway linking adverse social conditions with activation of cell signal transduction in immune cells (i.e., leukocytes).
Figure 1. Mindfulness state mediates social signal transduction at CNS phase.
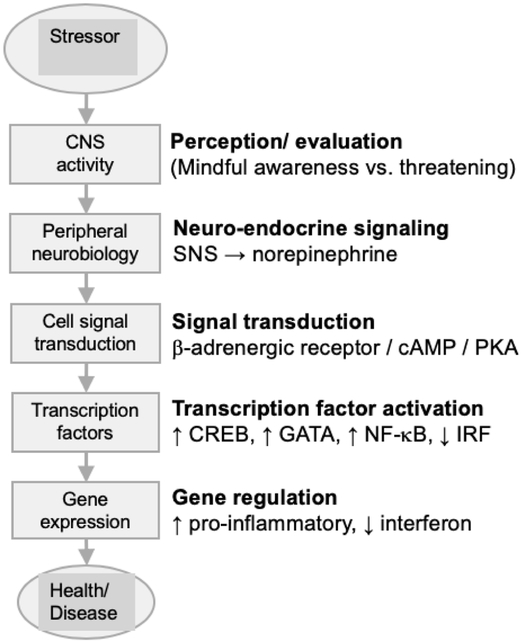
Brain-mediated attention and awareness serve as the detection filter for cognitive evaluation of threat associated with a stimuli (potential stressor) arising either from an actual challenge in the environment or one mentally imagined. Perception of the stimuli as a threat can activate the sympathetic nervous system (SNS), leading to release of norepinephrine (NE) at SNS nerve terminals, activation of b-adrenergic receptors on adjacent cells, and the cAMP/PKA signaling pathway that ultimately regulates gene expression by stimulating transcription factors such as cAMP response element-binding protein (CREB), GATA, and nuclear factor kappa-light-chain-enhancer of activated B cells (NF-kB). We speculate that the conscious state of mindfulness cultivated during mindfulness meditation and carried forth in daily living functions as a filter in attention and awareness that functions to reduce the valence of an imagined threat, resulting in reduced activation of the transcription factors that influence gene expression, and so improve health via reduction in chronic diseases and illnesses such as cardiovascular disease (CVD), cancer, and depression. Figure adapted with permission (Cole, 2014). We acknowledge that SNS activation caused by acute environmental challenges, such as escaping from danger, are short lived and adaptive to human health and survival. Therefore, it is reasonable that mindfulness would be more health protective, and relevant from a social genomics perspective, for stressors that are chronic in nature (e.g., social isolation, chronic pain, cancer survivorship, caregiving, insomnia) or elicited mentally from previous experience or future concern (e.g., burnout, post-traumatic stress disorder, social phobias and anxieties, job loss, financial concerns)
The paramount role of subjective perception in human social signal transduction stems from the fact that central nervous system–mediated experiences of social-environmental conditions can trigger the release of neural and endocrine response molecules that proximally regulate gene expression (Irwin and Cole, 2011; McEwen and Gianaros, 2010; Slavich and Cole, 2013; Slavich et al., 2010a; Slavich etal., 2010b). Put another way, many effects of social experiences on gene expression are neuro-cognitively mediated. The same social-environmental conditions can be appraised in different ways and therefore have different gene expression consequences for different people depending on factors such as individuals’ sensitivity to social threat (Gyurak et al., 2012; O'Donovan et al., 2013), or the propensity to view stressful circumstances as challenging versus threatening (Blascovich et al., 1999). Thus, we hypothesize that the heightened attention, awareness, and self-regulation that emerge from mindfulness meditation functions at the level of perception/evaluation of a stressor. As a mental stance and intervention approach we argue that mindfulness may mitigate interpretations of threat and thus reduce activity of threat-responsive signal transduction process and downstream gene regulation.
Conclusion
Modern life is complex and often fear-arousing, thus gearing our brain activity toward the fight-or-flight stress response. Here we offer hypothesis-generating arguments to stimulate research on the mechanistic process by which mindfulness meditation affects the process linking brain/mental activity with gene expression in the context of such an environment. We hypothesize that mindfulness is a level of heightened attention, awareness, and self-regulation that can mitigate stressor/threat evaluation and thus can quiet downstream molecular defense programs (i.e., gene expression profiles) that, when chronically activated, can contribute to inflammation-related diseases and vulnerability to viral infections. Our speculative mind-body conceptual framework is informed by the field of social genomics, and provides an empirically testable framework for future research mapping the relationships between mindfulness meditation, gene expression, and disease.
Acknowledgments
Funding:
Funding support was provided by the National Institute on Aging USC/UCLA Center on Biodemography and Population Health grant (P30AG017265 to D.B.) and database resources were used from the American Mindfulness Research Association.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest:
The authors declare no conflicts of interest with this study.
References
- 1.Levitin DJ: The Organized Mind: Thinking Straight in the Age of Information Overload. New York, NY: Plume/Penguin Random House; 2014. [Google Scholar]
- 2.American Psychological Association: Stress in America: The State of Our Nation [Internet]; URL:http://www.apa.org/news/press/releases/stress/2017/state-nation.pdf.
- 3.Walsh R: The consciousness disciplines and the behavioral sciences: questions of comparison and assessment. Am J Psychiatry 1980, 137:663–673. [DOI] [PubMed] [Google Scholar]
- 4.Dahl CJ, Lutz A, Davidson RJ: Reconstructing and deconstructing the self: cognitive mechanisms in meditation practice. Trends Cogn Sci 2015, 19: 515–523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kabat-Zinn J: Mindfulness-Based Interventions in Context: Past, Present, and Future. Clinical Psychology: Science & Practice 2003, 10:144–156. [Google Scholar]
- 6.Arnsten AF: Stress signalling pathways that impair prefrontal cortex structure and function. Nat Rev Neurosci 2009, 10:410–422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gianaros PJ, Sheu LK, Matthews KA, Jennings JR, Manuck SB, Hariri AR: Individual differences in stressor-evoked blood pressure reactivity vary with activation, volume, and functional connectivity of the amygdala. J Neurosci 2008, 28:990–999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Holzel BK, Carmody J, Evans KC, Hoge EA, Dusek JA, Morgan L, Pitman RK, Lazar SW: Stress reduction correlates with structural changes in the amygdala. Soc Cogn Affect Neurosci 2010, 5:11–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ditto B, Eclache M, Goldman N: Short-term autonomic and cardiovascular effects of mindfulness body scan meditation. Ann BehavMed 2006, 32:227–234. [DOI] [PubMed] [Google Scholar]
- 10.Thayer JF, Lane RD: A model of neurovisceral integration in emotion regulation and dysregulation. J Affect Disord 2000, 61:201–216. [DOI] [PubMed] [Google Scholar]
- 11. *.Creswell JD, Taren AA, Lindsay EK, Greco CM, Gianaros PJ, Fairgrieve A, Marsland AL, Brown KW, Way BM, Rosen RK, et al. : Alterations in Resting-State Functional Connectivity Link Mindfulness Meditation With Reduced Interleukin-6: A Randomized Controlled Trial. Biol Psychiatry 2016, 80:53–61. [DOI] [PubMed] [Google Scholar]
- 12.Cole SW: Human social genomics. PLoS Genet 2014, 10:e1004601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Slavich GM, Cole SW: The Emerging Field of Human Social Genomics. Clin Psychol Sci 2013, 1:331–348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Webster JI, Tonelli L, Sternberg EM: Neuroendocrine regulation of immunity. Annu Rev Immunol 2002, 20:125–163. [DOI] [PubMed] [Google Scholar]
- 15.Irwin MR, Cole SW: Reciprocal regulation of the neural and innate immune systems. Nat Rev Immunol 2011, 11:625–632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.McEwen BS, Gianaros PJ: Central role of the brain in stress and adaptation: links to socioeconomic status, health, and disease. Ann N Y Acad Sci 2010, 1186:190–222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Slavich GM, O'Donovan A, Epel ES, Kemeny ME: Black sheep get the blues: a psychobiological model of social rejection and depression. Neurosci Biobehav Rev 2010, 35:39–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Slavich GM, Way BM, Eisenberger NI, Taylor SE: Neural sensitivity to social rejection is associated with inflammatory responses to social stress. Proc Natl Acad Sci U S A 2010, 107:14817–14822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gyurak A, Hooker CI, Miyakawa A, Verosky S, Luerssen A, Ayduk ON: Individual differences in neural responses to social rejection: the joint effect of self-esteem and attentional control. Soc Cogn Affect Neurosci 2012, 7:322–331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.O’Donovan A, Slavich GM, Epel ES, Neylan TC: Exaggerated neurobiological sensitivity to threat as a mechanism linking anxiety with increased risk for diseases of aging. Neurosci Biobehav Rev 2013, 37:96–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Blascovich J, Mendes WB, Hunter SB, Salomon K: Social "facilitation" as challenge and threat. J Pers Soc Psychol 1999, 77:68–77. [DOI] [PubMed] [Google Scholar]
- 22. *.Black DS, O'Reilly GA, Olmstead R, Breen EC, Irwin MR: Mindfulness meditation and improvement in sleep quality and daytime impairment among older adults with sleep disturbances: a randomized clinical trial. JAMA Intern Med 2015,175:494–501.This randomized control trial revealed that a low-cost, community-accessible mindful awareness practices program led to significant improvement in sleep quality among older adults compared to a sleep hygiene education program. Significant reductions in NF-κB were found in both intervention groups.
- 23.Creswell JD, Irwin MR, Burklund LJ, Lieberman MD, Arevalo JM, Ma J, Breen EC, Cole SW: Mindfulness-Based Stress Reduction training reduces loneliness and pro-inflammatory gene expression in older adults: a small randomized controlled trial. Brain Behav Immun 2012, 26:1095–1101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. *.Bower JE, Crosswell AD, Stanton AL, Crespi CM, Winston D, Arevalo J, Ma J, Cole SW, Ganz PA: Mindfulness meditation for younger breast cancer survivors: a randomized controlled trial. Cancer 2015, 121:1231–1240.This randomized control trial revealed the short-term efficacy of a mindful awareness practices intervention among breast cancer survivors in reducing self-reported stress, depression, and pro-inflammatory signaling. This was the first study to examine the effects of a minduflness-based program on cytokine production and positive psyhcological outcomes among younger breast cancer survivors.
- 25. *.García-Campayo J P-GM, Labarga A, Urdánoz A, Roldán M, Pulido L, Martínez de Morentin X, Perdones-Montero A, Montero-Marín J, Mendioroz M: Epigenetic response to mindfulness in peripheral blood leukocytes involves genes linked to common human diseases. Mindfulness 2017, 9:1146–1159.This genome study was conduced to assess the epigenetic effects of mindfulness among expert meditators. Researchers discovered sixty-four differentially mythelated regions (associated with 43 genes-including those of common human diseases) in meditators compared to controls, and defined the regulating role of tumor necrosis factor (TNF) and NF-κB signaling on genes associated with mindfulness.
- 26.Kaliman P, Alvarez-Lopez MJ, Cosin-Tomas M, Rosenkranz MA, Lutz A, Davidson RJ: Rapid changes in histone deacetylases and inflammatory gene expression in expert meditators. Psychoneuroendocrinology 2014, 40:96–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. *.Lim HW, Saw WY, Feng L, Lee YK, Mahendran R, Cheah IK, Rawtaer I, Kumar AP, Kua EH, Mahendran R, et al. : Dataset on gene expression in the elderly after Mindfulness Awareness Practice or Health Education Program. Data Brief 2018,18:902–912.This randomized control trial revealed the effects of a mindful awareness practices program versus a health education program on gene expression among elderly Asian adults with cognitive impairment. Observed differentiating genes and pathways can be utilzied as identifying markers in studies examining the effects mindfulness-based interventions.
- 28. *.Wang X, Sundquist K, Palmer K, Hedelius A, Memon AA, Sundquist J: Macrophage Migration Inhibitory Factor and microRNA-451a in Response to Mindfulness-based Therapy or Treatment as Usual in Patients with Depression, Anxiety, or Stress and Adjustment Disorders. Int J Neuropsychopharmacol 2018, 21:513–521.This randomized control trial revealed the effects of a mindfulness-based therapy versus treatment as usual on cytokine activity among a group of patients with psychiatric disorders. Researchers found a reduction in macrophage migration inhibitory factor among both groups post-intervention.


