Significance
Phosphorylation (P)-type ATPases act to maintain and modulate charge distribution across membranes and these proteins have been coopted for electrical signaling in animals. When wounded, membranes in the leaves of Arabidopsis thaliana depolarize rapidly. This is followed by a slower repolarization phase. We found that the proton pump AHA1 acted to control membrane potential when these plants were wounded. Specifically, the repolarization phase in aha1 mutants is prolonged relative to that in wild-type plants. In parallel, the jasmonate defense pathway is activated strongly and the mutant plants are better defended against herbivores than the wild type. We reveal that plants, like animals, use P-type ATPases in electrical signaling and show that AHA1 couples membrane potential to anti-herbivore defense.
Keywords: jasmonate, proton ATPase, wound, vasculature, defense
Abstract
Electrogenic proton pumps have been implicated in the generation of slow wave potentials (SWPs), damage-induced membrane depolarizations that activate the jasmonate (JA) defense pathway in leaves distal to wounds. However, no defined H+-ATPases have been shown to modulate these electrical signals. Pilot experiments revealed that the proton pump activator fusicoccin attenuated SWP duration in Arabidopsis. Using mutant analyses, we identified Arabidopsis H+-ATPase 1 (AHA1) as a SWP regulator. The duration of the repolarization phase was strongly extended in reduced function aha1 mutants. Moreover, the duration of SWP repolarization was shortened in the presence of a gain-of-function AHA1 allele. We employed aphid electrodes to probe the effects of the aha1 mutation on wound-stimulated electrical activity in the phloem. Relative to the wild type, the aha1-7 mutant increased the duration and reduced the amplitudes of electrical signals in sieve tube cells. In addition to affecting electrical signaling, expression of the JA pathway marker gene JAZ10 in leaves distal to wounds was enhanced in aha1-7. Consistent with this, levels of wound-response jasmonoyl-isoleucine were enhanced in the mutant, as was defense against a lepidopteran herbivore. The work identifies a discrete member of the P-type ATPase superfamily with a role in leaf-to-leaf electrical signaling and plant defense.
Found in all domains of life, phosphorylation (P)-type ATPases power proton, ion, and molecule movements across membranes (1, 2). ATPases from this superfamily help to determine transmembrane potentials; their activities have been widely co-opted in electrical signaling. For example, Na+/K+ATPases are essential components of axonal action potential signaling throughout the animal kingdom (3). Unlike in animals, no defined P-type ATPase gene has an attributed function in the formation of any organ-to-organ electrical signal in plants. Nevertheless, the action of proton pumps has been implicated repeatedly in the production of slow wave potentials (SWPs), which are electrical signals that are generated in response to severe wounds (4). SWPs occur across the plant kingdom (4, 5) and earlier studies strongly suggest that proton gradients and, specifically, H+-ATPases within the P-type superfamily underlie part of the SWP mechanism in numerous plants (6–9). These studies were largely pharmacology based. Previous work used uncouplers (6, 7), proton pump activators (e.g., the fungal effector fusiccocin; ref. 7), chemical inhibitors such as cyanide (7, 8), orthovanadate (9), or apoplastic dyes (10) to investigate the relationship of ATP-driven proton pumping and SWP generation. Through the use of orthovanadate or fusicoccin, H+-ATPases have also been implicated in the generation of signals that are distinct from SWPs: system potentials (11).
The principal electrical events in the SWP are generally monitored with noninvasive surface electrodes. Measured this way, SWPs involve rapid (<2 s) and massive membrane depolarizations (>50 mV) and sustained repolarization phases lasting up to several minutes (4). In Arabidopsis, these events take place within the wounded leaf and, less than one minute later, in distal leaves that share direct vascular connections with the wounded leaf (12). Earlier studies specifically implicated transient H+ pump inactivation in the depolarization phase of the SWP followed by proton pump reactivation/stimulation to enable repolarization (e.g., ref. 7). That is, wound-response H+-ATPase regulation could operate in both the depolarization phase and during membrane repolarization in the recovery phase. Despite decades of research, the only molecular components so far identified as necessary for SWP propagation are several clade 3 glutamate receptor-like (GLR) proteins (12). Genetic analyses indicated that GLRs 3.1, 3.2, 3.3, and 3.6 are regulators of membrane potential in wounded plants and single mutants in each of these genes reduce the duration of the SWP (13). That is, clade 3 glr mutants affect the SWP repolarization phase, making it shorter than that of the wild type (WT). If H+-ATPases act in the SWP, in which phase might they operate? Also, how would proton pump/glr double mutants respond to wounds? Here, we first set out to test whether fusicoccin, an activator of plasma membrane proton pumps including Arabidopsis H+-ATPases (AHAs; refs. 14–17) affected SWP generation in Arabidopsis. To do this, we exploited vein exposure procedures (13, 18) to facilitate the direct treatment of the primary vasculature.
Double mutants in certain clade 3 GLRs that impact SWPs also reduce the wound-stimulated activity of the jasmonate pathway (12). These mutants show a reduced capacity to defend themselves against insect herbivores relative to the WT (13). For example, levels of wound-response jasmonic acid (JA) and jasmonoyl-isoleucine (JA-Ile) were reduced in glr3.3 glr3.6 double mutants as were wound-induced levels of transcripts for jasmonate pathway marker genes such as JAZ10 (12). Therefore, if H+-ATPase mutants that affect wound-response membrane potentials could be found, they might also be potential regulators of the jasmonate pathway. Indeed, proton pumps were implicated previously in jasmonate pathway regulation (19–21). Also, mutation of a subunit of a trans-Golgi network H+-ATPase caused an increase in the accumulation of the JA precursor oxo-phytodienoic acid (OPDA; ref. 22). Our strategy was therefore to assay proton pump contributions to the propagation of SWPs. In a second approach, we examined whether or not these mutants affected jasmonate synthesis, jasmonate signaling, and herbivore defense.
Results
Proton Pump Modulation Affects Wound-Induced Electrical Signals.
Based on evidence that plasma membrane proton pumps participate in SWP production in other species (6–9), we set out to identify a discrete proton pump that regulates the Arabidopsis SWP. Each of the two initial methods we used was nonspecific in that each potentially targeted multiple AHAs within the primary vein, which is an established route for SWP propagation (13). In the first approach, the adaxial side of the primary vein in leaf petioles was surgically exposed so as leave the abaxial side supported by extravascular tissue. Fusicoccin (FC), which activates plasma membrane H+-ATPases (14, 23), or carrier solution alone was then applied to the exposed vein. Surface electrodes were placed on leaf 8 and leaf 13 as shown in SI Appendix, Fig. S1A, and leaf 8 was then wounded. Under these conditions, FC treatment attenuated the SWP duration in distal leaf 13 without affecting signal on leaf 8 (SI Appendix, Fig. S1 B–D).
The second, essentially reverse approach involved expressing a protein phosphatase (PP2C-D1) that is negative regulator of AHA2 (24) and possibly other AHAs. In those experiments, PP2C-D1 expression driven by the 35S promoter led to a dwarf plant phenotype. With the goal of having a less severe impact on rosette growth, we employed a tissue-specific promoter. The phloem was targeted since GLR3.3, which is important for SWP production and defense against herbivores, is expressed in this tissue (13). However, GLR3.3 is also expressed in some epidermal cells. We therefore chose the phloem-specific promoter SUC2 that is active in companion cells (25). SUC2p::PP2C-D1 plants displayed smaller rosettes than did the WT (SI Appendix, Fig. S2A). Leaf 8 of both genotypes was wounded and SWPs were quantitated on distal leaf 13 using previously defined parameters (12). In contrast to FC treatments, plants expressing PP2C-D1 showed SWP durations that were significantly longer than those in the WT (SI Appendix, Fig. S2B).
AHA1 Acts in Long-Distance Electrical Signaling.
Since FC treatment reduced SWP duration and PP2C-D1 expression increased SWP duration, we investigated the roles of specific AHA genes. The Arabidopsis genome carries 11 members of the AHA family (AHA1-11). Transcriptome studies indicate that AHA1 and AHA2 are the most highly expressed members of this family throughout the plant’s life cycle (26). Phylogenetic studies also indicate that they share a common ancestor: AHA3 which is expressed in phloem (27). However, homozygous loss-of-function alleles in this gene cause pollen lethality (28), so aha3 mutants were not investigated in our study. Similarly, and indicating functional redundancy, aha1 aha2 double mutants are embryo lethal (26). Since, aha1 and aha2 single mutants are viable, SWP production was tested using aha1 and aha2 mutants. SWP production was compared in the WT and in heterozygous and homozygous aha1-7 plants (26). In the AHA1 gene (SI Appendix, Fig. S3A), the aha1-7 allele produces a truncated mRNA (26). We confirmed that this was the case and found that the truncation occurred upstream (5′) to the last three predicted transmembrane helices (SI Appendix, Fig. S3B). Then, using a pH indicator-based assay, we compared the ability of WT, the ost2-2D gain-of-function mutant in AHA1 (29), and two reduced function alleles of AHA1: aha1-6 (26) and aha1-7 (26) roots to acidify growth medium. The ost2-2D mutant strongly acidified the growth medium. The aha1-6 and aha1-7 mutants, in contrast, failed to acidify the growth medium to the extent of that caused by the WT (SI Appendix, Fig. S3C). Since our work was focused on leaves from 5 to 6 wk-old plants, and the SWP is transmitted from leaf to leaf through the primary vasculature (13), we verified our results for medium acidification in aha1-7 with leaf midveins isolated according to Kurenda and Farmer (18). Primary veins from aha1-7 were less effective in acidifying the growth medium than were WT veins (SI Appendix, Fig. S3D).
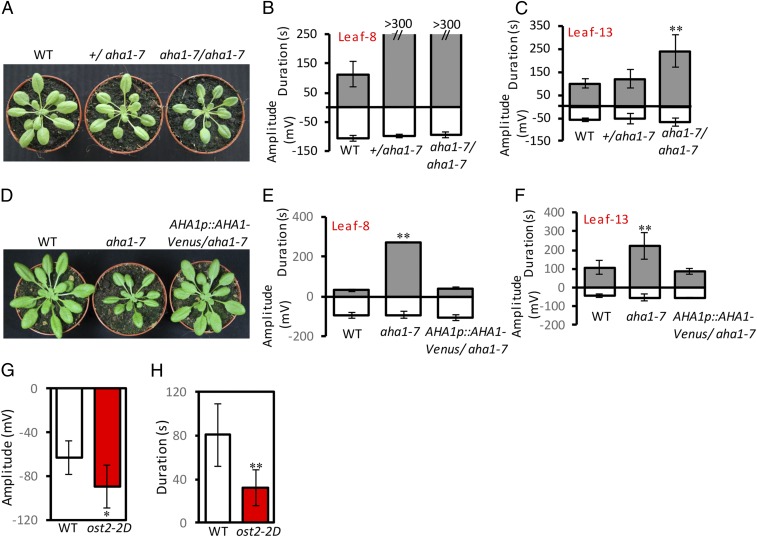
Under our growth conditions, aha1-7 showed a weak growth reduction phenotype (Fig. 1A). In each case, leaf 8 was wounded and SWPs were monitored on this leaf and on distal leaf 13. The duration of the repolarization phase of the SWP in wounded leaf 8 was extended in both +/aha1-7 and in aha1-7/aha1-7 plants (Fig. 1B). Additionally, the aha1-7 homozygote increased SWP duration in leaf 13 (Fig. 1C). To confirm that mutations in AHA1 were responsible for altering wound-activated electrical signals, the aha1-7 mutant was complemented with an AHA1-encoding genomic fragment to which a Venus fluorescent protein tag (30) was added to the C terminus. Examination of third generation (T3) transformants showed that AHA1 restored the WT growth phenotype which, prior to rescue, was smaller than the WT (Fig. 1D). Moreover, WT-like SWPs were observed in both wounded leaf 8 and in distal leaf 13 of complemented plants (Fig. 1 E and F). We then tested the ost2-2D mutant in which AHA1 is constitutively active (29). We found that ost2-2D increased SWP depolarization amplitudes (Fig. 1G) and reduced SWP repolarization durations in leaf 13 when leaf 8 was wounded (Fig. 1H).
Fig. 1.
Phenotype and wound-activated surface potential measurements in aha1-7, complemented aha1-7, and ost2-2D. (A) Five weeks-old wild-type (WT) rosette, +/aha1-7, and aha1-7/ aha1-7 rosettes. (B) Surface potential changes on leaf 8. Broken columns indicate measurements after wounding where repolarization taking more than 300 s was not quantified. (C) Surface potentials on distal leaf 13. (D) Five weeks-old WT, aha1-7, and aha1-7 complemented with AHA1p::AHA1-Venus. (E) Wound-activated surface potential changes on leaf 8, and (F) on distal leaf 13 (n = 10–12). (G and H) Wound-activated surface potential changes in distal leaf 13 of ost2-2D. (G) Amplitude and (H) duration, (n = 15). Data shown are means ± SD. Asterisks indicate a significant difference with WT. Student t test: *P < 0.05, **P < 0.01.
To investigate a second reduced function aha1 allele, we tested aha1-6 (26). In wounded aha1-6, and consistently with what we observed with aha1-7, we detected substantial increases in SWP durations compared to the WT in distal leaf 13 when leaf 8 was wounded (SI Appendix, Fig. S4A). In contrast, no significant differences in SWP characteristics were found when the WT and the aha2-4 mutant were compared (SI Appendix, Fig. S4B). Together, these results indicated a potentially stronger role of AHA1 than AHA2 in SWP generation. At this point our results strongly suggested a role of AHA1 in membrane potential regulation in the SWP. However, assessing the effects of proton pump mutants on the SWP are potentially complicated due to possible effects on plant development. We therefore examined sections of the petiolar primary veins of the WT and aha1-7 using transmission electron microscopy. No readily detectable differences were observed between the two genotypes (SI Appendix, Fig. S5).
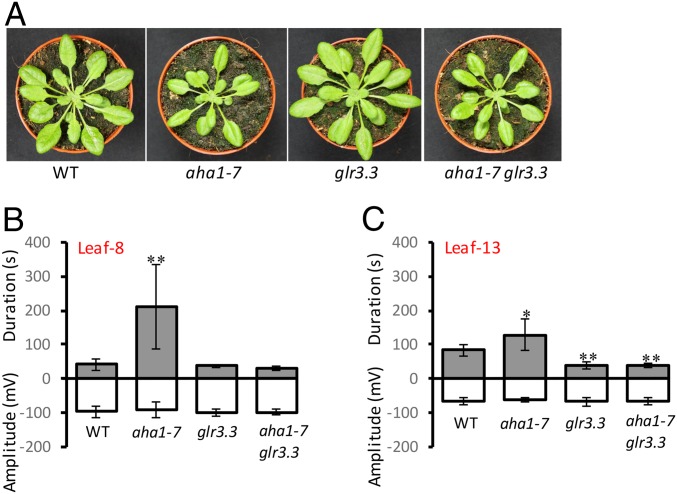
Proton pumps are major regulators of membrane potential in plants (16, 17) and SWPs are detected as strong and prolonged changes in membrane potential that are triggered by wounding (4). So far, the only gene products that have been found to regulate the Arabidopsis SWP are several clade 3 GLRs. These proteins act as regulators of membrane potential during the wound response (12, 13). While glr double mutants carrying loss-of-function glr3.3 and glr3.6 alleles largely or completely abolish the SWP detected in a leaf distal to a wound, the glr3.3 mutant has a weak attenuating effect on the SWP without eliminating it (12). We investigated whether the highly prolonged repolarization phases seen in both wounded leaf 8 and distal leaf 13 of aha1-7 were affected in a glr3.3 background. The increased duration of the repolarization phase seen in aha1-7 was reduced to a shorter-than-WT duration in glr3.3 aha1-7; i.e., glr3.3 was epistatic to aha1-7 (Fig. 2).
Fig. 2.
The glr3.3 mutation attenuates the effect of aha1-7 on slow wave potentials. (A) Five weeks-old rosettes of the WT, aha1-7, glr3.3, and aha1-7 glr3.3. (B and C) Surface potential changes on leaf 8 and leaf 13 after wounding (n = 12). Data shown are means ± SD. Asterisks indicate significant difference to WT. Student t test: *P < 0.05, **P < 0.01.
aha1-7 Alters Sieve Element Depolarization Signals.
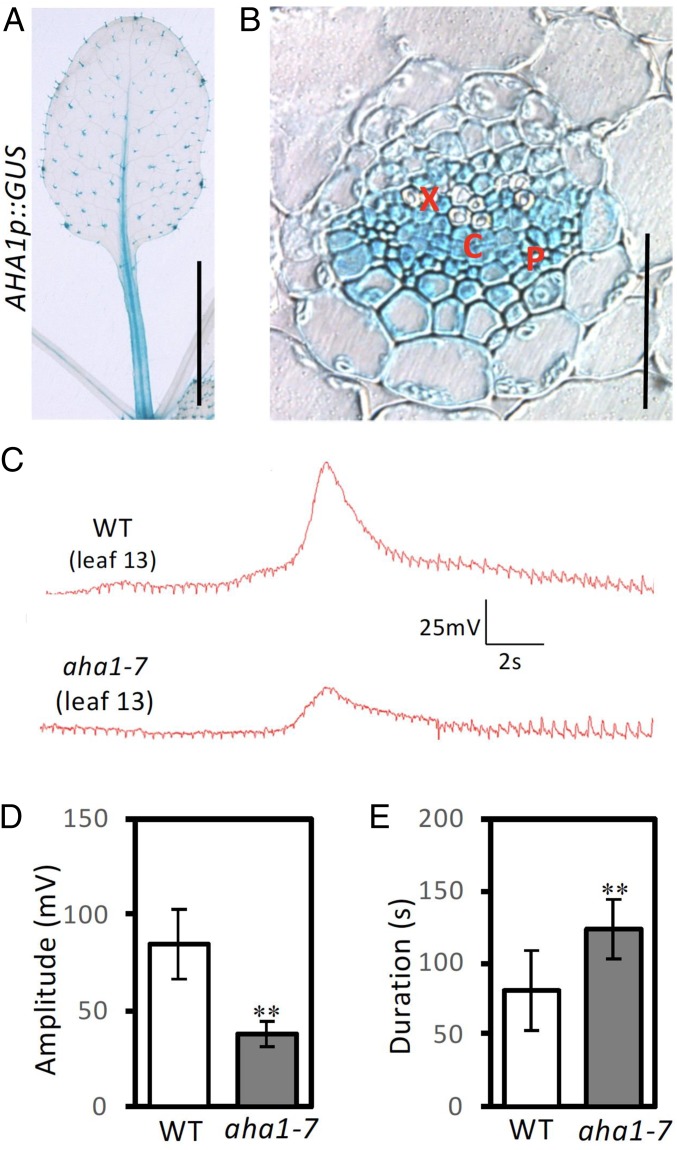
To identify cells in which the AHA1 promoter was active, we used an AHA1p::GUS (β-glucuronidase) fusion gene. GUS staining was mostly restricted to the vasculature and to trichomes (Fig. 3A). The AHA1 promoter was active in the core vasculature; i.e., within cells associated with both the phloem and xylem (Fig. 3B). This raised the possibility that AHA1 affects electrical signaling in these cells and prompted us to use cell-specific electrodes based on live insects. Living aphids can be used as intracellular electrodes to detect plant cell-specific electrical signaling (31). This method revealed that wounding of the WT caused a prolonged multiphasic depolarization of phloem sieve elements (SEs). In the WT, a fast, spike-like depolarization signal is embedded in a long duration (slower) depolarization (31). Here, we wounded leaf 8 of 5.5 wk-old plants and, using aphid electrodes, examined electrical events in SEs in leaf 13 of the WT and aha1-7. The amplitudes of the spike signals as well as the overall durations of the depolarization phases were quantitated according to ref. 31 as shown in SI Appendix, Fig. S6. Only the fast spike-like signal is shown in Fig. 3C. We found that aha1-7 attentuated the amplitudes of these rapid signals (Fig. 3D) whereas the duration of the full depolarization phase was increased in the mutant compared to the WT (Fig. 3E). This prompted us to perform additional experiments since, when measured with surface electrodes, the rapid SWP depolarization phase is followed by increases in cytosolic Ca2+ (13). Using the approach outlined in ref. 13, we tested whether aha1-7 affected cytosolic Ca2+ transients in the petiole of leaf 13 following wounding of leaf 8. Consistent with the prolonged repolarization phase observed in aha1-7, we found prolonged Ca2+ transients compared to the WT (SI Appendix, Fig. S7). The averaged mean duration of the WT calcium transient at half maximum amplitude was 66 s. This value in aha1-7 was 112 s. We also noted that the amplitude of GCaMP3 fluorescence (ΔF/F) was somewhat higher in aha1-7 than in the WT.
Fig. 3.
AHA1 promoter activity associated with the vasculature, and electrical penetration recordings from sieve elements. (A) GUS staining pattern for an AHA1p::GUS transcriptional reporter; Leaf 6 of 4 wk-old plant. (Scale bars, 0.5 cm.) (B) Transversal petiole cross section from leaf 6. (Scale bar, 50 μm.) (C–E) Potential changes recorded on leaf 13 after wounding leaf 8. (C) Traces from WT and aha1-7. Only the region of fast spike signal is shown, see SI Appendix, Fig. S6 for details of the overall signal structure and quantification. (D) SWP amplitude and (E) duration, see EPG recording methods. X, xylem region. P, phloem region, and C, cambium region. Data shown are means ± SD. Asterisks indicate significant difference with WT. Student t test: **P < 0.01, n = 10.
Increased Jasmonate Accumulation and Anti-Herbivore Defense in aha1-7.
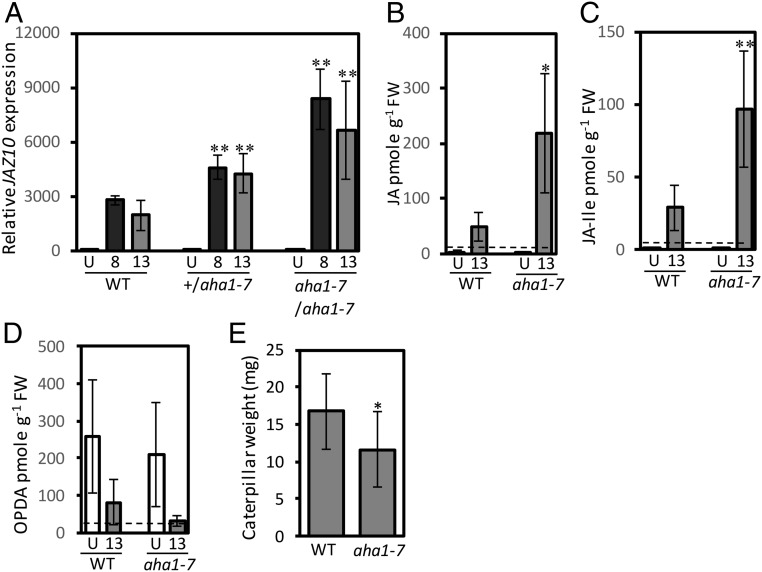
Proton pump activity has been implicated in the regulation of jasmonate signaling (19). To test whether various aha1 alleles altered the activity of this pathway, we measured levels of the JAZ10 jasmonate signaling marker gene (32) in distal leaf 13 after wounding leaf 8. In these experiments the ost2-2D gain-of-function allele of AHA1, which shortens the SWP duration, showed a reduced capacity to accumulate JAZ10 transcript in a leaf distal to a wound (SI Appendix, Fig. S8A) whereas aha1-6 mutant that increases the SWP duration displayed higher than WT JAZ10 transcript levels in leaf 13 1 h after wounding leaf 8 (SI Appendix, Fig. S8B). To verify whether this was due to AHA1 mutation, we measured JAZ10 levels in aha1-7 and in aha1-7 complemented with AHA1p::AHA1-Venus. Complementation restored wound-induced JAZ10 transcripts in distal leaf 13 to levels similar to those in the wounded WT (SI Appendix, Fig. S8C). Next, JAZ10 transcript levels were compared in heterozygous and homozygous aha1-7 plants. Consistent with the AHA1 gene-dosage effect on SWP duration, the wounded heterozygous plants displayed higher-than-WT JAZ10 transcript levels in both leaves 8 and 13 relative to those in the wounded WT (Fig. 4A).
Fig. 4.
Wound-induced up-regulation of JAZ10, accumulation of jasmonic acid (JA), jasmonoyl-isoleucine (JA-Ile), oxo-phytodienoic acid (OPDA), and reduced insect growth in the aha1-7 mutant. (A) JAZ10 transcript levels in leaves 8 and 13 1 h after wounding (n = 4), (B) JA, (C) JA-Ile, and (D) OPDA in connected leaf 13, 45 min after wounding (n = 4). Cognate internal standards were used for JA and JA-Ile quantification. OPDA levels were assessed with the internal standard for JA-Ile. (E) Weight of S. littoralis larvae grown for 12 d on the WT and/or on the aha1-7 mutant. Each replicate consisted of 11 plants and 44 larvae. Data shown are combined from two replicated bioassays. Data shown are means ± SD. Asterisks refer to data significantly different from wounded WT: Student t test: *P < 0.05, **P < 0.01. For statistics in B–D, only data above the limit of quantification (LOQ) (dashed line) were considered as relevant. U, unwounded.
To test whether the increased levels of JAZ10 messenger RNAs were associated with altered levels of jasmonates, plants were wounded on leaf 8 and, 45 min later, leaf 13 was harvested. Levels of jasmonic acid (JA), jasmonoyl-isoleucine (JA-Ile) and the precursor 12-oxo-phytodienoic acid (OPDA) were assessed by liquid chromatography/mass spectrometry. A significant up-regulation of JA and JA-Ile levels in leaf 13 was seen after wounding leaf 8 (Fig. 4 B and C). Consistent with this, a decreased amount of the OPDA relative to the WT was observed in the leaf distal to a wound at this 45 min time-point (Fig. 4D). To test the effect of AHA1 mutation on defense responses, the performance of larvae of Spodoptera littoralis on aha1-7 mutants and WT plants (WT) was tested. Fig. 4E shows that the larvae gained less weight on aha1-7 relative to the WT (P = 0.024). Another output of jasmonate signaling is wound-stimulated rosette growth restriction (33). A serial wounding assay was performed starting with 2 wk-old plants on which leaf 1 was wounded. Consecutive leaves were then wounded at 3 d intervals over a 3 wk period. Control plants were manipulated (touched) but not wounded. As expected (33), the serially wounded WT showed strong growth restriction: the fresh mass of the wounded WT plant was reduced by 66% relative to the unwounded WT control. In aha1-7, the effect of wounding was slightly enhanced and serial wounding caused a 74% reduction in fresh weight relative to unwounded aha1-7 (SI Appendix, Fig. S9).
Discussion
In 1982 Chastain and Hanson (34) revealed that wounding caused the modulation of electrogenic plasma membrane H+ transport in wheat. Later, using other plants, the first experimental evidence for transient H+-ATPase inhibition followed by stimulation of H+ extrusion during the SWP was reported (6, 7). Following this, modulation of H+-ATPase activity and H+ gradients using a variety of inhibitors, activators, and ionophores then linked proton pump activity to JA-controlled defenses (19, 20, 35). Our results confirm the importance of H+-ATPases in the wound response by identifying a discrete pump, AHA1, that acts to regulate membrane repolarization and JA synthesis in wounded Arabidopsis. An initial link to much previous literature was the use of the H+-ATPase activator FC. Perhaps due to low penetrability of FC into veins, it was necessary in our experiments to use relatively high FC concentrations (25 μM) in order to modify the SWP. Therefore, our FC pilot experiments were considered indicative of roles of proton pumps in the SWP. The same was true when we employed the elegant approach of Spartz et al. (24) to inhibit AHAs by expressing the regulatory gene PP2C-D1, in the phloem. Consistent with a role of H+-ATPases in the SWP repolarization processes, those experiments caused a prolongation of the SWP repolarization phase in leaves distal to wounds. However, both these experiments did not identify any discrete proton pump as playing a role in the SWP. Further experiments based on mutant analysis were therefore initiated.
Here we provide genetic evidence that the P-type ATPase AHA1 acts in leaf-to-leaf wound signaling in a plant. Experiments with the AHA1 alleles aha1-7 and ost2-2D provide insights into H+-ATPase action in the SWP. The experiments with reduced function aha1 mutants including aha1-7 revealed a role of AHA1 in the SWP repolarization phase. The aha1-7 mutant prolonged this phase of the SWP significantly relative to the WT. This leads us to expect AHA-controlled extracellular pH changes in the leaf during the SWP. Consistent with the effects of reduced function aha1 mutants that extend the SWP repolarization phase, the gain-of-function mutant ost2-2D mutant shortened the duration of SWP relative to the WT. Further examination of SWPs in ost2-2D revealed a weakly increased amplitude of the initial SWP membrane depolarization. However, in aha1-7, no significant effects on initial SWP depolarization were observed. Further investigation will therefore be needed to establish definitively whether there is transient H+-ATPase inactivation during the SWP as was proposed previously (6, 7). Together, our findings raise the question of whether other types of electrical signals may be affected by proton pump mutations. For example, system potentials are systemically transmitted plasma membrane hyperpolarizations that can be induced by herbivore feeding (11, 36). The stimulation of H+-ATPase activity was proposed to underlie this form of wound-induced electrical signaling (36). It will therefore be of interest to test whether the functional AHA1 gene is required for system potential formation in Arabidopsis. Having found that the aha1-7 mutation causes an extended duration of the SWP phase in both leaf 8 and in leaf 13, we attempted to reverse the effect of the mutation. To do this, we generated an aha1-7 glr3.3 double mutant. Wounding this plant indeed showed that the extended membrane repolarizations seen in aha1-7 were reversed in both the wounded and distal leaves of the double mutant. AHA1 likely acts downstream of GLR3.3 in the SWP.
Also known to occur downstream of GLR3.3 action in the SWP are wound-response changes in cytosolic calcium levels (13). In that previous study, clade 3 glr mutants, which reduced the duration of the SWP in leaves distal to wounds, also reduced the levels of cytosolic Ca2+ in those leaves. Consistent with a role of AHA1 in the repolarization phase of the SWP, we found that cytosolic Ca2+ transients were of prolonged duration and increased amplitude in aha1-7 relative to the WT. It is conceivable that this larger-than-WT Ca2+ transient is associated with increased activity of the JA pathway in wounded aha1-7.
Monitoring slow wave potentials with surface electrodes does not give information on individual cell types that contribute to electrical activities in the wounded plant. Having found evidence for AHA1 promoter activity throughout the core primary vasculature, we then probed wound-stimulated electrical signals in a specific cell type: the sieve element. The signal characteristics we recorded from WT leaves distal to wounds were similar to those observed previously (31). However, the amplitude of spike-like signals observed in wounded aha1-7 were attenuated whereas the durations of the full wound-induced signal; i.e., the slow depolarization and repolarization phase with the superimposed fast spike depolarization/ repolarization (31), were extended. Sieve elements, sites of clade 3 GLR protein expression, play key roles in SWP propagation (13). The present findings with aha1-7 further support an important role of the phloem in leaf-to-leaf electrical signaling. Moreover, the identification of AHA1 as a SWP regulator will now allow mechanistic studies of its regulation during wounding. For this, increasing information on the action of the peptide elicitor systemin from plants in the tomato family (37) may provide valuable insights. In tomato, systemin elicits the synthesis of proteinase inhibitors that are jasmonate inducible (38). A recent proteomics study found that systemin elicited the dephosphorylation of threonine 995 in the C-tail of the H+-ATPase LHA1 in tomato, and this reduced LHA1 activity (39). Phosphorylation sites at the C-tail of Arabidopsis AHA1 are candidates for regulation of this protein during SWP formation. Mechanisms that link systemin perception (40) to H+-ATPase activity may provide further clues about potential modes of wound-associated AHA1 regulation.
Here we investigated the roles of proton pumps in JA pathway function and not the function of the JA pathway in regulating proton pumps. We found that AHA1 regulates jasmonate synthesis and signaling in wounded plants, and acts as a suppressor of herbivore defense. Viewed from a genetic perspective, AHA1 in our study is a negative regulator of JA synthesis and signaling. This contrasts with a report that AHA1 positively regulates JA signaling by enhancing the interaction of JA receptor components (21). The mechanism we are studying therefore appears to differ from that studied by Zhou et al. (21). Prolonging the repolarization phase of the SWP may enhance JAZ10 expression and plant defense. This would be consistent with genetic evidence that the activity of the jasmonate pathway is controlled in part by wound-response membrane potential changes (12, 13). Additionally, JA pathway activity is up-regulated in a gain-of-function ion channel mutant that may cause endomembrane depolarization (41). Summarizing, AHA1 acts as a negative regulator of SWP duration. We leave open the possibility that other AHAs participate in SWP generation either as positive or negative regulators. Clade 3 GLRs were previously identified as SWP regulators (12, 13). The present study identifies AHA1 as a second element in SWP generation. This protein powers proton transfer necessary to restore membrane potential after the SWP. Plants, like animals, use P-type ATPases in electrical signaling.
Materials and Methods
All plants used were in the Columbia-0 background. Generation of transgenics, JA quantifications, insect bioassays, surface electrophysiology, aphid electrophysiology, acidification assays, and gene expression analysis techniques are described in the SI Appendix, SI Materials and Methods.
Supplementary Material
Acknowledgments
We thank S. Stolz (University of Lausanne) for help with SUC2 promoter cloning, A. Kurenda (University of Lausanne) for help with image analysis, B. Fauvet (University of Lausanne) for advice on statistics, and the Electron Microscopy Facility, University of Lausanne, for micrographs. Jasmonate quantifications were performed under the direction of Dr. G. Glauser, platform of Analytical Chemistry, University of Neuchâtel. S. Kellenberger, C. Hardtke, and P. Reymond (University of Lausanne) provided critical comments on the manuscript. This work was funded by Swiss National Science Foundation grants (31003A-138235 and 31003A-175566) to E.E.F.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1907379116/-/DCSupplemental.
References
- 1.Kühlbrandt W., Biology, structure and mechanism of P-type ATPases. Nat. Rev. Mol. Cell Biol. 5, 282–295 (2004). [DOI] [PubMed] [Google Scholar]
- 2.Thever M. D., Saier M. H. Jr, Bioinformatic characterization of p-type ATPases encoded within the fully sequenced genomes of 26 eukaryotes. J. Membr. Biol. 229, 115–130 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Liebeskind B. J., Hofmann H. A., Hillis D. M., Zakon H. H., Evolution of animal neural systems. Annu. Rev. Ecol. Evol. Syst. 48, 377–398 (2017). [Google Scholar]
- 4.Stahlberg R., Cleland R. E., Van Volkenburgh E., “Slow wave potentials—A propagating electrical signal unique to higher plants” in Plant Electrophysiology: Theory and Methods, Volkov A. G., Ed. (Communication in Plants, Springer, 2006), pp. 291–308. [Google Scholar]
- 5.Boari F., Malone M., Wound-induced hydraulic signals: Survey of occurrence in a range of species. J. Exp. Bot. 44, 741–746 (1993). [Google Scholar]
- 6.Julien J. L., Desbiez M. O., De Jaegher G., Frachisse J. M., Characteristics of the wave of depolarization induced by wounding in Bidens pilosa L. J. Exp. Bot. 42, 131–137 (1991). [Google Scholar]
- 7.Julien J. L., Frachisse J. M., Involvement of the proton pump and proton conductance change in the wave of depolarization induced by wounding in Bidens pilosa. Can. J. Bot. 70, 1451–1458 (1992). [Google Scholar]
- 8.Stahlberg R., Cosgrove D. J., Rapid alterations in growth rate and electrical potentials upon stem excision in pea seedlings. Planta 187, 523–531 (1992). [DOI] [PubMed] [Google Scholar]
- 9.Katicheva L., Sukhov V., Akinchits E., Vodeneev V., Ionic nature of burn-induced variation potential in wheat leaves. Plant Cell Physiol. 55, 1511–1519 (2014). [DOI] [PubMed] [Google Scholar]
- 10.Stahlberg R., Cosgrove D. J., Induction and ionic basis of slow wave potentials in seedlings of Pisum sativum L. Planta 200, 416–425 (1996). [DOI] [PubMed] [Google Scholar]
- 11.Zimmermann M. R., Maischak H., Mithöfer A., Boland W., Felle H. H., System potentials, a novel electrical long-distance apoplastic signal in plants, induced by wounding. Plant Physiol. 149, 1593–1600 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mousavi S. A., Chauvin A., Pascaud F., Kellenberger S., Farmer E. E., GLUTAMATE RECEPTOR-LIKE genes mediate leaf-to-leaf wound signalling. Nature 500, 422–426 (2013). [DOI] [PubMed] [Google Scholar]
- 13.Nguyen C. T., Kurenda A., Stolz S., Chételat A., Farmer E. E., Identification of cell populations necessary for leaf-to-leaf electrical signaling in a wounded plant. Proc. Natl. Acad. Sci. U.S.A. 115, 10178–10183 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Würtele M., Jelich-Ottmann C., Wittinghofer A., Oecking C., Structural view of a fungal toxin acting on a 14-3-3 regulatory complex. EMBO J. 22, 987–994 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pedersen B. P., Buch-Pedersen M. J., Morth J. P., Palmgren M. G., Nissen P., Crystal structure of the plasma membrane proton pump. Nature 450, 1111–1114 (2007). [DOI] [PubMed] [Google Scholar]
- 16.Haruta M., Gray W. M., Sussman M. R., Regulation of the plasma membrane proton pump (H(+)-ATPase) by phosphorylation. Curr. Opin. Plant Biol. 28, 68–75 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Falhof J., Pedersen J. T., Fuglsang A. T., Palmgren M., Plasma membrane H+-ATPase regulation in the center of plant physiology. Mol. Plant 9, 323–337 (2016). [DOI] [PubMed] [Google Scholar]
- 18.Kurenda A., Farmer E. E., Rapid extraction of living primary veins from the leaves of Arabidopsis thaliana. Protocol Exchange, 10.1038/protex.2018.119 (2018). [DOI] [Google Scholar]
- 19.Schaller A., Oecking C., Modulation of plasma membrane H+-ATPase activity differentially activates wound and pathogen defense responses in tomato plants. Plant Cell 11, 263–272 (1999). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Frick U. B., Schaller A., cDNA microarray analysis of fusicoccin-induced changes in gene expression in tomato plants. Planta 216, 83–94 (2002). [DOI] [PubMed] [Google Scholar]
- 21.Zhou Z., et al. , An Arabidopsis plasma membrane proton ATPase modulates JA signaling and is exploited by the Pseudomonas syringae effector protein AvrB for stomatal invasion. Plant Cell 27, 2032–2041 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Brüx A., et al. , Reduced V-ATPase activity in the trans-Golgi network causes oxylipin-dependent hypocotyl growth inhibition in Arabidopsis. Plant Cell 20, 1088–1100 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Marre E., Fusicoccin: A tool in plant physiology. Annu. Rev. Plant Physiol. 30, 273–288 (1979). [Google Scholar]
- 24.Spartz A. K., et al. , SAUR inhibition of PP2C-D phosphatases activates plasma membrane H+-ATPases to promote cell expansion in Arabidopsis. Plant Cell 26, 2129–2142 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Truernit E., Sauer N., The promoter of the Arabidopsis thaliana SUC2 sucrose-H+ symporter gene directs expression of β-glucuronidase to the phloem: Evidence for phloem loading and unloading by SUC2. Planta 196, 564–570 (1995). [DOI] [PubMed] [Google Scholar]
- 26.Haruta M., et al. , Molecular characterization of mutant Arabidopsis plants with reduced plasma membrane proton pump activity. J. Biol. Chem. 285, 17918–17929 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.DeWitt N. D., Sussman M. R., Immunocytological localization of an epitope-tagged plasma membrane proton pump (H(+)-ATPase) in phloem companion cells. Plant Cell 7, 2053–2067 (1995). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Robertson W. R., Clark K., Young J. C., Sussman M. R., An Arabidopsis thaliana plasma membrane proton pump is essential for pollen development. Genetics 168, 1677–1687 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Merlot S., et al. , Use of infrared thermal imaging to isolate Arabidopsis mutants defective in stomatal regulation. Plant J. 30, 601–609 (2002). [DOI] [PubMed] [Google Scholar]
- 30.Nagai T., et al. , A variant of yellow fluorescent protein with fast and efficient maturation for cell-biological applications. Nat. Biotechnol. 20, 87–90 (2002). [DOI] [PubMed] [Google Scholar]
- 31.Salvador-Recatalà V., Tjallingii W. F., Farmer E. E., Real-time, in vivo intracellular recordings of caterpillar-induced depolarization waves in sieve elements using aphid electrodes. New Phytol. 203, 674–684 (2014). [DOI] [PubMed] [Google Scholar]
- 32.Acosta I. F., Farmer E. E., Jasmonates. Arabidopsis Book 8, e0129 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yan Y., et al. , A downstream mediator in the growth repression limb of the jasmonate pathway. Plant Cell 19, 2470–2483 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chastain C. J., Hanson J. B., Control of proton efflux from corn root tissue by an injury-sensing mechanism. Plant Sci. Lett. 24, 97–104 (1982). [Google Scholar]
- 35.Schaller A., Frasson D., Induction of wound response gene expression in tomato leaves by ionophores. Planta 212, 431–435 (2001). [DOI] [PubMed] [Google Scholar]
- 36.Zimmermann M. R., Mithöfer A., Will T., Felle H. H., Furch A. C., Herbivore-triggered electrophysiological reactions: Candidates for systemic signals in higher plants and the challenge of their identification. Plant Physiol. 170, 2407–2419 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Pearce G., Strydom D., Johnson S., Ryan C. A., A polypeptide from tomato leaves induces wound-inducible proteinase inhibitor proteins. Science 253, 895–897 (1991). [DOI] [PubMed] [Google Scholar]
- 38.Farmer E. E., Ryan C. A., Regulation of expression of proteinase inhibitor genes in plant leaves. Plant Physiol. 98, 995–1002 (1992). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ahmad FH., Wu X., Stintzi A., Schaller A., Schulze W.X., The systemin signaling cascade as derived from time course analyses of the systemin-responsive phosphoproteome. Mol. Cell Proteomics 18, 1526–1542 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wang L., et al. , The systemin receptor SYR1 enhances resistance of tomato against herbivorous insects. Nat. Plants 4, 152–156 (2018). [DOI] [PubMed] [Google Scholar]
- 41.Lenglet A., et al. , Control of basal jasmonate signalling and defence through modulation of intracellular cation flux capacity. New Phytol. 216, 1161–1169 (2017). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.