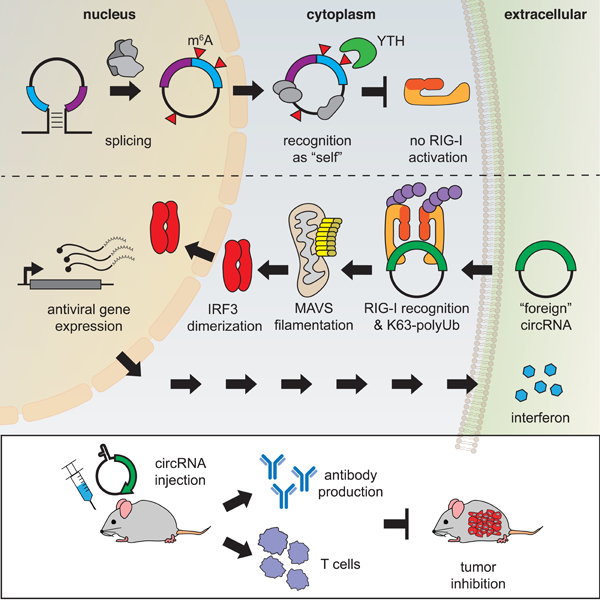
SUMMARY
Circular RNAs (circRNAs) are prevalent in eukaryotic cells and viral genomes. Mammalian cells possess innate immunity to detect foreign circRNAs, but the molecular basis of self vs. foreign identity in circRNA immunity is unknown. Here we show that N6-methyladenosine (m6A) RNA modification on human circRNAs inhibits innate immunity. Foreign circRNAs are potent adjuvants to induce antigen-specific T cell activation, antibody production, and anti-tumor immunity in vivo, and m6A modification abrogates immune gene activation and adjuvant activity. m6A reader YTHDF2 sequesters m6A-circRNA and is essential for suppression of innate immunity. Unmodified circRNA, but not m6A-modified circRNA, directly activates RNA pattern recognition receptor RIG-I in the presence of lysine-63-linked polyubiquitin chain to cause filamentation of the adaptor protein MAVS and activation of the downstream transcription factor IRF3. CircRNA immunity has considerable parallel to prokaryotic DNA restriction modification system that transforms nucleic acid chemical modification into organismal innate immunity.
Keywords: Circular RNA, RIG-I, N6-methyladenosine, self/non-self, vaccine, cancer immunotherapy
Graphical Abstract
eTOC:
Mammalian cells distinguish between foreign and endogenous circular RNAs (circRNAs), but the molecular basis is unknown. Chen et al identifies N6-methyladenosine (m6A) RNA modification as marker for “self”. Unmodified circRNA but not m6A-modified circRNA activates RIG-I in the presence of K63-polyubiquitin to cause MAVS filamentation, IRF3 dimerization, and interferon production.
INTRODUCTION
Tens of thousands of circular RNAs (circRNAs) have been identified in eukaryotes (Hansen et al., 2013; Memczak et al., 2013). Viruses like the hepatitis delta virus and plant viroids possess circRNA genomes, and many viruses produce circRNAs in their replication cycle (Kos et al., 1986; Sanger et al., 1976; Toptan et al., 2018). Recent studies suggest an emerging picture of an innate immune system based on circRNAs. Introduction of certain exogenous circRNAs can activate an antiviral and immune gene expression program (Chen et al., 2017), while endogenous circRNAs can collectively inhibit protein kinase R and set the threshold for innate immunity upon virus infection (Liu et al., 2019). The mammalian innate immune system depends on pattern recognition receptors (PRRs) recognizing pathogen-associated molecular patterns (PAMPs) that are common among viruses and bacteria. RIG-I and MDA5 are PRRs found in the cytosol that sense foreign nucleic acids. MDA5 is known to detect long dsRNA whereas RIG-I has been characterized to recognize 5’ triphosphate on short dsRNAs (Hornung et al., 2006; Reikine et al., 2014). Although linear RNA ligands for RIG-I activation have been extensively characterized (Schlee and Hartmann, 2010), RIG-I interaction with circRNAs has not been investigated, especially in the context of foreign circRNA detection.
N6-methyladenosine (m6A) is one of the most abundant RNA modifications, present on 0.4–0.6% of all cellular RNAs including mRNAs, long noncoding RNAs, and circRNAs (Dominissini et al., 2012; Meyer et al., 2012). On mRNAs, m6A has been demonstrated to regulate different functions including splicing (Xiao et al., 2016), translation (Meyer et al., 2015; Wang et al., 2015), and degradation (Wang et al., 2014), which have cell- and tissue-wide effects. Previous studies suggest that m6A are also present on circRNA, and has the potential to initiate cap-independent translation (Legnini et al., 2017; Yang et al., 2017). However, the scope of m6A on circRNA function and the role in RIG-I detection of circRNAs are not known. m6A modification of 5’-triphosphate linear RNA ligands abrogates RIG-I binding and activation (Durbin et al., 2016; Peisley et al., 2013). However, these prior studies employed 100% m6A modified short linear RNA ligands in vitro. Whether physiologic levels of RNA modification can be sensed by RIG-I, its relevance to other types of RNAs, nor its biological impact in vivo are not known.
Here we investigate the role of m6A modification in innate immunity induced by exogenous circRNA. We evaluate circRNA immunogenicity in vivo, and test the ability of circRNA induction of innate immunity to cross prime adaptive immunity in the contexts of vaccination and tumor immunity. We identify the m6A reader YTHDF2 on endogenous human circRNA, and dissect the functional roles of YTHDF2 and RIG-I as the pattern recognition receptor for self vs. foreign circRNAs.
RESULTS
In vitro production and characterization of immunogenic circRNA
A circularized Green Fluorescent Protein (GFP) mRNA via permuted td intron from T4 bacteriophage, termed “circFOREIGN” hereafter, is highly immunogenic in cultured mammalian cells (Chen et al., 2017). td introns program autocatalytic splicing during in vitro transcription to form circFOREIGN. Prolonged treatment (>2 hours) of circFOREIGN with exonuclease RNase R degraded linear RNA byproducts and yielded enriched circRNAs (Chen et al., 2017, Methods). Subsequent alkaline phosphatase treatment removes 5’ phosphate from free ends. Delivery of foreign circRNAs into mammalian cells potently stimulated immune gene expression and protected against subsequent viral infection (Chen et al., 2017). A recent report suggests that exogenous circRNAs are not immunostimulatory, but 5’ triphosphorylated linear RNA contaminants due to incomplete RNase R digestion trigger immune response (Wesselhoeft et al., 2019). Wesselhoeft et al. used a short (30 min) RNase R treatment, no phosphatase treatment, and then performed HPLC to remove linear 5’ phosphorylated RNAs from circRNA. We have previously shown the immune stimulation by circFOREIGN synthesized in vitro in the presence of phosphatase and treated with RNase R for 2 hours is comparable to the circFOREIGN treated with a second round of phosphatase to remove triphosphates on contaminating linear RNA, whereas linear RNA with phosphatase treatment has greatly reduced immune activation (Chen et al., 2017). This demonstrates that the circFOREIGN stimulation is independent of the presence of aberrant 5’ triphosphates in the sample. However, to confirm that 5’ triphosphates are not stimulating immune gene expression, we treat all circFOREIGN synthesized with phosphatase in this report.
We probed if gel purification of the circFOREIGN treated with RNase R altered the circFOREIGN immune stimulation. We reasoned that if there are contaminating linear RNA components that are contributing to the circFOREIGN’s immunogenicity, then gel extraction would eliminate these contaminants, which have different molecular weights. The nicked-circRNA products in the gel are not immunostimulatory, since they become linear. We compared gel purified circFOREIGN treated with RNase R and alkaline phosphate vs. the same circFOREIGN preparation that underwent gel purification. Each RNA preparation was transfected into HeLa cells, followed by qRT-PCR analysis of innate immunity genes 24 hours later. The gel purified circFOREIGN stimulated innate immune genes with similar potency compared to a standard preparation of circFOREIGN (Figure S1A–B).
We also subjected the synthesized circRNA treated with RNase R to HPLC fractionation. Size exclusion chromatography resolved the RNase-R-treated circRNA into two fractions (Figure S1C). Concentration and TapeStation analysis of each fraction reflected that the HPLC peak 1 and 2 mirror the results from gel electrophoresis of RNase R-treated circFOREIGN (Figure S1C). We noted that our HPLC purification chromatogram and resulting fractions differ from previously reported separation (Wesselhoeft et al., 2019) due to differences in instrumentation. Transfection of each fraction into HeLa cells followed by qRT-PCR revealed that the fraction with circRNA retained immune response (Figure S1D). Although peak 1 includes smaller degraded RNA and un-digested introns, this fraction was still immunogenic. This result is consistent with the interpretation that phosphatase treatment throughout the sample preparation had inactivated immunogenic linear RNAs. We found that circFOREIGN integrity was better-preserved in gel purification over HPLC purification with less degradation into smaller RNA fragments in the former, which correlates with better preservation of circFOREIGN immunogenicity.
We concluded that smaller linear RNA resulting from incomplete RNase R digestion was not responsible for circRNA immunogenicity in our preparation. Differences in circRNA sequence and cell types tested may also contribute to apparent different findings from Weseslhoeft et al. (more below). We found that our enzymatic purification process appeared to preserve circFOREIGN integrity the best, and thus continued with our enzymatic purification process circFOREIGN subjected to RNase R enrichment and phosphatase treatment.
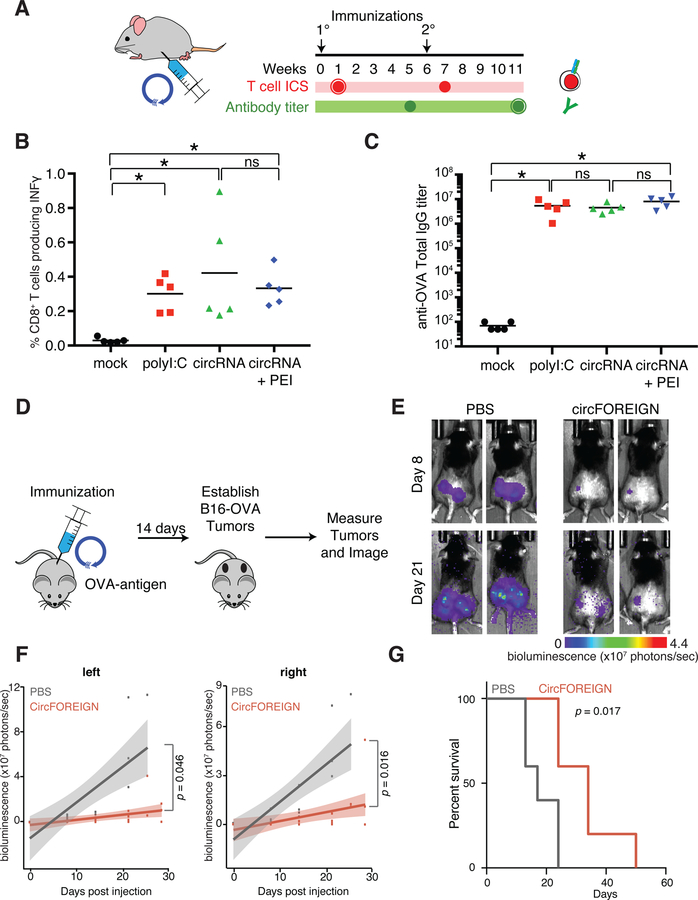
CircFOREIGN acts as vaccine adjuvant in vivo
CircFOREIGN has previously been shown to potently stimulate immune gene expression (Chen et al., 2017), but its behavior in vivo is not known. We reasoned that circFOREIGN has the potential to activate innate immunity and thus act as a vaccine adjuvant to increase the efficacy of the vaccine. CircFOREIGN was in vitro transcribed, purified, and delivered in combination with chick ovalbumin (OVA) into C57BL/6J mice by subcutaneous injection. PolyI:C served as a positive control for RNA adjuvant. CircFOREIGN was delivered as naked RNA or after packaging in a transfection agent, polyethylenimine (PEI). We collected T cells and performed intracellular cytokine staining (ICS) seven days following primary or secondary vaccinations and collected serum and measured antibody responses five weeks after vaccinations (Figure 1A). Induction of OVA-specific, interferon gamma-positive (Ifnγ+) activated CD8 T cells required adjuvant such as polyI:C, as expected (Figures 1B and S2A–C). Notably, coinjection of circFOREIGN induced potent anti-OVA T cell to comparable levels as polyI:C, either with naked circRNA (p=0.0088 compared to mock, Anova-Tukey’s test) or in PEI nanoparticles (p=0.0039 compared to mock, Anova-Tukey’s test, Figure 1B). Measurement of OVA-specific antibodies revealed that circFOREIGN alone stimulates antibody production to comparable levels as the positive control polyI:C (Figures 1C and S2B). CircFOREIGN did not require a transfection reagent in order for stimulation of OVA-specific CD8+ T cells and antibodies. In fact, the CD8+ T cell responses were higher in injections without PEI, and we omitted PEI in subsequent experiments.
Figure 1. CircFOREIGN induces immune response but m6A-modified circFOREIGN attenuates stimulation in vivo.
A. Agonist RNA in conjunction with OVA is delivered by subcutaneous injection. T cell ICS and antibody titers are measured at the indicated times following primary and secondary immunizations.
B. CircRNA stimulates anti-OVA T cell responses independent of transfection agent following primary vaccination. Means are shown (n = 5), *p < 0.05, Kruskal-Wallis test.
C. CircRNA stimulates anti-OVA antibody titers independent of transfection agent following secondary vaccination. Means are shown (n = 5), *p < 0.05, Anova-Tukey’s test.
D. CircFOREIGN vaccination in conjunction with OVA is delivered by subcutaneous injection. 14 days later, OVA-expressing B16-melanoma cells are established in right and left flanks. Tumors are measured and imaged.
E. Quantification of bioluminescence measurements in left and right tumors for mice vaccinated with PBS or circFOREIGN prior to tumor establishment. p value calculated by Wilcoxon signed-rank test. n=5 mice in each group.
F. Quantification of bioluminescence measurements in left and right tumors for mice vaccinated with PBS or circFOREIGN prior to tumor establishment. p value calculated by Wilcoxon signed-rank test. n=5 mice in each group.
G. Mice vaccinated with circFOREIGN survive twice as long as negative control mice. Survival curves for mice vaccinated with PBS or circFOREIGN prior to tumor establishment. p value calculated by log-rank test. n=5 mice in each group.
After immunization of mice with circFOREIGN or control, we isolated dendritic cells (DCs) from draining lymph nodes. We found that circFOREIGN adjuvant activated both cDC1 and cDC2 subsets, as judged by increased cell surface expression of the costimulatory molecule CD86 over control (Figure S2D–E). These results provide direct evidence in vivo that circRNA inoculation activated DCs. DC activation can in principle facilitate antigen cross presentation and activation of CD4+ Tfh cells and CD8+ T cells. However, circRNA may also directly affect T cells and other immune cell types, which remain to be explored.
CircFOREIGN can induce anti-tumor immunity
Because the delivery of circFOREIGN induces CD8+ T cell responses, we hypothesized that mice that were exposed to circFOREIGN and OVA would have adaptive immunity against OVA-expressing tumors. Thus, we vaccinated mice with circFOREIGN and OVA and two weeks later, implanted OVA-expressing B16 melanoma cells into the right and left flanks of the mice (Figure 1D). The OVA-B16 melanoma model is immune restricted largely through CD8+ effector T cells (Budhu et al., 2010). Mice receiving circFOREIGN have lower tumor growth compared to negative control mice receiving PBS (Figures 1E–F and S2F). The left and right tumors in each mouse are correlated with each other, demonstrating that there is a systemic-wide effect from the vaccination. The mice that were vaccinated with circFOREIGN just once have nearly doubled overall survival compared to the negative control mice (p=0.0173, log-rank test, Figure 1G), and were comparable to the mice receiving positive control polyI:C HMW (Figure S2G). These results indicate that circRNA immunity can be harnessed toward potential therapeutic ends.
Endogenous circRNA associate with m6A machinery
Given that mammalian cells have endogenous circRNAs, their immune response to circFOREIGN suggests that they distinguish between self and non-self circRNAs. CircRNAs are produced through back-splicing to covalently join the 3’ and 5’ ends of RNA exons. Because intron identity dictates circRNA immunity (Chen et al., 2017) but is not part of the final circRNA product, we reasoned that introns may direct the deposition of one or more covalent chemical marks onto the circRNA. Among the over 100 known RNA chemical modifications, m6A is the most abundant modification on linear mRNAs and long noncoding RNAs, and is present for 0.2% to 0.6% of all adenosines in mammalian polyA-tailed transcripts (Roundtree et al., 2017). m6A has recently been detected on mammalian circRNAs (Zhou et al., 2017).
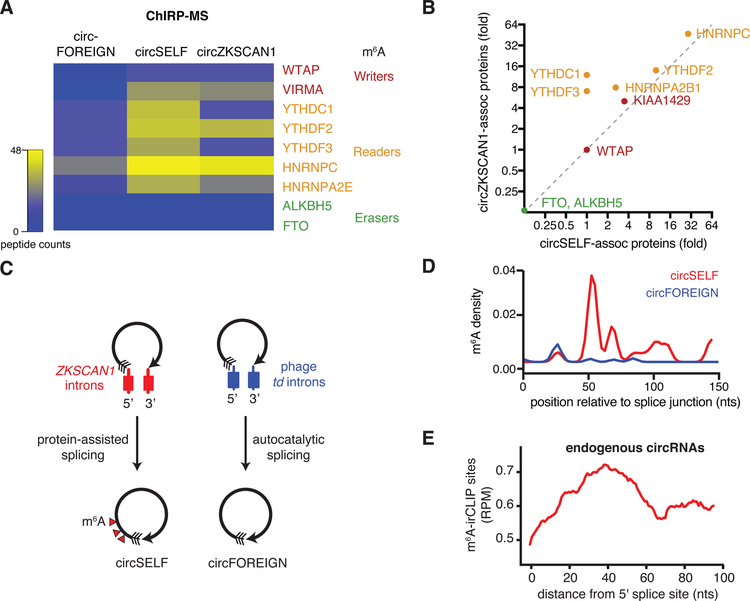
CircZKSCAN1 is a human circRNA produced by its endogenous introns and not immunogenic when expressed in human cells. We used the ZKSCAN1 introns to program the production of circGFP, termed “circSELF”. We transfected DNA plasmids encoding circRNAs generated by protein-assisted (circSELF) or autocatalytic splicing (circFOREIGN) into HeLa cells and performed comprehensive identification of RNA binding proteins by mass spectrometry (ChIRP-MS) experiments (Chen et al., 2017). We analyzed writers, readers, and erasers of covalent m6A modification (Roundtree et al., 2017) in association with circRNAs (Figure 2A). We found that circZKSCAN1, but not circFOREIGN, is associated with components of the m6A writer complex, such as WTAP and VIRMA (also known as Virilizer homolog or KIAA1429) as well as the m6A reader proteins YTHDF2, HNRNPC, and HNRNPA2B1 (Figure 2A). Neither circRNA is associated with putative m6A demethylases (“erasers”), such as FTO and ALKBH5. Importantly, circSELF is the same circRNA sequence as circFOREIGN, but is no longer immunogenic (Chen et al., 2017), and is now associated with m6A writer and reader proteins (Figure 2A). Two different circRNAs (circSELF and circZKSCAN1) programmed by human introns achieve similar levels of association with m6A writer and reader proteins, including WTAP, VIRMA, and YTHDF2 (Figure 2B). These results suggest that m6A modification machinery is transferred to exonic circRNAs as a memory of the introns that mediate their biogenesis, which occurs independently of circRNA sequence.
Figure 2. CircSELF but not circFOREIGN associate with m6A machinery and are modified by m6A.
A. Heatmap of peptide counts from ChIRP-MS of circZKSCAN1, circSELF, and circFOREIGN. Enzymes are classified as m6A writers, readers, and erasers.
B. m6A machinery associates with circZKSCAN1 and circSELF but not circFOREIGN, as indicated by ChIRP-MS. Fold enrichment over RNase-treated control is shown.
C. Model showing ZKSCAN1 introns directing protein-assisted splicing to yield m6A-modified circSELF and phage td introns directing autocatalytic splicing to form unmodified circFOREIGN.
D. m6A-irCLIP identifies high-confidence m6A positions proximal to circRNA splice junction. ZKSCAN1 introns suffice to direct m6A modification on circSELF (red) compared with td intron-directed circFOREIGN (blue). Density of m6A-irCLIP reads normalized to reads per million.
E. m6A-irCLIP read density near circRNA splice junction of endogenous human circRNAs in HeLa cells. Density of m6A-irCLIP reads normalized to reads per million for reads proximal to circRNA splice junctions.
Self and foreign circRNAs have different m6A modification patterns
We next defined the m6A modification patterns of human and foreign circRNAs. In human cells programmed to express the appropriate circRNAs, we used RNase R treatment to enrich for circRNAs, and then performed m6A-UV-C crosslinking and m6A immunoprecipitation (m6A-irCLIP) (Zarnegar et al., 2016) to map the sites of m6A modification with high sensitivity (Figure 2C). m6A-irCLIP of circSELF vs. circFOREIGN revealed that circSELF gained m6A modification within 50–100 nucleotides (nt) at the 3’ side of the circularization junction (Figure 2D). We did not observe other significant differences in modification through the rest of the transcript (Figure S3A). Because circSELF and circFOREIGN are the same exonic sequence circularized by a human (self) or phage (foreign) intron, this result indicates that human introns are sufficient to place m6A modification on the resulting circRNA. Moreover, comparison of endogenous circRNAs subjected to m6A-irCLIP to model human-programmed circRNA indicates that both have similar patterns of m6A modification (Figure 2E). m6A is enriched in the +40–100 nt window 3’ of the back-splice junction on endogenous circRNAs transcriptome-wide (Figure 2E). m6A is known to be enriched at the last exon of linear mRNA and lncRNAs (Figure S3B) (Dominissini et al., 2012; Ke et al., 2015; Meyer et al., 2012). Our finding of m6A modification 3’ of the back-splice junction is consistent with this pattern. Splicing occurs co-transcriptionally from 5’ to 3’, and the 3’ to 5’ back splice expected is the last splicing event on a circRNA (i.e. no intron remains to be spliced out).
m6A modification marks circRNA as “self”
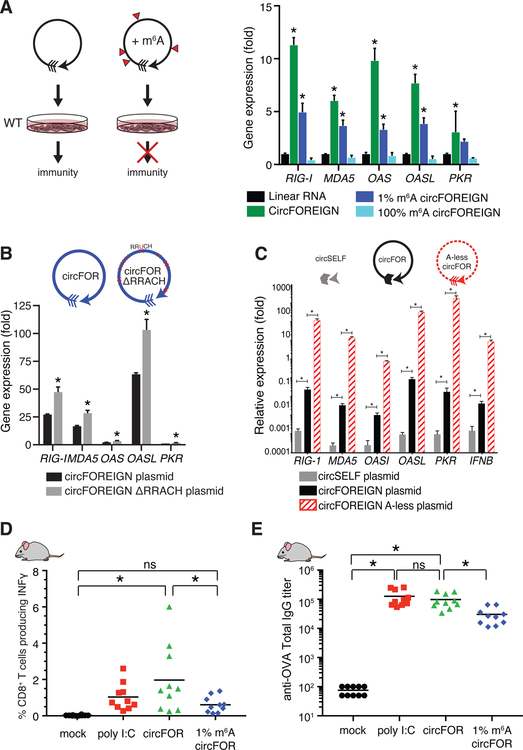
We hypothesized that the chemical modification itself or in combination with the recognition of the m6A by RNA-binding proteins allows marking of “self” circRNA. First, we tested whether incorporation of m6A into circFOREIGN would mask the “non-self” identity and decrease the immunogenicity of the circFOREIGN. We synthesized unmodified or m6A-modified circFOREIGN by in vitro transcription (Chen et al., 2017) and purified the circRNA by RNase R treatment. Incorporation of m6A modification into circRNA does not affect splicing to form circRNA and RNase R treatment enriches for circRNA (Figure S3C–D). We then transfected the circFOREIGN into recipient cells and measured anti-viral gene expression. We note that circRNA m6A modification in cells is concentrated at specific positions along the transcript, whereas m6A incorporation during in vitro transcription is random. Thus, we chose to replace all adenosines with m6A (100% m6A) or add in just 1% m6A into the circRNA to yield an average of 3 m6A modifications for each circRNA. We reasoned that 100% m6A is supra-physiologic but models the consecutive occurrence of m6A observed in vivo. 1% m6A models the overall level of m6A ratio on endogenous RNA but not their modification pattern. CircFOREIGN potently induced a panel of antiviral genes, including RIG-I, MDA5, OAS, OASL, and PKR, and anti-viral gene induction was completely abrogated when all of the adenosines were replaced with m6A modification (Figure 3A, 100% m6A). 1% m6A incorporation significantly reduced but did not eliminate anti-viral gene induction (Figure 3A). Thus, m6A modification sufficed to reduce the immunogenicity of a foreign circRNA in cultured cells.
Figure 3. m6A-modified circFOREIGN attenuates immune response in vitro and in vivo.
A. (Left) Model of unmodified and m6A-modified circFOREIGN effects of immunogenicity. (Right) Transfection of unmodified circFOREIGN into wild-type HeLa cells stimulates immune response, but m6A-modified circFOREIGN does not. Graph showing gene expression of innate immune genes 24 hours following RNA transfection. Relative expression of the indicated mRNA and transfected RNA are measured by qRT-PCR, results normalized to expression following mock transfection. Means ± SEM are shown (n = 3), *p < 0.05, Student’s t-test, comparing gene stimulation of linear RNA to indicated RNA.
B. Transfection of circFOREIGN plasmid lacking RRACH m6A consensus motifs stimulates immune response at a greater level than circFOREIGN. RRACH motifs (n = 12 sites) were mutated to RRUCH throughout the exon sequence. Mutating every adenosine to uracil within the first 200 bases (n = 37 sites) after the splice junction further increased immunogenicity. Graph showing gene expression of innate immune genes following DNA plasmid transfection. Relative expression of the indicated mRNA and transfected RNA are measured by qRT-PCR, results normalized to expression following mock transfection. Means ± SEM are shown (n = 3), **p < 0.01, ***p < 0.001, Student’s t-test, comparing circFOREIGN to indicated RNA transfection.
C. Transfection of circFOREIGN plasmid with all adenosines replaced by uracil results in elevated immunogenicity. Relative expression of the indicated mRNA and transfected RNA are measured by qRT-PCR, results normalized to expression following mock transfection. Means ± SEM are shown (n = 3), *p<0.01, Student’s t-test, comparing circFOREIGN to indicated RNA transfection.
D. m6A-modified circFOREIGN attenuates anti-OVA T cell responses following primary vaccination. Means are shown (n = 10), *p < 0.05, Anova-Tukey’s test.
E. m6A-modified circRNA attenuates anti-OVA antibody titers following secondary vaccination. Means are shown (n = 10), *p < 0.05, ANOVA-Tukey’s test.
We then modified the circFOREIGN plasmid, eliminating m6A consensus motifs (Dominissini et al., 2012) by mutating all instances (n=12) of RRACH sequence to RRUCH in GFP exon. We hypothesized that when circFOREIGN is transcribed in the nucleus of human cells, METTL3/14 may modify circFOREIGN at low level, which would be abrogated in the ∆RRACH mutant. We transfected plasmids encoding wild type or mutant circRNA into HeLa cells, and then quantified circRNA levels and innate immunity gene induction by qRT-PCR. The gene induction is then normalized to the level of the measured circRNA. We found that RRACH site mutation significantly increased circRNA induction of anti-viral genes by approximately two-fold (Figure 3B). Because m6A is enriched on but not exclusively present at RRACH motif, we next constructed a modified circFOREIGN plasmid where all adenosines are mutated to uracil in the GFP exons (A-less circFOREIGN, Figure 3C). Transfection of plasmids encoding the A-less circRNA led to ~100 fold-increase in anti-viral gene induction over circFOREIGN. These results provide the first evidence that specific circRNA exonic sequences impact immunity, and specifically suggest endogenous m6A modification dampens innate immunity.
m6A modification of circRNA blunts vaccination response in vivo
m6A modification also decreased the immunogenicity of circRNA as adjuvant in vivo. When we used 1% m6A-modified circFOREIGN in the same adjuvant regime as unmodified circFOREIGN, we found that 1% m6A modification substantially reduced both the activated CD8 T cell response (Figure 3D vs. Figure 1B) and antibody titers (Figure 3E vs. Figure 1C). We observed that repeated immunizations with 1% m6A-modified circFOREIGN induced an immune response that is diminished but not null (Figure S4). These results show that circFOREIGN is a potent immune stimulant in vivo, and 1% m6A modification is sufficient to blunt circRNA immunity.
YTHDF2 reader protein and suppression of circRNA immunity
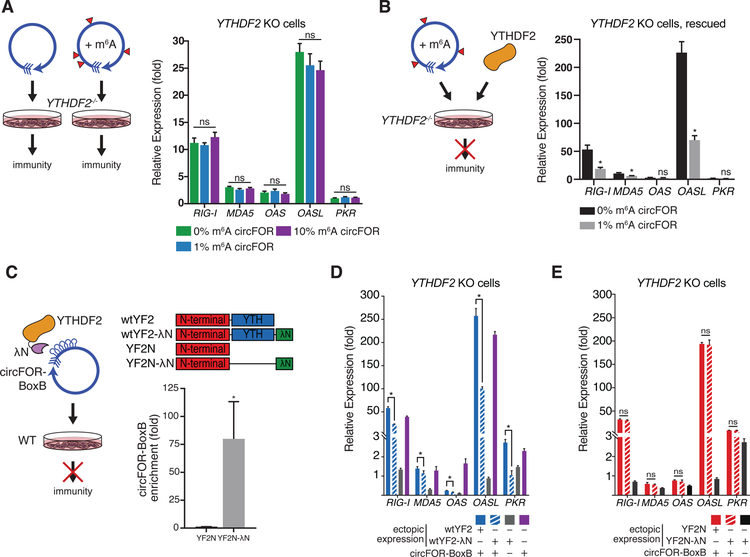
We next sought to define the mechanisms of m6A suppression of circRNA immunity. m6A is recognized by a family of reader proteins, the most prominent of which are the YTH-domain containing RNA binding proteins (Dominissini et al., 2012; Edupuganti et al., 2017). We focused on YTHDF2 because (i) it is the main m6A reader we detected in association with endogenous circRNA or circSELF (Figure 2A–B), and (ii) YTHDF2 is cytoplasmic, the same compartment as endogenous and transfected circRNAs (Chen et al., 2017; Rybak-Wolf et al., 2015; Salzman et al., 2012). CircFOREIGN transfection into YTHDF2−/− HeLa cells (Figure S5A) led to potent induction of anti-viral genes (Figure 4A). Moreover, incorporation of 1% or 10% m6A into circFOREIGN no longer suppressed the antiviral gene induction in YTHDF2−/− cells (Figure 4A). An independent YTHDF2−/− clone gave very similar results (Figure S5B). Furthermore, ectopic expression of YTHDF2 in YTHDF2−/− cells rescued the suppression of immune gene induction in response to m6A-modified circFOREIGN (Figure 4B), indicating that YTHDF2 is required for mediating the “self” identity of m6A-marked circRNAs.
Figure 4. m6A-modified circFOREIGN evades non-self detection through the binding of YTHDF2.
A. YTHDF2 is necessary for differential response to unmodified vs. m6A-modified circFOREIGN. Transfection of unmodified or m6A-modified circFOREIGN into YTHDF2−/− HeLa cells stimulate immune response. Left: Model showing the responses to unmodified or m6A-modified circFOREIGN. Right: Graph showing gene expression of innate immune genes 24 hours following RNA transfection. Relative expression of the indicated mRNA and transfected RNA are measured by qRT-PCR, results normalized to expression following mock transfection. Means ± SEM are shown (n = 3). Student’s t-test, comparing circFOREIGN with 0% m6A to indicated RNA transfection.
B. Ectopic expression of YTHDF2 rescues response to unmodified vs. m6A-modified circFOREIGN in YTHDF2 KO HeLa cells. Left: Model showing response to m6A-modified circFOREIGN following rescue. Right: Graph showing gene expression of innate immune genes 24 hours following RNA transfection. Relative expression of the indicated mRNA and transfected RNA are measured by qRT-PCR, results normalized to expression following mock transfection. Means ± SEM are shown (n = 3). *p<0.05, Student’s t-test, comparing 0% m6A circFOREIGN to 1% m6A circFOREIGN.
C. Tethering of YTHDF2 to unmodified circFOREIGN mask circRNA immunity. Left: Model showing in vivo tethering of protein to RNA via lambdaN and BoxB leading to attenuation of immunogencity. Right top: Protein domain architecture of full-length wild-type YTHDF2 with and without lambdaN tethering tag, and YTHDF2 N-terminal domain with and without lambdaN tethering tag. Right bottom: RIP-qPCR enrichment of indicated YTH protein followed by qRT-PCR of circRNA-BoxB or control actin RNA. Means ± SEM are shown (n = 3). *p<0.05, Student’s t-test, comparing YTHDF2 N-terminus with lambdaN tethering to YTHDF2 N-terminus without tethering.
D. Transfection of unmodified circBoxB tethered to full length wild-type YTHDF2 into wild-type HeLa cells attenuates immune response. Graph showing gene expression of innate immune genes 24 hours following RNA transfection. Relative expression of the indicated mRNA and transfected RNA are measured by qRT-PCR, results normalized mock transfection. Wild-type YTHDF2-lambdaN (grey) was ectopically expressed as immunogenicity negative control. Transfection with solely circBoxB (purple) serves as immunogenicity positive control. Means ± SEM are shown (n = 3). *p<0.05, Student’s t-test, comparing circBoxB with wild-type YTHDF2 with lambdaN tethering to wild-type YTHDF2 without tethering.
E. Transfection of unmodified circBoxB tethered to N-terminal domain of YTHDF2 into YTHDF2 KO cells is insufficient to attenuate immune response. Graph showing gene expression of innate immune genes 24 hours following RNA transfection. Relative expression of the indicated mRNA and transfected RNA are measured by qRT-PCR, results normalized mock transfection. N-terminal domain of YTHDF2-lambdaN (black) was ectopically expressed as immunogenicity negative control. Means ± SEM are shown (n = 3). Student’s t-test, comparing circBoxB with YTHDF2 N-terminus with lambdaN tethering to YTHDF2 N-terminus without tethering.
We next tested which domains of YTHDF2 are necessary for suppressing immune stimulation by circFOREIGN. We first artificially tethered full-length YTHDF2 (Figure 4C) to unmodified circFOREIGN and tested whether the proximity of m6A reader proteins can bypass the need for m6A modification to suppress circRNA immunity. We introduced five consecutive BoxB stem loop RNA elements into circFOREIGN immediately after the splice junction, which we termed “circFOREIGN-BoxB” (Baron-Benhamou et al., 2004). Additionally, we cloned C-terminal lambdaN peptide tags into proteins we tested and confirmed their expression via western blot (Figures S5C–D). The BoxB stem loops and lambdaN system enabled recruitment of YTH proteins fused to a lambdaN peptide to circFOREIGN-BoxB RNA, as confirmed by RIP-qPCR (Figures 4C and S5E). Transfection of circFOREIGN-BoxB RNA alone strongly stimulated antiviral genes, and tethering of full-length YTHDF2 significantly diminished antiviral gene induction (Figure 4D).
To establish if the N-terminal domain of YTHDF2, termed YTHDF2N, is sufficient for immune evasion of circFOREIGN, we tethered YTHDF2 N-terminus to unmodified circFOREIGN-BoxB. The N-terminus was not sufficient to suppress immune response to circFOREIGN-BoxB (Figure 4E). Since the N-terminal domain is responsible for cellular localization of YTHDF2-RNA complex and the C-terminal domain selectively binds to m6A-modified RNA (Wang et al., 2014), the C-terminal domain is likely required for diminishing antiviral gene induction by circFOREIGN-BoxB.
We then probed if the YTH domain is capable of marking circFOREIGN-BoxB as self by joining YTH to unmodified circFOREIGN-BoxB (Figure S5F). There was no significant change in RIG-I gene expression between circFOREIGN alone and circFOREIGN tethered to the YTH domain. However, tethering circFOREIGN-BoxB and YTH significantly increased the expression of MDA5 and OAS1. Since full-length YTHDF2 protein is larger than each of the separate domains, we probed if tethering circFOREIGN-BoxB to Red Fluorescent Protein (RFP) would affect cellular recognition of unmodified circRNA (Figure S5G). There is a modest decrease in stimulation of RIG-I gene expression, but none of the other immune sensors tested changed expression. This suggests that full suppression of circFOREIGN-BoxB immunogenicity requires all of the YTHDF2 domains and greater interaction between another protein and circRNA is not sufficient.
To test if other members of the YTH family are involved in immune suppression of circFOREIGN, we probed the effects of tethering YTHDF1, another cytoplasmic m6A reader protein to circFOREIGN-BoxB. Following transfection of DNA plasmids, the N-terminus of YTHDF1 also failed to diminish antiviral gene induction, similar to YTHDF2 (Figure S5H). Taken together, these results demonstrate that the full-length m6A reader protein is necessary to mask circRNA immunity and circRNA requires either the m6A chemical modification or m6A reader protein to distinguish between “self” and “foreign” circRNAs.
m6A writer METTL3 needed for self/non-self recognition of circRNA
To probe the necessity of m6A in conveying a “self” mark on the circRNAs, we investigated the role of METTL3, the catalytic subunit of the writer complex for installing the m6A modification. METTL3 is essential for embryonic development due to the critical role of m6A in timely RNA turnover (Batista et al., 2014). METTL3 depletion in many human cancer cell lines leads to cell death. We surmised that one possible consequence of METTL3 depletion is a deficit of m6A modification of endogenous circRNA, leading to immune activation. RIG-I is a RNA binding and signaling protein that senses viral RNA for immune gene activation (Wu and Hur, 2015). We previously showed that foreign circRNAs co-localize with RIG-I in human cells, and RIG-I is necessary and sufficient for circRNA immunity (Chen et al., 2017). Thus, if m6A is required to prevent cells from recognizing their own circRNA as foreign and initiating an immune response, then concomitant RIG-I inactivation should ameliorate the response. Indeed, METTL3 depletion in wild-type HeLa cells led to widespread cell death, but RIG-I inactivation in HeLa (Chen et al., 2017) rescued the cell death (Figure S6). These results suggest that one function of m6A is to prevent RIG-I activation by self RNAs, although we cannot rule out indirect effects due to other RNA targets of METTL3.
CircFOREIGN recognition by RIG-I is distinct from linear RNA ligand
To probe the mechanism of how circRNA stimulates an innate immune response, we turned to biochemical reconstitution with purified components. First, we tested the ability of circFOREIGN to induce ATP hydrolysis by RIG-I. When RIG-I recognizes the 5’ ppp dsRNA agonist, the protein’s helicase domain hydrolyzes ATP (Hornung et al., 2006; Schlee et al., 2009). Exposure of RIG-I to circFOREIGN or 5’ hydroxyl linear RNA did not stimulate its ATPase activity, whereas a 512 base pair 5’-triphosphate dsRNA induced ATP hydrolysis by RIG-I (Figure S7A). Next, we tested for the ability of circFOREIGN to activate purified RIG-I by forming filaments directly on circFOREIGN. Electron microscopy imaging of RIG-I, circFOREIGN, and ATP did not reveal the obvious formation of filaments, whereas the positive control 5’ ppp dsRNA induced RIG-I polymerization (Figure S7B). Thus, circFOREIGN does not interact with nor activate RIG-I in the same manner as 5’ ppp RNA ligands, as expected.
CircFOREIGN directly binds RIG-I and K63-polyubiquitin chain
An alternate mechanism of RIG-I activation involves lysine 63 (K63)-linked polyubiquitin chains (K63-Ubn), which interact with and stabilize RIG-I 2CARD domain oligomers (Jiang et al., 2012; Peisley et al., 2014; Zeng et al., 2010). We probed the ability of RIG-I to bind unmodified and m6A-modified circFOREIGN and the dependency of the interaction on K63-polyubiquitin chains. Using a native gel shift binding assay with purified RIG-I and circFOREIGN, we find that RIG-I binds positive control 5’ ppp 162bp dsRNA both in the absence (Figure S7C, lane 2) and presence (lanes 3–4) of K63-polyubiquitin. We find that RIG-I binds both unmodified and m6A-modified circFOREIGN (Figure S7C, lanes 5–16). Although K63-polyubiquitin chains do not seem to be necessary for RIG-I binding to circFOREIGN, there is greater binding of RIG-I to circFOREIGN when the concentrations of K63-polyubiquitin chains are high (Figure S7C, lane 7 vs. 8, 11 vs. 12, 15 vs. 16). Because RIG-I binds to both unmodified and m6A-modified circRNA but is activated only by the former, these results suggest that RIG-I discriminates between unmodified and m6A-modified circRNA at the level of conformational change, rather than binding. These results further support that RIG-I binding to circRNA is different than 5’ ppp dsRNA ligands.
PRRs like RIG-I and MDA5 survey many RNAs, but only selectively undergo conformational change for oligomerization upon interaction with immunogenic RNA ligands (Ahmad et al., 2018). Similarly, the selectivity of RIG-I for 5’ triphosphate (present on viral RNAs) over m7Gppp cap (present on all mRNAs) is due to conformational change rather than ligand binding (Devarkar et al., 2016). Therefore, we next examine whether RIG-I discriminates against m6A-modified circRNA at the level of binding vs. conformational change.
CircFOREIGN sensing: RIG-I and K63-polyubiquitin chain activate MAVS and discriminates m6A
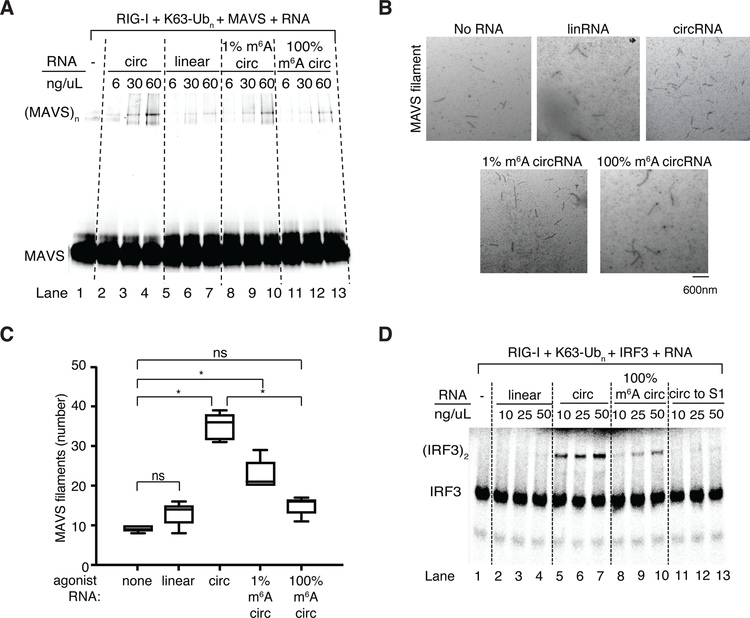
When RIG-I is activated, oligomerized RIG-I templates the polymerization of Mitochondrial Anti-Viral Signaling protein (MAVS, also known as IPS-1, Cardif, and VISA) into filaments, creating a platform for subsequent signal transduction that culminates in the activation and dimerization of IRF3 transcription factor. We reconstituted purified circFOREIGN, RIG-I, K63-polyubiquitin, and MAVS in vitro, and we monitored MAVS transition from monomer into filament by gel shift (Figure 5A) or electron microscopy (Figure 5B). Unmodified circFOREIGN strongly stimulated MAVS polymerization in a concentration-dependent manner in the presence of K63-polyubiquitin (Figure 5B). Importantly, when m6A modification is incorporated onto circFOREIGN at 1% or 100%, the MAVS filamentation is substantially decreased or fully abrogated, respectively (Figure 5B–C). In the absence of K63-polyubiquitin, none of the circRNA substrates induced MAVS polymerization, indicating that polyubiquitin is necessary to stabilize activated RIG-I conformation in order for subsequent MAVS polymerization and signaling to occur (Figure S7D). Quantification of MAVS filaments by electron microscopy confirmed that unmodified circFOREIGN strongly induced MAVS filamentation, whereas m6A modification of circFOREIGN suppressed the ability of MAVS to oligomerize (Figure 5B–C).
Figure 5. RIG-I directly distinguishes unmodified vs. m6A-circFOREIGN and initiates innate immune signaling cascade.
A. In vitro reconstitution with purified RIG-I, MAVS, K63-Ubn and the indicated RNA ligands. Native gel of fluorescently-labeled MAVS 2CARD domain is shown.
B. Representative electron microscopy images of MAVS filaments after MAVS polymerization assay with indicated RNAs. Scale bar indicates 600 nm.
C. Quantification of the total number of MAVS filaments observed in five electron microscopy images for each agonist RNA. *p<0.05, Student’s t-test.
D. In vitro reconstitution of the circRNA-mediated induction of IRF3 dimerization. Native gel of radiolabeled-IRF3 with the indicated RNA ligands is shown. S1 is cellular extract.
These in vitro results with purified components demonstrate that unmodified circFOREIGN can directly activate RIG-I in the presence of K63-polyubiquitin and activate MAVS, in the absence of any other enzyme or RNA binding proteins. Although RIG-I binding fails to distinguish between unmodified and m6A-modified circRNA (Figure S7C), only unmodified circFOREIGN initiates MAVS filamentation in the presence of K63-polyubiquitin (Figure 5A–C). These results suggest that m6A discrimination occurs in the MAVS monomer to filament transition and dependent on RIG-I conformational change, and not in RIG-I binding.
CircFOREIGN activates IRF3 dimerization
Following MAVS filamentation, dimerization of the downstream transcription factor IRF3 completes the innate immune signaling to the genome. To test the ability of circFOREIGN to activate IRF3, we performed a cell free assay by first forming the RIG-I, RNA, K63-polyubiquitin complex, and then incubating with radio-labeled IRF3 in the presence of cellular extract (S1) containing both cytosolic and mitochondrial fractions. CircFOREIGN strongly induced IRF3 dimerization in a concentration-dependent manner, whereas m6A-modified circFOREIGN led to substantially less IRF3 dimerization (Figure 5D, lanes 5–7 vs. lanes 8–10). The known agonist 5’ ppp 162 bp dsRNA stimulated RIG-I-mediated IRF3 dimerization better when it is present at a substoichiometic amount and increased dsRNA prevented effective oligomerization of RIG-I on RNA (Figure S7D). 5’-hydroxyl linear RNA did not stimulate IRF3 dimerization, as expected of the negative control (Figure 5D, lanes 2–4).
CircFOREIGN requires proper complex formation prior to activation
To understand the requirements of RIG-I oligomerization and activation, we examined the order of addition of specific components for the in vitro assay. 5’ ppp RNA showed a more potent response when pre-incubated with RIG-I and K63-polyubiquitin prior to the supplementation of S1 lysate. However, addition of 5’ ppp dsRNA after the introduction of S1 lysate resulted in a reduced, albeit significant, stimulatory activity (Figure S7E, lanes 2 and 5 vs. 8–9.). Adding K63-polyubiquitin at the S1 stage is not active since there is no difference between whether or not poly-ubiquitin is present (Figure S7E, lanes 2 and 5 vs. 10 and 11). When we added circFOREIGN to S1 cellular lysate and then mixed with RIG-I and polyubiquitin, no IRF3 dimerization activity resulted (Figure 5D, lanes 11–13). This result suggests that poly-ubiquitin needs to interact with and stabilize RIG-I in the presence of agonist circRNA first, possibly due to rapid degradation or destabilization of free K63-polyubiquitin chain in cellular lysate. Therefore, the signaling complex needs to be pre-formed before the addition of S1 cellular lysate. Additional experiments rule out the role of endogenous RNAs in circFOREIGN-mediated activation of IRF3 (Figure S7F). Together, our biochemical reconstitution demonstrated that circFOREIGN, RIG-I, and K63-Ubn form a three-component signaling-competent complex for immune signaling.
Distinct localization of unmodified vs. m6A-marked circRNAs in cells
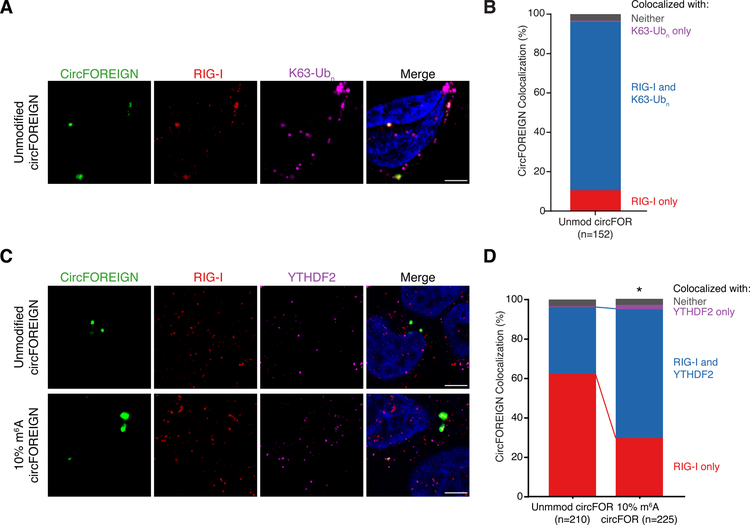
To validate the in vitro assay of RIG-I directly sensing circFOREIGN, we performed immunofluorescence microscopy. HeLa cells were transfected with FITC-labeled circFOREIGN, fixed with formaldehyde, and RIG-I and K63-polyubiquitin were labeled (Figure 6A). The vast majority of unmodified circFOREIGN-FITC co-localized with both RIG-I and K63-polyubiquitin (Figure 6B, 85.5%). Interaction with unmodified circFOREIGN activates RIG-I when K63-polyubiquitin is present in the complex, which allows subsequent stimulation of MAVS filamentation.
Figure 6. Immunofluorescence reveals circFOREIGN co-localization with RIG-I and K63-Ubn and YTHDF2 recruitment to m6A-modified circFOREIGN.
A. CircFOREIGN co-localizes with RIG-I and K63-polyubiquitin chain. Representative field of view is shown.
B. Quantification of circFOREIGN colocalization with RIG-I and K63-Ubn (n = 152). Foci were collected across 10 fields of view across biological replicates and representative of replicate experiments.
C. 10% m6A circFOREIGN has increased co-localization with YTHDF2. Representative field of view is shown. Foci were collected across >10 fields of view and representative of replicate experiments.
D. Quantification of circFOREIGN and 10% m6A circFOREIGN colocalization with YTHDF2 and RIG-I. *p<0.05, Pearson’s χ² test.
Since RIG-I activation discriminates between unmodified and m6A-modified circRNA, we reasoned that YTHDF2 participates in the complex that either inhibits RIG-I activation or decreases RIG-I binding. We had previously used 1% m6A modification on circRNA, but we anticipate that the m6A level at RRACH consensus motif is much lower than 1% because the m6A is randomly incorporated. YTHDF2 binds m6A at RRACH motifs (Dominissini et al., 2012; Meyer et al., 2012). Thus, we incorporated 10% m6A into circFOREIGN for better modeling of m6A placement at consensus sequences. We performed immunofluorescence microscopy with unmodified or 10% m6A-modified circFOREIGN, RIG-I, and YTHDF2 (Figure 6C). The percentage of circRNA co-localization with RIG-I and YTHDF2 is more than doubled when m6A-modification is present on circFOREIGN (33.8% to 65.3%), whereas the percentage of circFOREIGN interacting with RIG-I alone decreased (Figure 6D, 61.9% to 29.3%). The results demonstrate that m6A modification recruits YTHDF2 to the same complex with RIG-I. These immunofluorescence studies provide orthogonal and spatial information for the distinct fates of unmodified vs. m6A-modified circRNAs in cells.
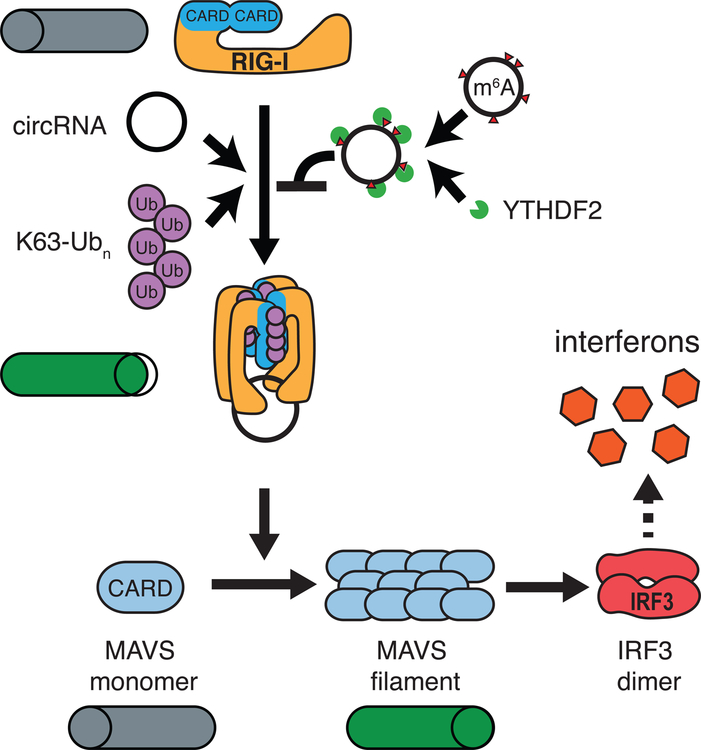
Taken together, the data suggest that RIG-I recognizes foreign circRNA through a mechanism that is dependent on K63-polyubiquitin (Figure 7). Forming the complex of RIG-I, unmodified RNA, and K63-polyubiquitin triggers MAVS filamentation and IRF3 dimerization to stimulate interferon production downstream. m6A-modified circFOREIGN also binds RIG-I but suppresses RIG-I activation, and thus self circRNAs that carry the m6A modification can be safely ignored. In cells, YTHDF2 acts with m6A to inhibit immune signaling.
Figure 7. Model of circRNA induction of innate immune response through RIG-I.
Unmodified circRNA interacts with RIG-I in the presence of K63-Ubn to induce MAVS filamentation, which triggers IRF3 dimerization and interferon production. m6A-modified circRNAs together with YTHDF2 repel RIG-I and does not initiate signaling.
DISCUSSION
The definition of self vs. foreign is the fundamental challenge of any system of immunity. Mammalian cells possess elaborate mechanisms to monitor the ends of RNA transcripts. All transcripts generated by RNA polymerase II bear a 5’ m7G cap, and RIG-I sense 5’-triphosphate for immune monitoring. In principle, circRNAs can bypass the end monitoring system because circRNAs have no ends. Although the functions of endogenous circRNAs are mostly not known, their large number and the presence of viral circRNA genomes necessitate a system of circRNA immunity. The recent discoveries of human circRNA modulation of viral resistance through regulation of NF90/NF110 (Li et al., 2017) and autoimmunity through PKR regulation (Liu et al., 2019) highlight this concept. We provide in vivo evidence that circRNA acts as potent adjuvants to induce specific T and B cell responses. We show that circRNA can induce both innate and adaptive immune responses and has the ability to inhibit the establishment and growth of tumors. The ability of circRNA to enter cells in vivo allows for potential development of immunotherapies that harness the stability, immunogenicity, and ability to encode proteins in circRNA sequences.
Although our results appear to contradict those of Wesselhoeft et al. at first glance, they may in fact converge on several common lessons. We found that exogenous circRNAs can activate RIG-I in comparison to phosphatase-treated linear RNA (Chen et al., 2017; this work). Wesselhoeft et al. reported that in the absence of phosphatase treatment, 5’ triphosphate-containing linear RNAs present in circRNA preparations are more immunogenic than circRNAs. Both results are consistent with our findings using purified RIG-I pathway components and dose titration of RNA ligands, quantifying MAVS polymerization or IRF3 dimerization as readouts. That is, circRNA is RIG-I-stimulatory, but it does not appear to be as strong as dsRNA with 5’ppp. Thus, care should be taken for applications of circRNA as vehicles for gene delivery, but the immunogenicity of circRNA may also be exploited for therapeutic applications such as against cancer.
Our results suggest that human circRNAs are marked at birth, based on the introns that program their back splicing, by the covalent m6A modification. We find that RIG-I discriminates between unmodified and m6A-modified circRNAs, and is only activated by the former. RIG-I is necessary and sufficient for innate immunity to foreign circRNA (Chen et al., 2017) while toll-like receptors are not responsive to circRNAs (Wesselhoeft et al., 2019). In contrast, foreign circRNA lacking RNA modification is recognized by RIG-I and K63-Ubn, and m6A modification of foreign circRNA suffice to mark them as “self” to prevent immune activation. Mutation of all adenosines, or just the adenosines in RRACH, the canonical m6A motif, in a model circRNA substantially increased circRNA induction of anti-viral genes. These results provide the first evidence that specific circRNA exonic sequences impact immunity, and specifically suggest endogenous m6A modification dampens innate immunity. We speculate that an RRACH-less or A-less circSELF would also activate innate immunity, although this remains to be shown. m6A modification of 5’-triphosphate linear RNA ligands also abrogates RIG-I binding and activation (Durbin et al., 2016; Peisley et al., 2013). Hence, RIG-I appears to be a general reader of circRNAs and its activation is suppressed by RNA modification, a predominant feature of eukaryotic RNAs. Both unmodified and m6A circRNA can bind RIG-I, but only unmodified circRNA activates RIG-I to initiate MAVS filamentation. These results suggest RIG-I conformational changes are necessary to induce MAVS filamentation. This observation is analogous to the selectivity of RIG-I for 5’ triphosphate (present on viral RNAs) over m7Gppp cap (present on all mRNAs) due to conformational change rather than ligand binding (Devarkar et al., 2016). Co-crystal structure and biochemical analyses show that 5’ triphosphate and m7Gppp both bind RIG-I with the same affinity, but the latter triggers a distinct conformational change and causes RIG-I to filter against endogenous mRNAs and lowers ATPase activity (Devarkar et al., 2016). In living cells, YTHDF2 may inhibit the RIG-I conformational transitions necessary for downstream signaling of immune genes (Figure 6).
We systematically addressed the necessity, sufficiency, and domain requirements of YTHDF2-mediated suppression of circRNA immunity. The requirement of full-length YTHDF2 is consistent with recent model that YTH-proteins recruit m6A-modified RNAs into phase-separated condensates via their N-terminal disordered domains, i.e. both domains are needed for higher-order RNA-protein interactions (Luo et al., 2018). Our results extend prior knowledge about YTHDF function. Although tethering just the effector domain is sufficient to induce RNA decay or translation (Wang et al., 2014; Wang et al., 2015), the full-length protein is needed for self-foreign discrimination of circRNAs. These results suggest a double-layered system for m6A to both sequester and block endogenous circRNAs from activating the RIG-I antiviral pathway. These results have substantially increased our mechanistic understanding of the role of YTHDF2 in circRNA immunity. In addition to YTHDF2, there may also be other sensors and receptors involved in identifying endogenous circRNAs as self. Moreover, m6A may also not be the only RNA modification that impacts circRNA immunity, as other RNA modifications also modulate circRNA immunity (Figure S7G). Since over a hundred different RNA modifications have been discovered and characterized, endogenous circRNAs may contain more than one modification that regulates the cell’s ability to identify self/non-self RNA. The logic of using nucleic acid chemical modification to distinguish self vs. foreign genes, independent of sequence content, is strikingly similar for DNA modification-restriction systems recognized since the 1970s and circRNA immunity that we uncovered here.
STAR METHODS
CONTACT FOR REAGENT AND RESOURCE SHARING
Further information and requests for resources and reagents should be directed to and will be fulfilled by the Lead Contact, Howard Y. Chang (howchang@stanford.edu).
EXPERIMENTAL MODEL AND SUBJECT DETAILS
Cell lines and maintenance
Human HeLa (cervical adenocarcinoma, ATCC CCL-2), human HEK293T (embryonic kidney, ATCC CRL-3216) cells, mouse B16/OVA (provided by Dr. Edith Lord, University of Rochester), and mouse B16/OVA/luciferase cells were grown in Dulbecco’s modified Eagle’s medium (DMEM, Invitrogen, 11995–073) supplemented with 100 units/ml penicillin-streptomycin (Gibco, 15140–163) and 10% (v/v) fetal bovine serum (Thermo Fisher Scientific, SH30071.03). Cell growth was maintained at 37 °C in a 5% CO 2 atmosphere, passaging at a 1:10 dilution every 2–3 days. B16/OVA/luciferase cells were generated by integrating a luciferase expression cassette into B16/OVA cells.
Cell culture and transient transfection
Cells were plated 24 hours prior to transfection. Cells were at 70 to 80% confluence and 500 ng of RNA was transfected into one well of a 24-well plate using Lipofectamine 3000 (Thermo Fisher Scientific, L3000008). The nucleic acids with P3000 and Lipofectamine 3000 were diluted in Opti-MEM (Thermo Fischer Scientific, 31985088) per manufacturer’s instructions, and incubated for 3 minutes at room temperature. The nucleic acids and Lipofectamine 3000 were the mixed together, incubated for 20 minutes at room temperature and then the nucleic acids-Lipofectamine 3000 complexes were applied dropwise to the monolayer cultures.
When ectopic protein expression prior to RNA transfection was necessary, cells were electroporated with NEON Transfection System (Thermo Fisher Scientific MPK5000S) per manufacturer’s instructions. In most cases, cells were resuspended in buffer R at 2×107/mL and 5 μg of DNA plasmid was electroporated with a 100 μL NEON tip. Electroporation parameters were 1,000V, 35ms pulse, and 2 pulses for HeLa cells. 12 hours later, cells were passaged and plated such that 24 hours later they would be at 70 to 80% confluent. 24 hours later, cells were then transfected with RNA with Lipofectamine 3000 as described above. 24 hours after transfection, cells were washed once with PBS and 300 μL of TRIzol reagent was added per 24-well. RNA was harvested with Direct-zol kit (Zymo Research).
Immunization of mice (for CD8+ T-cell FACS analysis, antibody ELISA, and Dendritic cell analysis)
Eight-to-twelve-week-old female C57BL/6 mice purchased from Jackson Laboratories were immunized subcutaneously at the base of the tail with 100 μg per mouse of OVA protein (Invivogen, vac-pova) adjuvanted with 25 μg of HMW vaccine grade Poly I:C (Invivogen, vac-pic), 25 μg of circular RNA alone or with in vivo-jetPEI (Polyplus Transfection, 201–10G), 25 μg of modified RNA alone or with in vivo-jet PEI. PEI/RNA complexes were formulated as per the manufacturer’s instructions. Mice were bled via the lateral tail or facial vein at regular intervals for analysis of CD8+ T cell and antibody responses after vaccination as indicated in the figures. A booster vaccination after 5 weeks of primary vaccination was given where indicated. For tumor establishment and proliferation studies, 0.5 million OVA-expressing B16 melanoma cells with an integrated luciferase expression cassette (B16/OVA/luciferase) were delivered with matrigel (Corning, 356231) in the right and left flanks of mice fourteen days after a single RNA vaccination. Tumors were measured twice a week and bioluminescence was measured once a week. Bioluminescence was measured by injecting 3 mg per 20 g mouse of D-luciferin (Biosynth International, L-8220) intraperitoneally and imaged at 20 second to 1 minute range of exposure using Ami HT imager (Spectral Instruments). All animal procedures were performed in accordance with guidelines established by Stanford university institutional animal care and use committee guidelines.
METHOD DETAILS
Plasmids
Plasmid encoding phage introns that express circRNA through autocatalytic splicing was previously described in (Chen et al., 2017). In-Fusion HD assembly (Takara Bio, 638910) was used to construct the plasmid encoding phage introns expressing foreign circGFP with BoxB motif (GCCCTGAAGAAGGGC) separated with short 9–44bp variable spacer sequences to prevent recombination. The full sequence of the 5 BoxB cassette is as follows:
ttccctaagtccaactactaaactggggattcctggGCCCTGAAGAAGGGCccctcgactaagtccaa ctactaaactggGCCCTGAAGAAGGGCccatataggGCCCTGAAGAAGGGCcctatcgag gatattatctcgactaagtccaactactaaactggGCCCTGAAGAAGGGCccatataggGCCCTG AAGAAGGGCcctatcgaggatattatctcgag.
Plasmids expressing YTHDF1N and YTHDF2N with and without lambdaN (DAQTRRRERRAEKQAQWKAAN) were provided by Dr. Chuan He (University of Chicago). Plasmids expressing YTHDF2 protein domain truncations were constructed with In-Fusion HD assembly. All plasmids were propagated in NEB Turbo Competent E. coli cells (NEB, C2984H) grown in LB medium and purified using the ZymoPURE II Plasmid Prep Kits (Zymo Research, D4200). Plasmids are available upon request.
RNA synthesis and purification
RNA was synthesized by in vitro transcription using MEGAscript T7 transcription kit (Ambion, AM1334) following the manufacturer’s instructions and incubation at 37 °C overnight, or for at least 8 hours. m6A-labeled RNA was synthesized in the same way by in vitro transcription using MEGAscript T7 transcription kit (Ambion, AM1334) and adding m6ATP (Trilink, N-1013) in the specified ratio with the transcription kit’s ATP. Transcribed circFOREIGN was purified by RNeasy Mini column (Qiagen, 74106), then treated with RNase R (Epicenter, RNR07250) in the following manner: circFOREIGN secondary structure was denatured at 72°C for 5 minutes follo wed by 2 minutes on ice; RNaseR was added at a ratio of 1U: 1 ug of RNA and incubated at 37 °C for 2–3 hours. CircRNALinear RNA was not treated with RNase R. CircFOREIGN was then purified by RNeasy column. CircFOREIGN or linear RNA were then phosphatase treated by FastAP in the following manner: FastAP was added at a ratio of 1U: 1ug of circFOREIGN, incubated at 37 °C for 2 hours, then purified by RN easy column. RNA quality was assessed by Tapestation analysis (Agilent, 5067–5576).
CircFOREIGN was gel purified by first denaturing at 72 °C for 3 minutes followed by 2 minutes on ice. RNA loading dye (New England Biolabs, B0363S) was then added and the RNA-dye mix was then loaded on a 1% low melting point agarose gel. Gel extraction was done on a blue light transilluminator (Clare Chemical) followed by Zymoclean Gel Recovery Kit (Zymo Research, R1011) purification following the manufacturer’s instructions except for melting, which was done rotating at room temperature for 10 minutes to better preserve RNA integrity.
HPLC fractionation was performed with a 4.6×300mm s ize exclusion column (Sepax Technologies, 215980P-4630) with particle size of 5um and pore size of 2000 Å. Nuclease-free TE buffer was used as the mobile phase at a flow rate of 0.3ml/minute. RNA fractions were manually collected, lyophilized, and then cleaned with RNA Clean & Concentrator-5 (Zymo Research, R1013) prior to subsequent quality control and experimental use.
m6A-irCLIP
10 µg of total RNA was enriched for circRNA by removing mRNAs (polyA-) using the Poly(A)Purist MAG Kit (Thermo Fisher Scientific, AM1922) and removing ribosomal RNAs (ribo-) using the RiboMinus Eukaryote System v2 kit (Thermo Fisher Scientific, A15026). The resulting polyA-/ribo- RNA was then fragmented to 35–100 nt sizes using the RNA Fragmentation Buffer (Thermo Fisher Scientific, AM8740) at 75 °C for 12 minutes. Fragmented RNA was denatured and then incubated with anti-m6A antibody (Synaptic Systems, 202003) for 2 hours at 4 °C in I PP buffer (50 mM Tris-HCl, pH 7.4; 100 mM NaCl; 0.05% NP-40; 5 mM EDTA). The RNA and antibody were then crosslinked using UV light (254 nm) using 2 rounds of crosslinking at 0.15J (Stratalinker 2400). The crosslinked RNA and antibody were then incubated with Protein A Dynabeads (Thermo Fisher Scientific, 10002D) for 2 hours at 4 °C. The beads were then washed with once with IPP buffer for 10 minutes at 4 °C with rotation, once with low salt buffer (50 mM Tris, pH 7.4; 50 mM NaCl; 1 mM EDTA; 0.1% NP-40) for 10 minutes at 4 °C with rotation, once with high salt buffer (50 mM Tris–HCl pH 7.4, 1M NaCl, 1% NP-40, 0.1% SDS) for 10 minutes at 4 °C with rotation, transferred to a new 1.5 mL tube, and washed twice with PNK buffer (20 mM Tris-HCl, pH 7.4; 10 mM MgCl2; 0.2% Tween 20). Libraries were then prepared using the irCLIP method (Zarnegar et al., 2016). Libraries were checked for quality by Bioanalyzer and submitted for sequencing on NextSeq 500 with custom sequencing primer P6_seq, as described in the irCLIP method. Reads were mapped to the hg38 and subsequently to a custom assembly of the circGFP sequence and PCR duplicates were removed using UMI-tools(Smith et al., 2017). Reproducible RT stops were identified using the FAST-iCLIP pipeline (Flynn et al., 2015).
m6A-RIP-seq
10 µg of total RNA was enriched for circRNA by removing mRNAs (polyA-) using the Poly(A)Purist MAG Kit (Thermo Fisher Scientific) and removing ribosomal RNAs (ribo-) using the RiboMinus Eukaryote System v2 kit (Thermo Fisher Scientific). The remaining RNA was then treated with RNase R to remove residual linear RNAs. The polyA-/ribo-RNase R+ RNA was then fragmented for 12 minutes at 75 °C with RNA Fragmentation Buffer (Thermo Fisher Scientific). 3 µg anti-m6A (Synaptic Systems) was bound to Protein A Dynabeads (Thermo Fisher Scientific) for 2 hours at room temperature. Antibody bound beads were then washed with IPP buffer (50mM Tris-HCl, pH 7.4; 100mM NaCl; 0.05% NP-40; 5mM EDTA) and resuspend in IPP with 1 µL RiboLock (Thermo Fisher Scientific, EO0382). Fragmented RNA in IPP buffer was incubated with antibody and beads for 2 hours at 4 °C with rotatio n. RNA-bound beads were then washed once with IPP buffer for 10 minutes at 4 °C with rotation, once with low-salt buffer (50 mM Tris, pH 7.4; 50 mM NaCl; 1 mM EDTA; 0.1% NP-40) for 5 minutes at 4 °C with rotation, once with high-salt buffer (50 mM Tris–HCl pH 7.4, 1M NaCl, 1% NP-40, 0.1% SDS) for 5 minutes at 4 °C with rotation. Beads were then resuspended in 300 µL high-salt buffer and transfer to a new 1.5 mL tube. Beads were washed with PNK buffer (20 mM Tris-HCl, pH 7.4; 10 mM MgCl2; 0.2% Tween 20) and then resuspended in 500 µL Trizol and incubated for 5 minutes at 25 °C. 150 µL chloroform:isoamyl alcohol was added and mixed before incubating at 25 °C for 2 minutes. After spinning at 13,000xg at 4 °C for 10 minutes, the aqueous layer was transferred to a new 1.5 mL tube and cleaned up with RNA Clean & Concentrator-5 (Zymo Research, R1013), R1013. RNA was eluted in 10 µL nuclease-free water. To eluted RNA and 10 % input RNA, 10 µL of end-repair mix was added (4 µL 5X PNK buffer; 1 µL RiboLock, 1 µL FastAP; 2 µL T4 PNK, 2 µL nuclease-free water). Reaction was incubated at 37 °C for 1 h. 20 µL of linker ligation mix (2 µL 10X RNA ligation buffer; 2 µL 100 mM DTT; 2µL L3 linker (Zarnegar et al., 2016); 2 µL T4 RNA ligase buffer; 12 µL PEG8000 50% w/v). Reaction was incubated for 3 hours at 25 °C and the n cleaned up with an RNA Clean & Concentrator-5 column. Processed RNA was eluted in 10 µL of nuclease-free water. Libraries were prepared using the irCLIP method(Zarnegar et al., 2016) and sequenced on a NextSeq 500 using a custom sequencing primer (P6_seq, (Zarnegar et al., 2016)). Reads were aligned to hg38 and circGFP sequence. Bam files were normalized to genome mapped reads.
Reverse transcription and real time PCR analysis (qRT-PCR)
Total RNA was isolated from cells using TRIzol (Invitrogen, 15596018) and Direct-zol RNA Miniprep (Zymo Research, R2052) with on-column DNase I digestion, following the manufacturer’s instructions. qRT-PCR analysis was performed in triplicate using Brilliant II SYBR Green qRT-PCR Master Mix (Agilent, 600825) and a LightCycler 480 (Roche). qRT-PCR primers used are found in Table S1. mRNA levels were normalized to beta-actin. Relative expression of indicated mRNA genes for RNA transfection are normalized by level of transfected RNA and plotted as the fold change to the expression level of cells with mock transfection.
Western blot analysis
Cells were collected and lysed 24 hours after transfection to extract total proteins. Bioruptor sonication with RIPA buffer (150 mM NaCl, 1% Triton X-100, 0.5% sodium deoxycholate, 0.1% SDS, 50 mM Tris, pH 8.0) was used to lyse the cells. Proteins were fractionated by sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE), transferred to nitrocellulose membranes, blocked in phosphate-buffer saline containing 4% (wt/vol) nonfat milk for 1 hour rocking at room temperature, and then incubated overnight at 4 °C with the indicated primary antibo dy. YTHDF2−/− KO cells were validated with 1:250 YTHDF2 polyclonal antibody (Thermo Fisher Scientific, PA5–63756). Ectopic protein expression was validated with 1:1000 Flag M2 monoclonal antibody (Sigma-Aldrich F1804). Secondary antibodies were incubated for 1 hour at 1:15,000, in pairs depending on the primary antibody identities: IRDye® 800CW Goat anti-Mouse IgG (Li-COR Biosciences, 926–32210), IRDye® 800CW Goat anti-Rabbit IgG (Li-COR Biosciences, 926–32211), IRDye® 680RD Goat anti-Mouse IgG (Li-COR Biosciences, 926–68070), IRDye® 680RD Goat anti-Rabbit IgG (Li-COR Biosciences, 926–68071). Western blot detection and quantification was done using an Odyssey infrared imaging system (Li-COR Biosciences).
YTHDF2 rescue and YTHDF1/2 Tethering to circFOREIGN-BoxB
As described above, plasmids expressing YTHDF1N or YTHDF2N with and without λN were electroporated into cells via NEON Transfection System. After 12 hours later, cells were passaged and plated such that 24 hours later they would be at 70 to 80% confluent. 24 hours after this, 500 ng of circBoxB (circRNA with 5 BoxB sites) was transfected with Lipofectamine 3000. RNA was harvested and qRT-PCR was performed with Brilliant II SYBR Green qRT-PCR Master Mix and a LightCycler 480 as described above. Extra duplicates were set aside for protein lysate collection and ectopic protein expression in these conditions was simultaneously confirmed via western blot.
RNA immunoprecipitation-qPCR
Plasmids expressing Flag-tagged YTHDF1N or YTHDF2N with and without a lambdaN peptide tag were electroporated into cells via NEON Transfection System, then later passaged into 6-well format in a timeline described above. Approximately 3 million cells were harvested with 0.25% Trypsin-EDTA (Thermo Fisher Scientific, 25200056), then washed with PBS. Cells were then lysed in cell lysis buffer (50 mM Tris pH 8.0, 100 mM NaCl, 5 mM EDTA, 0.5% NP-40 with proteinase inhibitor) by Covaris Ultrasonicator with the following settings: Fill Level 10, Duty Cycle 5%, Peak Incident Power 140 W, Cycles/Burst 200, time per tube 300s. Cell lysate was pelleted for 15 minutes at 16,000 rcf. Supernatant was collected and incubated with 100 µL of Anti-FLAG M2 Magnetic Beads (Sigma-Aldrich, M8823–5ML) for 2 hours rotating at room temperature to pull down YTHDF1N or YTHDF2N. Beads were washed three times with cell lysis buffer and one time with PBS. Beads were resuspended in 500 µL of TRIzol and total RNA was extracted using an RNeasy Mini kit (Qiagen, 74106). qRT-PCR was performed with Brilliant II SYBR Green qRT-PCR Master Mix and a LightCycler 480 as described above. RNA levels were normalized as percent of input within each biological replicate. Results are presented as the fold change of the enrichment of circRNA over actin.
METTL3 siRNA FACS analysis
Cells were seeded into 24-well format at 60,000 cells per well in DMEM with FBS without antibiotics. After 24 hours, cells were transfected with siRNA per manufacturer’s recommendations. Dharmafect SMARTpool ON-TARGETplus METTL3 siRNA (Dharmacon, L-005170–02-0005) was used as the knockdown siRNA and ON-TARGETplus Non-targeting Control siRNAs (Dharmacon, D-001810–01-05) was used as the non-targeting siRNA. Media was refreshed at 12 and 36 hours following transfection. 48 hours after transfection, cells were collected via 0.25% Trypsin-EDTA, stained with Annexin V-647 (Thermo Fisher Scientific, A23204) in Annexin Binding Buffer (BD Biosciences, 556454) for 15 minutes. Cells were then spun down and re-suspended into DAPI in Annexin Binding Buffer for 5 minutes. Cells were then resuspended in Annexin Binding Buffer without stain and passed through Round-Bottom Tubes with Cell Strainer Cap (Corning, 352235). Flow analysis was done on a special order FACS Aria II (BD Biosciences, Stanford Shared FACS Facility). Cells subject to the same transfection as above were collected and protein lysate was collected. METTL3 knockdown was confirmed via western blot using 1:1500 anti-METTL3 antibody (Abcam, ab195352). RIG-I protein expression was assayed via western blot using 1:1000 RIG-I monoclonal antibody (Abcam, ab180675).
CD8+ T-cell FACS analysis
Primary and memory CD8+ T-cell responses were evaluated at day 7 after primary and secondary immunizations. Briefly, peripheral blood mononuclear cells (PBMCs) were enriched using a Histopaque-1083 sucrose density gradient separation (Sigma-Aldrich 10831) and cultured with OVA-specific MHC class I restricted peptide at 1 µg/mL (SIINFEKL) (Invivogen, vac-sin) for restimulation ex-vivo in the presence of BD Golgi Plug TM (BD Biosciences, 555029) for 5 hours. Stimulated cells were first stained for surface markers anti-mouse CD8α (Biolegend, 100747), anti-mouse-CD3 (Biolegend, 100231), anti-mouse CD4 (Biolegend, 100555) followed by fixation in BD cytofix/cytoperm and intracellular staining (BD Biosciences, 554714) with anti-mouse IFN-γ (BD Biosciences, 554409), in BD cytoperm buffer. Dead cell was excluded using live/dead aqua stain (Thermo Fisher Scientific, L23101). Labeled cells were acquired on a special order FACS Aria II (BD Biosciences, Stanford Shared FACS Facility) and data were analyzed using Flow JO software (TreeStar).
Antibody ELISA
Nunc MaxiSorp 96 -well plates (Thermo Fisher Scientific, 44–2404-21) were coated with 100 µl of 20 µg/mL of OVA protein (Invivogen) overnight at 4 °C. Plates were washed 3 times with PBS/0.5% Tween-20 using a Bio-Rad auto plate washer and blocked with 200 µl of 4% BSA (Sigma Aldrich, A9418–5G) for 2 h at room temperature. Serum samples from immunized mice at the indicated time points were serially diluted in 0.1% BSA in PBS/0.5% Tween-20 and incubated on blocked plates for 2 h at room temperature. Wells were washed and incubated with 1:5000 anti-mouse IgG-HRP (Southern Biotech, 1036–05), 1:5000 anti-mouse IgG1–HRP conjugate (Southern Biotech, 1071–05) and 1:2000 anti-mouse IgG2c–HRP conjugate (Southern Biotech, 1078–05) in PBS/0.5% Tween-20 for 2 h at room temperature. Plates were washed and developed using 100 µl per well of tetramethylbenzidine (TMB) substrate (Thermo Fisher Scientific, 34028), followed by stopping the reaction using stop solution (Thermo Fisher Scientific, N600). Plates were analyzed using a Bio-Rad plate reading spectrophotometer at 450 nm with correction at 595 nm. Antibody titers were represented as serum reciprocal dilution yielding a >0.3 optical density (OD) value at 450 nm.
Dendritic cell analysis
Mice were immunized with PBS (control) or circular RNA (25ug /mice) subcutaneously at base of the tail, 24 hours after the immunization mice were euthanized and skin draining inguinal lymph nodes were excised. Skin draining inguinal lymph nodes were gently busted with a 3mL syringe plunger thumb rest, and digested with 1mg/mL collagenase type 4 for 20–25 minutes at 37°C. Reactions were st opped with 2mM EDTA and single cell suspension were prepared by passing through 40µm cell strainers (Corning, 352340). The following staining antibodies were used:
| Antibody | Clone | fluorochrome |
| CD11c | N418 | BV421 |
| CD11b | M1/70 | BV650 |
| CD103 | 2E7 | PE/BB710 |
| MHCII | M5/114.15.2 | Alexa 700 |
| CD86 | PO3 | FITC |
| CD45 | 30-F11 | BV605 |
RIG-I ATPase assay
0.1 µM RIG-I was pre-incubated with the specified circular RNA or 512 bp 5’ ppp dsRNA (0.4 ng/ µl) in buffer B (20 mM HEPES pH 7.5, 150 mM NaCl, 1.5 mM MgCl2). The reaction was initiated by adding 2 mM ATP at 37 °C. Aliquots (10 µl) were withdrawn at 2, 4, or 8 minutes after ATP addition, and immediately quenched with 100 mM EDTA. The ATP hydrolysis activity was measured using BIOMOL® Green Reagent (Enzo Life Sciences, AK111–0250). The Green Reagent (90 µl) was added to the quenched reaction at a ratio of 9:1, and OD650 was measured using a Synergy2 plate reader (BioTek).
RIG-I native gel shift assay
RNA (1 ng/µL) was incubated with RIG-I (500nM) in buffer A (20 mM HEPES pH 7.5, 50 mM NaCl, 1.5 mM MgCl2, 2 mM ATP, and 5 mM DTT) at room temperature for 15 min. Poly-ubiquitin was then added at the indicated concentration and incubated at room temperature for 5 minutes. The complex was analyzed on Bis-Tris native PAGE gel (Life Technologies) and was stained with SybrGold stain (Thermo Fisher Scientific, S11494). SybrGold fluorescence was recorded using the scanner FLA9000 (Fuji) and analyzed with Multigauge (GE Healthcare).
RIG-I polymerization assay
0.4 µM RIG-I was incubated with the specified circular RNAs (1 ng/ µl) in buffer A (20 mM HEPES pH 7.5, 50 mM NaCl, 1.5 mM MgCl2, 2 mM ATP, and 5 mM DTT) at room temperature for 15 minutes. Prepared samples were adsorbed to carbon-coated grids (Ted Pella) and stained with 0.75% uranyl formate. Images were collected using a Tecnai G2 Spirit BioTWIN transmission electron microscope at 30,000x or 49,000x magnification.
Protein Preparation
Human RIG-I was expressed as previously reported (Peisley et al., 2013). Briefly, the proteins were expressed in BL21(DE3) at 20°C for 16–20 hours following induction with 0.5 mM IPTG. Cells were homogenized using an Emulsiflex C3 (Avestin), and the protein was purified three-step protocol including Ni-NTA, heparin affinity chromatography and size exclusion chromatography (SEC) in 20 mM HEPES, pH 7.5, 150 mM NaCl and 2 mM DTT.
K63-Ubn was synthesized as previously reported (Dong et al., 2011). Briefly, mouse E1, human Ubc13, Uev1a and ubiquitin were purified from BL21(DE3), and were mixed in a reaction containing 0.4 mM ubiquitin, 4 µM mE1, 20 µM Ubc13 and 20 µM Uev1a in a buffer, 10 mM ATP, 50 mM Tris pH 7.5, 10 mM MgCl2, 0.6 mM DTT. After incubating the reaction overnight at 37 °C, synthesized K63-Ubn chains were diluted 5-fold into 50 mM ammonium acetate pH 4.5, 0.1 M NaCl and separated over a 45 mL 0.1–0.6 M NaCl gradient in 50 mM ammonium acetate pH 4.5 using a Hi-Trap SP FF column (GE Healthcare). High molecular weight fractions were applied to an S200 10/300 column equilibrated in 20 mM HEPES pH 7.5, 0.15 M NaCl.
MAVS CARD was expressed as a fusion construct with the SNAP tag (CARD-S) in BL21(DE3) at 20 °C for 16–20 hours following induction with 0.4 mM IPTG. The SNAP tag allows fluorescent labeling of MAVS CARD. MAVS CARD-S fusion was purified using Ni-NTA affinity chromatography as described(Wu et al., 2016), with the exception of using 0.05% NP-40 instead of CHAPS. Purified CARD-S was denatured in 6 M guanidinium hydrochloride for 30 minutes at 37 °C with constant shaking, followed by dialysis against refolding buffer (20 mM Tris, pH 7.5, 500 mM NaCl, 0.5 mM EDTA and 20 mM BME) at 4 °C for 1 h. Refolded CARD-S was pas sed through a 0.1µ filter and subsequently fluorescently labeled with Alexa647-benzylguanine (New England Biolabs, S9136S) on ice for 15 minutes according to the manufacturer’s instructions. The labeled MAVS CARD-S was immediately used for polymerization assay.
MAVS polymerization assay
The MAVS filament formation assay was performed as previously reported (Wu et al., 2013). MAVS CARD fused to SNAP (CARD-S) was labeled with BG-Alexa 647 (New England Biolabs) on ice for 15 minutes. RIG-I (1 µM) was pre-incubated with various concentrations of RNA and 2 mM ATP in the presence or absence of 6 µM K63-Ubn in (20 mM HEPES pH 7.5, 150 mM NaCl, 1.5 mM MgCl2, 2 mM DTT) for 15 minutes at room temperature. Subsequently, labeled monomeric MAVS CARD-S (10 µM) was added to the mixture and incubated further for 1 hour at room temperature. MAVS filament formation was detected by native PAGE analysis or by negative-stain EM. Prior to running on Bis-Tris gel (Life Technologies Corp.), all the samples were subjected to one round of freeze-thaw cycle by incubating on dry ice for 5 minutes followed by incubation at room temperature for 5 minutes. Fluorescent gel images were scanned using an FLA9000 scanner (Fuji). Samples from MAVS polymerization assay were adsorbed to carbon-coated grids (Ted Pella) and stained with 0.75% uranyl formate as described previously (Ohi et al., 2004). Images were collected using a Tecnai G2 Spirit BioTWIN transmission electron microscope at 9,300x magnification.
Immunofluorescence and Quantification
FITC-labeled RNA was synthesized as described above, except for the substitution of 5% Fluorescein 12 UTP (Thermo Fisher Scientific, 11427857910) for 100% UTP in the in vitro transcription reaction mix. 10% m6A FITC-labeled RNA was synthesized with the additional substitution of 10% m6A for 100% ATP in the in vitro transcription reaction mix. RNaseR and FastAP treatment were carried out as described. RNA quality was assessed via Tapestation.
HeLa cells were seeded on 22×22mm #1.5 thickness cover slips in 6-well format. After 12 hours, transient transfection of FITC-labeled circRNA was performed with Lipofectamine 3000 (Thermo Fisher Scientific, L3000015). After 12 hours, cells were fixed with 1% formaldehyde in PBS (Thermo Fisher Scientific, 28908) for 10 minutes at RT. The formaldehyde fixed slide was rinsed in PBS and permeabilized in 0.5% Triton X-100 in PBS for 10 minutes at RT. After the permeabilization solution was rinsed, the slide was blocked with antibody diluent (Thermo Fisher Scientific, 003118) for 1 hour at room temperature. Anti-RIG-I rabbit polyclonal primary antibody (Cell Signaling Technology, 3743S) and anti-Ub-K63 mouse monoclonal antibody (eBioscience, 14–6077-82) were diluted at 1:200 in antibody diluent and incubated overnight at 4°C. After washing with PBS, slides were incubated with goat anti-rabbit IgG highly cross-adsorbed-Alexa594 (Thermo Fisher Scientific, A-11037) and goat anti-mouse IgG highly cross-adsorbed Alexa647 (Thermo Fisher Scientific, A-21236) were diluted at 1:1000 in the antibody diluent and incubated for 2 hours at room temperature. The slides were washed with PBS, mounted using Vectashield with DAPI (Vector Labs, H-1200) and imaged with a Zeiss LSM 880 confocal microscope (Stanford Microscopy Facility). Colocalization of RIG-I and K63-polyUb were counted if foci were directly overlapping with FITC-circRNA and/or each other.
Anti-RIG-I rabbit polyclonal primary antibody (Cell Signaling Technology, 3743S) and anti-YTHDF2 mouse polyclonal antibody (USBiological, 135486) were diluted at 1:200 each in antibody diluent. The rest of the immunofluorescence including secondary staining, mounting, and imaging were done as detailed above. Colocalization of RIG-I and YTHDF2 were counted if foci were directly overlapping with FITC-circRNA and/or each other.
IRF3 dimerization assay
This assay was performed as described previously (Ahmad et al., 2018). Briefly, HEK293T cells were homogenized in hypotonic buffer (10 mM Tris pH 7.5, 10 mM KCl, 0.5 mM EGTA, 1.5 mM MgCl2, 1 mM sodium orthovanadate, 1X mammalian Protease Arrest (GBiosciences, 786–108)) and centrifuged at 1000g for 5 minutes to pellet the nuclei. The supernatant (S1), containing the cytosolic and the mitochondrial fractions, was used for in vitro IRF3 dimerization assay. The stimulation mix containing 10 ng/ µl RIG-I, 2.5 ng/ µl K63-Ubn along with the indicated amounts of RNA was pre-incubated at 4°C for 30 minutes in (20 mM HEPES pH 7.4, 4 mM MgC l2 and 2 mM ATP). 35S-IRF3 was prepared by in vitro translation using TnT T7 Coupled Reticulocyte Lysate System (Promega, L4610) according to manufacturer’s instructions. The IRF3 activation reaction was initiated by adding 1.5 µl of pre-incubated stimulation mix to 15 µl reaction containing 10 µg/ µl of S1, 0.5 µl 35S-IRF3 in (20 mM HEPES pH 7.4, 4 mM MgCl2 and 2 mM ATP) and incubated at 30°C for 1 h. Subsequently, the samples were centrifuged at 18,000g for 5 minutes and the supernatant was subjected to native PAGE analysis. IRF3 dimerization was visualized by autoradiography and phosphorimaging (Fuji, FLA9000).
Statistical Analyses
All statistical analysis was done with the software Graphpad Prism (GraphPad Software, La Jolla, CA). Student’s t-, Kruskal-Wallis, or Anova-Tukey test was used whenever appropriate. p values less than 0.05 were considered statistically significant.
Supplementary Material
Table S1. Related to STAR Methods. qRT-PCR primers.
KEY RESOURCES TABLE
| REAGENT or RESOURCE | SOURCE | IDENTIFIER |
|---|---|---|
| Antibodies | ||
| β-Actin (8H10D10) Mouse mAb (1:9000 for WB) | Cell Signaling Technology | 3700S RRID: AB_2242334 |
| Anti-beta Actin antibody (1:1000 for WB) | Abcam | ab8227 RRID: AB_230518: |
| Monoclonal ANTI-FLAG® M2 antibody produced in mouse (1:1000 for WB) | Sigma-Aldrich | F1804 RRID: AB_262044 |
| Anti-YTHDF1 antibody (1:1000 for WB) | Abcam | ab157542 RRID: N/A |
| YTHDF2 Polyclonal Antibody (1:250 for WB) | Thermo Fisher Scientific | PA5–63756 RRID: AB_2649742 |
| Recombinant Anti-METTL3 antibody [EPR18810] (1:1500 for WB) | Abcam | ab195352 RRID: AB_2721254 |
| Rig-I (D14G6) Rabbit mAb (1:200 for IF) | Cell Signaling Technology | 3743S RRID: AB_2269233 |
| Ub-K63 Monoclonal Antibody (HWA4C4) (1:200 for IF) | Thermo Fisher Scientific | 14–6077-82 RRID: AB_1257213 |
| Mouse Anti-YTHDF2 polyclonal antibody (1:200 for IF) | USBiological Life Sciences | 135486 RRID: N/A |
| IRDye® 800CW Goat anti-Mouse IgG Secondary Antibody (1:15,000 for WB) | Li-COR Biosciences | 926–32210 RRID: AB_621842 |
| IRDye® 800CW Goat anti-Rabbit IgG Secondary Antibody (1:15,000 for WB) | Li-COR Biosciences | 926–32211 RRID: AB_621843 |
| IRDye® 680RD Goat anti-Mouse IgG Secondary Antibody (1:15,000 for WB) | Li-COR Biosciences | 926–68070 RRID: AB_10956588 |
| IRDye® 680RD Goat anti-Rabbit IgG Secondary Antibody (1:15,000 for WB) | Li-COR Biosciences | 926–68071 RRID: AB_10956166 |
| Goat anti-Rabbit IgG (H+L) Highly Cross-Adsorbed Secondary Antibody, Alexa Fluor Plus 594 (1:2000 for IF) | Thermo Fisher Scientific | A32740 RRID: AB_2762824 |
| Goat anti-Mouse IgG (H+L) Highly Cross-Adsorbed Secondary Antibody, Alexa Fluor Plus 647 (1:2000 for IF) | Thermo Fisher Scientific | A32728 RRID: AB_2633277 |
| m6A Polyclonal rabbit purified antibody | Synaptic Systems | 202 003 RRID: AB_2279214 |
| Anti-FLAG® M2 Magnetic Beads | Sigma-Aldrich | M8823 RRID: AB_2637089 |
| Brilliant Violet 711™ anti-mouse CD8a Antibody (clone 53–6.7) | Biolegend | 100747 RRID: AB_11219594 |
| Brilliant Violet 785™ anti-mouse CD3 Antibody (clone 17A2) | Biolegend | 100231 RRID: AB_11218805 |
| Brilliant Violet 650™ anti-mouse CD4 Antibody (clone RM4–5) | Biolegend | 100555 RRID: AB_2562529 |
| Purified Rat Anti-Mouse IFN-γ (clone XMG1.2) | BD Biosciences | 554409 RRID: AB_398550 |
| Brilliant Violet 421™ anti-mouse CD11c Antibody (clone N418) | Biolegend | 117329 RRID: AB_10897814 |
| Brilliant Violet 650™ anti-mouse/human CD11b Antibody (clone M1/70) | Biolegend | 101239 RRID: AB_11125575 |
| Alexa Fluor® 700 anti-mouse I-A/I-E Antibody (clone M5/114.15.2) | Biolegend | 107621 RRID: AB_493726 |
| FITC anti-mouse CD86 Antibody (clone PO3) | Biolegend | 105109 RRID: AB_313162 |
| Brilliant Violet 605™ anti-mouse CD45 Antibody (clone 30-F11) | Biolegend | 103155 RRID: AB_2650656 |
| Goat Anti-Mouse IgG(H+L)-HRP | Southern Biotech | 1036–05 RRID: AB_2794348 |
| Goat Anti-Mouse IgG1-HRP | Southern Biotech | 1071–05 RRID: AB_2794426 |
| Goat Anti-Mouse IgG2c-HRP | Southern Biotech | 1078–05 RRID: AB_2794462 |
| Bacterial and Virus Strains | ||
| NEB® Turbo Competent E. coli (High Efficiency) | New England Biolabs | C2984H |
| Biological Samples | ||
| Chemicals, Peptides, and Recombinant Proteins | ||
| In-Fusion® HD Cloning Kit | Takara Bio | 638909 |
| Fluorescein-12-UTP | Sigma-Aldrich | 11427857910 |
| N6-Methyladenosine-5’-Triphosphate | TriLink Biotechnologies | N-1013 |
| Pseudouridine-5’-Triphosphate | TriLink Biotechnologies | N-1019 |
| Inosine-5’-Triphosphate | TriLink Biotechnologies | N-1020 |
| FastAP | Thermo Fischer Scientific | EF0652 |
| RNaseR | Lucigen | RNR07250 |
| RNA Loading Dye, (2X) | New England Biolabs | B0363S |
| GeneRuler 1 kb DNA Ladder, ready-to-use | Thermo Fischer Scientific | SM0313 |
| RiboRuler Low Range RNA Ladder, ready-to-use | Thermo Fischer Scientific | SM1833 |
| T4 DNA Ligase | New England Biolabs | M0202M |
| DNase I | Ambion | AM2222 |
| T4 PNK | New England Biolabs | M0201S |
| RNA Fragmentation Reagents | Thermo Fischer Scientific | AM8740 |
| Ribolock RNase Inhibitor | Thermo Fischer Scientific | EO0382 |
| TRIzol | Thermo Fischer Scientific | 15596018 |
| Phenol:Chloroform:Isoamyl Alcohol 25:24:1 Saturated with 10 mM Tris, pH 8.0, 1 mM EDTA | Sigma-Aldrich | P2069 |
| cOmplete™ Protease Inhibitor Cocktail | Sigma-Aldrich | 11697498001 |
| Dynabeads™ Protein A for Immunoprecipitation | Thermo Fischer Scientific | 10002D |
| PEG 8000, Molecular Biology Grade (Polyethylene Glycol 8000) | Promega | V3011 |
| DAPI | Thermo Fischer Scientific | D1306 |
| Annexin V Binding Buffer, 10X concentrate | BD Biosciences | 556454 |
| Annexin V, Alexa Fluor™ 647 conjugate | Thermo Fischer Scientific | A23204 |
| Falcon® 5mL Round Bottom Polystyrene Test Tube, with Cell Strainer Snap Cap | Corning | 352235 |
| Falcon® 40 µm Cell Strainer, Blue, Sterile, | Corning | 352340 |
| Polyinosine-polycytidylic acid -TLR3 agonist | InvivoGen | vac-pic |
| EndoFit Ovalbumin | InvivoGen | vac-pova |
| Two OVA peptide standards for ELISPOT | InvivoGen | vac-sin |
| in vivo-jetPEI | Polyplus transfection | 201–10G |
| Corning® Matrigel® Growth Factor Reduced (GFR) Basement Membrane Matrix | Corning | 356231 |
| D-Luciferin Firefly, potassium salt | Biosynth International | L-8220 |
| Histopaque®–1083 | Sigma-Aldrich | 10831 |
| BD GolgiPlug™ Protein Transport Inhibitor (Containing Brefeldin A) | BD Biosciences | 555029 |
| Nunc MaxiSorp™ flat-bottom | Thermo Fischer Scientific | 44–2404-21 |
| Bovine Serum Albumin | Sigma-Aldrich | A9418–5G |
| 1-Step™ Ultra TMB-ELISA Substrate Solution | Thermo Fischer Scientific | 34028 |
| Stop Solution for TMB Substrates | Thermo Fischer Scientific | N600 |
| Antibody Diluent | Thermo Fischer Scientific | 3118 |
| VECTASHIELD® Antifade Mounting Medium with DAPI | Vector Laboratories | H-1200 |
| Square Cover Glasses No. 1 1/2, Corning® | VWR | 89239–698 |
| Lipofectamine 3000 | Thermo Fischer Scientific | L3000008 |
| Opti-MEM™ I Reduced Serum Medium | Thermo Fischer Scientific | 31985088 |
| DMEM, high glucose, pyruvate | Thermo Fischer Scientific | 11995–073 |
| Trypsin-EDTA (0.25%), phenol red | Thermo Fischer Scientific | 25200056 |
| Penicillin-Streptomycin | Thermo Fischer Scientific | 15140–163 |
| HyClone Characterized Fetal Bovine Serum (FBS), U.S. Origin | Thermo Fisher Scientific | SH30071.03 |
| NuPAGE™ 4–12% Bis-Tris Protein Gels, 1.0 mm, 15-well | Thermo Fisher Scientific | NP0323BOX |
| NuPAGE™ MOPS SDS Running Buffer (20X) | Thermo Fisher Scientific | NP0001 |
| 2x Laemmli Sample Buffer | Bio-Rad Laboratories | 161–0737 |
| Phosphate Buffered Saline with Tween® 20 (PBST-20X) | Cell Signaling Technologies | 9809 |
| milliTUBE 1ml AFA Fiber | Covaris | 520130 |
| RIG-I purified protein | Peisley et al., 2013 | N/A |
| K63-Ubn purified protein | Dong et al., 2011 | N/A |
| MAVS CARD-S purified protein | Wu et al., 2016 | N/A |
| BIOMOL® Green | Enzo Life Sciences | BML-AK111–0250 |
| SNAP-Surface® Alexa Fluor® 647 | New England Biolabs | S9136S |
| 35S-IRF3 | Ahmad et al., 2018 | N/A |
| SYBR™ Gold Nucleic Acid Gel Stain (10,000X Concentrate in DMSO) | Thermo Fisher Scientific | S11494 |
| Critical Commercial Assays | ||
| ZymoPURE II Plasmid Prep Kits | Zymo Research | D4200 |
| MEGAscript™ T7 Transcription Kit | Thermo Fisher Scientific | AM1334 |
| Direct-zol RNA Miniprep | Zymo Research | R2051 |
| RNeasy Mini Kit | Qiagen | 74106 |
| RNA Clean & Concentrator-5 | Zymo Research | R1013 |
| Zymoclean Gel Recovery Kit | Zymo Research | R1011 |
| RiboMinus Eukaryote System v2 kit | Thermo Fisher Scientific | A15026 |
| Poly(A)Purist™ MAG Kit | Thermo Fisher Scientific | AM1922 |
| Brilliant II QRT-PCR Master Mix Kit, 1-Step | Agilent | 60080 |
| Neon™ Transfection System 100 µL Kit | Thermo Fisher Scientific | MPK10025 |
| RNA ScreenTape | Agilent | 5067–5576 |
| RNA ScreenTape Sample Buffer | Agilent | 5067–5577 |
| LIVE/DEAD™ Fixable Green Dead Cell Stain Kit, for 488 nm excitation | Thermo Fisher Scientific | L23101 |
| BD Cytofix/Cytoperm Fixation/Permeabilization Solution Kit | BD Biosciences | 554714 |
| Deposited Data | ||
| m6A-irCLIP sequencing data | This paper | GEO: GSE116007 |
| ChIRP-MS data | Chen et al., 2017 | N/A |
| Experimental Models: Cell Lines | ||
| HeLa | ATCC | CCL-2 |
| HEK293T | ATCC | CRL-3216 |
| RIG-I KO HeLa | Chen et al., 2017 | N/A |
| YTHDF2 KO HeLa | Dr. Chuan He | N/A |
| Experimental Models: Organisms/Strains | ||
| C57BL/6J mice | Jackson Laboratories | 664 |
| Oligonucleotides | ||
| qRT-PCR primers | This paper | Table S1 |
| irCLIP sequencing primers | Zarnegar et al., 2016 | N/A |
| SMARTpool ON-TARGETplus METTL3 siRNA | Dharmacon | L-005170–02-0005 |
| ON-TARGETplus Non-targeting Control siRNA #1 | Dharmacon | D-001810–01-05 |
| Recombinant DNA | ||
| Plasmid: autocatalytic-splicing linear GFP-IRES (circGFPd2IRES) | Chen et al., 2017 | N/A |
| Plasmid: circGFPd2IRES_N’5BoxB | This paper | pRC0050 |
| Plasmid: pPB-CAG-Flag-GGS-lambda | Dr. Chuan He | N/A |
| Plasmid: pPB-CAG-Flag-YTHDF1N | Dr. Chuan He | N/A |
| Plasmid: pPB-CAG-Flag-YTHDF1N-lambda | Dr. Chuan He | N/A |
| Plasmid: pPB-CAG-Flag-YTHDF2N | Dr. Chuan He | N/A |
| Plasmid: pPB-CAG-Flag-YTHDF2N-lambda | Dr. Chuan He | N/A |
| Plasmid: pCAGGS-Flag-YTHDF1N | This paper | pRC0070 |
| Plasmid: pCAGGS-Flag-YTHDF1N-GS-lambda | This paper | pRC0071 |
| Plasmid: pCAGGS-Flag-YTHDF2N | This paper | pRC0103 |
| Plasmid: pCAGGS-Flag-YTHDF2N-GS-lambda | This paper | pRC0104 |
| Plasmid: pCAGGS-3xFlag-YTHdomain | This paper | pRC0151 |
| Plasmid: pCAGGS-3xFlag-mRuby3-YTHdomain | This paper | pRC0152 |
| Plasmid: pCAGGS-3xFlag-YTHdomain-GS-lambdaN | This paper | pRC0164 |
| Plasmid: pCAGGS-3xFlag-mRuby3-YTHdomain-GS-lambdaN | This paper | pRC0165 |
| Software and Algorithms | ||
| ZEN (blue edition) | Carl Zeiss Microscopy | zeiss.com/microscopy/us/products/microscope-software/zen.html |
| FlowJo_V10 | FlowJo, LLC | flowjo.com/solutions/flowjo/downloads |
| Other | ||
HIGHLIGHTS.
Unmodified circRNA adjuvant induces antigen-specific T and B cell responses.
m6A RNA modification marks self circRNAs and abrogates circRNA immunity.
Unmodified circRNA and K63-polyubiquitin activate RIG-I and innate immune signaling.
YTHDF2 binding of m6A-circRNA additionally suppresses circRNA immunity.
ACKNOWLEDGEMENTS
We thank members of our labs for discussion, C. He (U Chicago/HHMI) for YTHDF2−/−cells and DNA constructs, M. G. Mohsen and E. Kool (Stanford) for HPLC usage, and the Transgenic, Knockout, and Tumor Model Center of Stanford Cancer Institute at Stanford University for the tumor establishment experiments. FACS data was collected on an instrument in the Shared FACS Facility obtained using NIH S10 Shared Instrument Grant S10RR027431–01. Microscopy was performed at the Cell Sciences Imaging Facility. Supported by NIH 5K12CA215110 and American Cancer Society IRG17–172-57 (to Y.G.C); NIH 5T32GM008412 (to R.C.); Cancer Research Institute Post-Doctoral Fellowship (to S.A.); Parker Institute for Cancer Immunotherapy, NIH 2R37DK057665, 2R37AI048638, and U19AI057266 (to B.P.); NIH R01AI106912 and R01AI111784 (to S.H.); and Parker Institute for Cancer Immunotherapy, NIH R35CA209919, and R01HG004361 (to H.Y.C.). H.Y.C. is an Investigator of the Howard Hughes Medical Institute.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
DECLARATION OF INTERESTS
Stanford University and Emory University have filed patent applications based on this work, on which H.Y.C., Y.G.C., R.C., L.A., B.P. and S.K. are named as co-inventors. H.Y.C. is a co-founder and advisor of Accent Therapeutics and Pretzel Therapeutics. H.Y.C. is an advisor of 10X Genomics, Arsenal Biosciences, and Spring Discovery.
Data Availability
m6A-irCLIP sequencing data is deposited in Gene Expression Omnibus under the accession number GSE116007.
REFERENCES
- Ahmad S, Mu X, Yang F, Greenwald E, Park JW, Jacob E, Zhang C-Z, and Hur S (2018). Breaching Self-Tolerance to Alu Duplex RNA Underlies MDA5-Mediated Inflammation. Cell 172, 797–810.e713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baron-Benhamou J, Gehring NH, Kulozik AE, and Hentze MW (2004). Using the λN Peptide to Tether Proteins to RNAs. In mRNA Processing and Metabolism: Methods and Protocols, Schoenberg DR, ed. (Totowa, NJ: Humana Press; ), pp. 135–153. [DOI] [PubMed] [Google Scholar]
- Batista PJ, Molinie B, Wang J, Qu K, Zhang J, Li L, Bouley DM, Lujan E, Haddad B, Daneshvar K, et al. (2014). m(6)A RNA modification controls cell fate transition in mammalian embryonic stem cells. Cell stem cell 15, 707–719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Budhu S, Loike JD, Pandolfi A, Han S, Catalano G, Constantinescu A, Clynes R, and Silverstein SC (2010). CD8+ T cell concentration determines their efficiency in killing cognate antigen–expressing syngeneic mammalian cells in vitro and in mouse tissues. The Journal of Experimental Medicine 207, 223–235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen YG, Kim MV, Chen X, Batista PJ, Aoyama S, Wilusz JE, Iwasaki A, and Chang HY (2017). Sensing Self and Foreign Circular RNAs by Intron Identity. Molecular cell 67, 228–238.e225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devarkar SC, Wang C, Miller MT, Ramanathan A, Jiang F, Khan AG, Patel SS, and Marcotrigiano J (2016). Structural basis for m7G recognition and 2′-O-methyl discrimination in capped RNAs by the innate immune receptor RIG-I. Proceedings of the National Academy of Sciences 113, 596–601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dominissini D, Moshitch-Moshkovitz S, Schwartz S, Salmon-Divon M, Ungar L, Osenberg S, Cesarkas K, Jacob-Hirsch J, Amariglio N, Kupiec M, et al. (2012). Topology of the human and mouse m6A RNA methylomes revealed by m6A-seq. Nature 485, 201–206. [DOI] [PubMed] [Google Scholar]
- Dong Ken C., Helgason E, Yu C, Phu L, Arnott David P., Bosanac I, Compaan Deanne M., Huang Oscar W., Fedorova Anna V., Kirkpatrick Donald S., et al. (2011). Preparation of Distinct Ubiquitin Chain Reagents of High Purity and Yield. Structure 19, 1053–1063. [DOI] [PubMed] [Google Scholar]
- Durbin AF, Wang C, Marcotrigiano J, and Gehrke L (2016). RNAs Containing Modified Nucleotides Fail To Trigger RIG-I Conformational Changes for Innate Immune Signaling. mBio 7, e00833–00816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edupuganti RR, Geiger S, Lindeboom RGH, Shi H, Hsu PJ, Lu Z, Wang S-Y, Baltissen MPA, Jansen PWTC, Rossa M, et al. (2017). N6-methyladenosine (m6A) recruits and repels proteins to regulate mRNA homeostasis. Nature Structural & Molecular Biology 24, 870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flynn RA, Martin L, Spitale RC, Do BT, Sagan SM, Zarnegar B, Qu K, Khavari PA, Quake SR, Sarnow P, et al. (2015). Dissecting noncoding and pathogen RNA–protein interactomes. RNA (New York, N.Y.) 21, 135–143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansen TB, Jensen TI, Clausen BH, Bramsen JB, Finsen B, Damgaard CK, and Kjems J (2013). Natural RNA circles function as efficient microRNA sponges. Nature 495, 384–388. [DOI] [PubMed] [Google Scholar]
- Hornung V, Ellegast J, Kim S, Brzózka K, Jung A, Kato H, Poeck H, Akira S, Conzelmann K-K, Schlee M, et al. (2006). 5’-Triphosphate RNA Is the Ligand for RIG-I. Science (New York, N.Y.) 314, 994–997. [DOI] [PubMed] [Google Scholar]
- Jiang X, Kinch L, Brautigam CA, Chen X, Du F, Grishin N, and Chen ZJ (2012). Ubiquitin-Induced Oligomerization of the RNA Sensors RIG-I and MDA5 Activates Antiviral Innate Immune Response. Immunity 36, 959–973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ke S, Alemu EA, Mertens C, Gantman EC, Fak JJ, Mele A, Haripal B, Zucker-Scharff I, Moore MJ, Park CY, et al. (2015). A majority of m(6)A residues are in the last exons, allowing the potential for 3′ UTR regulation. Genes & Development 29, 2037–2053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kos A, Dijkema R, Arnberg AC, van der Meide PH, and Schellekens H (1986). The hepatitis delta ([delta]) virus possesses a circular RNA. Nature 323, 558–560. [DOI] [PubMed] [Google Scholar]
- Legnini I, Di Timoteo G, Rossi F, Morlando M, Briganti F, Sthandier O, Fatica A, Santini T, Andronache A, Wade M, et al. (2017). Circ-ZNF609 Is a Circular RNA that Can Be Translated and Functions in Myogenesis. Molecular cell 66, 22–37.e29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X, Liu C-X, Xue W, Zhang Y, Jiang S, Yin Q-F, Wei J, Yao R-W, Yang L, and Chen L-L (2017). Coordinated circRNA Biogenesis and Function with NF90/NF110 in Viral Infection. Molecular cell 67, 214–227.e217. [DOI] [PubMed] [Google Scholar]
- Liu C-X, Li X, Nan F, Jiang S, Gao X, Guo S-K, Xue W, Cui Y, Dong K, Ding H, et al. (2019). Structure and Degradation of Circular RNAs Regulate PKR Activation in Innate Immunity. Cell 177, 865–880.e821. [DOI] [PubMed] [Google Scholar]
- Luo Y, Na Z, and Slavoff SA (2018). P-Bodies: Composition, Properties, and Functions. Biochemistry 57, 2424–2431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Memczak S, Jens M, Elefsinioti A, Torti F, Krueger J, Rybak A, Maier L, Mackowiak SD, Gregersen LH, Munschauer M, et al. (2013). Circular RNAs are a large class of animal RNAs with regulatory potency. Nature 495, 333–338. [DOI] [PubMed] [Google Scholar]
- Meyer Kate D., Patil Deepak P., Zhou J, Zinoviev A, Skabkin Maxim A., Elemento O, Pestova Tatyana V., Qian S-B, and Jaffrey Samie R. (2015). 5’ UTR m6A Promotes Cap-Independent Translation. Cell 163, 999–1010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer KD, Saletore Y, Zumbo P, Elemento O, Mason CE, and Jaffrey SR (2012). Comprehensive Analysis of mRNA Methylation Reveals Enrichment in 3’ UTRs and Near Stop Codons. Cell 149, 1635–1646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohi M, Li Y, Cheng Y, and Walz T (2004). Negative staining and image classification — powerful tools in modern electron microscopy. Biological Procedures Online 6, 23–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peisley A, Wu B, Xu H, Chen ZJ, and Hur S (2014). Structural basis for ubiquitin-mediated antiviral signal activation by RIG-I. Nature 509, 110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peisley A, Wu B, Yao H, Walz T, and Hur S (2013). RIG-I Forms Signaling-Competent Filaments in an ATP-Dependent, Ubiquitin-Independent Manner. Molecular cell 51, 573–583. [DOI] [PubMed] [Google Scholar]
- Reikine S, Nguyen JB, and Modis Y (2014). Pattern Recognition and Signaling Mechanisms of RIG-I and MDA5. Frontiers in Immunology 5, 342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roundtree IA, Evans ME, Pan T, and He C (2017). Dynamic RNA Modifications in Gene Expression Regulation. Cell 169, 1187–1200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rybak-Wolf A, Stottmeister C, Glažar P, Jens M, Pino N, Giusti S, Hanan M, Behm M, Bartok O, Ashwal-Fluss R, et al. (2015). Circular RNAs in the Mammalian Brain Are Highly Abundant, Conserved, and Dynamically Expressed. Molecular cell 58, 870–885. [DOI] [PubMed] [Google Scholar]
- Salzman J, Gawad C, Wang PL, Lacayo N, and Brown PO (2012). Circular RNAs Are the Predominant Transcript Isoform from Hundreds of Human Genes in Diverse Cell Types. PLoS ONE 7, e30733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanger HL, Klotz G, Riesner D, Gross HJ, and Kleinschmidt AK (1976). Viroids are single-stranded covalently closed circular RNA molecules existing as highly base-paired rod-like structures. Proceedings of the National Academy of Sciences of the United States of America 73, 3852–3856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlee M, and Hartmann G (2010). The Chase for the RIG-I Ligand—Recent Advances. Molecular Therapy 18, 1254–1262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlee M, Roth A, Hornung V, Hagmann CA, Wimmenauer V, Barchet W, Coch C, Janke M, Mihailovic A, Wardle G, et al. (2009). Recognition of 5′-triphosphate by RIG-I helicase requires short blunt double-stranded RNA as contained in panhandle of negative strand virus. Immunity 31, 25–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith T, Heger A, and Sudbery I (2017). UMI-tools: modeling sequencing errors in Unique Molecular Identifiers to improve quantification accuracy. Genome Research 27, 491–499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toptan T, Abere B, Nalesnik MA, Swerdlow SH, Ranganathan S, Lee N, Shair KH, Moore PS, and Chang Y (2018). Circular DNA tumor viruses make circular RNAs. Proceedings of the National Academy of Sciences [DOI] [PMC free article] [PubMed]
- Wang X, Lu Z, Gomez A, Hon GC, Yue Y, Han D, Fu Y, Parisien M, Dai Q, Jia G, et al. (2014). m(6)A-dependent regulation of messenger RNA stability. Nature 505, 117–120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, Zhao BS, Roundtree IA, Lu Z, Han D, Ma H, Weng X, Chen K, Shi H, and He C (2015). N(6)-methyladenosine Modulates Messenger RNA Translation Efficiency. Cell 161, 1388–1399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wesselhoeft RA, Kowalski PS, Parker-Hale FC, Huang Y, Bisaria N, and Anderson DG (2019). RNA Circularization Diminishes Immunogenicity and Can Extend Translation Duration In Vivo. Molecular cell [DOI] [PMC free article] [PubMed]
- Wu B, Huoh Y-S, and Hur S (2016). Measuring Monomer-to-Filament Transition of MAVS as an In Vitro Activity Assay for RIG-I-Like Receptors. In Toll-Like Receptors: Practice and Methods, McCoy CE, ed. (New York, NY: Springer New York; ), pp. 131–142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu B, and Hur S (2015). How RIG-I like receptors activate MAVS. Current Opinion in Virology 12, 91–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu B, Peisley A, Richards C, Yao H, Zeng X, Lin C, Chu F, Walz T, and Hur S (2013). Structural Basis for dsRNA Recognition, Filament Formation, and Antiviral Signal Activation by MDA5. Cell 152, 276–289. [DOI] [PubMed] [Google Scholar]
- Xiao W, Adhikari S, Dahal U, Chen Y-S, Hao Y-J, Sun B-F, Sun H-Y, Li A, Ping X-L, Lai W-Y, et al. (2016). Nuclear m6A Reader YTHDC1 Regulates mRNA Splicing. Molecular cell 61, 507–519. [DOI] [PubMed] [Google Scholar]
- Yang Y, Fan X, Mao M, Song X, Wu P, Zhang Y, Jin Y, Yang Y, Chen L, Wang Y, et al. (2017). Extensive translation of circular RNAs driven by N6-methyladenosine. Cell Res [DOI] [PMC free article] [PubMed]
- Zarnegar BJ, Flynn RA, Shen Y, Do BT, Chang HY, and Khavari PA (2016). irCLIP platform for efficient characterization of protein-RNA interactions. Nat Meth 13, 489–492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeng W, Sun L, Jiang X, Chen X, Hou F, Adhikari A, Xu M, and Chen ZJ (2010). Reconstitution of the RIG-I Pathway Reveals a Pivotal Role of Unanchored Polyubiquitin Chains in Innate Immunity. Cell 141, 315–330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou C, Molinie B, Daneshvar K, Pondick JV, Wang J, Van Wittenberghe N, Xing Y, Giallourakis CC, and Mullen AC (2017). Genome-Wide Maps of m6A circRNAs Identify Widespread and Cell-Type-Specific Methylation Patterns that Are Distinct from mRNAs. Cell Reports 20, 2262–2276. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Related to STAR Methods. qRT-PCR primers.