Significance
Unlike other great apes, humans evolved multisystem capabilities for moderate-intensity EPA, but it is unknown if selection acted similarly on the heart. We present data from a sample of humans, chimpanzees, and gorillas showing that the human (LV) evolved numerous features that help to augment stroke volume (SV), enabling moderate-intensity EPA. We also show that phenotypic plasticity of the human LV trades off pressure adaptations for volume capabilities, becoming more similar to a chimpanzee-like heart in response to physical inactivity or chronic pressure loading. Consequently, the derived human heart appears partly dependent upon moderate EPA and its absence, in combination with a highly processed diet, likely contributes to the modern epidemic of hypertensive heart disease.
Keywords: blood pressure, physical activity, left ventricle, trade-off, human evolution
Abstract
Chimpanzees and gorillas, when not inactive, engage primarily in short bursts of resistance physical activity (RPA), such as climbing and fighting, that creates pressure stress on the cardiovascular system. In contrast, to initially hunt and gather and later to farm, it is thought that preindustrial human survival was dependent on lifelong moderate-intensity endurance physical activity (EPA), which creates a cardiovascular volume stress. Although derived musculoskeletal and thermoregulatory adaptations for EPA in humans have been documented, it is unknown if selection acted similarly on the heart. To test this hypothesis, we compared left ventricular (LV) structure and function across semiwild sanctuary chimpanzees, gorillas, and a sample of humans exposed to markedly different physical activity patterns. We show the human LV possesses derived features that help augment cardiac output (CO) thereby enabling EPA. However, the human LV also demonstrates phenotypic plasticity and, hence, variability, across a wide range of habitual physical activity. We show that the human LV’s propensity to remodel differentially in response to chronic pressure or volume stimuli associated with intense RPA and EPA as well as physical inactivity represents an evolutionary trade-off with potential implications for contemporary cardiovascular health. Specifically, the human LV trades off pressure adaptations for volume capabilities and converges on a chimpanzee-like phenotype in response to physical inactivity or sustained pressure loading. Consequently, the derived LV and lifelong low blood pressure (BP) appear to be partly sustained by regular moderate-intensity EPA whose decline in postindustrial societies likely contributes to the modern epidemic of hypertensive heart disease.
Humans have distinctive physical activity patterns and endurance capabilities compared to most mammals including the other great apes, our closest evolutionary relatives. Chimpanzees typically walk less than 4 km/d (1, 2) and spend most of the day feeding and resting interspersed with occasional bursts of intense RPA, such as climbing and fighting. At some point, hominins are thought to have evolved to engage in comparatively greater amounts of low-to-moderate-intensity EPA necessary to facilitate hunting and gathering (3). Hunter–gatherers in tropical habitats walk an average of 9–15 km/d (4), sometimes run long distances (LDs) (5), and have been measured to spend on average ∼3–6 h a day performing light-intensity EPAs, such as preparing food, 2–4 h doing moderate-intensity EPAs, such as walking and digging, and between 20–72 min a day performing vigorous EPAs, such as running (6, 7). Low-to-moderate-intensity EPA capabilities are equally critical for other preindustrial humans including subsistence farmers who spend many hours plowing, planting, and harvesting (8). Although musculoskeletal, thermoregulatory, and central nervous system adaptations that enable EPA are well documented in humans (5, 9), it is unknown whether the heart has been similarly selected, and if so, whether this selection involved trade-offs with implications for the susceptibility to heart disease in contemporary populations.
RPA and EPA impart markedly different physiological demands on the cardiovascular system. RPA is characterized by relatively brief but high-intensity contractions of large skeletal muscle groups and is accompanied by surges in arterial BP (10). During RPA, the LV must overcome this pressure challenge in order to sustain CO (the product of LV SV and heart rate), perfuse the brain, and maintain consciousness. The law of Laplace (wall tension is proportional to pressure times radius) dictates that LV wall stress, the primary determinant of cardiac work, is minimized in a LV with small chamber volume, thick walls, and spherical geometry. In contrast, EPA, even at moderate intensities, requires a sustained increase in CO to meet the body’s increased metabolic and thermoregulatory demands (11) and, thus, represents a volume stress for the LV. Accordingly, LVs with larger chambers, thinner walls, and an ability to fill quickly and completely at high heart rates are favorable for EPA. To date, the degree to which these principles apply to the evolutionary history of the human heart has not been rigorously examined.
We hypothesized that our closest ape relatives, mammals that regularly engage in RPA, would have LVs adapted to preferentially manage pressure stress while humans, a species selected for high levels of lifelong moderate-intensity EPA, would have LVs differentially adapted to accommodate volume stress. To test this hypothesis, we compared LV structure, LV function, and BP in African great apes and in a human sample with diverse patterns of habitual physical activity (SI Appendix). Specifically, our human sample included sedentary disease-free adults from a western population, Native American subsistence farmers (Tarahumara), who engage primarily in moderate-intensity EPA, and 2 groups of highly trained competitive athletes: resistance-trained American-style football linemen and endurance-trained LD runners (LDRs). Since humans and chimpanzees are sister taxa that share a last common ancestor, we focus primarily on the comparison between these 2 species but also include gorillas as an outgroup (SI Appendix). We further hypothesized that phenotypic plasticity of the human heart, which remodels in response to both sustained pressure and volume challenges (12), comes with physiologic trade-offs that can affect susceptibility to hypertensive heart disease. To test this secondary hypothesis, we coupled a series of physiologic provocation experiments with a comparative analysis of life span arterial BP data from the Tarahumara subsistence farmers and data collected through the U. S. National Health and Nutrition Examination Survey (NHANES) (SI Appendix).
Results
Comparative LV Structure and Function.
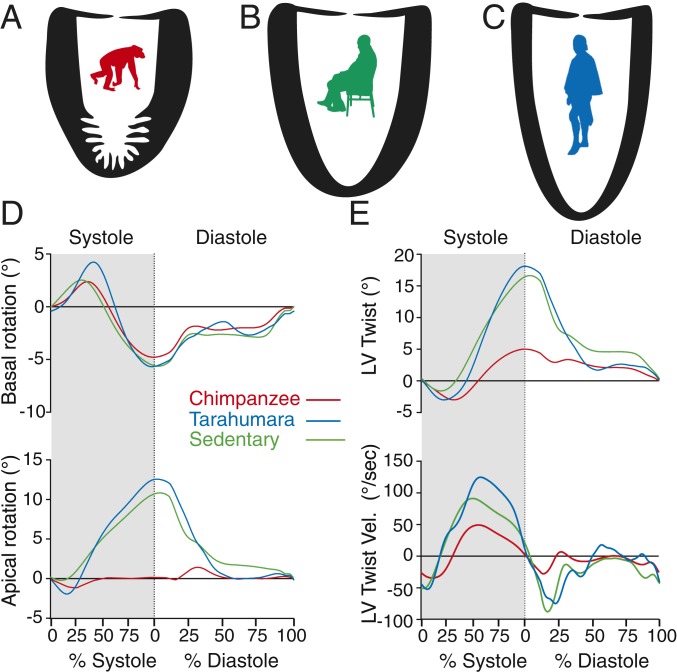
Data describing LV structure and function in adult chimpanzees, sedentary adult humans, subsistence farmers, highly trained American-style football linemen, and endurance runners are shown in Table 1 and SI Appendix, Table S1. Consistent with variable physical activity profiles, there was significant variation across the human groups with respect to absolute and scaled measures of left ventricular wall thickness (LV WT), ventricular volumes, and CO. Despite this intraspecies variation, all human groups differed similarly and significantly from chimpanzees across key cardiac features related to EPA (Tables 1, SI Appendix, Table S1, and Fig. 1 A–C). On average, human LVs were 16% longer, 10% less spherical, and lacked the prominent apical trabeculations observed in chimpanzees (SI Appendix and Fig. S1). In addition to these structural attributes, humans demonstrated enhanced early diastolic ventricular function as evidenced by higher early diastolic relaxation velocities (E′) (P < 0.001, Welch’s t test, Table 1). Another key functional difference observed among all human groups compared to chimpanzees was the significantly greater twisting (on average, 272%) during systole and more rapid untwisting during diastole (on average, 205%) (both P < 0.001, Welch’s t test, Table 1 and Fig. 1 D and E). In aggregate, these structural and functional differences translate into significantly larger LV SVs and COs in humans as compared to chimpanzees and, indeed, gorillas, who have cardiac attributes similar to chimpanzees (SI Appendix and Table S4).
Table 1.
Demographic, hemodynamic, and LV structure and function (scaled where appropriate) in semiwild sanctuary chimpanzees, Tarahumara, sedentary humans, LDRs, and American football linemen (AFL)
| Parameter assessed | Chimpanzeees (n = 43) | All humans (n = 164) | Tarahumara (n = 42) | Sedentary humans (n = 40) | LDRs (n = 42) | AFL (n = 40) | P value* |
| Demographics | |||||||
| Age (years) | 21 ± 5 | 25 ± 8 | 33 ± 8† | 27 ± 4† | 20 ± 2 | 19 ± 1 | 0.015 |
| Body Mass (kg) | 55 ± 8 | 76 ± 18 | 62 ± 8† | 75 ± 9† | 67 ± 5† | 102 ± 14† | <0.001 |
| Height (cm)‡ | 127 ± 9 | 176 ± 10 | 164 ± 5† | 176 ± 7† | 179 ± 6† | 185 ± 9† | <0.001 |
| Hemodynamics | |||||||
| SBP (mmHg) | 138 ± 21 | 116 ± 11 | 113 ± 11† | 115 ± 8† | 110 ± 8† | 127 ± 9 | <0.001 |
| DBP (mmHg) | 92 ± 17 | 67 ± 11 | 69 ± 10† | 67 ± 8† | 60 ± 8† | 74 ± 13† | <0.001 |
| Heart rate/mass−0.25 | 158 ± 28 | 178 ± 31 | 165 ± 26 | 179 ± 24† | 159 ± 24 | 209 ± 24† | 0.002 |
| CO (L/min)/mass0.75 | 0.147 ± 0.047 | 0.208 ± 0.049 | 0.181 ± 0.036† | 0.188 ± 0.035† | 0.250 ± 0.047† | 0.210 ± 0.045† | <0.001 |
| LV structure | |||||||
| Mid-LV WT (cm)/mass0.25 | 0.376 ± 0.089 | 0.307 ± 0.053 | 0.264 ± 0.033† | 0.323 ± 0.039† | 0.274 ± 0.026† | 0.372 ± 0.024 | <0.001 |
| Mid-LV RWT | 0.427 ± 0.113 | 0.366 ± 0.074 | 0.317 ± 0.058† | 0.378 ± 0.050 | 0.322 ± 0.035† | 0.452 ± 0.058 | 0.02 |
| EDV (ml)/mass1.0 | 1.8 ± 0.3 | 2.0 ± 0.5 | 1.7 ± 0.3 | 1.7 ± 0.3 | 2.8 ± 0.3† | 1.7 ± 0.2 | 0.201 |
| ESV (ml)/mass1.0 | 0.91 ± 0.24 | 0.79 ± 0.29 | 0.63 ± 0.14† | 0.63 ± 0.15† | 1.20 ± 0.18† | 0.68 ± 0.12† | 0.095 |
| LVL (cm)/mass0.25 | 2.80 ± 0.20 | 3.00 ± 0.29 | 3.11 ± 015† | 2.79 ± 0.20 | 3.31 ± 0.20† | 2.78 ± 0.16 | <0.001 |
| LV OT (cm)/mass0.25 | 0.720 ± 0.063 | 0.728 ± 0.055 | 0.755 ± 0.043 | 0.698 ± 0.046 | 0.768 ± 0.039† | 0.688 ± 0.043 | 1 |
| SI | 1.68 ± 0.15 | 1.85 ± 0.17 | 1.90 ± 0.16† | 1.84 ± 0.11† | 1.93 ± 0.15† | 1.72 ± 0.17 | <0.001 |
| LV Systolic function | |||||||
| SV (ml)/mass1.0 | 0.926 ± 0.219 | 1.192 ± 0.304 | 1.109 ± 0.194† | 1.057 ± 0.194 | 1.578 ± 0.205† | 1.006 ± 0.197 | <0.001 |
| S′ (cm/s) | 0.0624 ± 0.0134 | 0.1114 ± 0.0157 | 0.1015 ± 0.0157† | 0.1143 ± 0.0093† | 0.1067 ± 0.0146† | 0.1235 ± 0.0133† | <0.001 |
| Peak LV twist (°)§ | 6.4 ± 3.3 | 17.4 ± 6.2 | 18.0 ± 6.3† | 18.4 ± 5.7† | 14.4 ± 5.2† | 16.5 ± 7.4† | <0.001 |
| LV diastolic function | |||||||
| E (cm/s) | 0.77 ± 0.17 | 0.83 ± 0.14 | 0.85 ± 0.20 | 0.84 ± 0.11 | 0.88 ± 0.13 | 0.77 ± 0.09 | 0.241 |
| A (cm/s) | 0.39 ± 0.15 | 0.51 ± 0.15 | 0.51 ± 0.15† | 0.48 ± 0 0.11† | 0.39 ± 0.09 | 0.65 ± 0.14† | <0.001 |
| E/A | 2.21 ± 0.73 | 1.82 ± 0.69 | 1.79 ± 0.65 | 1.82 ± 0.44 | 2.38 ± 0.73 | 1.26 ± 0.38† | 0.029 |
| E′ (cm/s) | 0.101 ± 0.024 | 0.136 ± 0.032 | 0.152 ± 0.030† | 0.115 ± 0.014 | 0.168 ± 0.013† | 0.107 ± 0.018 | <0.001 |
| A′ (cm/s) | 0.062 ± 0.018 | 0.073 ± 0.025 | 0.102 ± 0.020† | 0.051 ± 0.012 | 0.065 ± 0.011 | 0.074 ± 0.020 | 0.017 |
| LV UTV (°/s)§ | −53 ± 23 | −109 ± 38 | −109 ± 39† | −121 ± 44† | −109 ± 27† | −96 ± 36† | <0.001 |
SBP, systolic blood pressure; DBP, diastolic blood pressure; CO, cardiac output; LV, left ventricular; WT, wall thickness; RWT, relative WT; EDV, end diastolic volume; ESV, end systolic volume; OT, outflow tract; SV, stroke volume; S′, systolic ventricular wall velocity; LVL, LV length; E, early diastolic transmitral filling velocity; A, late diastolic transmitral filling velocity; E:A, the ratio of early to late diastolic transmitral filling velocities; E′, early diastolic ventricular wall velocity; A′, late diastolic ventricular wall velocity; UTV, untwisting velocity.
Comparison of chimpanzees to all humans using 2-tailed unpaired Welch’s t tests for unequal variances. P values are adjusted for familywise error (37 tests) using the sequential Bonferroni method.
P < 0.05 for comparison of chimpanzees to individual human groups using 2-tailed unpaired Welch’s t tests for unequal variances. P values are adjusted for familywise error (148 tests) using the sequential Bonferroni method.
Calculated via the summation of lower limb and crown to rump measurements. n = 207 for all analyses except: EDV/mass1.0 (198); ESV/mass1.0 (198); SV/mass1.0 (198); CO/mass0.75 (198); sphericity index (SI) (204); MVE (206); MVA (206); MV E/A (206); mean E′ (206); mean A′ (206); mean S′ (205); twist peak (97); UTV (97). See SI Appendix for full table including all nonscaled variables.
For 2 angular variables (peak LV twist and LV UTV) a circular-linear model with a von Mises distribution for the response was used to compare means across groups.
Fig. 1.
Comparison of the LV structure and the function in chimpanzees and 2 representative human groups: sedentary Americans and Tarahumara subsistence farmers. (A–C) Scaled outlines of LVs among the 3 groups highlighting differences in trabeculation, WT, chamber size, and shape. (D) Basal and apical systolic (shaded) and diastole (unshaded) rotation. (E) Magnitude of LV twisting, untwisting, and the respective velocities during systole (shaded) and diastole (unshaded). While chimpanzees lack apical rotation and, thus, overall systolic twist, sedentary humans and subsistence farmers have similar levels of systolic twisting and early diastolic UTVs.
Physical Activity and LV Morphology.
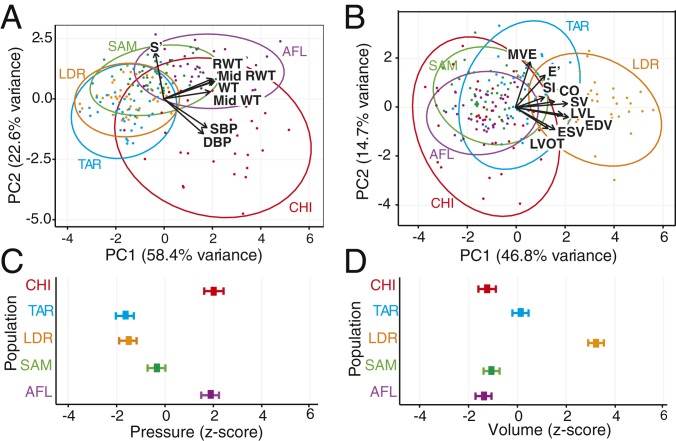
To examine the relationship between habitual physical activity patterns and structural and functional LV attributes within a phylogenetic context, we first performed principal component (PC) analyses on the combined chimpanzee and human samples using variables determined a priori to be associated with pressure or volume adaptation (Fig. 2 A and B and SI Appendix) and then used the PC scores in general linear models (GLMs) to assess differences between groups (Fig. 2 C and D). In the analysis of pressure-related variables in which the first PC primarily reflects absolute and relative LV WT (Fig. 2 A and C), highly trained AFL, who engage almost exclusively in RPA, differ significantly from the other human groups (P < 0.0001, GLM, SI Appendix and Table S5) and converge on the chimpanzee phenotype. In the analysis of volume-related variables in which the first PC primarily reflects LV chamber size, SV, and diastolic function (Fig. 2 B and D), highly trained endurance athletes and, to a lesser degree, the subsistence farmers (P < 0.0001, GLM), differ from chimpanzees, AFL, and sedentary humans who do not differ significantly from each other (P > 0.49, GLM, SI Appendix and Table S6). Importantly, the sedentary disease-free humans also demonstrate a shift toward a chimpanzee-like cardiac phenotype with moderate pressure adaptation and an absence of volume adaptation compared to humans, who engage in either routine moderate-intensity EPA (subsistence farmers) or high-intensity/high volume endurance training (runners). To summarize, these data suggest significant cross-species associations among physical activity patterns, LV structure, and function.
Fig. 2.
Comparison of the LV structure and the function among chimpanzees and 4 human groups with diverse physical activity histories. (A and B) Principal component analysis of LV variables determined a priori to be associated with either pressure (A) or volume (B) exposure. Principal component scores are expressed as standardized Z scores. (C and D) General linear model of first principal component scores for pressure (C) and volume (D) regressed on group identity with means and 95% confidence intervals. Groups analyzed: chimpanzees (CHI); sedentary Americans (SAM); American-style football linemen (AFL); long-distance runners (LDR); and Tarahumara subsistence farmers (TAR). Pressure variables are as follows: peak systolic LV tissue velocity (S′), Mid-LV Mid RWT, basal-LV RWT, Mid-LV Mid WT, SBP, DBP; volume variables are early diastolic transmitral valve blood flow velocity (MVE), peak early diastolic tissue velocity (E′), SI, CO, SV, LVL, LV EDV, LV ESV, LV OT diameter. All ventricular structural data entered into the analyses were scaled as per SI Appendix.
Pressure Versus Volume: Adaption with Trade-off.
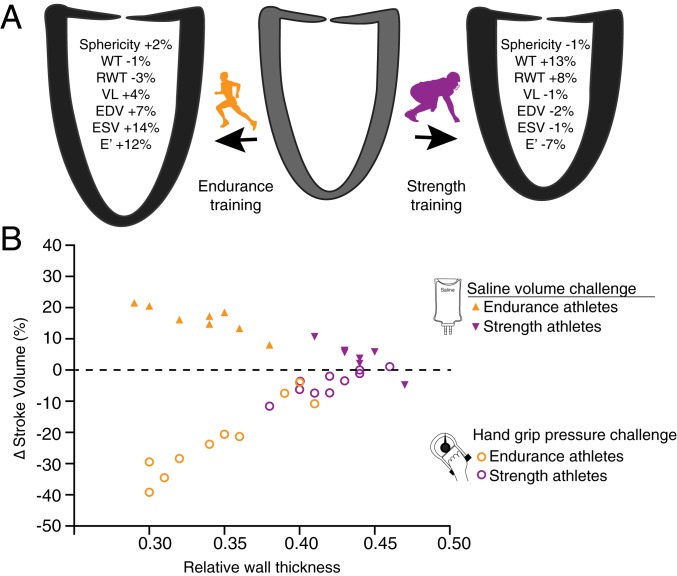
To test if adaptations associated with either high pressure or high volume compromise the ability of the LV to subsequently cope with a volume or pressure challenge, we examined the functional LV response to isolated pressure and volume challenges in highly trained LDRs and AFL (SI Appendix, SI Text). The structural and functional LV responses to a 90-d period of sport specific training intensification among LDRs and American-style football linemen are shown in (Fig. 3A and SI Appendix and Tables S7–S9). As previously reported in other cohorts participating in the same exercise training program (SI Appendix), highly trained LDRs demonstrated LV dilation with minimal wall thickening (eccentric remodeling) while trained American-style football linemen experienced LV wall thickening with no change in chamber size (concentric remodeling) together with an increase in resting BP. Then, to test if eccentric and concentric remodelings compromise the LV’s ability to handle the alternate hemodynamic stress, we examined LV functional responses to isolated pressure and volume challenges in both cohorts (Fig. 3B and SI Appendix and Tables S10 and S11). When pressure challenged with an isometric handgrip test, AFL maintained a nearly consistent LV SV (Δ = −4 ± 4%; P = 0.14, permutation test) while the LDRs experienced a 21 ± 11% decline (P = 0.074, permutation test), a 5.3-fold decrease compared with AFL (P = 0.0024, permutation test) (Fig. 3B and SI Appendix and Table S10). Conversely, when volume challenged with a large bolus of i.v. saline, the LDRs augmented LV SV by 16 ± 4% (P = 0.12, permutation test), 2.7 times (P = 0.0017, permutation test) more than the AFL (Δ = 6 ± 5%, P = 0.31, permutation test) (Fig. 3B and SI Appendix and Table S11). Thus, the divergent forms of LV remodeling stimulated by intense RPA and EPA training over a prolonged period of time, appear to be associated with significant reductions in the LV’s ability to handle the alternative form of intracardiac stress.
Fig. 3.
Trade-off between EPA and RPA training on the LV structure and function. (A) After 90 d of intensive training, the EPA athletes demonstrated eccentric LV remodeling characterized by increases in LV chamber volume (∆ EDV = 7%, ∆ RWT = −3%) and improved diastolic function (∆ E′ = 12%) while the RPA athletes demonstrated concentric remodeling characterized by (∆ WT = 13%, ∆ RWT = 8%) and reduced diastolic function (∆ E′ = −7%). (B) Relationship between RWT and LV SV in response to volume challenge (Top) and pressure challenge (Bottom) among these athletes after training. RPA-trained athletes with relatively thicker LV walls were less able than EPA-trained athletes to increase SV (∆SV = 6% vs. ∆SV = 16%) when challenged with rapid intravascular infusion of normal saline. In contrast, RPA-trained athletes were better able to preserve SV (∆SV = −4%) when pressure challenged with an isometric grip test than EPA-trained athletes (∆SV = −21%).
BP.
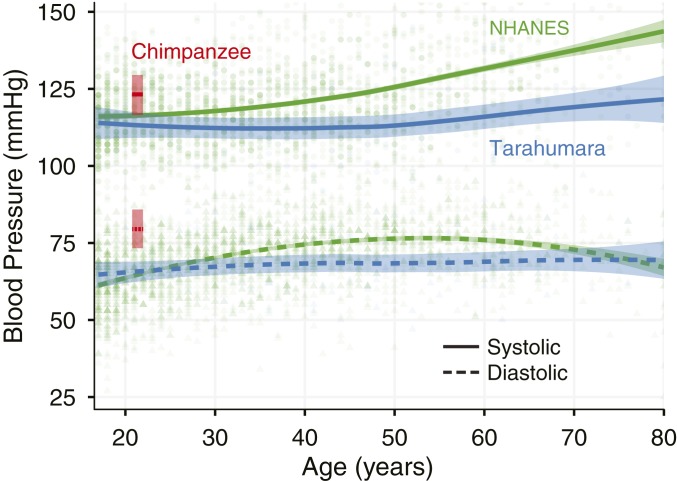
In a cross-sectional comparison, chimpanzees demonstrated significantly higher BPs (138 ± 21/92 ± 17 mmHg) than the combined human sample (116 ± 11/67 ± 11 mmHg, Table 1 and Fig. 4). Note that, within the human sample, Tarahumara subsistence farmers, young disease-free sedentary men, and highly trained LDRs demonstrated similar and significantly lower BPs than American-style football linemen (127 ± 9/74 ± 13 mmHg, P value for comparison with chimpanzees >0.05), who possessed values similar to those reported previously (13) and that approached those seen in chimpanzees. A comparative analysis of age-related BP trends between an expanded sample of Tarahumara subsistence farmers (n = 103, age range = 14–94 y) and a similarly age-diverse sample of postindustrial Americans (n = 3,495, age range = 8–80 y) from the NHANES database is shown in Fig. 4. As previously established in other postindustrial populations (14), systolic SBP among the NHANES sample were higher with advancing age with the values observed in the sixth decade of life similar to those observed in adult, but not elderly, sanctuary chimpanzees. In contrast, systolic and DBPs in the Tarahumara, such as other subsistence farmer and hunter–gatherer populations (6, 15, 16), was comparatively low and was not moderated by age.
Fig. 4.
Difference in SBP (solid line) and DBP (dashed line) in male chimpanzees, Tarahumara subsistence farmers, and sedentary Americans (NHANES data). Tarahumara subsistence farmers (blue) and sedentary Americans (green) are presented relative to age (shaded areas indicate 95% confidence interval of the mean), whereas a point estimate of mean BP relative to mean age is provided for the smaller chimpanzee cohort (red). Although BP increases with age in the NHANES sample, this is not apparent in the Tarahumara.
Discussion
The primary motivation for this study, which integrates comparative functional anatomical and physiological data with experimental data, was to test if the human heart evolved to facilitate regular bouts of sustained moderate-intensity EPA and, if so, to what degree this selection led to trade-offs relevant to the contemporary epidemic of heart disease. A first conclusion is that chimpanzees and gorillas, which represent the ancestral condition, possess thick-walled, spherical, hypertrabeculated LVs, and are, thus, well adapted to counter surges in BP during intense RPA. In contrast, the human LV, despite considerable within-species variation, is comparatively thin walled, elongated, and minimally trabeculated. The derived human LV structure likely improves ventricular compliance (17), untwisting velocity, and diastolic tissue velocities, thus, enhancing ventricular filling. These adaptations suggest selection for regular moderate-intensity EPA abilities, which require sustained increases in CO. Together, these findings are supportive of the hypothesis that, cardiac evolution among humans, in conjunction with other previously described musculoskeletal and thermoregulatory adaptations (5, 9), played a crucial role in enabling the human capability for moderate-intensity EPA. Second, our data suggest that the phenotypic plasticity of the LV—its propensity to differentially remodel to divergent hemodynamic stimuli—leads to an important trade-off. Among humans, LV remodeling in response to sustained pressure loading facilitates maintenance of CO during pressure stress. This remodeling however, comes at the cost of a reduced CO augmentation during volume stress. In contrast, LV remodeling in response to sustained volume loading produces the opposite result. Although we demonstrate the physiology of this trade-off among highly trained athletes exposed to the extremes of the pressure-volume spectrum, its implications are most notable when applied to the rapidly growing global population of sedentary humans. Specifically, we found, in relatively young disease-free men, that the absence of routine moderate-intensity EPA is associated with a shift toward an apelike LV phenotype which occurs independently of hypertension. As corroborated by abundant data on the effects of physical activity on the cardiovascular system (18), EPA beginning early in life and maintained into old age helps to maintain normal BP. Our data further emphasize the cardiovascular importance of regular EPA by showing that the maintenance of the derived human LV phenotype may also require routine bouts of moderate-intensity EPA across the life span. Thus, the independent and combined effects of routine lifelong moderate EPA on the derived LV and arterial BP may help to reduce the corollary risk of hypertensive heart disease.
Comparing the cardiac phenotype of the Tarahumara subsistence farmers, who engage in lifelong low-to-moderate-intensity EPA and, thus, represent typical preindustrial humans as well as the average human LV phenotype to both chimpanzees and gorillas (Fig. 1, Table 1, and SI Appendix, Table S1), supports the inference of a transition over the course of human evolution from hearts adapted to handle high pressures to hearts generally adapted to handle higher volumes but with the capacity to remodel in response to chronically elevated hemodynamic demands. Although the human LV may have evolved to facilitate several hours a day of regular moderate-intensity EPA, presumably the dominant form of physical activity in preindustrial human populations, the heart’s ability to withstand and remodel in response to variable and occasional levels of RPA, could also have been advantageous. RPAs demand strength and power rather than endurance and are not dependent on CO augmentation. Instead, the primary role of the LV during RPA is to maintain CO in the face of high downstream arterial resistance caused by intense skeletal muscle contraction (10), thus, avoiding rapid reductions in blood flow to the brain. Although RPA may have been less frequent than EPA during human evolution, activities, such as lifting or fighting, which impose substantial physiological challenges to the heart, would likely have been important for survival. Thus, conservation of LV phenotypic plasticity in response to sustained elevations in pressure would likely have been advantageous and, thus, selected for.
Within this evolutionary context, the LV variation we observed among the diverse human groups sampled here sheds a different perspective on differences in LV structure and function between sedentary adults and athletes who engage in extreme degrees of either chronic EPA or RPA. AFL experience LV remodeling that better enables them to cope with the acute and chronic increases in pressure observed in response to RPA training, while highly trained LDRs’ LVs remodel differentially to meet the extreme volume demand of competitive high-intensity EPA (11). Such plastic responses to sustained changes in the prevailing hemodynamic load were likely conserved because they would have been advantageous during hominin evolution. Hemodynamically mediated variations in LV structure and function, however, come with a fundamental trade-off that we document in humans. As shown in Fig. 3, decreases in the RWT of the LV augments the ability to increase CO, the requisite response for EPA at the expense of the ability to cope with the high-pressure loads associated with RPA. Similarly, increases in relative LV wall thickness compromise CO augmentation in the setting of increased preload but enhance the heart’s ability to maintain CO during surges in afterload. Because of remodeling, the LVs of humans who engage primarily in EPA develop less capacity to tolerate acute surges in pressure during RPA, while the LVs of humans who engage primarily in RPA become less responsive to volume loading and, thus, less capable of augmenting CO during EPA.
It is important to note that we do not know and are unable to test whether chimpanzees also possess the same type and degree of LV phenotypic plasticity evident among humans. chimpanzees, however, do not and are probably unable to engage in significant levels of sustained EPA due to an inability to cool effectively by sweating (9, 19). In addition, their lower percentage of slow-twitch muscle fibers and 2-fold higher metabolic cost of locomotion in comparison to humans limits their capacity for endurance (9, 20). These physiologic limitations, combined with the ecological niche inhabited by most chimpanzees, raise the hypothesis that there was little selection for endurance in this species and that they rarely experience the physiological stimuli, such as blood volume expansion and sustained periods of elevated heart rate required for LV volume adaptation. In contrast, selection for LVs with thinner walls, less apical trabeculation, and a resultant increase in compliance (17), suggest there may have been an evolutionary shift in ventricular structure at some point in hominin evolution following the divergence from chimpanzees.
The combined effects of selection and phenotypic plasticity on the LV, which trade off LV pressure adaptation for volume capabilities, provide different insight into the ultimate evolutionary bases for why regular exposure to moderate-intensity EPA helps mitigate against age-related increases in BP (18) and ultimately hypertensive heart disease. Unlike chimpanzees and gorillas, almost all humans prior to the industrial era regularly engaged throughout adult life in, at least, several hours per day of low-to-moderate-intensity EPA including during postreproductive years (4, 6, 7). However, we show that, in the setting of either chronic pressure stimuli (American-style football linemen) or an absence of sustained EPA (sedentary humans), the phenotypically plastic human LV remodels away from the derived phenotype. In both cases, the absence of sustained EPA results in a mismatch condition to which humans are poorly adapted resulting in a more chimpanzee-like LV phenotype. We show that, in the football linemen, this remodeling is coupled with a relative loss in the ability to augment SV in response to a volume challenge and is likely to reduce the capacity to enhance CO in response to EPA. As such, routine moderate-intensity EPA throughout the life span, which up until the Industrial Revolution, was fundamental to human survival, may be essential in helping to maintain the derived human LV.
It is well documented that western individuals who engage in lifelong EPA are less susceptible to age-related increases in BP (18) and, thus, hypertensive remodeling of the LV. In addition to influencing the heart, lifelong EPA also influences the systemic circulation by promoting growth and elasticity of the peripheral arterial vasculature thereby maintaining low systemic resistance (21). People who are physically inactive or engage wholly in RPA (22), develop blood vessels with reduced elasticity, dimensions, and vasodilatory capacity (21), thus, setting the stage for hypertension. It has long been known that chronic pressure loading on the heart and blood vessels perpetuates adverse cardiovascular remodeling with a shift toward a thicker-walled LV (12). However, a critical finding from our analysis is the intermediate LV phenotype observed among sedentary adults with normal BP (Fig. 2C). While further confirmatory work is needed, the observation that the LV remodels early in life in the absence of EPA and that this occurs prior to the onset of hypertension, challenges the conventional paradigm that it is increased in BP that initiate adverse cardiac remodeling.
The sanctuary chimpanzees we assessed had significantly higher BPs than similar-aged humans (Table 1 and Fig. 4). In captivity, chimpanzees begin to experience age-related increases in BP by the second decade of life (23). As noted above, in the absence of data on cardiovascular phenotypic plasticity in chimpanzees, which is likely constrained by their physiology, we do not know if high levels of habitual EPA would result in lower BP and more volume adapted LVs. Irrespective, in chimpanzees and almost all other mammals excluding humans, the onset of chronic diseases, such as hypertension after the age of reproductive capability is evolutionarily irrelevant. Whereas, chimpanzees in the wild rarely live beyond the age of female fecundity (∼50 y), the modal age of death among human foragers who survive childhood is 68–78 y (16, 24). Among preindustrial humans, this enhanced longevity comes with minimal age-related decline in low-moderate-intensity EPA because postreproductive foragers play a key role in gathering and hunting surplus food for their children and grandchildren (6, 16). As such, longevity, coupled with stably low BP, is distinctively evolved in humans and partly dependent on EPA.
Finally, our comparative analyses shed light on how and why low levels of regular EPA contribute to the growing global burden of hypertensive heart disease (25, 26). For millions of years, hominins engaged in daily low-moderate-intensity EPA, including during middle and old age, leading to selection for capacities that favor increased CO, and an absence of selection for coping with the effects of cardiac remodeling in response to inactivity. Today, however, postindustrial conditions encourage physical inactivity, which in synergy with easy access to highly processed high sodium diets (27), initiates pathologic cardiac remodeling, and hypertension, often at an early age. We hypothesize that this process begins with a shift toward a thicker less compliant cardiac phenotype, which then impedes the ability to augment CO and, hence, EPA capability. This response, which is then followed by the onset of hypertension, sets up a deleterious pathophysiologic cycle in which persistently inadequate EPA leads to further remodeling and marked hypertension. If left uninterrupted over the course of a normal human life span, this cascade of events culminates in hypertensive heart disease, one of the most common causes of morbidity and mortality in the developed world (28). Critically, this evolutionary perspective presents clear targets for intervention and subsequent disease reduction.
Materials and Methods
All animals included in this study were cared for in line with recommended guidelines, and procedures involving animals received approval from the Cardiff Metropolitan University Ethics Committee. All aspects of recruitment, enrollment, and data collection for the human groups were approved by the Partners Human Research Committee (Somerville, MA, USA) and Harvard University, and all participants provided informed consent as outlined in the SI Appendix. A single cardiac ultrasound protocol as outlined previously (29) and in line with the recommendations of the American Society of Echocardiography (30), was used for the assessment of cardiac structure and function in all groups studied. Full details of the echocardiographic protocol, the assessment of BP, the hemodynamic pressure and volume challenges, as well as the statistical methods employed are provided in the SI Appendix. All datasets generated during and/or analyzed during the current study are available from the corresponding authors on reasonable request.
Supplementary Material
Acknowledgments
We thank the staff at the sanctuaries and zoos that care for the animals included in this study. For assistance in Mexico, we thank M. Mahaffey, S. Cubesare, C. Arzate, E. Rubio, and H. Ramos. For assistance with the NHANES data extraction we thank Bryony Curry. For comments and feedback, we thank A. Garber, E. Hart, N. Holowka, G. Howatson, D. Oxborough, H. Pontzer, D. Raichlen, C. Sharp, E. Stöhr, I. Wallace, and R. Wrangham.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1906902116/-/DCSupplemental.
References
- 1.Pontzer H., Wrangham R. W., Climbing and the daily energy cost of locomotion in wild chimpanzees: Implications for hominoid locomotor evolution. J. Hum. Evol. 46, 317–335 (2004). [DOI] [PubMed] [Google Scholar]
- 2.Pontzer H., Wrangham R. W., Ontogeny of ranging in wild chimpanzees. Int. J. Primatol. 27, 295–309 (2006). [Google Scholar]
- 3.Kelly R. L., Kelly R. L., The Lifeways of Hunter-Gatherers: The Foraging Spectrum (Cambridge University Press, Cambridge, ed. 2, 2013), pp. xix, 362 pp.
- 4.Marlowe F. W., Hunter-gatherers and human evolution. Evol. Anthropol. 14, 54–67 (2005). [DOI] [PubMed] [Google Scholar]
- 5.Bramble D. M., Lieberman D. E., Endurance running and the evolution of Homo. Nature 432, 345–352 (2004). [DOI] [PubMed] [Google Scholar]
- 6.Raichlen D. A., et al. , Physical activity patterns and biomarkers of cardiovascular disease risk in hunter-gatherers. Am. J. Hum. Biol. 29, e22919 (2017). [DOI] [PubMed] [Google Scholar]
- 7.Gurven M., Jaeggi A. V., Kaplan H., Cummings D., Physical activity and modernization among Bolivian Amerindians. PLoS One 8, e55679 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.James W. P. T., Schofield E. C., Human Energy Requirements: A Manual for Planners and Nutritionists (Oxford University Press, Oxford, UK, 1990). [Google Scholar]
- 9.Lieberman D. E., Human locomotion and heat loss: An evolutionary perspective. Compr. Physiol. 5, 99–117 (2015). [DOI] [PubMed] [Google Scholar]
- 10.MacDougall J. D., Tuxen D., Sale D. G., Moroz J. R., Sutton J. R., Arterial blood pressure response to heavy resistance exercise. J. Appl. Physiol. 58, 785–790 (1985). [DOI] [PubMed] [Google Scholar]
- 11.Levine B. D., et al. , Eligibility and disqualification recommendations for competitive athletes with cardiovascular abnormalities: Task force 1: Classification of sports: Dynamic, static, and impact: A scientific statement from the American heart association and American college of cardiology. J. Am. Coll. Cardiol. 66, 2350–2355 (2015). [DOI] [PubMed] [Google Scholar]
- 12.Grossman W., Jones D., McLaurin L. P., Wall stress and patterns of hypertrophy in the human left ventricle. J. Clin. Invest. 56, 56–64 (1975). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hedman K., et al. , Blood pressure in athletic preparticipation evaluation and the implication for cardiac remodelling. Heart 105, 1223–1230 (2019). [DOI] [PubMed] [Google Scholar]
- 14.Franklin S. S., et al. , Hemodynamic patterns of age-related changes in blood pressure. The Framingham Heart Study. Circulation 96, 308–315 (1997). [DOI] [PubMed] [Google Scholar]
- 15.Truswell A. S., Kennelly B. M., Hansen J. D., Lee R. B., Blood pressures of kung bushmen in Northern Botswana. Am. Heart J. 84, 5–12 (1972). [DOI] [PubMed] [Google Scholar]
- 16.Kaplan H., Hill K., Lancaster J., Hurtado A. M., A theory of human life history evolution: Diet, intelligence, and longevity. Evol. Anthropol. 9, 156–185 (2000). [Google Scholar]
- 17.Halaney D. L., et al. , The effect of trabeculae carneae on left ventricular diastolic compliance: Improvement in compliance with trabecular cutting. J. Biomech. Eng. 139, 031012 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Liu J., et al. , Effects of cardiorespiratory fitness on blood pressure trajectory with aging in a cohort of healthy men. J. Am. Coll. Cardiol. 64, 1245–1253 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kamberov Y. G., et al. , Comparative evidence for the independent evolution of hair and sweat gland traits in primates. J. Hum. Evol. 125, 99–105 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sockol M. D., Raichlen D. A., Pontzer H., Chimpanzee locomotor energetics and the origin of human bipedalism. Proc. Natl. Acad. Sci. U.S.A. 104, 12265–12269 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Green D. J., Hopman M. T., Padilla J., Laughlin M. H., Thijssen D. H., Vascular adaptation to exercise in humans: Role of hemodynamic stimuli. Physiol. Rev. 97, 495–528 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Miyachi M., et al. , Greater age-related reductions in central arterial compliance in resistance-trained men. Hypertension 41, 130–135 (2003). [DOI] [PubMed] [Google Scholar]
- 23.Eichberg J. W., Shade R. E., “Normal” blood pressure in chimpanzees. J. Med. Primatol. 16, 317–321 (1987). [PubMed] [Google Scholar]
- 24.Wood B. M., Watts D. P., Mitani J. C., Langergraber K. E., Favorable ecological circumstances promote life expectancy in chimpanzees similar to that of human hunter-gatherers. J. Hum. Evol. 105, 41–56 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Global Burden of Disease Cancer Collaboration , Fitzmaurice C., et al. ; Global, regional, and national cancer incidence, mortality, years of life lost, years lived with disability, and disability-adjusted life-years for 32 cancer groups, 1990 to 2015: A systematic analysis for the global burden of disease study. JAMA Oncol. 3, 524–548 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lieberman D. E., The Story of the Human Body: Evolution (Health and Disease, Pantheon, New York, 2013). [PubMed] [Google Scholar]
- 27.Mozaffarian D., et al. ; Global Burden of Diseases Nutrition and Chronic Diseases Expert Group , Global sodium consumption and death from cardiovascular causes. N. Engl. J. Med. 371, 624–634 (2014). [DOI] [PubMed] [Google Scholar]
- 28.Mora S., Cook N., Buring J. E., Ridker P. M., Lee I. M., Physical activity and reduced risk of cardiovascular events: Potential mediating mechanisms. Circulation 116, 2110–2118 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Shave R., et al. , Echocardiographic assessment of cardiac structure and function in great apes: A practical guide. Int. Zoo Yearb. 48, 218–233 (2014). [Google Scholar]
- 30.Lang R. M., et al. , Recommendations for cardiac chamber quantification by echocardiography in adults: An update from the American society of Echocardiography and the european association of cardiovascular imaging. Eur. Heart J. Cardiovasc. Imaging 16, 233–270 (2015). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.