Significance
Heme proteins have several well-established functions in biology, from oxygen transport to electron transfer and catalysis. But in addition, there is new evidence that heme has a wider regulatory role in the cell. This includes a role for heme in the regulation of circadian rhythm. In this work, we have examined the binding of heme to human CLOCK, one of the positive elements in the circadian autoregulatory feedback loop. We find evidence for a conformational mobility within the heme pocket, and that heme disrupts binding of CLOCK to its E-box DNA. These results provide a direct link between heme binding and DNA partner recognition that may form the basis for a mechanism of regulatory control.
Keywords: heme, circadian, CLOCK
Abstract
The circadian clock is an endogenous time-keeping system that is ubiquitous in animals and plants as well as some bacteria. In mammals, the clock regulates the sleep–wake cycle via 2 basic helix–loop–helix PER-ARNT-SIM (bHLH-PAS) domain proteins—CLOCK and BMAL1. There is emerging evidence to suggest that heme affects circadian control, through binding of heme to various circadian proteins, but the mechanisms of regulation are largely unknown. In this work we examine the interaction of heme with human CLOCK (hCLOCK). We present a crystal structure for the PAS-A domain of hCLOCK, and we examine heme binding to the PAS-A and PAS-B domains. UV-visible and electron paramagnetic resonance spectroscopies are consistent with a bis-histidine ligated heme species in solution in the oxidized (ferric) PAS-A protein, and by mutagenesis we identify His144 as a ligand to the heme. There is evidence for flexibility in the heme pocket, which may give rise to an additional Cys axial ligand at 20K (His/Cys coordination). Using DNA binding assays, we demonstrate that heme disrupts binding of CLOCK to its E-box DNA target. Evidence is presented for a conformationally mobile protein framework, which is linked to changes in heme ligation and which has the capacity to affect binding to the E-box. Within the hCLOCK structural framework, this would provide a mechanism for heme-dependent transcriptional regulation.
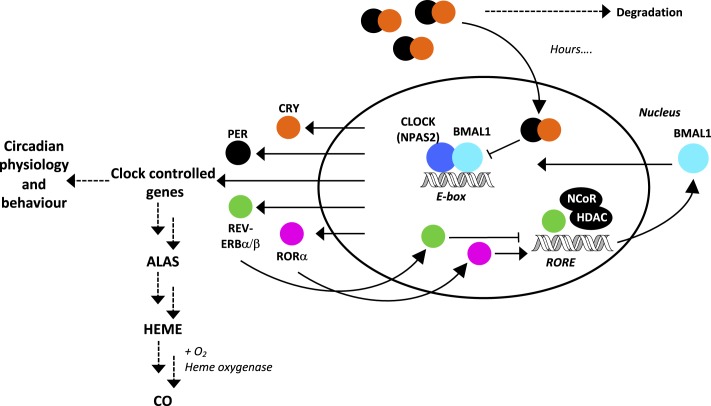
The circadian clock is the internal timekeeping system that generates a daily rhythm in physiology, biochemistry, and behavior of almost all higher organisms and some prokaryotes. In mammalian systems, as in insects, a transcriptional–translational feedback loop appears to be at the core of the oscillator (1), and this is coupled to an evolutionarily more primitive metabolic oscillator (2). The transcriptional–translational feedback loop has a number of positive and negative components that drive interconnected loops that generate the molecular clockworks. In mammals, NPAS2 (in the forebrain) and CLOCK (in the suprachiasmatic nucleus) form heterodimers with BMAL1 (brain and muscle arnt-like 1) and represent the positive limb of the feedback loop (Fig. 1). Both dimers bind to the same E-box DNA sequence (CANNTG) to activate expression of the negative autoregulators, the 3 Period (Per) and 2 Cryptochrome (Cry) genes (1). PER and CRY, after various posttranslational modifications that build delays into the loop, then interact with the BMAL1 complexes to negatively regulate their own genes, thereby closing the loop (Fig. 1 and ref. 1). REV-ERBα, a member of the nuclear receptor family, also functions in the cell to repress transcription of BMAL1.
Fig. 1.
Simplified figure of mammalian regulatory clockworks (52). In the positive loop, NPAS2 (dark blue circle) and CLOCK (ditto) form heterodimers with BMAL1 (light blue) which bind to the E-box to activate expression of PER (black) and CRY (orange). PER and CRY heterodimers then interact with the BMAL1 heterodimers to negatively regulate their own genes, thereby closing the loop (1). Other CLOCK-related genes are expressed, including the nuclear receptors REV-ERBα/β (green) and the retinoid receptor orphan receptor (ROR, pink). ALAS, which controls the synthesis (and hence the concentrations) of heme, is also clock regulated. See introductory text for details.
There is an accumulating body of evidence that indicates that heme is a regulator of the circadian oscillator (3), but the mechanisms of regulation are entirely unknown. Conceptually, heme binding provides a simple mechanism for signal transduction because regulation by heme can be coupled to its ability to bind other ligands—such as oxygen (O2), carbon monoxide (CO), and nitric oxide (NO)—which are themselves established as important signaling molecules in cellular control (4).
The 2 E-box binding proteins—NPAS2 and CLOCK—belong to the family of transcriptional regulators that contain both basic helix–loop–helix (bHLH) domains at the N terminus as well as 2 PAS domains. High sequence identity between these 2 proteins (5, 6) was noted before a direct link between heme and any circadian protein had been established. In this work we present an analysis of heme binding to the PAS-A domain of human CLOCK, we examine the effect of heme on the CLOCK–DNA binding interaction, and we present a structure for the PAS-A domain. We use this information to put forward ideas on how heme binding might be linked to regulation of circadian control.
Results
Crystal Structure of hCLOCK PAS-A.
A recombinant version (N-terminal His tagged) of the PAS-A domain of hCLOCK (hCLOCK PAS-A, Fig. 2) was expressed in Escherichia coli. The purified protein crystallized as a homodimer in the apo-form (i.e., without heme), consistent with behavior of the protein during size exclusion chromatography (SI Appendix, Fig. S2).
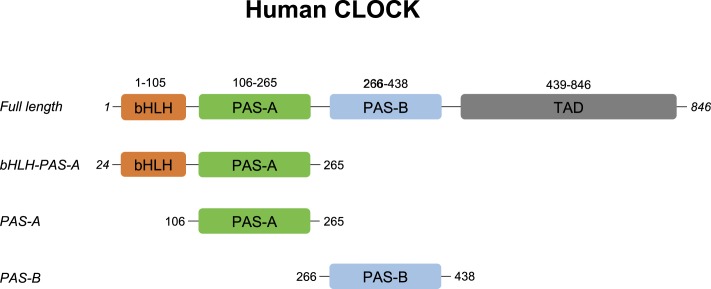
Fig. 2.
Domain structure in hCLOCK, showing the N-terminal HLH domains and the 2 PAS domains (PAS-A and PAS-B). The protein constructs used in this work are also shown. The vectors used for each construct design are shown in SI Appendix, Fig. S1.
The structure of hCLOCK PAS-A is presented in Fig. 3A. The tertiary structure of the overall PAS fold consists of a series of antiparallel β-sheets forming a hydrophobic core in which a signaling molecule can bind (7). A number of α-helices are in close proximity which are believed to be responsible for the propagation of structural changes to the rest of the protein or to a partner protein when in a complex.
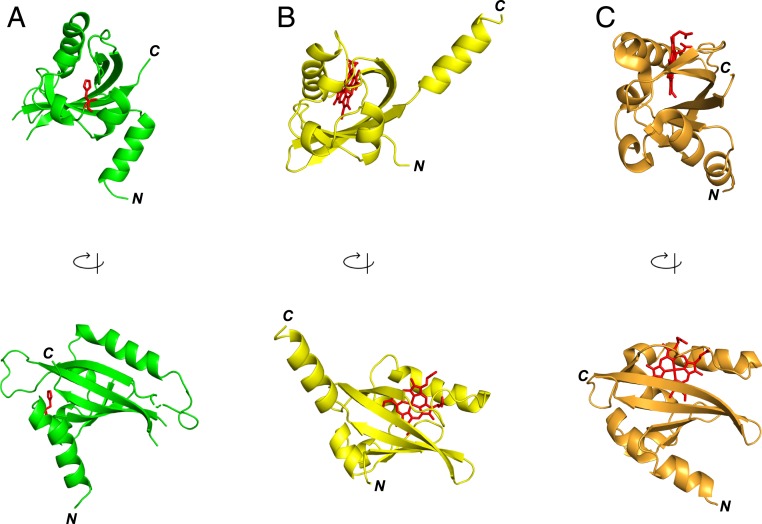
Fig. 3.
A comparison of the structure of PAS-A domains. (A) hCLOCK as presented in this work (PDB code 6QPJ). His144 is indicated in red. This is similar to the equivalent PAS-A domain of mouse CLOCK protein in the CLOCK:BMAL1 complex (53). (B) The O2 sensor protein from Rhizobium (FixL) (8). (C) The heme-regulated phosphodiesterase from E. coli (EcDOS, refs. 9 and 10). In A, and for mouse CLOCK, the protein crystallizes in the apo-form. In B and C, which both crystallize as the holo-protein, the heme molecule is indicated in red. The Upper figures highlight the binding pocket created by the β-sheets; the Lower structures (which are rotated by 90° about the indicated axis) show the similarity in overall arrangement of the domains. For direct comparison, an overlay of the hCLOCK and FixL structures is presented in SI Appendix, Fig. S3.
The hCLOCK structure has close similarity to that of the O2 sensor from Rhizobium (FixL, ref. 8) and the heme-regulated phosphodiesterase from E. coli (EcDOS) (9, 10), as shown in Fig. 3 B and C. A comparison of the heme binding locations in the 2 structures is informative (SI Appendix, Fig. S3). In the structure of hCLOCK PAS-A, histidine 144, which is implicated in heme binding as below, is found on a loop between 2 short helices and is oriented toward the β-sheets of the domain in a hydrophobic pocket. This hydrophobic core is a feasible heme binding pocket, as shown in SI Appendix, Fig. S3. From the hCLOCK structure, it appears that H144 is a realistic proximal residue for heme and is ideally situated at the mouth of this hydrophobic pocket and is surrounded by numerous hydrophobic residues (Phe121, Leu145, Leu149, Phe157, and Leu198) which leave adequate space for a heme molecule. A comparison with FixL shows that the heme in the crystal structure of FixL is located in a different location, at the opposite end of this hydrophobic pocket (SI Appendix, Fig. S3A).
Heme Binding to hCLOCK PAS-A.
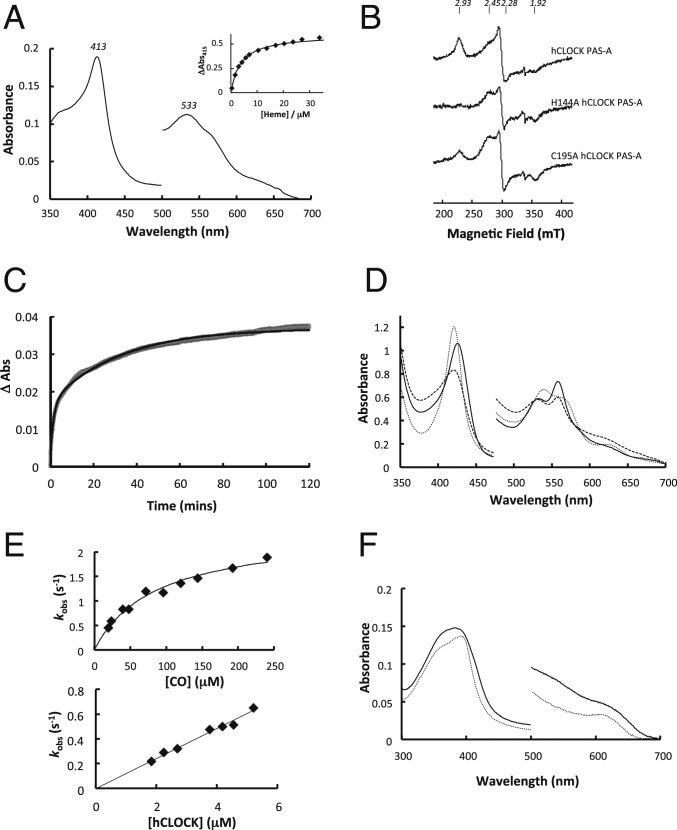
Although heme is not present in the structure of hCLOCK above, binding of heme to the PAS-A domain of hCLOCK is clearly evident from UV-visible spectra. The spectrum of the oxidized, ferric derivative of hCLOCK PAS-A (λmax = 413, 533 nm, Fig. 4A) is consistent with heme binding to the protein and with a 6-coordinate, low-spin heme species. Electron paramagnetic resonance (EPR) spectra are in agreement with this assignment. The spectrum of heme-bound hCLOCK at 20 K (Fig. 4B) reveal turning points at g1 = 2.93, g2 = 2.28, and g3 ≥ 1.7 typical of a low-spin, ferric (S = 1/2) heme species (11). These low-spin g-values are consistent with a bis-histidine heme coordination. Weak resonances were also observed at 4 K at geff ∼ 6.0 and geff ∼ 2, which are characteristic of high-spin ferric (S = 5/2) heme (SI Appendix, Fig. S4A). In addition, a shoulder is observed on the central line and attributed to overlap of a second (minor) low-spin heme species (g1 = 2.45, g2 = 2.28, and g3 = 1.92). These g-values are similar to those previously observed for the isolated PAS-A domain of mouse PER2 (12). Empirical g-values for heme-bound species are well studied (11). In this case the g-values for the second species in the EPR are unlike those reported for either His/His or His/Met ligation, and on this basis are assigned as arising from a Cys/His or Cys/OH− coordination.
Fig. 4.
(A) UV-visible absorption spectra of the ferric heme/CLOCK PAS-A complex. (Inset) Shows ΔAbs at 415 nm (obtained from difference spectra at various heme concentrations) as a function of heme concentration and fitted to a 1:1 binding event. (B) The 9 GHz EPR spectra of ferric heme bound to hCLOCK PAS-A (Top), the H144A variant of PAS-A (Middle), and the C195A variant of PAS-A (Bottom) in 50 mM Tris/HCl buffer at pH 7.0. (C) Absorbance change at 412 nm for the dissociation of heme from hCLOCK PAS-A (3 to 4 μM) on mixing with apo-H64Y/V68F myoglobin (80 μM). Data are fitted to a double exponential process. (D) Spectrum of the ferrous (solid line), CO-bound (dotted line), and NO-bound (dashed line) derivatives of heme-bound hCLOCK PAS-A. (E) Observed rate constants for the association of CO with hCLOCK PAS-A, shown as a function of [CO] (Top) and [hCLOCK PAS-A] (Bottom). (F) Spectrum of the ferric heme/CLOCK PAS-A H144A complex (solid line) compared to free ferric heme (dotted line). This spectrum resembles that of the related His335 variant of NPAS2 PAS-B (where H335 is assigned as 1 of the heme ligands) (21).
Heme binds to apo-hCLOCK with a 1:1 stoichiometry (KD = 4.2 ± 0.25 µM; Fig. 4A, Inset). Heme dissociation from hCLOCK PAS-A was measured at 412 nm in the presence of the H64Y/V68F mutant of sperm whale myoglobin which has a high affinity for heme (13) (Fig. 4C). A biphasic exponential change was observed giving rate constants of 8.9 × 10−3 s−1 and 4.5 × 10−4 s−1 for the fast and slow phases, respectively. This biphasic behavior is consistent with the concept of conformational mobility within the heme binding site. The rate constants for heme dissociation are consistent with those derived for other heme regulatory proteins, such as SOUL and p22HBP (kdiss ∼ 10−3 s−1) (14).
As for the ferric protein above, the ferrous derivative of hCLOCK PAS-A (λmax = 425, 533, and 558 nm; Fig. 4D), shows characteristic heme peaks, which is again consistent with 6-coordinate, low-spin heme. On the basis of similarities to ferrous NPAS2 (λmax = 423, 530, and 558 nm) (15), which is assigned as a His/His ligated heme species (16), we assign hCLOCK PAS-A as the same. Ferrous hCLOCK binds both CO (λmax = 421, 540, and 570 nm; Fig. 4D) and NO (λmax = 421, 531, and 556 nm; Fig. 4D), with a presumed His/CO ligation. In kinetic experiments measuring CO binding to hCLOCK PAS-A, pseudo first-order rate constants (kobs) showed a nonlinear dependence on the concentration of CO, but were linearly dependent on the protein concentration (Fig. 4E). This kinetic behavior is consistent with a dynamic equilibrium of the protein structure (P) prior to CO binding (17), presumably leading to loss of one of the axial His ligands,
| [1] |
and in the case where CO binding is irreversible (k-2<< k1k2/k-1), a hyperbolic dependence of kobs on CO concentration is observed,
| [2] |
The addition of oxygen to ferrous protein under anaerobic conditions gave no evidence of a ferrous-oxy intermediate—this might be linked to the low reduction potential of the heme in hCLOCK PAS-A, which has been previously determined (18, 19) as −111 mV vs. NHE.
Based on a sequence alignment with NPAS2 (SI Appendix, Fig. S5), we identified His144 as a possible ligand to the heme in hCLOCK (equivalent to the axial His119 ligand in NPAS2). The corresponding spectra of the ferric H144A mutant of hCLOCK PAS-A in the presence of heme showed a broad, strongly blue-shifted Soret band (λmax = 374 nm) which is different from that of the ferric wild-type protein and which is not consistent with a bona fide heme protein (Fig. 4F). On this basis, we assign His144 as one of the heme ligands in hCLOCK. EPR spectra confirm this assignment as the g1 = 2.93 resonance is missing in the spectrum of the H144A variant (Fig. 4B). The identity of the proposed Cys ligand seen only at low temperature in the EPR (above) was not established. The most likely candidate was considered to be Cys195 which points toward the heme pocket, but the EPR spectrum of the C195A variant (which corresponds to Cys170 in NPAS2 and has been suggested as a ligand) (20) was unchanged from that of the wild-type protein (Fig. 4B). The UV-visible spectrum of the C195A variant is also very similar to the wild type (SI Appendix, Fig. S4B) and thiol modification of apo-hCLOCK with DTNB (to modify all Cys residues) followed by reconstitution with heme did not lead to a change in the UV-visible spectrum. Mutation of the adjacent Cys194 residue gave a similar heme-bound spectrum (λmax = 414 nm) and was presumed to bind heme normally in solution by virtue of the His144 ligand. Together, we interpret this information to mean that the Cys-ligated species is only present at low (cryogenic) temperatures.
We also expressed a PAS-B fragment of hCLOCK (Fig. 2 and SI Appendix, Fig. S1), which was found to bind heme in the ferric form (λmax = 416 and 536 nm) (SI Appendix, Fig. S6) but with marginally weaker affinity (KD = 13.3 ± 0.97 µM) (SI Appendix, Fig. S6) than the PAS-A form of hCLOCK. The PAS-B domain of NPAS2 is also reported as binding heme more weakly than the corresponding PAS-A domain (21) but the functional consequences, if any, of this difference are not known because the role of PAS-B is not yet established. The ferrous (λmax = 424, 532, and 559 nm) (SI Appendix, Fig. S6) and CO-bound forms (λmax = 421, 541, and 569 nm) (SI Appendix, Fig. S6) also form normally in PAS-B. Like the hCLOCK PAS-A domain, these spectra are indicative of a 6-coordinate heme binding interaction but this was not examined further in this work.
Heme Affects Binding to the E-Box.
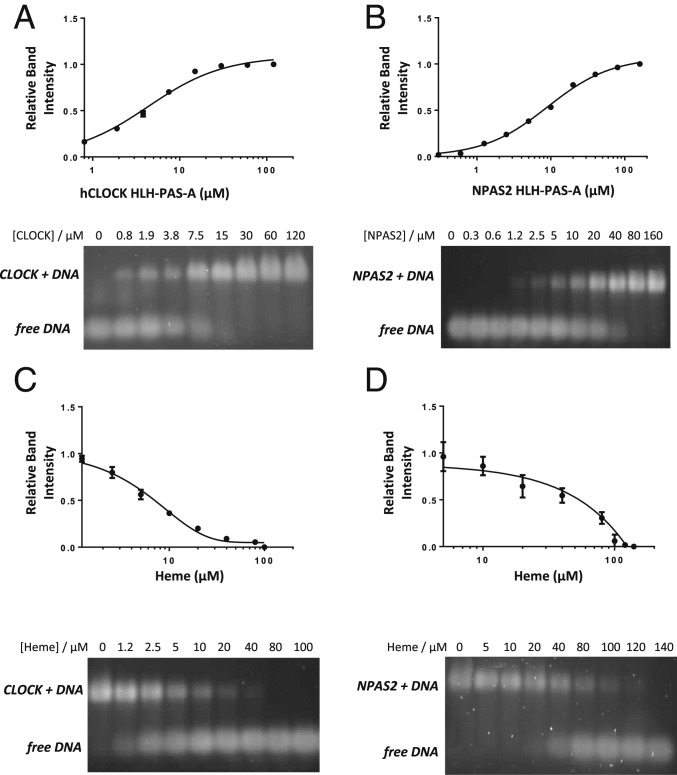
To assess the functional effect of heme on DNA binding, we expressed a construct of hCLOCK PAS-A containing an N-terminal HLH domain (Fig. 2 and SI Appendix, Fig. S7A). The hCLOCK HLH/PAS-A construct was demonstrated to bind to E-box DNA (Fig. 5A); a plot of the relative band intensity versus [hCLOCK HLH/PAS-A] gave a KD = 6.3 μM, which is similar to that determined (KD = 9 μM) for a different construct of CLOCK containing only the HLH domain (22). Control experiments (Fig. 5B) with the corresponding HLH/PAS-A construct of NPAS2 (SI Appendix, Figs. S7B and S8) similarly demonstrated binding of NPAS2 to E-box (KD = 11 μM, Fig. 5B). These HLH/PAS-A proteins are presumed to bind to DNA as homodimers, as HLH domains are unable to form a specific interaction with E-box DNA in the monomeric form.
Fig. 5.
Electrophoretic mobility shift assays showing DNA binding to the HLH/PAS-A domains of hCLOCK (A and C) and NPAS2 (B and D) in the absence (A and B) and presence (C and D) of heme.
Addition of heme inhibits DNA binding to the HLH domain of hCLOCK, as evidenced by the shift in the DNA band in electrophoretic mobility shift assays (EMSAs) (Fig. 5C); the same effect is observed for NPAS2 (Fig. 5D). From the intensities of the DNA–protein and the the free DNA bands, an IC50 of 7.4 μM was derived for the inhibitory effect of heme on DNA binding to hCLOCK; this value is in the same range as the KD for heme binding to the PAS-A domain.
Discussion
The Role of Heme in Circadian Regulation.
In addition to known roles as a prosthetic group in a multitude of heme proteins and enzymes, there is now emerging evidence that heme has a wider role to play in biology. A regulatory mechanism in cells that is linked to heme binding is, to our way of thinking, chemically logical and potentially versatile. Heme is redox active and, thus, responds to redox changes within the cell by modulating its oxidation state. Concentrations of heme in the cell are controlled by the balance of heme biosynthesis (to increase heme concentrations) and heme oxygenase activity (which degrades heme and produces CO as a byproduct). Cell-signaling gases (O2, NO, and CO) all bind to heme, which creates a possible mechanism of regulatory control. CO is itself produced by degradation of heme by the O2-dependent heme oxygenase enzyme, and NO is produced by the O2-dependent NO synthase enzyme. When one considers this in the context of circadian control, this means that the redox state of the cell, the oxidation state of heme, the balance between heme synthesis and degradation, and O2/NO/CO concentrations may all be interconnected. This is summarized in outline form in Fig. 1. This interconnectivity could form the basis for a powerful heme-dependent circadian control mechanism that reflects metabolic activity. The fact that transcription of ALAS1, the first enzyme in the heme synthesis pathway, and other genes involved in heme catabolism (such as heme oxygenase) and heme transport (23–29) are also clock regulated is also significant as a means of connecting heme synthesis, heme degradation and CO formation to circadian events. Indeed, it has previously been shown that heme-related genes in mouse are also implicated in the light resetting of the clock either as cause or effect (30).
In the case of particular circadian proteins, several—including REV-ERB, PER2, NPAS2—are shown to bind heme (12, 15, 20, 21, 31–40) and complex formation with partner proteins can be affected by heme or CO binding (34, 41, 42). And there are precedents for a circadian control mechanism that occurs through binding to PAS domain proteins, because of other regulatory PAS domain proteins that are known to bind heme [for example FixL in the histidine kinase family, the phosphodiesterases EcDOS and PDEA1 (43) and Burkholderia xenovorans RcoM (16)].
Structure and Heme Ligation in hCLOCK.
We have presented a structure for the PAS-A domain of hCLOCK. Heme is not bound to hCLOCK in this structure, although there is convincing evidence for noncovalent heme binding in the UV-visible and EPR spectra, as well as from the kinetic data. The absence of heme in our crystal structure does not imply a lack of specific heme binding—it may be that the particular conformation in which hCLOCK PAS-A crystallizes is not compatible with heme binding correctly in the cavity and thus heme is excluded during crystal formation. An absence of heme in the structure of a presumed heme binding protein has also been noted in the periplasmic heme transporter protein BhuT (44); this has been attributed to structural flexibility of the transporter complex.
Heme binds relatively weakly to the PAS-A domain of hCLOCK, and weak heme binding is a feature of a number of regulatory heme binding proteins (20). The heme is proposed to reside at the mouth of a hydrophobic cavity comprising residues Phe121, Leu145, Leu149, Phe157, and Leu198 (SI Appendix, Fig. S3). This is plausible in the sense that FixL also binds heme in a hydrophobic pocket (residues Val188, Met192, Tyr203, and Ile204) that helps to orient the heme and the His ligand (His200) in the correct location (8). Our comparison of the structures (SI Appendix, Fig. S3) indicates that the heme binds at different locations in hCLOCK and FixL—both locations appear to be viable but the biological relevance of these differences is not yet known.
From the available spectroscopic data for hCLOCK we assign the heme binding interaction in ferric hCLOCK as histidine ligated at room temperature in solution. Data for the His144 variant strongly implicate this residue as one of the heme ligands, and His144 is essential for heme binding. The hCLOCK protein appears to contain 6-coordinated (His/His) heme as evidenced by EPR, but appears able to adopt other distinct states with the axial ligand(s) being labile. The identity of the second histidine ligand is not known. There is evidence for a 5-coordinated ferric high-spin heme species from the EPR, again consistent with axial ligand lability (but His144 is presumed not to be labile, as it is essential for heme binding). These data are in agreement with those for NPAS2, which assign the equivalent residue (His119) as an axial ligand (20) (SI Appendix, Fig. S5). There is evidence from EPR only (empirical interpretation of the EPR g-values) for an additional Cys ligand to the heme in hCLOCK at 20 K. Cys ligation has been suggested in other heme regulatory proteins (16), but the spectroscopic evidence for it in the case of NPAS2 (where Cys170 has been proposed as a ligand, equivalent to Cys195 in hCLOCK) (SI Appendix, Fig. S5), and particularly in mouse PER2, is not totally conclusive. In the case of NPAS2, axial ligation might be affected by the presence of the N-terminal HLH domain (12, 20, 45). For hCLOCK, there are 3 Cys residues in the sequence and one of these (Cys250) is at >20 Å from His144. Cys195 points toward the pocket, but is still at >10 Å from the His144 ligand. The adjacent Cys194 residue is exposed and pointing away from the proposed heme pocket. A homology model of hCLOCK and NPAS2 (Fig. 6) indicates an overlay of the His144/His119 and Cys195/Cys170 residues. From the evidence available so far, our conclusions are that the axial ligand identities in hCLOCK—and, by analogy, in other related regulatory proteins—are not as straightforwardly defined as in other well-known heme proteins, such as myoglobin (His/H2O), cytochrome b5 (His/His), cytochrome c peroxidase (His/H2O), or cytochrome P450 (Cys/H2O) which have more rigidly coordinated heme ligands. Instead, we envisage these regulatory heme proteins as being much more fluid in their heme binding—and thus able to change their axial ligation in response to different stimuli, which could be binding to a protein partner, to DNA, to another heme ligand (such as CO), or to a change in oxidation state (16). This would require a degree of flexibility in the heme pocket which, in the case of the CLOCK protein, is consistent with the fact that PAS domains are known to be conformationally mobile (46). We have good evidence for conformational mobility of hCLOCK in our observations, namely the weak heme binding, the absence of heme in the crystal structure, and the required loss of the axial His ligand on CO binding to the ferrous form. This type of protein flexibility has been noted in other regulatory proteins (47) as well as in heme-based sensors (48), and could be a common feature of the way that these regulatory heme proteins operate. Further structural information will be important in resolving these questions.
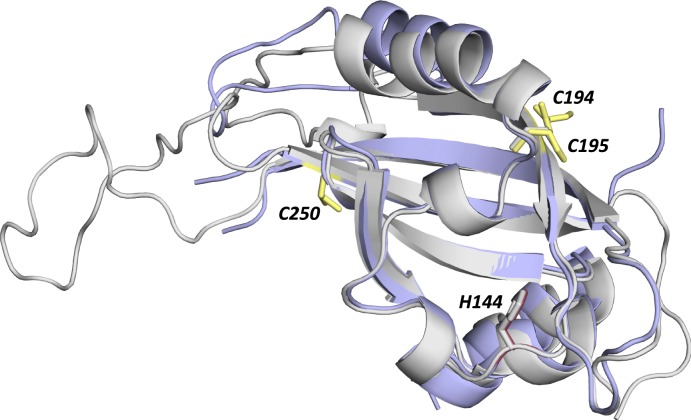
Fig. 6.
The hCLOCK PAS-A crystal structure as presented in this work (in pale blue, PDB code 6QPJ) superimposed onto a homology model of the PAS-A domain of NPAS2 (in light gray). Cys195 (equivalent to Cys171 in NPAS2, overlaid in gray) and Cys194 (equivalent to Tyr169 in NPAS2, not shown) in hCLOCK are shown in yellow; Cys250 in hCLOCK is also shown. His144 in hCLOCK is highlighted in red, overlaid with His119 in NPAS2 (in gray). The homology model was produced using SWISS-MODEL (Biozentrum, University of Basel) using the PAS-A domain of the mouse CLOCK structure (PDB code 4F3L) (54). The figure was created using Pymol (55).
Effect of Heme on DNA Binding and Functional Implications.
Our data demonstrate that heme inhibits binding of hCLOCK to DNA. This provides evidence for an influence of heme on the DNA binding ability of CLOCK in cells. How might this work and how might it affect signal transduction? We offer the following comments. If the proteins are conformationally mobile, for which there is evidence (as above), then it is plausible that heme binding to the PAS domain causes structural rearrangements that are transmitted and affect the interaction with the E-box. Based on the weak heme binding affinity, it is feasible that the heme binding event is itself reversible; reversible loss of heme has been noted in other systems recently (49) and could contribute to the regulatory control. The PAS domains that have been studied appear to initiate signaling from the central β-sheet region of the structure (50); the signal is then often propagated to the Cα helix of the PAS domain. His144, which binds to the heme in hCLOCK, is located on the Cα helix, and conformational changes linked to heme binding in this proposed signaling region would therefore provide a convenient mechanism for signal transduction. Binding of signaling gases to the heme (such as CO) might also lead to structural rearrangements in the heme pocket, and there is evidence for this in the related FixL sensor protein (51). How these signals are further propagated is not yet known.
Materials and Methods
Detailed methods on the following subjects are available in SI Appendix, SI Materials and Methods: cloning and mutagenesis, expression and purification, electronic spectroscopy, crystallization, kinetic measurements, and EMSAs.
Supplementary Material
Acknowledgments
We are grateful to the late Dr. X. Yang (University of Leicester PROTEX cloning facility) for providing the expression constructs used for this work and Dr. J. Olson (Rice University) for providing us with apo-H64Y/V68F SwMb. We thank Toru Shimizu for sharing unpublished spectra on NPAS2. This work was funded by the Biotechnology and Biological Sciences Research Council (grant BB/L006626/1 to E.L.R.). We thank the National Engineering and Physical Sciences Research Council EPR service and facility for support (NS/A000055/1) and Louise Bird and Raymond Owens (Oxford Protein Production Facility, Harwell) for help with cloning.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
Data deposition: The atomic coordinates and structure factors have been deposited in the Protein Data Bank, www.rcsb.org (PDB ID code 6QPJ).
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1905216116/-/DCSupplemental.
References
- 1.Gallego M., Virshup D. M., Post-translational modifications regulate the ticking of the circadian clock. Nat. Rev. Mol. Cell Biol. 8, 139–148 (2007). [DOI] [PubMed] [Google Scholar]
- 2.Edgar R. S., et al. , Peroxiredoxins are conserved markers of circadian rhythms. Nature 485, 459–464 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Shimizu T., et al. , Gaseous O2, NO, and CO in signal transduction: Structure and function relationships of heme-based gas sensors and heme-redox sensors. Chem. Rev. 115, 6491–6533 (2015). [DOI] [PubMed] [Google Scholar]
- 4.Verma A., Hirsch D. J., Glatt C. E., Ronnett G. V., Snyder S. H., Carbon monoxide: A putative neural messenger. Science 259, 381–384 (1993). [DOI] [PubMed] [Google Scholar]
- 5.King D. P., et al. , Positional cloning of the mouse circadian clock gene. Cell 89, 641–653 (1997). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Vitaterna M. H., et al. , Mutagenesis and mapping of a mouse gene, Clock, essential for circadian behavior. Science 264, 719–725 (1994). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Taylor B. L., Zhulin I. B., PAS domains: Internal sensors of oxygen, redox potential, and light. Microbiol. Mol. Biol. Rev. 63, 479–506 (1999). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gong W., et al. , Structure of a biological oxygen sensor: A new mechanism for heme-driven signal transduction. Proc. Natl. Acad. Sci. U.S.A. 95, 15177–15182 (1998). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kurokawa H., et al. , A redox-controlled molecular switch revealed by the crystal structure of a bacterial heme PAS sensor. J. Biol. Chem. 279, 20186–20193 (2004). [DOI] [PubMed] [Google Scholar]
- 10.Park H., Suquet C., Satterlee J. D., Kang C., Insights into signal transduction involving PAS domain oxygen-sensing heme proteins from the X-ray crystal structure of Escherichia coli Dos heme domain (Ec DosH). Biochemistry 43, 2738–2746 (2004). [DOI] [PubMed] [Google Scholar]
- 11.Walker F. A., Magnetic spectroscopic (EPR, ESEEM, Mossbauer, MCD and NMR) studies of low-spin ferriheme centers and their corresponding heme proteins. Coord. Chem. Rev. 185–186, 471–534 (1999). [Google Scholar]
- 12.Kitanishi K., et al. , Heme-binding characteristics of the isolated PAS-A domain of mouse Per2, a transcriptional regulatory factor associated with circadian rhythms. Biochemistry 47, 6157–6168 (2008). [DOI] [PubMed] [Google Scholar]
- 13.Hargrove M. S., et al. , His64(E7)–>Tyr apomyoglobin as a reagent for measuring rates of hemin dissociation. J. Biol. Chem. 269, 4207–4214 (1994). [DOI] [PubMed] [Google Scholar]
- 14.Sato E., et al. , SOUL in mouse eyes is a new hexameric heme-binding protein with characteristic optical absorption, resonance Raman spectral, and heme-binding properties. Biochemistry 43, 14189–14198 (2004). [DOI] [PubMed] [Google Scholar]
- 15.Mukaiyama Y., et al. , Spectroscopic and DNA-binding characterization of the isolated heme-bound basic helix-loop-helix-PAS-A domain of neuronal PAS protein 2 (NPAS2), a transcription activator protein associated with circadian rhythms. FEBS J. 273, 2528–2539 (2006). [DOI] [PubMed] [Google Scholar]
- 16.Smith A. T., et al. , Functional divergence of heme-thiolate proteins: A classification based on spectroscopic attributes. Chem. Rev. 115, 2532–2558 (2015). [DOI] [PubMed] [Google Scholar]
- 17.Badyal S. K., et al. , Conformational mobility in the active site of a heme peroxidase. J. Biol. Chem. 281, 24512–24520 (2006). [DOI] [PubMed] [Google Scholar]
- 18.Efimov I., et al. , The redox properties of ascorbate peroxidase. Biochemistry 46, 8017–8023 (2007). [DOI] [PubMed] [Google Scholar]
- 19.Efimov I., et al. , A simple method for the determination of reduction potentials in heme proteins. FEBS Lett. 588, 701–704 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Uchida T., et al. , CO-dependent activity-controlling mechanism of heme-containing CO-sensor protein, neuronal PAS domain protein 2. J. Biol. Chem. 280, 21358–21368 (2005). [DOI] [PubMed] [Google Scholar]
- 21.Koudo R., et al. , Spectroscopic characterization of the isolated heme-bound PAS-B domain of neuronal PAS domain protein 2 associated with circadian rhythms. FEBS J. 272, 4153–4162 (2005). [DOI] [PubMed] [Google Scholar]
- 22.Wang Z., Wu Y., Li L., Su X. D., Intermolecular recognition revealed by the complex structure of human CLOCK-BMAL1 basic helix-loop-helix domains with E-box DNA. Cell Res. 23, 213–224 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kaasik K., Lee C. C., Reciprocal regulation of haem biosynthesis and the circadian clock in mammals. Nature 430, 467–471 (2004). [DOI] [PubMed] [Google Scholar]
- 24.Guenthner C. J., Bickar D., Harrington M. E., Heme reversibly damps PERIOD2 rhythms in mouse suprachiasmatic nucleus explants. Neuroscience 164, 832–841 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ceriani M. F., et al. , Genome-wide expression analysis in Drosophila reveals genes controlling circadian behavior. J. Neurosci. 22, 9305–9319 (2002). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Panda S., et al. , Coordinated transcription of key pathways in the mouse by the circadian clock. Cell 109, 307–320 (2002). [DOI] [PubMed] [Google Scholar]
- 27.Zheng B., et al. , Nonredundant roles of the mPer1 and mPer2 genes in the mammalian circadian clock. Cell 105, 683–694 (2001). [DOI] [PubMed] [Google Scholar]
- 28.Damulewicz M., Loboda A., Jozkowicz A., Dulak J., Pyza E., Interactions between the circadian clock and heme oxygenase in the retina of Drosophila melanogaster. Mol. Neurobiol. 54, 4953–4962 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mandilaras K., Missirlis F., Genes for iron metabolism influence circadian rhythms in Drosophila melanogaster. Metallomics 4, 928–936 (2012). [DOI] [PubMed] [Google Scholar]
- 30.Ben-Shlomo R., et al. , Light pulse-induced heme and iron-associated transcripts in mouse brain: A microarray analysis. Chronobiol. Int. 22, 455–471 (2005). [DOI] [PubMed] [Google Scholar]
- 31.Yin L., et al. , Rev-erbalpha, a heme sensor that coordinates metabolic and circadian pathways. Science 318, 1786–1789 (2007). [DOI] [PubMed] [Google Scholar]
- 32.Raghuram S., et al. , Identification of heme as the ligand for the orphan nuclear receptors REV-ERBalpha and REV-ERBbeta. Nat. Struct. Mol. Biol. 14, 1207–1213 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lukat-Rodgers G. S., Correia C., Botuyan M. V., Mer G., Rodgers K. R., Heme-based sensing by the mammalian circadian protein CLOCK. Inorg. Chem. 49, 6349–6365 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Dioum E. M., et al. , NPAS2: A gas-responsive transcription factor. Science 298, 2385–2387 (2002). [DOI] [PubMed] [Google Scholar]
- 35.Shimizu T., Binding of cysteine thiolate to the Fe(III) heme complex is critical for the function of heme sensor proteins. J. Inorg. Biochem. 108, 171–177 (2012). [DOI] [PubMed] [Google Scholar]
- 36.Airola M. V., Du J., Dawson J. H., Crane B. R., Heme binding to the mammalian circadian clock protein period 2 is nonspecific. Biochemistry 49, 4327–4338 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ishida M., Ueha T., Sagami I., Effects of mutations in the heme domain on the transcriptional activity and DNA-binding activity of NPAS2. Biochem. Biophys. Res. Commun. 368, 292–297 (2008). [DOI] [PubMed] [Google Scholar]
- 38.Carter E. L., Ramirez Y., Ragsdale S. W., The heme-regulatory motif of nuclear receptor Rev-erbβ is a key mediator of heme and redox signaling in circadian rhythm maintenance and metabolism. J. Biol. Chem. 292, 11280–11299 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Carter E. L., Gupta N., Ragsdale S. W., High affinity heme binding to a heme regulatory motif on the nuclear receptor rev-erbβ leads to its degradation and indirectly regulates its interaction with nuclear receptor corepressor. J. Biol. Chem. 291, 2196–2222 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Minegishi S., Sagami I., Negi S., Kano K., Kitagishi H., Circadian clock disruption by selective removal of endogenous carbon monoxide. Sci. Rep. 8, 11996 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Masri S., Zocchi L., Katada S., Mora E., Sassone-Corsi P., The circadian clock transcriptional complex: Metabolic feedback intersects with epigenetic control. Ann. N. Y. Acad. Sci. 1264, 103–109 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Klemz R., et al. , Reciprocal regulation of carbon monoxide metabolism and the circadian clock. Nat. Struct. Mol. Biol. 24, 15–22 (2017). [DOI] [PubMed] [Google Scholar]
- 43.Gilles-Gonzalez M. A., Gonzalez G., Heme-based sensors: Defining characteristics, recent developments, and regulatory hypotheses. J. Inorg. Biochem. 99, 1–22 (2005). [DOI] [PubMed] [Google Scholar]
- 44.Naoe Y., et al. , Crystal structure of bacterial haem importer complex in the inward-facing conformation. Nat. Commun. 7, 13411 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Uchida T., Sagami I., Shimizu T., Ishimori K., Kitagawa T., Effects of the bHLH domain on axial coordination of heme in the PAS-A domain of neuronal PAS domain protein 2 (NPAS2): Conversion from His119/cys170 coordination to His119/His171 coordination. J. Inorg. Biochem. 108, 188–195 (2012). [DOI] [PubMed] [Google Scholar]
- 46.Vreede J., van der Horst M. A., Hellingwerf K. J., Crielaard W., van Aalten D. M., PAS domains. Common structure and common flexibility. J. Biol. Chem. 278, 18434–18439 (2003). [DOI] [PubMed] [Google Scholar]
- 47.Kobayashi K., et al. , Redox-dependent dynamics in heme-bound bacterial iron response regulator (irr) protein. Biochemistry 55, 4047–4054 (2016). [DOI] [PubMed] [Google Scholar]
- 48.Sasakura Y., Yoshimura-Suzuki T., Kurokawa H., Shimizu T., Structure-function relationships of EcDOS, a heme-regulated phosphodiesterase from Escherichia coli. Acc. Chem. Res. 39, 37–43 (2006). [DOI] [PubMed] [Google Scholar]
- 49.Nelp M. T., et al. , Immune-modulating enzyme indoleamine 2,3-dioxygenase is effectively inhibited by targeting its apo-form. Proc. Natl. Acad. Sci. U.S.A. 115, 3249–3254 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Möglich A., Ayers R. A., Moffat K., Structure and signaling mechanism of Per-ARNT-Sim domains. Structure 17, 1282–1294 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Key J., Moffat K., Crystal structures of deoxy and CO-bound bjFixLH reveal details of ligand recognition and signaling. Biochemistry 44, 4627–4635 (2005). [DOI] [PubMed] [Google Scholar]
- 52.Claudel T., Cretenet G., Saumet A., Gachon F., Crosstalk between xenobiotics metabolism and circadian clock. FEBS Lett. 581, 3626–3633 (2007). [DOI] [PubMed] [Google Scholar]
- 53.Huang N., et al. , Crystal structure of the heterodimeric CLOCK:BMAL1 transcriptional activator complex. Science 337, 189–194 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Waterhouse A., et al. , SWISS-MODEL: Homology modelling of protein structures and complexes. Nucleic Acids Res. 46, W296–W303 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.DeLano W. L., The PyMOL Molecular Graphics System (DeLano Scientific, San Carlos, CA, 2002). [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.