How do we come to experience our body as our own? When we look at our hands, for example, we immediately sense that they are part of our body. This experience of the limbs and other body parts being one’s own is referred to as the sense of “body ownership” (1). Body ownership allows us to quickly and accurately identify and localize our limbs in space and to discriminate between the physical self and the external world. However, how does the brain represent one’s own body, and what kind of processes mediate the sense of body ownership? An important realization is that we experience our body as a coherent combination of sensory experiences from our different sensory modalities (multisensory perception). When we move our hand in full view, for example, the visual impressions of the hand moving and the somatosensory sensations from the stretching skin and muscles are seemingly blended together into a unitary multisensory experience of a single hand. There is evidence that the multisensory coherence of bodily perception is critical for the sense of body ownership (1). A striking demonstration of this is the “rubber hand illusion” (2), in which the experimental manipulation of the spatial and temporal correspondences of visual and somatosensory information leads to the illusory sensation of a rubber arm being part of one’s own body. In its classic version, repeated synchronous strokes applied to a rubber hand, in full view, and to the participant’s real hand, which is hidden, elicit the illusion that the rubber hand is one’s own and that the model hand is capable of sensing the strokes one observes. It has been theorized that this illusion happens as a consequence of the brain’s perceptual systems, inferring that what one sees and what one feels are the same thing—one’s hand—leading to the fusion of visual and somatosensory impressions (3, 4). However, how does the brain “know” which sensory signals originate from one’s body and which do not? This is the multisensory binding problem, and behavioral studies on the perception of external events suggest that the brain solves this problem by a process of “causal inference” in which the brain infers the probability that sensory signals share a common cause (5, 6) (for studies on heading perception, see ref. 7). In this computational theoretical framework, the most likely causal structure of the different sensory events is estimated based on spatial proximity, simultaneity, temporal correlation, and prior perceptual experiences, and, in turn, this inferred causal structure determines to what extent the sensory signals should be fused. However, it is unclear whether the brain uses causal inference in the multisensory perception of the body and body ownership. In PNAS, Fang et al. (8) present evidence from electrophysiological recordings in awake-behaving nonhuman primates showing that the integration of visual and somatosensory information from the arm is determined by causal inference and that this dynamic process is implemented by neurons in the premotor cortex. Moreover, the probabilistic combination of vision and proprioception is systematically related to the subjective sense of arm ownership, as revealed in complementary behavioral experiments in humans. These results are important because they advance our understanding of causal inference in multisensory own-body perception and reveal the neuronal basis of this process.
When starting their project, the authors first had to overcome a substantial methodical challenge: Monkeys cannot tell the experimenters about their subjective experiences of arm ownership (8). The authors solved this problem by using an indirect behavioral proxy of body ownership: proprioceptive drift (2). Proprioceptive drift measures how strongly participants perceive their hand to be located closer to a visually presented (rubber) hand under conditions of visuoproprioceptive disparity, and the stronger the subjective illusion of hand ownership is, the higher the proprioceptive drift, in most cases (9). Fang et al. developed a version of the rubber hand illusion based on reaching movements toward external visual targets with visual feedback provided by a video-based system. This system could introduce spatial disparity between the image of the arm displayed on the screen and the subject’s real (unseen) arm under the screen, in line with the classic rubber hand illusion paradigm. The monkeys were trained to perform a reaching task toward a visually presented target and to hold the hand still in the target area for 0.5 s, during which neuronal activity uncontaminated by movement was registered. Importantly, the degree of mispointing served as a measure of proprioceptive drift. A separate experiment conducted with human participants validated these proprioceptive drift results, and further confirmed that the drift and the subjective ratings of hand ownership were systematically related.
The results from the analysis of behavioral data from experiments in monkeys and humans showed that hand ownership as indexed by proprioceptive drift could be explained by a causal inference model. In these trials, the spatial disparity between visual and proprioceptive information was systematically varied in steps (from + 40° to –40°). The rationale was that, for larger disparities, multisensory combination should not occur, due to the extremely low inferred probability of a common cause, and the hand ownership illusion should not be triggered; in contrast, smaller (or nonexistent) discrepancies should lead to the fusion of visual and proprioceptive signals, due to the causal inference of one’s own hand (Fig. 1). The results supported these predictions and demonstrate that a Bayesian causal inference (BCI) model can explain whether combination or separation of visual and proprioceptive signals occurs in line with a probabilistic approach to the multisensory binding problem. Moreover, the BCI model outperformed a simpler model in which the fusion of vision and proprioception is always instantiated (forced fusion model; Fig. 1). Importantly, the probability of a common cause (Pcom) inferred by the BCI model based on the drift data across the various disparities tested was systematically related to the way subjective hand ownership varied across disparities in human participants. This result provides evidence for a link between causal inference in visuoproprioceptive integration and body ownership.
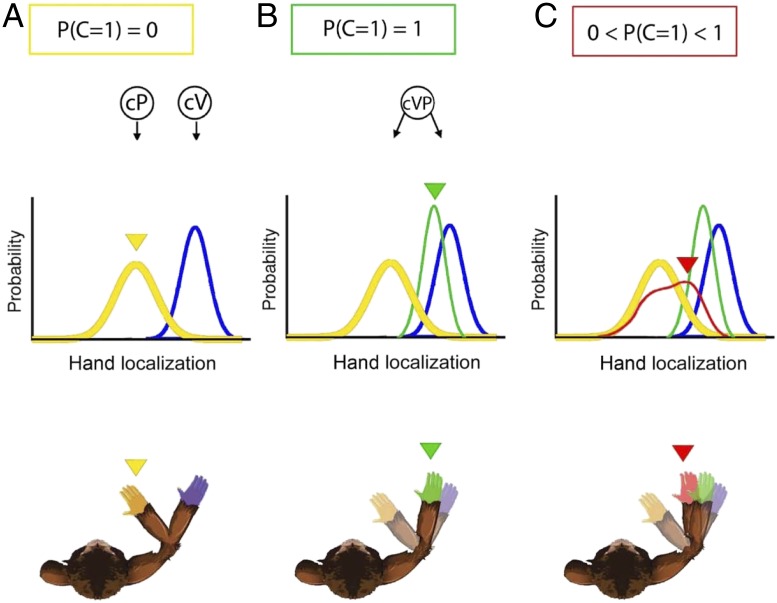
Fig. 1.
Schematic illustration of the BCI and the FF models used in Fang et al. (8). During the experiment, subjects received both visual and proprioceptive signals from the right arm. According to the BCI model, the final arm ownership percept depends on the probability of a common cause (P(C = 1)) for the visual and proprioceptive signals. (A) P(C = 1) = 0: Vision and proprioception seem to come from 2 different sources (as is typically the case for very large disparities); as a consequence, unisensory visual (blue) and proprioceptive (yellow) signals are not fused. (B) P(C = 1) = 1: In classical optimal integration—or a forced fusion model—a single source is always assumed, and unisensory visual (blue) and proprioceptive (yellow) signals are integrated into the visuoproprioceptive (green) estimate. Each unisensory signal contributes to the visuoproprioceptive estimate in proportion to the relative reliability of each signal. (C) 0 < P(C = 1) < 1: In the BCI model used by Fang et al., the 2 possible causal structures are combined according to their relative probabilities; thus, the final arm ownership percept (red) is a combination of the unisensory and fused estimates.
The main experiment then focuses on the neuronal basis of this causal inference process. The authors chose to study the lateral premotor cortex (8), an excellent candidate for several reasons. The premotor cortex is anatomically connected to both visual and somatosensory areas; this area contains neurons that respond to visual, tactile, and proprioceptive stimulation of the upper limb (10), and activity in this region correlates with the strength of subjective
In PNAS, Fang et al. present evidence from electrophysiological recordings in awake-behaving nonhuman primates showing that the integration of visual and somatosensory information from the arm is determined by causal inference and that this dynamic process is implemented by neurons in the premotor cortex.
hand ownership during the rubber hand illusion in human functional magnetic resonance imaging experiments (11). The authors thus probed this area in 2 macaques to analyze neuronal response profiles systematically related to the dynamic combination of visual and proprioceptive information, characteristic of causal inference. In the initial control experiments, they first established the ideal neuronal response profiles associated with maximal combination, when visual and proprioceptive information was perfectly aligned, and maximal segregation, when visual feedback was not available and only somatosensory information was available. In the main experiment, spatial discrepancy was systematically varied, and the neuronal responses from each trial could thus be characterized according to how similar they were to the ideal visuoproprioceptive combination or segregation profiles. Key analysis of 303 neurons during the target holding period showed dynamic shifts in response profiles, indicating that the balance between combination and segregation changed as a function of disparity, as expected. Most noteworthy was that a significant number of neurons (n = 79, 26.1%) showed responses that correlated with the inferred Pcom (obtained by fitting the drift with the causal inference model). This result implies that the characteristic feature of causal inference—the degree of the combination of vision and proprioception depending on the likelihood of a common source—is expressed by visuoproprioceptive neurons in the premotor cortex. Further analyses performed at the neuronal population level confirmed the association between the degree of visuoproprioceptive combination and the likelihood of a common cause. A number of control analyses ruled out trivial explanations for the changes in neuronal responses. Most importantly, when replacing the image of the hand with a rectangular block of wood, a well-established control condition that eliminates the rubber hand illusion (12) due to multisensory incongruence of hand shape and prior knowledge about the appearance of the human body, the authors observed a significant reduction in visuoproprioceptive combination at the neuronal level. This result suggests that dynamic neuronal effects related to multisensory combination are genuinely related to the hand illusion rather than other aspects of the task, such as the sense of being in voluntary control of the object seen on the screen (agency).
In sum, the study by Fang et al. (8) provides evidence that neurons in the premotor cortex implement visuoproprioceptive causal inference of the arm and that this higher-order multisensory process is related to the sense of body ownership. By explicitly modeling the BCI, the current behavioral results go beyond earlier studies of optimal integration of vision and proprioception for hand localization (13, 14). Similarly, the present neurophysiological findings extend our knowledge about the role of the premotor cortex in the multisensory representation of one’s upper arm (10, 11) by demonstrating that neurons in this region consider the probabilities that visual and proprioceptive information originate from the same source. Together with recent human magnetoencephalography data on visuoauditory inference of external events (15), the current results suggest a general role for the frontal cortex in multisensory causal inference. Future studies should clarify the relative roles and interactions between the premotor cortex and more-posterior brain regions in the causal inference of multisensory body ownership. Moreover, it will be important to test whether sensory uncertainty influences the causal inference process under discussion, as predicted by the theory. It is also important to develop new psychophysics methods to directly quantify body ownership perception to overcome the inherent limitations in relying on indirect measures such as proprioceptive drift (9). As a final note, we should emphasize that the present results have broad theoretical implications because body ownership is a central component of self-awareness and bodily self-consciousness (16), and body ownership can also influence a wide range of higher-order cognitive processes such as episodic memory (17), social identity (18), and self-location (19). Thus, speculatively, a probabilistic perceptual basis of body ownership could indicate that other higher-order aspects of self-representation are also determined by probabilistic computational principles.
Footnotes
The authors declare no conflict of interest.
See companion article on page 20151.
References
- 1.Ehrsson H. H., “The concept of body ownership and its relation to multisensory integration” in The New Handbook of Multisensory Processing, Stein B. E., Ed. (MIT Press, Cambridge, MA, 2012), pp. 775–792. [Google Scholar]
- 2.Botvinick M., Cohen J., Rubber hands ‘feel’ touch that eyes see. Nature 391, 756 (1998). [DOI] [PubMed] [Google Scholar]
- 3.Kilteni K., Maselli A., Kording K. P., Slater M., Over my fake body: Body ownership illusions for studying the multisensory basis of own-body perception. Front. Hum. Neurosci. 9, 141 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Samad M., Chung A. J., Shams L., Perception of body ownership is driven by Bayesian sensory inference. PLoS One 10, e0117178 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Körding K. P., et al. , Causal inference in multisensory perception. PLoS One 2, e943 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kayser C., Shams L., Multisensory causal inference in the brain. PLoS Biol. 13, e1002075 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dokka K., Park H., Jansen M., DeAngelis G. C., Angelaki D. E., Causal inference accounts for heading perception in the presence of object motion. Proc. Natl. Acad. Sci. U.S.A. 116, 9060–9065 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fang W., et al. , Statistical inference of body representation in the macaque brain. Proc. Natl. Acad. Sci. U.S.A. 116, 20151–20157 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Abdulkarim Z., Ehrsson H. H., No causal link between changes in hand position sense and feeling of limb ownership in the rubber hand illusion. Atten. Percept. Psychophys. 78, 707–720 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Graziano M. S., Where is my arm? The relative role of vision and proprioception in the neuronal representation of limb position. Proc. Natl. Acad. Sci. U.S.A. 96, 10418–10421 (1999). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ehrsson H. H., Spence C., Passingham R. E., That’s my hand! Activity in premotor cortex reflects feeling of ownership of a limb. Science 305, 875–877 (2004). [DOI] [PubMed] [Google Scholar]
- 12.Tsakiris M., Carpenter L., James D., Fotopoulou A., Hands only illusion: Multisensory integration elicits sense of ownership for body parts but not for non-corporeal objects. Exp. Brain Res. 204, 343–352 (2010). [DOI] [PubMed] [Google Scholar]
- 13.van Beers R. J., Wolpert D. M., Haggard P., When feeling is more important than seeing in sensorimotor adaptation. Curr. Biol. 12, 834–837 (2002). [DOI] [PubMed] [Google Scholar]
- 14.Reuschel J., Drewing K., Henriques D. Y., Rösler F., Fiehler K., Optimal integration of visual and proprioceptive movement information for the perception of trajectory geometry. Exp. Brain Res. 201, 853–862 (2010). [DOI] [PubMed] [Google Scholar]
- 15.Cao Y., Summerfield C., Park H., Giordano B. L., Kayser C., Causal inference in the multisensory brain. Neuron 102, 1076–1087.e8 (2019). [DOI] [PubMed] [Google Scholar]
- 16.Blanke O., Slater M., Serino A., Behavioral, neural, and computational principles of bodily self-consciousness. Neuron 88, 145–166 (2015). [DOI] [PubMed] [Google Scholar]
- 17.Bergouignan L., Nyberg L., Ehrsson H. H., Out-of-body−induced hippocampal amnesia. Proc. Natl. Acad. Sci. U.S.A. 111, 4421–4426 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Maister L., Slater M., Sanchez-Vives M. V., Tsakiris M., Changing bodies changes minds: Owning another body affects social cognition. Trends Cogn. Sci. (Regul. Ed.) 19, 6–12 (2015). [DOI] [PubMed] [Google Scholar]
- 19.Guterstam A., Björnsdotter M., Gentile G., Ehrsson H. H., Posterior cingulate cortex integrates the senses of self-location and body ownership. Curr. Biol. 25, 1416–1425 (2015). [DOI] [PubMed] [Google Scholar]