Significance
Cells maintain several mechanisms to ensure their survival, including the removal of old or damaged proteins and organelles. This process must be balanced: too little turnover results in the accumulation of cellular “junk,” while excessive removal can deplete the cell and organism of key components. Here, we show that Atp6ap2 in the pancreatic β cell is essential for insulin granule turnover, as its absence resulted in the accumulation of oversized cytosolic vacuoles which conceivably account for excessive granule degradation, and thereby leads to impaired insulin secretion and diabetes.
Keywords: autophagy, (pro)renin receptor, diabetes, vacuolar H+ ATPase
Abstract
Pancreatic β cells store insulin within secretory granules which undergo exocytosis upon elevation of blood glucose levels. Crinophagy and autophagy are instead responsible to deliver damaged or old granules to acidic lysosomes for intracellular degradation. However, excessive consumption of insulin granules can impair β cell function and cause diabetes. Atp6ap2 is an essential accessory component of the vacuolar ATPase required for lysosomal degradative functions and autophagy. Here, we show that Cre recombinase-mediated conditional deletion of Atp6ap2 in mouse β cells causes a dramatic accumulation of large, multigranular vacuoles in the cytoplasm, with reduction of insulin content and compromised glucose homeostasis. Loss of insulin stores and gigantic vacuoles were also observed in cultured insulinoma INS-1 cells upon CRISPR/Cas9-mediated removal of Atp6ap2. Remarkably, these phenotypic alterations could not be attributed to a deficiency in autophagy or acidification of lysosomes. Together, these data indicate that Atp6ap2 is critical for regulating the stored insulin pool and that a balanced regulation of granule turnover is key to maintaining β cell function and diabetes prevention.
Pancreatic β cells are exclusive in their role for producing insulin, and in response to increased extracellular glucose concentrations they rapidly synthesize, package, and export insulin into the systemic circulation. Proper control of each step is critical to maintain continuous insulin secretion throughout the day and is critical to preventing the onset of diabetes—a disease which affects approximately one-third of Western populations (1). Insulin is stored within secretory granules (SGs), which are small (∼250 nm), membrane-enclosed structures containing crystallized insulin (2). Maintaining the integrity of SGs and the removal of aged or defective SGs are also required for preserving β cell homeostasis (3).
Autophagy is one such mechanism involved in the intracellular turnover of SGs (4). This process entails the sequestration of cytoplasmic organelles, proteins, and other cellular constituents into double-membraned autophagosomes with a neutral luminal pH. Autophagosomes then fuse with lysosomes for degradation of their content within the acidic milieu of the autophagolysosome (5). While in general, autophagy is considered protective due to its ability to remove components which could perturb homeostasis, over- and uncontrolled degradation of cellular contents can also cause significant damage. Several pieces of evidence point to the relevance of balanced autophagy for β cell homeostasis. On one hand, absence of β cell autophagy in mice lacking autophagy related 7 (Atg7), a protein necessary for the formation of autophagosomes, results in endoplasmic reticulum stress, impaired insulin secretion, and hyperglycemia (6, 7). On the other hand, the occurrence of increased autophagy in human (8) and rodent (7, 9) islets is also associated with the development of diabetes. Likewise, hyperactivation of autophagy following treatment with rapamycin also impairs β cell function and viability, resulting in decreased insulin secretion and diabetes (10, 11).
As lysosomal acidification is required for the degradation of the autophagolysosomal content, understanding the biology of this process is of utmost importance. The vacuolar H+ (V)-ATPase is a large transmembrane protein complex responsible for establishing and retaining the pH gradient along the secretory, endocytic, and lysosomal pathways in all cell types. Complete loss of V-ATPase function is hence embryonic lethal (reviewed in ref. 12). The V-ATPase complex consists of 2 functional domains: V1, consisting of 8 subunits and responsible for ATP hydrolysis; and V0, with 6 to 7 membrane-embedded subunits and containing the proton-permeable pore. In yeast, V-ATPase activity is regulated by the reversible assembly and disassembly of these domains (12). In mice, loss of the V-ATPase a3 subunit causes diabetes due to impaired fusion of SGs with the plasma membrane and thus insulin release (13). Compared to yeast, higher-order eukaryotes have additional V-ATPase accessory proteins such as the ATPase H+ Transporting Accessory Protein 1 (Atp6ap1) and 2 (Atp6ap2). Conditional depletion of Atp6ap1 (also known as Ac45) impairs the processing of proinsulin to insulin in mouse insulinoma cells (14). Atp6ap2 was initially misnamed as the (pro)renin receptor (PRR), as it was thought to bind renin and contribute to the pathology of hypertension (15, 16). Atp6ap2 has since been shown to be essential for cellular homeostasis in mice, as its knockout is embryonic lethal (17), while conditional deletion impairs the function of a variety of cell types (18–24). Its role in Drosophila and Xenopus development is also established (25–27). Atp6ap2 colocalizes with V-ATPase subunits (28, 29), and overexpression studies showed its coprecipitation with V0 subunits (27) in association with V-ATPase dysfunction (30). Atp6ap2 has been proposed to chaperone V-ATPase assembly as its deletion causes reduced levels of V-ATPase subunits (18, 19). Other studies, however, could not show its effect on V-ATPase function (28). As deletion of Atp6ap2 resulted in the accumulation of autophagy markers (18, 20, 24), a link with autophagy has also been suggested (31). Taken together, the precise function of Atp6ap2 remains unclear due to the severe impact that its deletion has on cellular homeostasis, with gross developmental abnormalities and cell death hindering a deeper understanding of its activity. Since Atp6ap2 is highly expressed in pancreatic β cells (32), and V-ATPase activity is necessary for the acidification of the SG lumen and insulin processing, we hypothesized that its deletion in these cells could shed light on its function.
Results
Atp6ap2β-/y Islets Are Consumed by Large Vacuolated Structures.
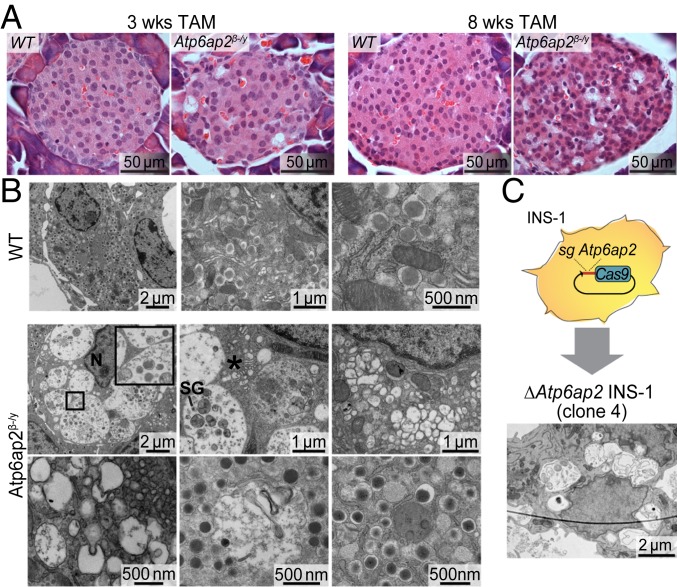
To study the specific role of Atp6ap2 in pancreatic β cells, female Atp6ap2fl/+ mice were mated with male mice expressing a Cre recombinase–estrogen receptor (ER) fusion protein under control of the rat insulin promoter (RIP-CreER) mice. Adult (8 wk) RIP-CreER;Atp6ap2fl/y male offspring were treated with tamoxifen (TAM) for 3 and 8 wk (SI Appendix, Fig. S1). Islets isolated after 3 wk TAM treatment from RIP-CreER;Atp6ap2fl/y mice (henceforth referred to as Atp6ap2β-/y mice) showed significantly decreased Atp6ap2 mRNA and protein levels, confirming the knockout of Atp6ap2 in β cells (SI Appendix, Fig. S1). The effect of Atp6ap2 ablation was unmistakable upon histological analysis of pancreases, with heavily vacuolated cells evident within islets of Atp6ap2β-/y mice after 3 wk and, to a much greater extent, 8 wk of TAM treatment (Fig. 1A). To analyze these phenotypic alterations in more detail, we performed transmission electron microscopy (TEM) of islets after 3 wk of TAM treatment. WT mice showed normal morphology, with most of the β cell cytoplasm being occupied by insulin SGs (Fig. 1B). By contrast, the cytoplasm of Atp6ap2-deficient β cells was filled with large vacuolated structures, with few to no insulin SGs (Fig. 1B). Vacuolated structures mostly had a single outer membrane (Fig. 1 B, Inset) and contained vesicles and remnants of degraded material, including insulin SGs (SI Appendix, Figs. S2 and S3). In addition, several very large vesicles were observed, some of which occupied an entire β cell (SI Appendix, Fig. S2). Other abnormalities included dilated cisternae of the Golgi complex and large regions with small electrolucent vesicles, possibly corresponding to SGs which have lost their “dense core” (Fig. 1B). To exclude the possibility that such alterations resulted from off-target recombination of CreER, we used CRISPR/Cas9 technology to generate Atp6ap2-deficient rat insulinoma INS-1 cells. Screening for Atp6ap2 expression by Western blotting allowed the identification of 2 Atp6ap2-deficient INS-1 clones (#4 and #7; SI Appendix, Fig. S4). TEM analysis of ΔAtp6ap2 INS-1 cells showed striking similarities to β cells of Atp6ap2β-/y mice, with their cytoplasm being almost entirely occupied by large, single-membraned vacuolated structures containing vesicles and debris, and their number of SGs being strongly reduced (Fig. 1C).
Fig. 1.
Atp6ap2 β cell deletion induces the accumulation of large vacuolated structures. (A) Morphological analysis by hematoxylin and eosin staining in pancreases from WT and Atp6ap2β-/y mice after TAM treatment for 3 and 8 wk. (B) Representative transmission electron microscopy images of β cells from WT and Atp6ap2β-/y mice after 3 wk of TAM. Inset shows a representative single-membraned vacuole in Atp6ap2β-/y islets. N, nucleus; SG, insulin secretory granule; *, dilated Golgi cisternae. (C) Rat insulinoma INS-1 cells were transfected with plasmid expressing Cas9-GFP and sgRNA targeting Atp6ap2. Successful clones deficient for Atp6ap2 (ΔAtp6ap2 INS-1 cells) were analyzed by transmission electron microscopy. Scale bars are indicated.
Atp6ap2β-/y Mice Have Severe Diabetes.
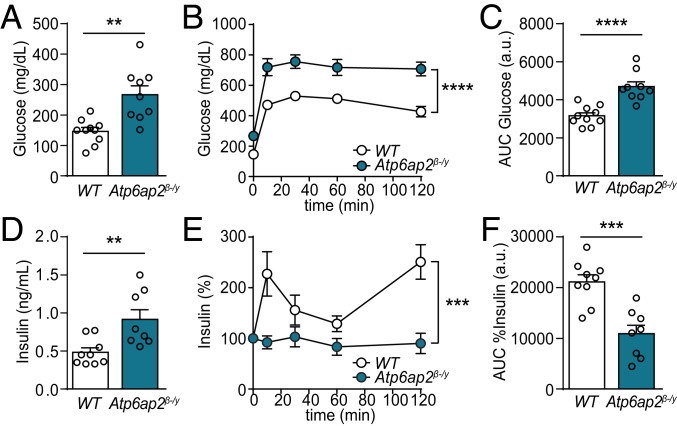
Next, we evaluated the effect of Atp6ap2 deletion in β cells on glucose homeostasis. Treatment of Atp6ap2β-/y mice with TAM for 8 wk did not alter their body weight compared to TAM-treated WT mice (SI Appendix, Fig. S1), while their fasting plasma glucose was significantly elevated (Fig. 2A). This alteration was accompanied by glucose intolerance during i.p. glucose tolerance tests (IPGTT) (Fig. 2 B and C). Specifically, in Atp6ap2β-/y mice, fasting plasma insulin levels were increased compared to controls (Fig. 2D), while glucose stimulation during IPGTT did not raise plasma insulin (Fig. 2 E and F), despite an increase of plasma glucose levels >700 mg/dL (Fig. 2B). These traits indicate the loss of glucose-stimulated insulin secretion (GSIS) in Atp6ap2β-/y mice and that knockout of Atp6ap2 in β cells causes severe diabetes.
Fig. 2.
Deletion of Atp6ap2 from β cells induces severe diabetes. (A) Fasting blood glucose of WT and Atp6ap2β-/y mice after 8 wk of TAM treatment. (B) Blood glucose levels during IPGTT on adult WT and Atp6ap2β-/y mice treated with TAM for 8 wk. (C) Area-under-the-curve (AUC) analysis of B. (D) Fasting insulin levels of WT and Atp6ap2β-/y mice after 8 wk of TAM treatment. (E) Insulin levels during IPGTT as in B. (F) AUC analysis of E. n = 9 to 10. **P < 0.01, ***P < 0.001, ****P < 0.0001.
Decreased Insulin Content in Atp6ap2 Deficient β-Cells.
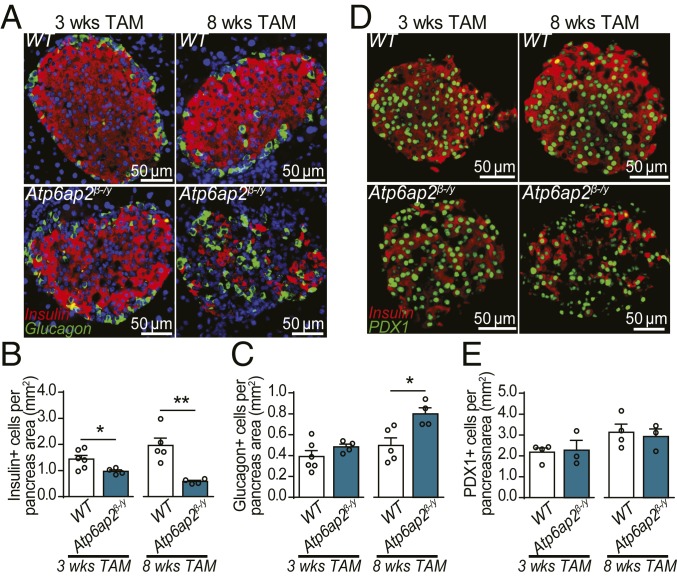
Atp6ap2-deficient β cells could lack GSIS due to decreased insulin content and/or an impaired ability to secrete insulin. Immunofluorescence of pancreas sections after 3 wk of TAM treatment revealed a reduction in the number of insulin+ cells in Atp6ap2β-/y mice, which was further reduced after 8 wk (Fig. 3 A and B). The decrease of insulin+ cells after 8 wk of TAM treatment was coupled with an increase in the number of glucagon+ cells (Fig. 3C). However, this increase did not originate from enhanced proliferation of α-cells, nor transdifferentiation of β cells as reporter Atp6ap2β-/y mice which also expressed YFP in β cells did not colocalize with glucagon (SI Appendix, Fig. S5). We next addressed whether there was β cell death but did not see any increase in TUNEL signal in WT or Atp6ap2β-/y islets (SI Appendix, Fig. S6). We then performed immunofluorescence for pancreatic and duodenal homeobox 1 (Pdx1), a β cell-specific transcription factor important for the maintenance of β cell identity, thus, an alternative, nucleus-specific marker for pancreatic β cells (Fig. 3D). No difference in Pdx1 staining was observed between pancreases of TAM-treated WT and Atp6ap2β-/y mice (Fig. 3E), despite the concurrent loss of insulin signal (Fig. 3D). This data suggests that the loss of insulin+ cells in TAM-treated Atp6ap2β-/y mice is not due to increased β cell death at this time point, but to depletion of the insulin stores.
Fig. 3.
Atp6ap2-deficient β cells have reduced insulin content. (A) Islet immunofluorescence for insulin (red) and glucagon (green) of mice after 3 and 8 wk of TAM treatment. Nuclear stain (DAPI) is shown in blue. (B and C) Quantification of A. n = 4 to 6. (D) Islet immunofluorescence for pancreatic and duodenal homeobox 1 (Pdx1; green) and insulin (red) from mice treated with TAM for 3 and 8 wk. (E) Quantification of D. n = 4 to 6. *P < 0.05, **P < 0.01. Scale bars are indicated.
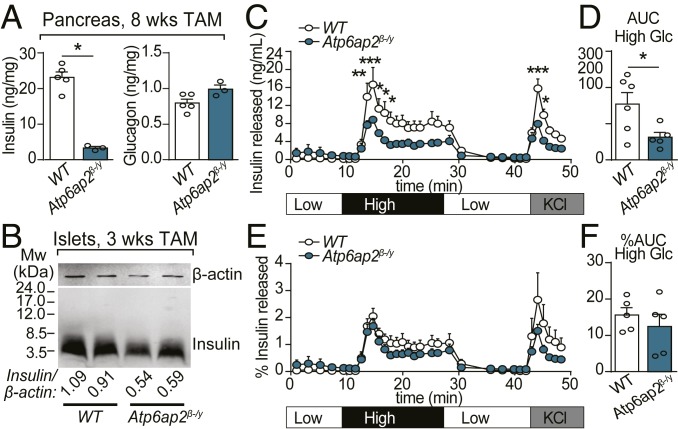
We then measured total insulin and glucagon content in whole pancreas extracts by radioimmunoassay (RIA). After TAM treatment for 8 wk the insulin content of Atp6ap2β-/y islets was significantly reduced, while glucagon content was unchanged (Fig. 4A). This finding was confirmed by Western blot of islets isolated from Atp6ap2β-/y mice after 3 wk of TAM treatment (Fig. 4B). Previous reports suggested that Atp6ap2 is associated with the V-ATPase complex (27, 29, 33). In β cells, this complex is enriched in SGs where it establishes the acidic pH necessary for proinsulin to insulin conversion (34). However, in islets of TAM-treated Atp6ap2β-/y mice, proinsulin processing was not affected, as the reduction of mature insulin (∼3.5 kDa) did not correlate with increased levels of proinsulin (∼7.6 kDa) (Fig. 4B).
Fig. 4.
Insulin processing and secretion is unaltered in Atp6ap2β-/y mice. (A) Insulin (Left) and glucagon (Right) content in whole pancreatic extracts from mice treated with TAM for 8 wk. n = 3 to 5. (B) Insulin processing was analyzed by Western blot in isolated islets from WT and Atp6ap2β-/y mice treated with TAM for 3 wk. Molecular weight markers are indicated. The ratio of insulin to loading control (β-actin) is shown normalized to WT. (C–F) Isolated islets from WT and Atp6ap2β-/y mice treated with TAM for 3 wk were perfused with low (3.3 mM), high (16.7 mM), and again low glucose, and then 60 mM KCl. (C) Amount of insulin secreted was measured by RIA. (D) AUC analysis of high glucose peak from C. (E) The percentage of insulin released (amount of insulin in C divided by total insulin content). (F) AUC analysis of high glucose peak from E. Three independent experiments with n = 2 of each genotype were pooled (n = 6 total). *P < 0.05, **P < 0.01, ***P < 0.001.
We next examined the role of Atp6ap2 in insulin SG exocytosis. Islets were isolated from TAM-treated WT and Atp6ap2β-/y mice and perfused ex vivo with low glucose (3.3 mM), high glucose (16.7 mM), and low glucose again (3.3 mM), followed by 60 mM KCl, which causes massive membrane depolarization and glucose-independent SG exocytosis. Insulin secretion was measured by RIA. In response to high glucose and KCl, Atp6ap2β-/y islets exhibited diminished insulin secretion compared to WT (Fig. 4 C and D). However, upon normalization for total insulin, the percentage of insulin released by islets from TAM-treated WT or Atp6ap2β-/y mice was comparable (Fig. 4 E and F). These findings indicate that Atp6ap2 deficiency reduces the insulin content of β cells, without affecting their machinery for SG exocytosis.
Vacuolated Bodies with Intact Acidic pH Consume Cellular Content, Including Insulin SGs.
Insulin content in Atp6ap2-deficient β cells could be decreased due to reduced insulin production or increased intracellular degradation of insulin. Since insulin mRNA levels in islets of WT and Atp6ap2β-/y mice were equivalent (SI Appendix, Fig. S1) and considering the extensive vacuolation of Atp6ap2-deficient β cells (Fig. 1), we turned our attention to mechanisms of degradation.
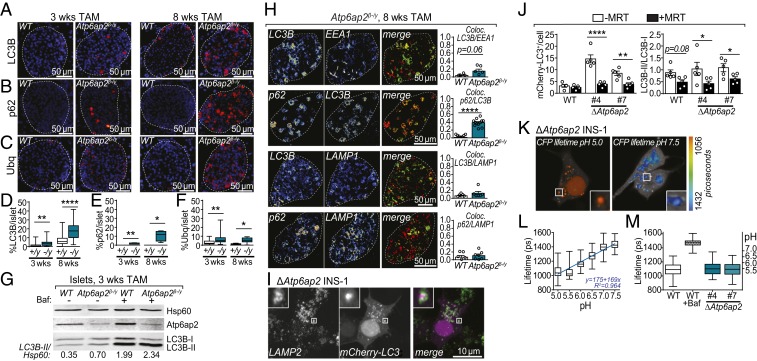
Autophagy is a key homeostatic mechanism by which cellular contents are sequestered by a membrane into large vacuoles which then fuse with lysosomes for degradation (5). This process therefore enables the removal of damaged proteins and organelles which could perturb cellular function. In conditions of nutrient deprivation, this degradative pathway is also used to supply amino acids to preserve essential housekeeping cellular activities. A link between Atp6ap2 and autophagy has been suggested because of the elevated expression of autophagy markers in Atp6ap2-deficient cells (18, 20, 24), including microtubule-associated protein 1A/1B light chain 3B (Map1Lc3b [LC3B]), sequestosome 1 (Sqstm1 or p62) and ubiquitin (Ubq). Lipidated LC3B binds to double-membraned autophagosomes, while p62 and Ubq bind to protein aggregates destined for encapsulation by autophagosomes. Immunostaining of pancreatic sections for autophagy markers revealed that in islets from 3 and 8 wk TAM-treated Atp6ap2β-/y mice areas positive for LC3B, p62, and Ubq were increased relative to islets from WT mice, suggesting an up-regulation of autophagy (Fig. 5 A–F). Following their formation, autophagosomes fuse with lysosomes, leading to the formation of autophagolysosomes in which engulfed proteins, organelles and portions of the cytosol are degraded (35). As the accumulation of autophagy markers can indicate an impairment in autophagolysosomal degradation, we next examined whether autophagic flux was impaired. Islets from Atp6ap2β-/y mice TAM treated for 3 wk were exposed ex vivo to the V-ATPase inhibitor bafilomycin A1 (BafA1). We determined the abundance of the free cytosolic form of LC3B (LC3B-I) and its lipidated form (LC3B-II), which associates with the autophagosome membrane. Autophagic flux is analyzed by comparing the levels of LC3B-II in cells treated with and without BafA1, as inhibition of the V-ATPase and thus lysosomal acidification leads to accumulation of LC3B-II. Evidence of increased LC3B-II levels prior to BafA1 treatment is indicative of a compromised autophagic flux. LC3B-II levels were slightly increased in islets of TAM-treated Atp6ap2β-/y mice (Fig. 5G). However, upon BafA1 treatment the accumulation of LC3B-II in islets of WT and Atp6ap2β-/y mice was comparable (Fig. 5G), suggesting that V-ATPase activity and lysosomal acidification is intact.
Fig. 5.
Atp6ap2 deletion induces the formation of large vacuoles without impairment of autophagy or lysosomal acidification. (A–C) Immunostaining of pancreatic sections from WT and Atp6ap2β-/y mice for LC3B (A), p62 (B), and ubiquitin (C) after 3 and 8 wk of TAM treatment. All proteins are depicted in red with a nuclear stain (DAPI) shown in blue. White dashed line indicates total islet area. (D–F) Quantification of A–C of the % area of positive staining within total islet area. WT are “+/y”; Atp6ap2β-/y as “−/y”. (G) Islets isolated from WT and Atp6ap2β-/y mice treated with TAM for 3 wk were cultured ±100 nM Bafilomycin A1 (Baf) for 120 min and then analyzed by Western blotting for expression of LC3B-I, LC3B-II, and Atp6ap2. The ratio of LC3B-II to loading control (Hsp60) is shown. Result is representative of 2 independent experiments. (H) Colocalization analysis of LC3B, EEA1, p62, and LAMP1 by immunofluorescence confocal microscopy of WT and Atp6ap2β-/y islets treated with TAM for 8 wk. Representative images of Atp6ap2β-/y islets are shown with quantitation of colocalization (Pearson’s). (I) Colocalization analysis of LC3B and LAMP2 by confocal microscopy of ΔAtp6ap2 INS-1 cells transfected with mCherry-LC3B. (J) WT and ΔAtp6ap2 INS-1 cells were transfected with mCherry-LC3B and incubated ±1 μM MRT 68921 for 12 h. mCherry-LC3B+ structures were counted (Left) and the ratio of LC3B-II/LC3B-I levels determined by Western blotting (Right). (K and L) LC3B+-vacuolar pH measurements in ΔAtp6ap2 INS-1 cells by FLIM with calibration to nigericin-buffered solutions from pH 5.0 to 7.5. (M) Vacuole pH measurements of WT and 2 independent ΔAtp6ap2 INS-1 clones were identical while addition of Baf increased vacuolar pH. For all panels, *P < 0.05; **P < 0.01; ****P < 0.0001.
We next employed confocal microscopy to more carefully analyze the identity and origin of the large vacuolated structures. We compared the colocalization of autophagosomal (LC3, p62), endosomal (early-endosome antigen 1, EEA1) and lysosomal (lysosomal-associated membrane protein, LAMP1) markers in islets of 8-wk TAM-treated Atp6ap2β-/y mice (Fig. 5H). LC3B exhibited partial but increased colocalization with the endosomal marker EEA1 and autophagosome marker p62. However, the colocalization of p62 and LC3B to LAMP1 was meager and unchanged compared to WT controls. Together, this analysis suggests that the abnormal vacuolated structures in Atp6ap2β-/y islets are likely to originate upstream of the induction of autophagy.
We next turned to ΔAtp6ap2 INS-1 cells to investigate the nature of the LC3+ structures and lysosomal acidification in more detail. Given the limited colocalization between LC3B and LAMP1 in Atp6ap2β-/y islets, we first asked whether fusion between autophagosomes and lysosomes to form an autolysosome is impaired. ΔAtp6ap2 INS-1 cells were transfected to express the fluorescent reporter mCherry-LC3B which localizes to the autophagosome lumen (Fig. 5I). This reporter colocalized extensively with an anti-LAMP2 antibody which recognizes a cytoplasmic epitope of the lysosomal marker, therefore accounting for the detection of a ring surrounding the luminal mCherry-LC3B (Fig. 5 I, Inset). These data imply that Atp6ap2 deficiency does not preclude the formation of autolysosomes (Fig. 5I). We then asked whether inhibiting the induction of autophagy would prevent the formation of these structures. WT and ΔAtp6ap2 INS-1 cells were treated with the ULK1 protein kinase complex inhibitor MRT 68921 (MRT), which prevents the lipidation of LC3B-II and hence the induction of autophagy. ΔAtp6ap2 INS-1 cells exhibited increased LC3+ structures which were rescued by treatment with MRT and associated with a decreased LC3B-II/LC3B-I ratio (Fig. 5J). We finally sought to measure the pH of LC3B+ structures by fluorescence lifetime imaging microscopy (FLIM) utilizing the pH sensitivity of CFP (36, 37). To this aim we generated a CFP-LC3B fusion protein and measured the CFP lifetime in ΔAtp6ap2 INS-1 cells cultured in nigericin-buffered solutions ranging from pH 5.0 to 7.5 (Fig. 5 K and L). During autophagosome formation, CFP-LC3B is lipidated with a fraction remaining attached to the luminal side of the autophagosome membrane. As the intracellular fluorescence lifetime of CFP undergoes large linear changes in this pH range, CFP-LC3B can be used to accurately measure the pH in the LC3B+ structures. As these LC3B+ structures also colocalize with LAMP2 (Fig. 5I), this method thus allows the measurement of autophagosome/autophagolysosome pH. The sensitivity of this reporter was confirmed by evidence that exposure of WT INS-1 cells to BafA1 increased the vacuolar pH from ∼5.5 to 7.0 (Fig. 5M). In both ΔAtp6ap2 INS-1 clones, LC3B+ structures had an acidic pH, with no significant difference to those in WT INS-1 cells (Fig. 5M). These data suggest that Atp6ap2 deletion does not perturb V-ATPase activity. We also measured more broadly the effect of ATP6AP2 silencing on lysosomal acidification in HeLa cells, by assessing EGF-induced EGFR recycling and cathepsin maturation (SI Appendix, Fig. S7). In all assays, control siRNA did not affect lysosomal acidification while the addition of BafA1 completely blocked V-ATPase activity as evidenced by a reduction in LysoTracker fluorescence intensity, inhibited EGFR degradation, and reduced Cathepsin L activity (SI Appendix, Fig. S7). By contrast, ATP6AP2-silenced cells did not exhibit any difference to control siRNA and further corroborating that ATP6AP2 deficiency does not affect lysosomal acidification (SI Appendix, Fig. S7).
Discussion
Disturbances in insulin SG trafficking and turnover can have profound consequences on cellular homeostasis of the β cell. Despite intact autophagy and acidification mechanisms in Atp6ap2β-/y islets, our results suggest that the major consequence of Atp6ap2 deficiency is the exacerbated generation and accumulation of multigranular bodies that consume the cytoplasm of β cells, ensnaring insulin SGs and thus causing insulin-deficient diabetes (SI Appendix, Fig. S8).
Atp6ap2 was discovered more than a decade ago and initially named the (pro)renin receptor, as it was thought to be functionally associated with the renin-angiotensin system (RAS) (15). It has subsequently been realized that Atp6ap2 is important for many other cellular functions independent of the RAS, and it is now becoming clear that the primary function of Atp6ap2 is not directly associated with this system; rather, it has a ubiquitous and essential role in maintaining cellular homeostasis (16, 38). In the years since its discovery, much effort has been directed toward the characterization of signaling pathways that are “controlled” by Atp6ap2. These include Wnt (25–27) and promyelocytic leukemia zinc finger (PLZF) (39) signaling, despite a lack of similarity between the respective in vivo models (21), and that employed concentrations of its “ligands” renin and (pro)renin were several orders of magnitude above circulating levels (16). Abnormal vacuolar phenotypes are evident upon conditional Atp6ap2 knockout from cardiomyocytes (19), podocytes (18, 20), and hepatocytes (24). Considering this literature with the results presented here, an explanation for why loss of Atp6ap2 affects so many pathways and cell types may be because of the cell “constipation” by vacuolated structures, with loss of homeostasis and thus inability to retain normal signaling function. This interpretation is supported by our finding that while Atp6ap2β-/y islets secrete reduced amounts of insulin in response to high glucose, this difference is lost when normalized for the total insulin content, indicating that what insulin is left can be secreted by the β cell. This essentiality of Atp6ap2 for cellular homeostasis should be carefully considered when evaluating its role in pathological processes. The fact that its deletion attenuates fibrosis in diabetic heart disease (40) and abolishes brain-RAS responses (41), may simply be due to the loss of homeostasis experienced by Atp6ap2-deficient cells, rendering them similarly unable to mount pathological responses. This may also explain why elevated expression of Atp6ap2 is associated with cellular insult (40, 42, 43) as subsequent increases in homeostatic mechanisms are thereafter induced to prevent disease.
Our study identifies Atp6ap2 as an important player in insulin homeostasis. While Atp6ap2 has been found to colocalize with the V-ATPase (28, 29), findings by us and others (28) suggest that this association is not required for the V-ATPase-driven acidification of SGs and lysosomes. This conclusion is supported by comparison of the phenotypic traits observed here in Atp6ap2-deficient β cells with those of V-ATPase-deficient β cells, which reported impaired processing of proinsulin to insulin (14) and decreased plasma insulin, attenuated glucose- and potassium-stimulated insulin secretion with normal islet and β cell morphology (13, 14). In stark contrast, we show that deletion of Atp6ap2 in β cells results in slightly increased fasted plasma insulin, no abnormalities in proinsulin processing, decreased insulin content with no impairment in SG release, and a severely disturbed vacuolated β cell morphology. Moreover, using the newly generated pH reporter CFP-LC3B for FLIM we found that Atp6ap2-deficient β cell lysosomes were acidic, but still susceptible to alkalinization upon BafA1 treatment, which inhibits V-ATPase function. Recent high-resolution atomic models of the V0 subunit of the yeast V-ATPase complex by electron cryomicroscopy has given new molecular insight into its proton translocation mechanism; notably, ATP6AP2 was absent from both structures as there is no known yeast homolog (44, 45). Elucidation of the fully assembled V0V1-ATPase from higher order eukaryotes will be important to determine if Atp6ap2 directly associates with this complex and if so, to reveal the functional implications of its association.
While markers of autophagy are increased in Atp6ap2β-/y cells, we find mechanisms underpinning autophagy induction and flux to be functional here, although grossly overloaded. Atp6ap2 has been proposed to modulate the activity of autophagy-regulator mammalian target of rapamycin (mTOR) (31). However, mTOR complex 1 (MTORC1)-deficient β cells of βra−/− mice display a very different phenotype from the Atp6ap2-deficient β cells described here. In particular they accumulate lysosomes filled with SGs, with apoptosis and loss of β cell mass (46). Likewise, Atg7Δβ mice which are unable to generate LC3B-II and therefore have a deficiency in autophagosome formation, show decreased serum insulin and compromised GSIS (6). By contrast, the phenotype of Atp6ap2β-/y mice is more reminiscent of that of Rab3A−/− mice, which lack a GTP-binding protein important for vesicular trafficking (47). Rab3A−/− β cells exhibit increased insulin degradation, with the accumulation of vacuolated structures and up-regulation of both autophagy and crinophagy (47). Similarly, podocyte-specific deletion of vacuolar protein sorting 34 (Vps34) resulted in the accumulation of large, single-membraned vacuoles due to impaired vesicle fusion and maturation (48). Our analyses of the identity of Atp6ap2β-/y vacuolated structures indicated that they originate upstream of the induction of autophagy, as evidenced by partial colocalization with endosomal and early autophagy markers and that the formation of LC3+ vacuoles was prevented in Atp6ap2-deficient INS-1 cells treated with an ULK1 inhibitor. That these structures contain luminal LC3 and are prevented by ULK1 inhibition also rules out their identity as LC3-associated phagocytosis (LAP) phagosomes (5). Taken together, and because of the disparities between V-ATPase- and autophagy-deficient mice, we hypothesize that Atp6ap2 is important for restraining SG fusion or trafficking upstream of the induction of autophagy. Accordingly, its deletion in β cells, which wholly focus on insulin SG exocytosis and recycling, leads to a very severe phenotype in which large vacuoles accumulate that feed into the autophagy pathway and results in the degradation of insulin. In line with this view, Kissing et al. observed increased fusion rates between phagosomes with lysosomes in Atp6ap2-deficient macrophages which were not associated with impaired lysosomal acidification (49). Further, Atp6ap2 was recently found to coimmunoprecipitate with sortilin-1, a protein required for transport between the endoplasmic reticulum, the Golgi complex, and endolysosomal compartments (50). While distinct from acidification, it may still be that Atp6ap2’s role in vesicular trafficking is driven by an association with the V-ATPase.
Methods
Detailed descriptions are provided in SI Appendix, SI Materials and Methods.
Animal experiments were approved by the Landesamt für Gesundheit und Soziales, Berlin, Germany. Atp6ap2β-/y mice (male) were generated by crossing female Atp6ap2 floxed mice (Atp6ap2flox/+) with male RIP-CreER mice. Tamoxifen pellets (25 mg/pellet, Innovative Research of America) were inserted s.c. IPGTTs were performed as previously described (51). Whole pancreases were dissected, fixed, embedded, and stained for TEM and immunofluorescence microscopy. Islets were isolated from the pancreases of WT and Atp6ap2β-/y mice treated with TAM for 3 wk by collagenase digestion and subsequent centrifugation through a Histopaque gradient. Isolated islets were then cultured overnight in media (RPMI containing 10% FCS and 1% penicillin/streptomycin) at 37 °C with 5% CO2. Islets were then assayed for insulin secretion, Western blotting, or real-time PCR. Atp6ap2 INS-1 knockout clones were generated as described (52) and assayed by microscopy, Western blotting, and for vacuole pH measurements.
Supplementary Material
Acknowledgments
We thank Jana Czychi, Ilona Kamer, Francesca Spagnoli, and Thomas Rathjen (all MDC-Berlin), Prof. Gil Leibowitz (The Hebrew University of Jerusalem), Prof. Paul Gleeson (The University of Melbourne), and Anthony Rousselle for generously providing the Atp6ap2flox/+;R26YFPflox/flox mice (MDC-Berlin). K.J.B. was funded for this study by an Australian National Health Medical Research Council (NHMRC) fellowship (APP1037633). A.L.B. and D.M.W. were supported by the German Research Foundation (BI1292/4-2, BI1292/9-1, BI1292/10-1, BI1292/12-1, and IRTG2251). D.N.M. was supported by the German Research Foundation (DFG; 394046635 - SFB1365) and the German Center for Cardiovascular Research. M.N. was supported by a Dresden International Graduate School for Biomedicine and Bioengineering (DIGS-BB) PhD fellowship. M.S. was supported by the German Federal Ministry of Education and Research (BMBF) to the German Center for Diabetes Research and by the German–Israeli Foundation (I-1429-201.2/2017).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission. D.C.R. is a guest editor invited by the Editorial Board.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1903678116/-/DCSupplemental.
References
- 1.Weir G. C., Bonner-Weir S., Five stages of evolving beta-cell dysfunction during progression to diabetes. Diabetes 53 (suppl. 3), S16–S21 (2004). [DOI] [PubMed] [Google Scholar]
- 2.Fava E., et al. , Novel standards in the measurement of rat insulin granules combining electron microscopy, high-content image analysis and in silico modelling. Diabetologia 55, 1013–1023 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Müller A., Mziaut H., Neukam M., Knoch K.-P., Solimena M., A 4D view on insulin secretory granule turnover in the β-cell. Diabetes Obes. Metab. 19 (suppl. 1), 107–114 (2017). [DOI] [PubMed] [Google Scholar]
- 4.Orci L., et al. , Insulin, not C-peptide (proinsulin), is present in crinophagic bodies of the pancreatic B-cell. J. Cell Biol. 98, 222–228 (1984). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cadwell K., Debnath J., Beyond self-eating: The control of nonautophagic functions and signaling pathways by autophagy-related proteins. J. Cell Biol. 217, 813–822 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jung H. S., et al. , Loss of autophagy diminishes pancreatic beta cell mass and function with resultant hyperglycemia. Cell Metab. 8, 318–324 (2008). [DOI] [PubMed] [Google Scholar]
- 7.Ebato C., et al. , Autophagy is important in islet homeostasis and compensatory increase of beta cell mass in response to high-fat diet. Cell Metab. 8, 325–332 (2008). [DOI] [PubMed] [Google Scholar]
- 8.Masini M., et al. , Autophagy in human type 2 diabetes pancreatic beta cells. Diabetologia 52, 1083–1086 (2009). [DOI] [PubMed] [Google Scholar]
- 9.Wang S., Sun Q.-q., Xiang B., Li X.-J., Pancreatic islet cell autophagy during aging in rats. Clin. Invest. Med. 36, E72–E80 (2013). [DOI] [PubMed] [Google Scholar]
- 10.Bell E., et al. , Rapamycin has a deleterious effect on MIN-6 cells and rat and human islets. Diabetes 52, 2731–2739 (2003). [DOI] [PubMed] [Google Scholar]
- 11.Tanemura M., et al. , Rapamycin causes upregulation of autophagy and impairs islets function both in vitro and in vivo. Am. J. Transplant. 12, 102–114 (2012). [DOI] [PubMed] [Google Scholar]
- 12.Oot R. A., Couoh-Cardel S., Sharma S., Stam N. J., Wilkens S., Breaking up and making up. The secret life of the vacuolar H+ -ATPase. Protein Sci. 26, 896–909 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sun-Wada G.-H., et al. , The a3 isoform of V-ATPase regulates insulin secretion from pancreatic beta-cells. J. Cell Sci. 119, 4531–4540 (2006). [DOI] [PubMed] [Google Scholar]
- 14.Louagie E., et al. , Role of furin in granular acidification in the endocrine pancreas. Identification of the V-ATPase subunit Ac45 as a candidate substrate. Proc. Natl. Acad. Sci. U.S.A. 105, 12319–12324 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nguyen G., et al. , Pivotal role of the renin/prorenin receptor in angiotensin II production and cellular responses to renin. J. Clin. Invest. 109, 1417–1427 (2002). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Campbell D. J., Critical review of prorenin and (pro)renin receptor research. Hypertension 51, 1259–1264 (2008). [DOI] [PubMed] [Google Scholar]
- 17.Burckle C., Bader M., Prorenin and its ancient receptor. Hypertension 48, 549–551 (2006). [DOI] [PubMed] [Google Scholar]
- 18.Oshima Y., et al. , Prorenin receptor is essential for normal podocyte structure and function. J. Am. Soc. Nephrol. 22, 2203–2212 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kinouchi K., et al. , The (pro)renin receptor/ATP6AP2 is essential for vacuolar H+-ATPase assembly in murine cardiomyocytes. Circ. Res. 107, 30–34 (2010). [DOI] [PubMed] [Google Scholar]
- 20.Riediger F., et al. , Prorenin receptor is essential for podocyte autophagy and survival. J. Am. Soc. Nephrol. 22, 2193–2202 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Geisberger S., et al. , New role for the (pro)renin receptor in T-cell development. Blood 126, 504–507 (2015). [DOI] [PubMed] [Google Scholar]
- 22.Song R., Preston G., Ichihara A., Yosypiv I. V., Deletion of the prorenin receptor from the ureteric bud causes renal hypodysplasia. PLoS One 8, e63835 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Trepiccione F., et al. , Renal Atp6ap2/(Pro)renin receptor is required for normal vacuolar H+-ATPase function but not for the renin-angiotensin system. J. Am. Soc. Nephrol. 27, 3320–3330 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kissing S., et al. , Disruption of the vacuolar-type H+-ATPase complex in liver causes MTORC1-independent accumulation of autophagic vacuoles and lysosomes. Autophagy 13, 670–685 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hermle T., Saltukoglu D., Grünewald J., Walz G., Simons M., Regulation of Frizzled-dependent planar polarity signaling by a V-ATPase subunit. Curr. Biol. 20, 1269–1276 (2010). [DOI] [PubMed] [Google Scholar]
- 26.Hermle T., Guida M. C., Beck S., Helmstädter S., Simons M., Drosophila ATP6AP2/VhaPRR functions both as a novel planar cell polarity core protein and a regulator of endosomal trafficking. EMBO J. 32, 245–259 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cruciat C.-M., et al. , Requirement of prorenin receptor and vacuolar H+-ATPase-mediated acidification for Wnt signaling. Science 327, 459–463 (2010). [DOI] [PubMed] [Google Scholar]
- 28.Daryadel A., et al. , Colocalization of the (Pro)renin Receptor/Atp6ap2 with H+-ATPases in mouse kidney but prorenin does not acutely regulate intercalated cell H+-ATPase activity. PLoS One 11, e0147831 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Advani A., et al. , The (Pro)renin receptor. Site-specific and functional linkage to the vacuolar H+-ATPase in the kidney. Hypertension 54, 261–269 (2009). [DOI] [PubMed] [Google Scholar]
- 30.Guida M. C., et al. , ATP6AP2 functions as a V-ATPase assembly factor in the endoplasmic reticulum. Mol. Biol. Cell 29, 2156–2164 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Binger K. J., Muller D. N., Autophagy and the (Pro)renin receptor. Front. Endocrinol.(Lausanne) 4, 155 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Schvartz D., et al. , Improved characterization of the insulin secretory granule proteomes. J. Proteomics 75, 4620–4631 (2012). [DOI] [PubMed] [Google Scholar]
- 33.Ludwig J., et al. , Identification and characterization of a novel 9.2-kDa membrane sector-associated protein of vacuolar proton-ATPase from chromaffin granules. J. Biol. Chem. 273, 10939–10947 (1998). [DOI] [PubMed] [Google Scholar]
- 34.Orci L., et al. , Conversion of proinsulin to insulin occurs coordinately with acidification of maturing secretory vesicles. J. Cell Biol. 103, 2273–2281 (1986). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Eskelinen E.-L., Saftig P., Autophagy: A lysosomal degradation pathway with a central role in health and disease. Biochim. Biophys. Acta 1793, 664–673 (2009). [DOI] [PubMed] [Google Scholar]
- 36.Poëa-Guyon S., et al. , The V-ATPase membrane domain is a sensor of granular pH that controls the exocytotic machinery. J. Cell Biol. 203, 283–298 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Neukam M., Soenmez A., Solimena M., FLIM-based pH measurements reveal incretin-induced rejuvenation of aged insulin secretory granules. bioRxiv:10.1101/174391 (9 November 2017).
- 38.Batenburg W. W., Danser A. H. J., (Pro)renin and its receptors. Pathophysiological implications. Clin. Sci. 123, 121–133 (2012). [DOI] [PubMed] [Google Scholar]
- 39.Schefe J. H., et al. , A novel signal transduction cascade involving direct physical interaction of the renin/prorenin receptor with the transcription factor promyelocytic zinc finger protein. Circ. Res. 99, 1355–1366 (2006). [DOI] [PubMed] [Google Scholar]
- 40.Yu S., et al. , (Pro)renin receptor RNAi silencing attenuates diabetic cardiomyopathy pathological process in rats. Hum. Gene Ther. 30, 727–739 (2019). [DOI] [PubMed] [Google Scholar]
- 41.Li W., et al. , Neuron-specific (pro)renin receptor knockout prevents the development of salt-sensitive hypertension. Hypertension 63, 316–323 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wilkinson-Berka J. L., et al. , RILLKKMPSV influences the vasculature, neurons and glia, and (pro)renin receptor expression in the retina. Hypertension 55, 1454–1460 (2010). [DOI] [PubMed] [Google Scholar]
- 43.Gonzalez A. A., Lara L. S., Luffman C., Seth D. M., Prieto M. C., Soluble form of the (pro)renin receptor is augmented in the collecting duct and urine of chronic angiotensin II-dependent hypertensive rats. Hypertension 57, 859–864 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Roh S.-H., et al. , The 3.5-Å CryoEM structure of nanodisc-reconstituted yeast vacuolar ATPase Vo proton channel. Mol. Cell 69, 993–1004.e3 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Mazhab-Jafari M. T., et al. , Atomic model for the membrane-embedded VO motor of a eukaryotic V-ATPase. Nature 539, 118–122 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Blandino-Rosano M., et al. , Loss of mTORC1 signalling impairs β-cell homeostasis and insulin processing. Nat. Commun. 8, 16014 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Marsh B. J., et al. , Regulated autophagy controls hormone content in secretory-deficient pancreatic endocrine beta-cells. Mol. Endocrinol. 21, 2255–2269 (2007). [DOI] [PubMed] [Google Scholar]
- 48.Bechtel W., et al. , Vps34 deficiency reveals the importance of endocytosis for podocyte homeostasis. J. Am. Soc. Nephrol. 24, 727–743 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kissing S., et al. , Vacuolar ATPase in phagosome-lysosome fusion. J. Biol. Chem. 290, 14166–14180 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lu X., et al. , Identification of the (Pro)renin receptor as a novel regulator of low-density lipoprotein metabolism. Circ. Res. 118, 222–229 (2016). [DOI] [PubMed] [Google Scholar]
- 51.Birkenfeld A. L., et al. , Deletion of the mammalian INDY homolog mimics aspects of dietary restriction and protects against adiposity and insulin resistance in mice. Cell Metab. 14, 184–195 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ran F. A., et al. , Genome engineering using the CRISPR-Cas9 system. Nat. Protoc. 8, 2281–2308 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.