Abstract
The applications of three-dimensional (3D) printing, or additive manufacturing, to the field of spine surgery continue to grow in number and scope especially in recent years as improved manufacturing techniques and use of sterilizable materials have allowed for creation of 3D printed implants. While 3D printing in spine surgery was initially limited to use as visual aids in preoperative planning for complex pathology, it has more recently been used to create intraoperative patient-specific screw guides and templates and is increasingly being used in surgical education and training. As patient-specific treatment and personalized medicine gains popularity in medicine, 3D printing provides a similar option for the surgical fields, particularly in the creation of customizable implants. 3D printing is a relatively new field as it pertains to spine surgery, and as such, it lacks long-term data on clinical outcomes and cost effectiveness; however, the apparent benefits and seemingly boundless applications of this growing technology make it an attractive option for the future of spine surgery.
Keywords: Three-dimensional printing (3D printing), spine surgery, technology, patient-specific guides, preoperative planning, custom implants, biotissues
Introduction
The surgical applications of three-dimensional (3D) printing and tissue engineering have been investigated since the early 2000s, though only recently has its use in spine surgery begun to be explored (1-3). The current, more common applications of 3D technology in spine surgery include models for use in preoperative planning, patient-specific surgical guides and templates, and teaching tools. However, the ability of 3D printed implants to effectively address variations in anatomy, size, bone quality and pathology in the population is now beginning to be appreciated. Expanding techniques in spine surgery require uniquely shaped implants and materials that are biocompatible. This review will discuss the current uses and future applications of 3D printing in spine surgery, highlighting the potential benefits as well as pitfalls and challenges to more widespread application.
Background
In 1984, Charles Hull patented the first device currently in use for additive manufacturing, also known as rapid prototyping or, more commonly, 3D printing (4,5). This device was called stereolithography (SLA), and the method of creating a 3D prototype is still utilized in 3D printing today. Additive manufacturing utilizes sequential two-dimensional slices, similar to cuts in cross sectional imaging such as computer tomography or magnetic resonance imaging, to lay down a 3D model. Each slice of the 3D model is laid down one after another, until a full prototype is created. In contrast, conventional manufacturing of prototypes is generally created through subtractive manufacturing with material waste as a natural byproduct of the manufacturing process. Subtractive manufacturing consists of raw material which is then fashioned into the final product via manual removal or with computer guidance (6).
Although 3D printing has been utilized in many disciplines and has been improved and refined since its inception, 3 main techniques in rapid prototyping exist. Fused deposition modeling (FDM) utilizes a heated polymer that is sequentially layered with a computer controlled extrusion nozzle (7). FDM is widely used in economic printers and is cost-effective compared to other techniques. However, use of materials that require heat softening often preclude their use in surgery, as these materials are difficult to sterilize without significant compromise. They have been examined in other medical disciplines, such as patient-specific drug delivery (8).
SLA and selective laser sintering (SLS) are the other 2 techniques commonly used in 3D printing. These techniques are more accurate than FDM, although they are more cost, labor, and training-intensive (9). SLA and SLS differ from FDM in the type of material used and the technique with which the material is fashioned and fused into the final model. SLA utilizes a light-curable resin to sequentially add layers. This resin undergoes a process called photopolymerization, in which the areas to be hardened are exposed to an optical light source, causing liquid monomers to become linked in the final polymer. SLS utilizes a focused energy source, such as a laser or electron beam, to sinter fine powder sequentially into a 3D model. The materials used with this technique are those suitable for implants, such as titanium alloys. Both SLS and SLA are more valuable in the setting of surgical disciples for their ability to withstand sterilization techniques, as well as their increased accuracy (7).
Applications to spine surgery
Given its breadth of potential applications, there has been a surge of interest in the utilization of 3D printing in medical disciplines, with early adaptors in oral and maxillofacial surgery as well as orthopaedic surgery (10). Combinations of biomaterials with organic tissues have been explored in 3D printing as well, with authors describing the generation and transplantation of skin, vascular tissue, cardiac tissue, and tracheal splints (11). The advancement of this technology has spurred increased interest in its use in spine surgery, with approximately 8% of all literature concerning rapid prototyping describing its use specifically for spine surgery (12). Potential applications of 3D printing include (I) creation of patient-specific models for educational or pre-operative planning purposes, (II) creation of patient-specific jigs or guides to optimize instrumentation placement, (III) creation of instrumentation or implants to fit a patient-specific or goal-specific need, and (IV) optimization of structure of off-the-shelf implants (6,7).
Preoperative planning
Among the first applications of 3D printing in spine surgery was the use of 3D models to assist in preoperative visualization and surgical planning (Figure 1). A number of studies have noted how physical 3D models—especially as 3D printing technology and the sophistication of models continue to advance—provide an improved sense of unique or complex surgical pathology which may be underappreciated or missed entirely when evaluated with preoperative imaging alone (13-15).
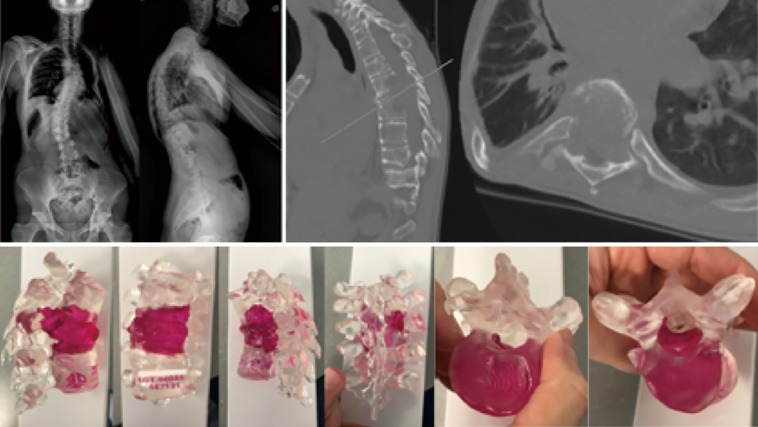
Figure 1.
Imaging studies (top) of a 30-year-old male with an undifferentiated high-grade post-radiation sarcoma involving the thoracic spine. A 3D printed model (bottom) was valuable in conceptualizing the full extent of the tumor. The patient ultimately underwent a T6-T8 en bloc vertebrectomy and reconstruction with an expandable interbody cage. 3D, three-dimensional.
Preoperative 3D modeling has been shown to improve surgical accuracy and improve intraoperative speed while decreasing blood loss in spine surgery for complex deformity. A retrospective single-surgeon study from 2018 compared screw accuracy in a group of 23 patients with complex spinal deformity for whom 3D models were used in preoperative surgical planning to evaluate and mark pedicle screw starting points and trajectories to a historical cohort of 20 patients where pedicle screws were placed freehand without the aid of a 3D printed spine model. They saw no difference in rates of screw accuracy between the two groups [494/513 accurately placed screws (96.3%) when 3D model-assisted vs. 339/352 (96.3%) freehand] despite a higher number of revision cases and subjectively more severe deformities in the 3D model-aided cohort (15). In the setting of congenital abnormalities secondary to myelomeningocele where distorted anatomy makes traditional imaging modalities an inadequate means of evaluating global and intersegmental relationships, Karlin et al. report on their experience of using 3D models to assist in preoperative planning. The authors compared surgical outcomes in a cohort of seven patients who had 3D models created to aid in preoperative planning and also served as a surgical reference intraoperatively versus a historical cohort of ten patients where only traditional imaging was used, finding that despite a greater degree of surgical complexity in the 3D group, the fluoroscopic use and blood loss were similar between the two groups (14). Among the benefits afforded by 3D modeling, the authors noted that in several cases they were able to appreciate anomalous anatomy which was undiagnosed on official imaging reports and were able to pre-contour the rods based on the 3D model, saving operative time as a result. In all cases they made changes to the operative plan as a result of the model.
Given the complex anatomy of the cervical spine, 3D models have been used to aid in preoperative planning with the goal of minimizing morbidity while still obtaining tumor free margins. Xiao et al. reported a series of five patients undergoing en bloc resection of primary malignant bone tumors of the cervical spine in which 3D models were used during preoperative surgical planning to assess the extent of the tumor and its proximity to vital structures (16). Use of sterilizable 3D models has also been described in upper cervical tumor surgery. In cases where tumor capsule may not be readily appreciable on imaging studies and irregular lesions may need to be resected without direct visualization, the study notes that the ability to reference a model of the tumor intraoperatively can minimize surgical morbidity while improving the surgical team’s ability to achieve negative margins (17).
Pedicle screw guides
One of the initial surgical applications of 3D printing in spine surgery was creation of patient-specific drill guides and templates meant to address some of the drawbacks of early image-guided navigation systems, including cumbersome stereotactic arrays, high technology startup cost, potential for surgeon interference, and increased surgical time among others. Proponents of 3D printed guides tout that they serve a similar purpose as computer navigated techniques without reliance on expensive technology, which may not be available or practical for use in underdeveloped countries (18). Use of pedicle screw guides was first reported in 2005 in a cadaveric study by Berry et al. in which personalized, 3D printed drill guides in 4 different designs were used in cadaveric specimens with varying degrees of success. Since that time, with changes to design and improvement in manufacturing processes, 3D printed pedicle screw guides have since become more accurate and precise, expanding their applications in spine surgery (19).
In a 2009 in vivo proof of concept study, computer-based 3D models of patient vertebral bodies with virtual pedicle screws were used to reverse engineer patient-specific drill templates which were subsequently sterilized and used to accurately place lumbar pedicle screws in a cohort of six patients. The authors note that the technique was time-intensive and thorough preparation of the bony surfaces was necessary to ensure an adequate fit between template and patient anatomy (20). This same group subsequently applied these techniques to a cohort of 25 patients undergoing cervical pedicle screw placement. Of the 88 screws placed, there were no pedicle violations, and only 1 screw had deviated greater than 2 mm from the planned trajectory (21).
Since their initial reported use in patients, 3D printed patient-specific guides of varying designs have been utilized successfully in C1-C2 transarticular screw placement (22), C1 lateral mass and C2 pedicle and laminar screws (23), mid- and lower-cervical pedicle screws (24,25), thoracolumbar cortical screws (26) and throughout the spine in severe congenital scoliosis (27). Typically, 3D guides are utilized as a means of improving screw accuracy in patients with complex anatomy or in regions where screw inaccuracy may lead to significant patient morbidity. Though relatively accurate, screw trajectory deviation has been reported to occur up to 17% of the time with use of 3D printed guides, likely due to a poor fit between template and bone or play within the screw trajectory guide during starting point placement and screw tract preparation (21,28). A randomized controlled trial comparing pedicle screw accuracy using 3D printed guides versus free hand technique in 29 patients undergoing surgery for spinal deformity found that 3D guides provided significantly greater screw accuracy and decreased total radiation dose, though notably the authors still reported that 9.8% of screws implanted using 3D guides were found to be malpositioned (29).
Several studies suggest, however, that these inaccuracies are typically relatively small and infrequently of clinical significance. Recently, Sugawara et al. reported results of a prospective, multicenter trial of a 3-template 3D printed pedicle screw guides. Of 813 screws inserted in 103 patients, 98.5% were found to be grade 0 (completely contained within the pedicle), while 1.5% were grade 1 (pedicle breech with >50% of the screw contained) based on postoperative CT imaging. Additionally, there was no report of vascular or neural injury (18). In another study comparing 3D template-assisted versus freehand pedicle screw accuracy in complex spinal deformity, there was a significantly higher number of screws with “perfect screw placement” and significantly fewer medial violations (P=0.005) when 3D templates were used. Moreover, surgical time was significantly shorter (P=0.03) and need for fluoroscopy was decreased in the 3D group (19). The authors advocate for use of 3D templates in less developed areas of the world where complex spinal deformity is relatively common and advanced technology (i.e., computer assisted navigation) may not be available.
Osteotomy guides
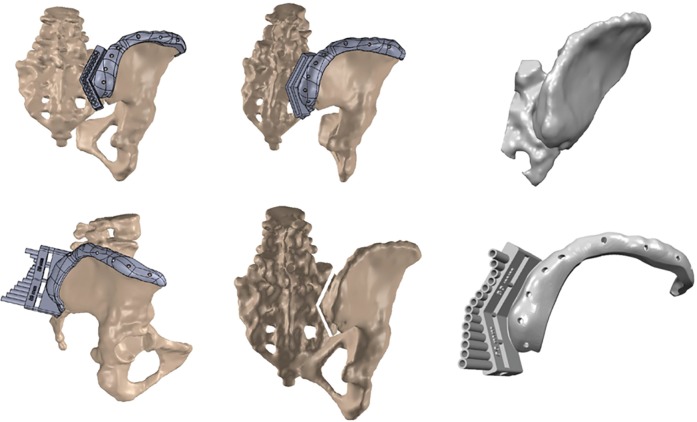
As an extension of the success noted with 3D printed guides for pedicle screw placements, 3D guides have recently been created to aid in osteotomies in both adult and pediatric patients (Figure 2). Tu et al. report on a cohort of patients with severe kyphoscoliosis secondary to ankylosing spondylitis who underwent software-aided simulated correction from which osteotomy guides were printed in titanium and secured to the spine with pre-planned pedicle screw guides. In 83 screws placed in nine patients undergoing osteotomies, they reported 94% screw accuracy and no serious neurovascular complications (30). A case report from Pijpker et al. from 2018 which described the first such use of osteotomy guides noted that while the guides were useful for the initial stages of the pedicle subtraction osteotomy (PSO), providing a template for resection of the posterior elements in the planned asymmetric PSO, they were not able to be used as the osteotomy progressed to the apex necessitating placement of stabilizing rods (31).
Figure 2.
Computer rendering of an osteotomy guide for use in the resection of an intraosseous high-grade iliac chondrosarcoma involving the sacroiliac joint.
Patient-specific implants
In cases of abnormal anatomy, 3D printing can afford the surgeon the opportunity to customize the device to the patient rather than surgically remodeling the patient to fit the implant, potentially limiting intraoperative trauma and surgical morbidity. Further, customized implants allow the surgeon to avoid destabilizing structural anatomic features of a patient’s particular anatomy, e.g., maintaining cortical bone and endplates for interbody devices (13). Patient-specific implant creation and preoperative modeling additionally allows for finite element analysis to predict and address stresses through the implant as well as those seen by adjacent structures, and can be further used to compare potential stresses in the setting of use of a custom implant versus generic devices (13,32).
Tumor
3D printed vertebrae have been used in revision surgery after tumor recurrence in the lumbar spine. In one case report of recurrent giant cell tumor after previous decompression and instrumented fusion of T12-L4 necessitating en bloc spondylectomy of L1-L3, 3D reconstruction afforded the ability to not only create an implant which effectively filled the gap created by the en bloc spondylectomy, but also achieve both osseointegration at the cranial and caudal levels of the construct, as well as fixation to posterior spinal instrumentation which may mitigate the risk of cage migration and subsidence (33). Choy et al. report successful implantation of a T9 vertebral body reconstruction cage for a hemangioendothelioma in a 14-year-old girl which was designed to accommodate the kyphoscoliotic nature of her deformity while maximizing endplate contact and providing fixation holes for incorporation into posterior instrumentation. The authors reported no complications and evidence of integration into the adjacent endplates at 6 months follow-up (34).
Long term clinical outcomes are lacking as a result of the relative infancy of this technology. However, Girolami et al. reported on radiographic outcomes ranging between 5 and 18 months in a series of 13 thoracolumbar and lumbar tumors resected through a single posterior or combined anterior-posterior approach and reconstructed using 3D printed cages. While the authors noted subsidence in all of their patients at both the proximal and distal levels, they reported progressive, symptomatic global sagittal imbalance requiring revision in only one. Of the four patients in their series with segmental kyphosis, the average correction was 72% (range, 31–97%) at last follow-up (35).
Li et al. utilized a 3D printed cage for reconstruction of the anterior cervical spine in a patient with metastatic papillary thyroid carcinoma in her C2-C4 vertebrae. In this case, the authors report that a custom 3D cage allowed them to match the footprint of the remaining osseous structures and provide custom points of fixation to the atlas and axis which would not have been possible through use of standard conventional titanium mesh cages (36). Similarly, Xu et al. report a case of C2 spondylectomy and reconstruction with a 3D printed self-stabilizing titanium vertebral body in a 12-year-old boy with Ewing’s sarcoma. Notably, the implant was designed to maximize surface area contact between C1 as well as provide a zero-profile ventral surface to minimize the risk of dysphagia. At 1 year follow up, the patient had evidence of implant osseointegration without displacement or subsidence and an improvement of his JOA score to 16/17 from a preoperative score of 8/17 (37).
Anatomy/biology
3D implants can be used to address not only anatomic considerations, but in some cases may be used to augment biological deficiencies as well. In osteoporotic patients, 3D custom implants have been used to accommodate mismatch between the contour of the cage and the endplate which may be deformed as a result of previous insufficiency fracture. In one case report, Siu et al. fabricated custom 3D printed titanium lateral lumbar interbody fusion (LLIF) for a 74-year-old female with degenerative scoliosis at levels of previous vertebral compression fractures, incorporating large graft windows to facilitate bony fusion, tapered ends for increased ease of insertion, and biconcave geometry to accommodate endplate changes (38).
Mobbs et al. report their experience with creation of custom 3D interbody cage for use in an anterior lumbar interbody fusion (ALIF) for a patient with complex sacral endplate anatomy. In addition to the custom implant, a 3D model of the patient was created allowing the surgical team to make a hands-on preoperative evaluation and identification of potential surgical pitfalls based on aberrant anatomy (13). Creation of 3D printed, patient-specific implants to accommodate anatomic variability can increase the bone-implant interface with the potential to positively impact fusion rates.
Biotissues
The application of 3D printing to biotissues is an expanding field. As it applies to bone and cartilage tissue engineering, 3D printing has been used to create biocompatible structural bone substitutes in rabbit and porcine models which provides an osteoconductive scaffolding much like a structural allograft (39,40). However, engineering soft tissues for clinical transplantation also typically requires having a structural scaffold in order to be physiologically useful. As it relates to spine surgery, one recent study details the creation of 3D printed matrices which provide a favorable scaffold for the ingrowth and viability of articular chondrocytes and nucleus pulposus cells (41). Additionally, a recent case report describing use of artificial 3D printed dura mater in the treatment of sacral canal cysts, primarily as a means of augmenting the dural plication, demonstrates an example of the early application of this rapidly developing technology to spine surgery (42).
Education
Among the more common applications of 3D printing technology to spine surgery is the use of 3D models for education and clinical training. As 3D printing techniques have advanced so too have their ability to accurately and realistically recreate normal and pathologic anatomy for teaching purposes. As highlighted by Weiss et al., while cadavers have long been used as training tools, they are expensive, require special preparation and storage, lack pathology, do not bleed, and may pose a biohazardous risk to trainees (43,44). Similarly, though virtual reality simulators continue to improve, they may be prohibitively expensive and lack the high fidelity of real or synthetic surgical training tools. As a result, 3D printed surgical trainers fill a unique surgical training need and afford surgeon educators the opportunity to effectively teach advanced or uncommonly encountered surgical techniques to trainees.
In response to the growing field of minimally invasive spine surgery and the search for new ways to safely and effectively teach these techniques outside the operating theatre, Weinstock et al. describe their experience of the creation of a scale reproduction of an adolescent with hydrocephalus to be used for training endoscopic third ventriculostomy. Their model featured “Hollywood special effects techniques” providing a highly realistic and valid training model for surgical residents, which is an increasingly common feature of 3D training “phantoms” (45). Similar surgical training models include a vertebral model which accurately mimics the tactile feedback of cortical versus cancellous bone in normal and osteoporotic bone (46), 3D printed pelvic models for placing S2-Alar-Iliac screws which accurately represent normal anatomy on fluoroscopy (44), and realistic 3D printed simulators, or “phantoms” for teaching freehand C2 laminar screw placement and cervical laminectomies (43,47) among others.
Pitfalls and challenges
3D implants limit the modularity inherent in typical implant systems which include, for example in interbody fusions—varying cage widths, heights and degrees of lordosis provides the surgeon with a degree of intraoperative flexibility to accommodate structural variation that occurs after bony decompression or soft tissue manipulation. With 3D implants, multiple patient-specific implants of various degrees of modularity would need to be produced, which may be cost-prohibitive. Chin et al. detailed additional issues in using patient-specific implants for tumor reconstruction, noting variability between medical imaging and actual surgical anatomy, intraoperative findings which change the surgical plan that cannot be accommodated by the implant, and inability to use a modular 3D system to address these issues as this technology simply does not yet exist (33). Several studies note that in order to overcome this potential pitfall, multiple implants and duplicates were manufactured in preparation for the case incorporating various cage heights and angles to allow for some degree of intraoperative customization (13,34).
At this point, given that this is an emerging technology with applications which are still being explored, the duration of follow-up for most case reports which expand the scope and application of 3D implants is relatively short. As the technology grows, mid- and long-term patient outcomes and studies comparing outcomes in patients receiving custom versus generic implants will be helpful in determining the limitations and broader application of this relatively new technology.
Cost
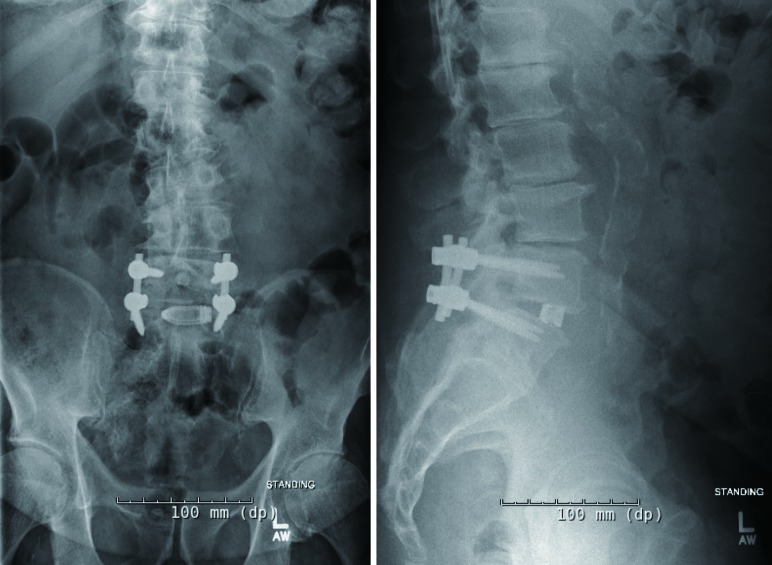
The potential cost savings with 3D printing stems from the ability to create implants where the manufacturing cost is related primarily to the amount of material printed rather than the complexity of the design. As Mobbs et al. note, the cost-burden is shifted to the design rather than the physical production of these devices as skilled labor is required in earlier stages of the custom 3D device workflow (13). While processes continue to improve, creation of custom implants is restricted by the time-intensive design processes required to create them. Additional considerations include the potential need to create multiple implants to impart a degree of modularity to the custom devices and provide the surgeon intraoperative alternatives to using a single implant which may not adequately achieve the intended surgical goals. Currently, mass-produced 3D printed implants are available for use, which defray the design and labor costs associated with custom implants, but sacrifices customizability for modularity (Figure 3).
Figure 3.
Postoperative X-ray images of a patient who underwent an L4-5 transforaminal lumbar interbody fusion and posterior spinal decompression and fusion using a mass-produced 3D printed cage (Stryker, Kalamazoo, MI, USA). 3D, three-dimensional.
With regards to surgical pedicle screw and osteotomy guides, several studies report on actual cost. Sugawara reported a total manufacturing cost for 3D templates which included a vertebral model and three templates per level to range from $12 to $28 per level (18). PIjpker et al. reported a total production cost of $175 for the manufacture of a polyamide osteotomy guide, though they did not comment on the additional manpower cost associated with the actual template design. At that cost, they note that this would likely not be considered cost-effective.
Regulatory considerations
Government agency regulation and potential risk to patients is an additional consideration in the manufacture and use of 3D printed devices and implants. In order to ensure patient safety under current FDA Quality Assurance practices for new devices, customized 3D printed implants require proof of adequate safety for both the design as well as the materials used to produce them. Morrison et al. note that, with regards to design safety, demonstrating compatibility between a patient and a custom-designed implant using 3D modeling software may be adequate. Among the additional device safety concerns specific to 3D devices are the use of recycled substrates in the manufacturing process which may be a potential source of contamination, the reproducibility of the physical characteristics between devices, and post-manufacture quality assurance including cleaning and sterilizing the device without significantly altering its physical and structural characteristics (48).
Benefits
One of the notable benefits of 3D printed interbody cages is the observed increased early stability secondary to robust osseointegration of the implant. The larger pore size of Ti6Al4V implants promote bony ingrowth into the implant providing rapid stability at the bone-implant interface, and by nature of the manufacturing methods, 3D printing imparts a rough surface to titanium implants which can increase the coefficient of friction and initial purchase of an interbody cage (49). Additional benefits of titanium include good biocompatibility and sufficient strength to withstand physiologic loads of the lumbar spine, though at the risk of increased cage subsidence when used as interbody implants due to an increased Young’s modulus as well as limitations when attempting to image the fusion mass within the cage. In a comparative animal study evaluating lumbar fusion between three cage materials (PEEK, plasma sprayed porous titanium coated PEEK, and 3D printed porous titanium alloy cage) in an ovine model, the 3D printed titanium cage demonstrated a significant decrease in motion and increase in stiffness and total bone volume within the graft window at 8 and 16 week timepoints (50). A similar study in canine models demonstrated similar rates of osseointegration into 3D printed porous implants at 4 and 12 weeks (51). 3D printed titanium cages may ultimately provide improved osseointegration into recipient tissues, though the ultimate impact on patient outcomes remains unclear as this technology continues to be evaluated.
Conclusions
There has been increasing interest in 3D printing in spine surgery, particularly over the past decade. Applications of 3D technology to the field have evolved from simple models for use in preoperative planning and education to creation of custom implants to fill bony defects and accommodate irregular anatomy. With continued improvement in manufacturing techniques, further development of bio-printing technology, and an increase in the body of literature to better define its indications and outcomes, 3D printing in spine surgery will continue to grow and evolve in the coming years.
Acknowledgments
None.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Footnotes
Conflicts of Interest: This manuscript has not been submitted or published elsewhere. Dr. Sheha and Dr. Gandhi have no conflicts of interest to declare. Dr. Colman has the following disclosures: K2M—medical education; Spinal Elements—royalties; Orthofix—royalties; CSRS—committee membership; AO spine—committee membership.
References
- 1.Sherwood JK, Riley SL, Palazzolo R, et al. A three-dimensional osteochondral composite scaffold for articular cartilage repair. Biomaterials 2002;23:4739-51. 10.1016/S0142-9612(02)00223-5 [DOI] [PubMed] [Google Scholar]
- 2.Melican MC, Zimmerman MC, Dhillon MS, et al. Three-dimensional printing and porous metallic surfaces: a new orthopedic application. J Biomed Mater Res 2001;55:194-202. [DOI] [PubMed] [Google Scholar]
- 3.Lalan S, Pomerantseva I, Vacanti JP. Tissue engineering and its potential impact on surgery. World J Surg 2001;25:1458-66. 10.1007/s00268-001-0131-3 [DOI] [PubMed] [Google Scholar]
- 4.Hull CW. Apparatus for production of three-dimensional objects by stereolithography. Washington DC: U.S. Patent and Trademark Office. U.S. Patent, 1986:4,575,330.
- 5.Gross BC, Erkal JL, Lockwood SY, et al. Evaluation of 3D printing and its potential impact on biotechnology and the chemical sciences. Anal Chem 2014;86:3240-53. 10.1021/ac403397r [DOI] [PubMed] [Google Scholar]
- 6.Hsu MR, Haleem MS, Hsu W. 3D printing applications in minimally invasive spine surgery. Minim Invasive Surg 2018;2018:4760769. [DOI] [PMC free article] [PubMed]
- 7.Garg B, Mehta N. Current status of 3D printing in spine surgery. J Clin Orthop Trauma 2018;9:218-25. 10.1016/j.jcot.2018.08.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Long J, Gholizadeh H, Lu J, et al. Application of fused deposition modelling (FDM) method of 3D printing in drug delivery. Curr Pharm Des 2017;23:433-9. [DOI] [PubMed] [Google Scholar]
- 9.Malik HH, Darwood AR, Shaunak S, et al. Three-dimensional printing in surgery: a review of current surgical applications. J Surg Res 2015;199:512-22. 10.1016/j.jss.2015.06.051 [DOI] [PubMed] [Google Scholar]
- 10.Hoang D, Perrault D, Stevanovic M, et al. Surgical applications of three-dimensional printing: a review of the current literature & how to get started. Ann Transl Med 2016;4:456. 10.21037/atm.2016.12.18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Murphy SV, Atala A. 3D bioprinting of tissues and organs. Nat Biotechnol 2014;32:773-85. 10.1038/nbt.2958 [DOI] [PubMed] [Google Scholar]
- 12.Tack P, Victor J, Gemmel P, et al. 3D-printing techniques in a medical setting: a systematic literature review. Biomed Eng Online 2016;15:115. 10.1186/s12938-016-0236-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mobbs RJ, Parr WCH, Choy WJ, et al. Anterior lumbar interbody fusion using a personalized approach: is custom the future of implants for anterior lumbar interbody fusion surgery? World Neurosurg 2019. [Epub ahead of print]. 10.1016/j.wneu.2018.12.144 [DOI] [PubMed] [Google Scholar]
- 14.Karlin L, Weinstock P, Hedequist D, et al. The surgical treatment of spinal deformity in children with myelomeningocele: the role of personalized three-dimensional printed models. J Pediatr Orthop B 2017;26:375-82. 10.1097/BPB.0000000000000411 [DOI] [PubMed] [Google Scholar]
- 15.Tan LA, Yerneni K, Tuchman A, et al. Utilization of the 3D-printed spine model for freehand pedicle screw placement in complex spinal deformity correction. J Spine Surg 2018;4:319-27. 10.21037/jss.2018.05.16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Xiao JR, Huang WD, Yang XH, et al. En bloc resection of primary malignant bone tumor in the cervical spine based on 3-dimensional printing technology. Orthop Surg 2016;8:171-8. 10.1111/os.12234 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ahmed AK, Pennington Z, Molina CA, et al. Multidisciplinary surgical planning for en bloc resection of malignant primary cervical spine tumors involving 3D-printed models and neoadjuvant therapies: report of 2 cases. J Neurosurg Spine 2019. [Epub ahead of print]. 10.3171/2018.9.SPINE18607 [DOI] [PubMed] [Google Scholar]
- 18.Sugawara T, Kaneyama S, Higashiyama N, et al. Prospective multicenter study of a multistep screw insertion technique using patient-specific screw guide templates for the cervical and thoracic spine. Spine (Phila Pa 1976) 2018;43:1685-94. 10.1097/BRS.0000000000002810 [DOI] [PubMed] [Google Scholar]
- 19.Garg B, Gupta M, Singh M, et al. Outcome and safety analysis of 3D-printed patient-specific pedicle screw jigs for complex spinal deformities: a comparative study. Spine J 2019;19:56-64. 10.1016/j.spinee.2018.05.001 [DOI] [PubMed] [Google Scholar]
- 20.Lu S, Xu YQ, Zhang YZ, et al. A novel computer-assisted drill guide template for lumbar pedicle screw placement: a cadaveric and clinical study. Int J Med Robot 2009;5:184-91. 10.1002/rcs.249 [DOI] [PubMed] [Google Scholar]
- 21.Lu S, Xu YQ, Lu WW, et al. A novel patient-specific navigational template for cervical pedicle screw placement. Spine (Phila Pa 1976) 2009;34:E959-66. 10.1097/BRS.0b013e3181c09985 [DOI] [PubMed] [Google Scholar]
- 22.Hu Y, Yuan ZS, Kepler CK, et al. Deviation analysis of C1-C2 transarticular screw placement assisted by a novel rapid prototyping drill template: a cadaveric study. J Spinal Disord Tech 2014;27:E181-6. 10.1097/BSD.0000000000000087 [DOI] [PubMed] [Google Scholar]
- 23.Sugawara T, Higashiyama N, Kaneyama S, et al. Accurate and simple screw insertion procedure with patient-specific screw guide templates for posterior C1-C2 fixation. Spine (Phila Pa 1976) 2017;42:E340-6. 10.1097/BRS.0000000000001807 [DOI] [PubMed] [Google Scholar]
- 24.Kaneyama S, Sugawara T, Sumi M. Safe and accurate midcervical pedicle screw insertion procedure with the patient-specific screw guide template system. Spine (Phila Pa 1976) 2015;40:E341-8. 10.1097/BRS.0000000000000772 [DOI] [PubMed] [Google Scholar]
- 25.Li F, Huang X, Wang K, et al. Preparation and assessment of an individualized navigation template for lower cervical anterior transpedicular screw insertion using a three-dimensional printing technique. Spine (Phila Pa 1976) 2018;43:E348-56. 10.1097/BRS.0000000000002341 [DOI] [PubMed] [Google Scholar]
- 26.Kim SB, Rhee JM, Lee GS, et al. Computer-assisted patient-specific prototype template for thoracolumbar cortical bone trajectory screw placement: a cadaveric study. Tech Orthop 2018;33:246-50. 10.1097/BTO.0000000000000285 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Luo M, Wang W, Yang N, et al. Does three-dimensional printing plus pedicle guider technology in severe congenital scoliosis facilitate accurate and efficient pedicle screw placement. Clin Orthop Relat Res 2019;477:1904-12. 10.1097/CORR.0000000000000739 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lamartina C, Cecchinato R, Fekete Z, et al. Pedicle screw placement accuracy in thoracic and lumbar spinal surgery with a patient-matched targeting guide: a cadaveric study. Eur Spine J 2015;24 Suppl 7:937-41. 10.1007/s00586-015-4261-y [DOI] [PubMed] [Google Scholar]
- 29.Cecchinato R, Berjano P, Zerbi A, et al. Pedicle screw insertion with patient-specific 3D-printed guides based on low-dose CT scan is more accurate than free-hand technique in spine deformity patients: a prospective, randomized clinical trial. Eur Spine J 2019;28:1712-23. 10.1007/s00586-019-05978-3 [DOI] [PubMed] [Google Scholar]
- 30.Tu Q, Ding HW, Chen H, et al. Three-dimensional-printed individualized guiding templates for surgical correction of severe kyphoscoliosis secondary to ankylosing spondylitis: outcomes of 9 cases. World Neurosurg 2019. [Epub ahead of print]. 10.1016/j.wneu.2019.07.047 [DOI] [PubMed] [Google Scholar]
- 31.Pijpker PAJ, Kuijlen JMA, Kraeima J, et al. Three-dimensional planning and use of individualized osteotomy-guiding templates for surgical correction of kyphoscoliosis: a technical case report. World Neurosurg 2018;119:113-7. 10.1016/j.wneu.2018.07.219 [DOI] [PubMed] [Google Scholar]
- 32.Serra T, Capelli C, Toumpaniari R, et al. Design and fabrication of 3D-printed anatomically shaped lumbar cage for intervertebral disc (IVD) degeneration treatment. Biofabrication 2016;8:035001. 10.1088/1758-5090/8/3/035001 [DOI] [PubMed] [Google Scholar]
- 33.Chin BZ, Ji T, Tang X, et al. Three-level lumbar en bloc spondylectomy with three-dimensional-printed vertebrae reconstruction for recurrent giant cell tumor. World Neurosurg 2019;129:531-7. 10.1016/j.wneu.2019.06.056 [DOI] [PubMed] [Google Scholar]
- 34.Choy WJ, Mobbs RJ, Wilcox B, et al. Reconstruction of thoracic spine using a personalized 3D-printed vertebral body in adolescent with t9 primary bone tumor. World Neurosurg 2017;105:1032.e13-1032.e17. 10.1016/j.wneu.2017.05.133 [DOI] [PubMed] [Google Scholar]
- 35.Girolami M, Boriani S, Bandiera S, et al. Biomimetic 3D-printed custom-made prosthesis for anterior column reconstruction in the thoracolumbar spine: a tailored option following en bloc resection for spinal tumors: preliminary results on a case-series of 13 patients. Eur Spine J 2018;27:3073-83. 10.1007/s00586-018-5708-8 [DOI] [PubMed] [Google Scholar]
- 36.Li X, Wang Y, Zhao Y, et al. Multilevel 3D printing implant for reconstructing cervical spine with metastatic papillary thyroid carcinoma. Spine (Phila Pa 1976) 2017;42:E1326-30. 10.1097/BRS.0000000000002229 [DOI] [PubMed] [Google Scholar]
- 37.Xu N, Wei F, Liu X, et al. Reconstruction of the upper cervical spine using a personalized 3D-printed vertebral body in an adolescent with ewing sarcoma. Spine (Phila Pa 1976) 2016;41:E50-4. 10.1097/BRS.0000000000001179 [DOI] [PubMed] [Google Scholar]
- 38.Siu TL, Rogers JM, Lin K, et al. Custom-made titanium 3-dimensional printed interbody cages for treatment of osteoporotic fracture-related spinal deformity. World Neurosurg 2018;111:1-5. 10.1016/j.wneu.2017.11.160 [DOI] [PubMed] [Google Scholar]
- 39.Tamimi F, Torres J, Gbureck U, et al. Craniofacial vertical bone augmentation: a comparison between 3D printed monolithic monetite blocks and autologous onlay grafts in the rabbit. Biomaterials 2009;30:6318-26. 10.1016/j.biomaterials.2009.07.049 [DOI] [PubMed] [Google Scholar]
- 40.Abarrategi A, Moreno-Vicente C, Martínez-Vázquez FJ, et al. Biological properties of solid free form designed ceramic scaffolds with BMP-2: in vitro and in vivo evaluation. PLoS One 2012;7:e34117. 10.1371/journal.pone.0034117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Rosenzweig DH, Carelli E, Steffen T, et al. 3D-printed ABS and PLA scaffolds for cartilage and nucleus pulposus tissue regeneration. Int J Mol Sci 2015;16:15118-35. 10.3390/ijms160715118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Liu B, Wang Z, Lin G, et al. Radiculoplasty with reconstruction using 3D-printed artificial dura mater for the treatment of symptomatic sacral canal cysts: two case reports. Medicine (Baltimore) 2018;97:e13289. 10.1097/MD.0000000000013289 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Weiss MY, Melnyk R, Mix D, et al. Design and validation of a cervical laminectomy simulator using 3D printing and hydrogel phantoms. Oper Neurosurg (Hagerstown) 2019. [Epub ahead of print]. [DOI] [PubMed]
- 44.Bow H, Zuckerman SL, Griffith B, et al. A 3D-printed simulator and teaching module for placing S2-alar-iliac screws. Oper Neurosurg (Hagerstown) 2019. [Epub ahead of print]. [DOI] [PubMed]
- 45.Weinstock P, Rehder R, Prabhu SP, et al. Creation of a novel simulator for minimally invasive neurosurgery: fusion of 3D printing and special effects. J Neurosurg Pediatr 2017;20:1-9. 10.3171/2017.1.PEDS16568 [DOI] [PubMed] [Google Scholar]
- 46.Burkhard M, Fürnstahl P, Farshad M. Three-dimensionally printed vertebrae with different bone densities for surgical training. Eur Spine J 2019;28:798-806. 10.1007/s00586-018-5847-y [DOI] [PubMed] [Google Scholar]
- 47.Clifton W, Nottmeier E, Edwards S, et al. Development of a novel 3D printed phantom for teaching neurosurgical trainees the freehand technique of C2 laminar screw placement. World Neurosurg 2019;129:e812-20. 10.1016/j.wneu.2019.06.038 [DOI] [PubMed] [Google Scholar]
- 48.Morrison RJ, Kashlan KN, Flanangan CL, et al. Regulatory considerations in the design and manufacturing of implantable 3D-printed medical devices. Clin Transl Sci 2015;8:594-600. 10.1111/cts.12315 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Shah FA, Snis A, Matic A, et al. 3D printed Ti6Al4V implant surface promotes bone maturation and retains a higher density of less aged osteocytes at the bone-implant interface. Acta Biomater 2016;30:357-67. 10.1016/j.actbio.2015.11.013 [DOI] [PubMed] [Google Scholar]
- 50.McGilvray KC, Easley J, Seim HB, et al. Bony ingrowth potential of 3D-printed porous titanium alloy: a direct comparison of interbody cage materials in an in vivo ovine lumbar fusion model. Spine J 2018;18:1250-60. 10.1016/j.spinee.2018.02.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Tanzer M, Chuang PJ, Ngo CG, et al. Characterization of bone ingrowth and interface mechanics of a new porous 3D printed biomaterial: an animal study. Bone Joint J 2019;101-B:62-7. 10.1302/0301-620X.101B6.BJJ-2018-1472.R1 [DOI] [PubMed] [Google Scholar]