Abstract
Compliant self-adjusting compression implants are a novel approach to increase the durability of megaprosthesis fixation. However, there is no report of current implant designs that documents the bone-prosthetic interface of this implant. A well-fixed compliant, self-adjusting distal femoral replacement was retrieved from a patient undergoing revision unrelated to fixation. The prosthesis-bone interface was preserved, embedded in poly(methyl methacrylate), and sectioned into 2–4-mm slices. Slices were then imaged using backscatter electron microscopy, and ongrowth and ingrowth were quantified using imaging software. The average percentage of bony ongrowth from five successive sections was 52.5%, and the average percentage of ingrowth into the porous titanium surface was 13.5%. We found that bone ongrowth on the cortex between anchor plug and spindle averages more than 50% and up to 70% depending upon the slice examined with backscatter electron microscopy. Bone ingrowth was consistently around 13% on every slice examined. This is a new finding compared with prior spindle designs, likely due to the addition of hydroxyapatite-coated porous metal titanium surface on the spindle. This report is an important step in understanding the mechanism of bony fixation generated by this implant and supports its increased use in oncological and complex reconstructive situations.
Keywords: megaprosthesis, osteointegration, compliant self-adjusting compression fixation, retrieval analysis, backscatter electron microscopy
Introduction
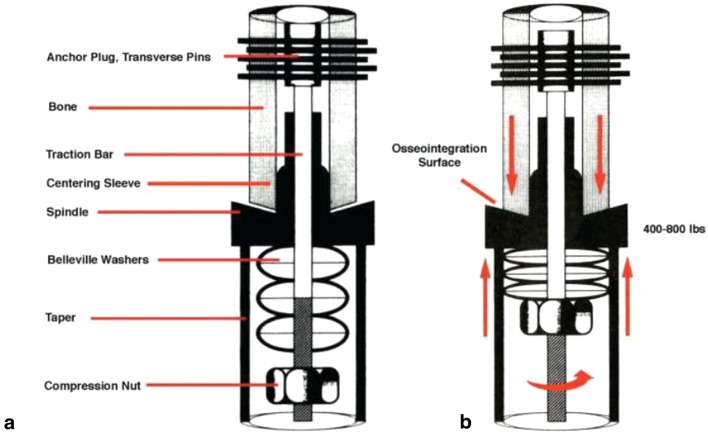
Young patients undergoing distal femoral replacement following tumor resection can have long life expectancies if cured of their disease, necessitating the use of implants with durable fixation. Overall failure rates of megaprostheses have been reported at 58 to 66% at 8 to 10 years, with aseptic loosening responsible for 3 to 19% of these failures [4, 7, 8]. Although modern uncemented stems appear to have better biomechanical properties and survivorship than older technologies, survivorship still hovers around 87% after 7 years [3, 5]. Compliant, self-adjusting compression implants (Compress®) are a novel approach to increasing the durability of megaprosthesis fixation. This technology uses stacked Belleville washers placed under compression to generate 400 to 800 lb of compressive force at the implant-bone interface (Fig. 1). The force is transmitted through a traction bar to an anchor plug, which is secured to the bone with five transverse pins. This compresses the cut bone surface and implant spindle, allowing the bone to share stress and enhance biologic fixation. Recent data demonstrates 85% survivorship at 5 years and 80% survivorship at 10 years of these prostheses for reconstruction after tumor resection around the knee—a significant improvement over previous press-fit and cemented technology [6, 9].
Fig. 1.
a Component diagram of the Compress device. b Diagram of the compressive forces being created at the osteointegration surface by tightening of the compression nut against the stacked Belleville washers. (Reprinted with permission from Avedian et al. [10]).
Although compression theoretically promotes bone growth and osteointegration, there is little evidence to support this in humans. The character of the bone hypertrophy across the junctional area in compliant, self-adjusting compression implants has been likened to an “elephant foot” deformity associated with fracture non-union, calling into question the presence and amount of osteointegration that actually occurs. Only one report of one patient with an earlier implant design documents a modest degree of osteointegration at the bone-prosthesis interface of this implant [2].
The purpose of this report is to quantify the extent, quality, and character of osteointegration at the bone-implant interface using backscatter electron microscopy (BSEM) in a well-fixed retrieved implant.
Case Report
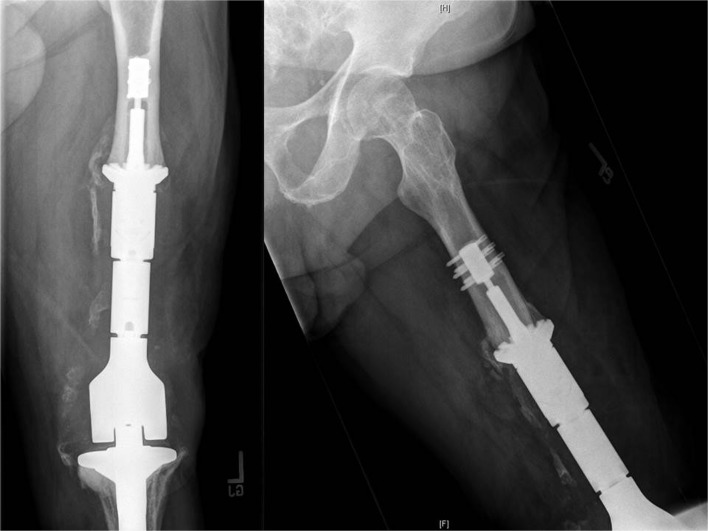
The implant specimen was retrieved from a 66-year-old man who had undergone implantation of the Compress implant with a porous titanium, hydroxyapatite-coated spindle under 600 lb of interface compression. He had undergone three prior complex revisions of a total condylar total knee replacement. He had good function and superior pain relief with the Compress implant than with his prior revisions but developed a recurrence of a chronic periprosthetic joint infection, despite multiple courses of intravenous antibiotics. The Compress implant had been in place for 6 years and appeared radiologically well fixed (Fig. 2) and was found to be well fixed during surgery.
Fig. 2.
Orthogonal radiographs of the implant-bone interface prior to removal.
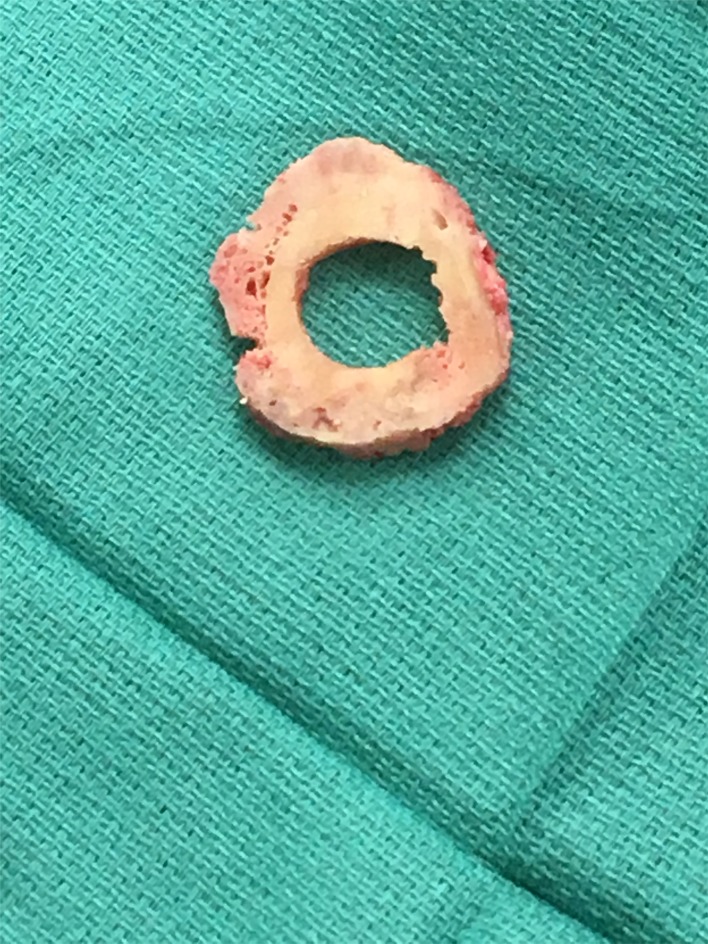
Removal of the prosthesis was carried out in a similar fashion to that described previously [1]. The modular implant was disengaged from the taper, and the compression nut was removed. The transfixation pins were also removed. A burr was then used to transect the femur just above the spindle surface. Finally, trephines were used to facilitate extraction of the anchor plug. A 2-cm ring of hypertrophic bone (Fig. 3) adjacent to the implant interface was removed to prepare the new femoral surface for prosthesis reimplantation. This preserved the prosthetic interface and adjacent bone. With institutional review board approval, the specimen was cleaned and catalogued in the biomechanics lab. The implant was then dehydrated, embedded in polymethyl methacrylate (PMMA) and sectioned in a parasagittal orientation into five slices, each 2 to 4 mm thick, using a diamond saw. Slices were then imaged using BSEM and analyzed using ImageJ software.
Fig. 3.
Proximal bone ring of the femoral diaphysis adjacent to the spindle.
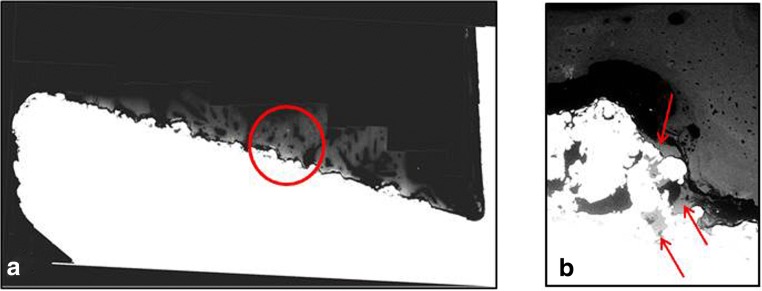
We sought to quantify the percentage of the implant spindle surface covered with bony ongrowth, as well as the percentage of the implant surface with bony ingrowth on each successive slice. Any bone seen above the implant surface was considered ongrowth, and bone deep to the implant surface was considered ingrowth (Fig. 4). We exported values for bone ongrowth distance (BD), metal surface distance (md), and length of implant surface and solved for bone ongrowth percentage using those values ([BD/md] × 100) taking into account the curvature of the implant surface. Bone ingrowth percentage was calculated in the same manner.
Fig. 4.
Cross-sectional backscatter electron microscopy images of bone-implant interface. A representative slice (a) shows the implant in white and the bone in light gray. An example of bone ongrowth is shown by the red circle. Bone ingrowth assessed at × 111 magnification is highlighted by the red arrows (b).
Results
The adjacent ring of bone demonstrated extra-cortical bone formation and hypertrophy consistent with what was seen on pre-operative radiographs. On BSEM, the percentage of bony ongrowth was 52.5% (SD, 16.4; range, 34.3–75.1), averaged from the five successive slices. The percentage of ingrowth into the porous titanium surface was 13.5% (SD, 4.3; range, 6.8–19.9).
Discussion
Endoprosthetic construction with Compress compliant, self-adjusting compression fixation is a durable option for limb salvage in the femur, harnessing Wolff’s law through consistent compression of 400 to 800 lb, based on the cortical thickness. This case report shows that there was substantial bony ongrowth and ingrowth at the bone-implant interface and bone hypertrophies over the intervening cortex from the anchor plug to the spindle, as distinct from the stress bypass bone loss that occurs around conventional stems. Heretofore, only one report in the literature has documented the bone-implant interface of this type of implants, and none has examined the available designs that have a spindle with porous titanium alloy and a hydroxyapatite coating.
This study has a number of limitations. First, this is a single retrieved implant, one that is relatively uncommon compared with most orthopedic implants, and a retrieved well-fixed Compress stem is even rarer. While greater numbers would help corroborate the data we describe here, our case is still important; waiting for further retrievals would likely just delay dissemination of this information. Second, the implant was retrieved in the setting of infection. However, the implant was stable for 6 years prior to removal, and we believe this case to be representative of the fixation these implants achieve. The implant displayed the characteristic radiographic appearance of bony hypertrophy at the spindle prior to removal, and the stem was well fixed at the time of surgical extraction. Although infection may alter the local milieu at the bone-implant interface, we do not believe it greatly affected the nature or extent of fixation in this case.
Despite these drawbacks, we believe this case constitutes an important addition to the literature on the advantages of compliant, self-adjusting compression fixation for megaprostheses.
We found that bone ongrowth on the cortex between anchor plug and spindle averages more than 50% and up to 70%, depending upon the slice examined with BSEM. It should be noted that the spindle must be greater than the dimensions of the adjacent bone, so the ongrowth percentage must be less than 100% by definition. Quantitatively, this amounts to a lower percentage of the overall spindle surface with bony ongrowth, but it provides a larger surface area for potential fixation. Therefore, it should not be interpreted as incomplete ongrowth over the spindle surface but rather a technique to increase overall fixation.
Critical bone ingrowth, defined as bone below or into the metal surface, was consistently about 13% of the bone-implant interface surface, on every slice examined. This is a new finding and is likely an important verification of the effect of the addition of a hydroxyapatite-coated porous titanium surface on the spindle. This additional degree of fixation contributes to implant stability and longevity and is likely why the Compress spindle was well fixed and difficult to remove, even in the setting of chronic infection. This report confirms the bony ongrowth achieved by compliant, self-adjusting compression fixation and demonstrates a new finding of additional bony ingrowth, perhaps related to the addition of hydroxyapatite coating on the porous titanium spindle. This finding is an important step in understanding the mechanism of bony fixation generated by compliant, self-adjusting compression implants and supports its increased use in oncological and complex reconstructive situations.
Compliance with Ethical Standards
Conflict of Interest
Alexander B. Christ, MD, and Elexis Baral, BS, declare that they have no conflicts of interest. Timothy M. Wright, PhD, reports research support from Stryker, stock options from Orthobond, intellectual property royalties from Mathys Ltd. and from Exactech, and intellectual property royalties and research support from Lima Corporate, as well as board membership in the Knee Society, outside the submitted work. John H. Healey, MD, reports being a paid consultant to Daiichi Sanyo and Stryker, outside the submitted work.
Human/Animal Rights
All procedures followed were in accordance with the ethical standards of the responsible committee on human experimentation (institutional and national) and with the Helsinki Declaration of 1975, as revised in 2013.
Informed Consent
Informed consent was waived from the patient for being included in this study.
Required Author Forms:
Disclosure forms provided by the authors are available with the online version of this article.
Footnotes
Work was performed at the Hospital for Special Surgery, New York, NY, and Memorial Sloan Kettering Cancer Center, New York, NY.
References
- 1.Abrams GD, Gajendran VK, Mohler DG, Avedian RS. Surgical technique: methods for removing a Compress compliant prestress implant. Clin Orthop Related Res. 2012;470(4):1204–1212. doi: 10.1007/s11999-011-2128-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bini SA, Johnston JO, Martin DL. Compliant prestress fixation in tumor prostheses: interface retrieval data. Orthopedics. 2000;23(7):702–707. doi: 10.3928/0147-7447-20000701-18. [DOI] [PubMed] [Google Scholar]
- 3.Bruns J, Delling G, Gruber H, Lohmann CH, Habermann CR. Cementless fixation of megaprostheses using a conical fluted stem in the treatment of bone tumours. J Bone Joint Surg Br. 2007;89(8):1084–1087. doi: 10.1302/0301-620X.89B8.19236. [DOI] [PubMed] [Google Scholar]
- 4.Capanna R, Scoccianti G, Frenos F, Vilardi A, Beltrami G, Campanacci DA. What was the survival of megaprostheses in lower limb reconstructions after tumor resections? Clin Orthop Related Res. 2014;473(3):820–830. doi: 10.1007/s11999-014-3736-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ferguson PC, Zdero R, Schemitsch EH, Deheshi BM, Bell RS, Wunder JS. A biomechanical evaluation of press-fit stem constructs for tumor endoprosthetic reconstruction of the distal femur. J Arthroplasty. 2011;26(8):1373–1379. doi: 10.1016/j.arth.2010.12.005. [DOI] [PubMed] [Google Scholar]
- 6.Healey JH, Morris CD, Athanasian EA, Boland PJ. Compress knee arthroplasty has 80% 10-year survivorship and novel forms of bone failure. Clin Orthop Related Res. 2013;471(3):774–783. doi: 10.1007/s11999-012-2635-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pala E, Trovarelli G, Calabrò T, Angelini A, Abati CN, Ruggieri P. Survival of modern knee tumor megaprostheses: failures, functional results, and a comparative statistical analysis. Clin Orthop Related Res. 2015;473(3):891–899. doi: 10.1007/s11999-014-3699-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Palumbo BT, Henderson ER, Groundland JS, et al. Advances in segmental endoprosthetic reconstruction for extremity tumors: a review of contemporary designs and techniques. Cancer Control. 2011;18(3):160–170. doi: 10.1177/107327481101800303. [DOI] [PubMed] [Google Scholar]
- 9.Zimel MN, Farfalli GL, Zindman AM, et al. Revision distal femoral arthroplasty with the Compress prosthesis has a low rate of mechanical failure at 10 years. Clin Orthop Related Res. 2016;474(2):528–536. doi: 10.1007/s11999-015-4552-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Avedian RS, Goldsby RE, Kramer MJ, O’Donnell RJ. Effect of chemotherapy on initial compressive osseointegration of tumor endoprostheses. Clin Orthop Relat Res. 2007;459:48–53. doi: 10.1097/BLO.0b013e3180514c66. [DOI] [PubMed] [Google Scholar]