Abstract
Throughout the years, colorectal cancer has steadily become a global health problem. While other types of cancers have seen a decline in cases because of screening and vaccination programs, colorectal cancer has risen become the third most diagnosed cancer worldwide and, more worryingly, the second leading cancer-related cause of death. The introduction of targeted therapy has been widely considered a major paradigm shift in the treatment of colorectal cancer, which agents such as bevacizumab and cetuximab quickly becoming mainstay options in the treatment of locally advanced or metastatic disease. However, this type of treatment has also shown its limitations, with limited or no benefit for a large portion of the patients. With more and more knowledge being gathered on the molecular mechanisms which govern the malignant phenotype presented by colorectal cancer, scientists are engaged in a continuous effort to develop new therapies based on these discoveries.
Keywords: Colorectal cancer, biomarkers, checkpoint inhibitors, immunohistochemistry
Introduction
With the advent of targeted therapy almost two decades ago, the landscape in oncology has changed dramatically.
Many forms of cancer which were previously considered very difficult to treat or even untreatable became easier to control with major benefits for patients in terms of overall survival (OS), progression-free survival (PFS) and quality of life (QoL).
More importantly, the existence of alternative options besides the classic approaches such as chemotherapy, radiotherapy and surgery meant that patients who were unable to follow these treatments due to added toxicities, treatment resistance or comorbidities were gifted with new treatment opportunities.
These new approaches could be used in monotherapy or combined with the aforementioned classic treatments, thus catering to a larger number of patients than previously available.
Colorectal cancer was one of the main beneficiaries of the introduction of targeted therapy, mainly because several discoveries were made about the molecular mechanisms which are largely responsible for the malignant phenotype displayed by the tumor (progression patterns, invasiveness, reduced apoptosis, angiogenesis and metastatic potential).
New laboratory techniques such as immunohistochemistry (IHC), quantitative polymerase chain reaction (qPCR), chromogenic/fluorescence in situ hybridization (CISH/FISH) and next generation sequencing (NGS) have proven extremely valuable for detecting these molecular aberrations.
Several biomarkers such as the tyrosine kinase receptors (TKR): Epithelial Growth Factor Receptor (EGFR), Vascular Endothelial Growth Factor Receptor (VEGFR) and signal transduction kinases such as K-RAS, BRAF or most recently the Microsatellite Instability (MSI)/DNA Mismatch Repair (MMR) status or expression of p53 and linked targeted genes have been confirmed to play major roles in the evolution of colorectal cancer and, as such, have been become the focal point of the development of novel drugs.
Despite this, colorectal cancer (CRC) continues to be one of the leading causes of cancer-related deaths (9.2%), with over 1.000.000 new cases being discovered each year and over 500.000 deaths in the year 2018 [1].
Out of all the newly discovered patients, 25% are diagnosed with a metastatic disease while 40% will develop metastasis within the following year [2].
Despite the addition of several new therapeutic agents, 5 year survival for patients with metastatic colon cancer remains very low, just under 15%.
These numbers underscore the importance of discovering new treatments options which could be used to improve survival rates and the QoL of patients suffering from this disease.
The purpose of this review is to analyze new treatments which have been recently added into clinical practice or are currently evaluated in phase I/II clinical trials.
Immunotherapy
Checkpoint inhibitors
Two anti PD-1 agents known as Nivolumab and Pembrolizumab have recently received approval for usage in patients suffering from mCRC with MSI-high (H) status which have previously progressed under oxaliplatin or irinotecan-based chemotherapy. The Checkmate 142 clinical trial has shown that 3mg/kg of Nivolumab biweekly produced an Overall Response Rate (ORR) of 31% and a stable disease rate (SDR) of 69% in a group of 72 heavily pretreated patients with mCRC [3].
Keynote 164 is a recent phase II clinical trial with ORR as its primary endpoint. In this study, 61 patients with mCRC with 2 previous lines of treatment were given 200mg of Pembrolizumab every three weeks. ORR was just reported in just over a quarter of the patients while SDR was 51% [4].
Given the encouraging results obtained in monotherapy several attempts were made to combine immunotherapeutic agents with other immune-based approaches or targeted therapy. As such, a study involving the combination between Nivolumab (3mg/kg) and the anti-CTLA 4 antibody Ipilimumab (1mg/kg) in patients with MSI-H mCRC presented an ORR of 50%, a SDR of 80% while the OS and PFS endpoints were not reached [5].
This shows that the combination between the two immunotherapeutic agents presented improved results when compared to the previous clinical trials where patients received Nivolumab in monotherapy.
Unfortunately, another arm of this study with the combination of Nivolumab+Ipilimumab on patients with MSS (microsatellite stability) presented no benefit in terms of ORR (0%) and similarly abysmal PFS (1.3 months) and OS (3.73 months) [5].
This further underscores the fact that immunotherapy caters for only a very specific niche of patients, with little to no benefit for the others which make up the bulk of the mCRC cases.
To counter this problem, a phase III study including 363 patients, 95% of them having MSS, investigated the effect of the combination between the immunotherapeutic agent atezolizumab and the MEK inhibitor cobimetinib versus atezolizumab in monotherapy versus regorafenib for locally advanced or mCRC.
Discouragingly, this combination failed to meets its endpoints in terms of OS, PFS and ORR [6].
The combination between atezolizumab and the well-studied VEGF inhibitor bevacizumab was also tested on 84 MSI-H patients to see if it’s safe and efficient. The results were encouraging with a 30% ORR and a 90% SDR. However, larger studies are required to confirm that this is, indeed, a strong option versus single immune therapy for MSI-H patients [7].
Other immunotherapeutic approaches
RG7802 is an antibody which simultaneously binds to CEA on CRC cells and the CD3 receptors found on the surface of T cells, thus increasing T cell infiltration in the tumor.
Two separate phase I trials investigating the effect of RG7802 alone or in combination with atezolizumab found out that the antibody was well tolerated with minimum side effects.
However, in terms of SDR, preliminary results have only shown a 25% ratio. As the trials are ongoing further details are expected to be presented this year (NCT02650713) (NCT02324257).
Viral therapy has also been widely explored in CRC throughout the last two decades.
The principle of genetically engineering a virus to detect and infect only specific cells of the body, in this case cancer cells, is not a new approach.
Several genetically engineered viruses designed to recognize different cancer-specific antigens (CEA, Ep-CAM) have been tested in phase I clinical trials.
The main viruses types used were adenoviruses [8], vaccinia viruses [9], retrovirus [10] or the avipox virus [11,12].
BRAF V600E
While the discovery of the role of the BRAF signaling pathway and BRAF V600E mutation and its subsequent inhibition through either monotherapy with dabrafenib and vemurafenib or alongside the MEK inhibitor trametinib has had a major impact on treatment of melanoma patients, this progress could not be replicated in the case of CRC patients harboring the same genetic alteration. Given that 5-10% of CRC patients present this mutation, several clinical trials have analyzed the potential of inhibiting this signaling pathway using anti-BRAF medication. A clinical trial from 2015 which enrolled 35 mCRC patients used the combination of Dabrafenib, Trametinib alongside the anti-EGFR antibody panitumumab [13]. The ORR for this study was 26% while SDR ratio was 83%. Another phase I clinical trial tested the efficacy of vemurafenib alongside cetuximab and the chemotherapeutic agent irinotecan versus cetuximab and irinotecan in 21 previously-treated patients who presented the BRAF V600E mutation. PFS was 4.4 months in the experimental arm versus 2 months in the control group [14]. ORR was 16% and SDR was 67% in the experimental group in stark comparison to the doublet arm where ORR was 4% and SDR was SDR 4%. A large phase III clinical trial named BEACON-CRC with 3 arms where patients received either encorafenib plus binimetinib plus cetuximab versus encorafenib and cetuximab versus irinotecan/FOLFIRI alongide cetuximab in patients with CRC. The preliminary results presented in 2019 showed a mOS of 15.3 months, while PFS was 8.0 months and ORR 48% making this triple combination substantially better in comparison to historical data [15].
RAS
Undoubtedly, given that almost 50% of CRC patients harbor the RAS mutation at diagnosis which renders anti-EGFR medication ineffective, large efforts have been made to overcome this major negative prognosis factor. Unfortunately, inhibiting the RAS gene has been widely unsuccessful mainly due to crosstalk between the RAS and MAPK pathways which would render RAS inhibition redundant [16]. Several clinical trials targeting well-known points in the MAPK pathways such as IP3K or mTOR have failed to present any clinically significant results [16]. A logical step for overcoming this is by inhibiting the MEK pathway which is situated downstream from the RAS one. However single MEK inhibition has presented non-superior clinical results in studies and as such, was not considered for induction into larger, phase II/III clinical trials [17,18]. Another strategy was to inhibit the CDK4/6 complex, responsible for regulating the FOXM1 transcription factor, which is also one of the key promoters of proliferation in KRAS-mutant CRCs [19,20,21]. The combination between a MEK inhibitor and a CDK4/6 palbociclib has, so far, produced encouraging results in a in vitro and in vivo preclinical study [18,22]. SDR was 33% and 9 of the 15 patients presented reduced uptake as a result of reduced metabolic activity, as observed by a PET-scan.
HER2
While anti-HER2 treatment is considered a staple in breast cancer where its amplification occurs more frequently, few studies have analyzed the extent and importance of HER2 in CRC. More recently, this subject has garnered attention due to the fact that around 3-5% patients present this amplification. A phase II study with 27 patients with immunohistochemical confirmation of HER2 who were previously treated with at least 2 lines of treatment were given a combination of lapatinib and trastuzumab. Almost three quarters of the patients presented no disease progression while ORR was 30% [23]. Another phase II study involving two anti-HER2 agents: tucatinib and trastuzumab has started in 2017 and is still ongoing (NCT03043313).
Natural compounds
CRC and, to a larger extent, gastrointestinal cancers are, undoubtedly, considered the most diet-linked types of cancer. Dietary habits consisting of high quantities of red meat, processed meat or low quantities of fibers derived from fruits or vegetables have been incriminated as key factors in the development of CRC for a long time [24] while diets rich in fruits, grains or vegetables have been regarded as being able to reduce CRC incidence. With the advent of globalization and an increase in availability of products which were previously part of ethnic or local diets, it stands to reason that the scientific community has invested a lot of effort in uncovering dietary elements with potential anti-cancer proprieties. Two natural compounds have stood out in the past years: curcumin and quercetin.
Curcumin is isolated from the Curcuma longa (turmeric) plant, an herb native to continental India. Curcumin has been used for over 2000 years as a spice, a dye for clothing and as an integral part of ayurvedic medicine for the treatment of different ailments such as infections, allergies, burns or rheumatoid arthritis [25]. In the past years, curcumin has been studied in many in vivo or in vitro trials for its antiseptic, antioxidant, anti-inflammatory or even antineoplastic properties [26,27]. The effect of curcumin was tested in a clinical setting alongside well established treatments such as chemotherapy. A study of 29 patients with mCRC involved the usage of MB-6 (a mixture of several plant extracts including curcumin) alongside 5-FU and oxaliplatin. The study reported an increase in PFS and a reduction in adverse effects for patients who received MB-6 [28]. Another double blind, randomized study analyzed the effect of curcumin as a dietary supplement before surgery for CRC. The experimental arm where curcumin was administered three times a day presented lower levels of p53, a depletion of TNF-α serum levels and increased apoptosis within the tumor [29]. Several clinical trials are currently underway to determine the efficacy of curcumin alongside irinotecan (NCT01859858) and even alongside targeted therapy with bevacizumab plus FOLFIRI for patients with inoperable mCRC (NCT02439385).
Quercetin (3,3',4', 5,7-pentahidroxyflavone) is another natural compound used in the treatment of cancer. The substance is capable of reducing mutant p53 levels, inducing cell death [30], can block cancer cell replication by arresting the process within the G1 phase [31], can bind estrogen receptors, and can reduce RAS and PI3K protein expression which are integral for downstream signaling in cancer cells, especially in CRC [32,33]. In a large study, 1456 patients receiving six flavonoids (including quercetin) experienced significantly lower levels of CRC at the end of the study in comparison to the patients in the control group [34]. Another study pointed out that increased intake of quercetin significantly reduces the risk of proximal colon cancer while reduced dietary intake increases the risk of distal CRC [35]. So far, no clinical trials evaluating the effect of quercetin for the treatment of colon cancer are available.
P53 gene mutation
Tp53, which encodes the p53 tumor suppressor protein, is the most common genetic mutation in neoplasia and is present in a proportion of 60% in colorectal cancer [36]. The presence of mutant p53 promotes the survival of cancer cells and is associated with ineffective and unfavorable therapeutic responses, playing a key role in the development and progression of neoplastic processes [37].
The majority of cancer do not express the p 53 function. This occurs due to either defects in the mechanisms involved in activating p53 or due to a mutation of the p53 gene itself. Even though reactivating p53 gene can restrict tumor growth, it has not yet been achieved therapeutically. A series of new studies have concentrated on discovering new pathways that can be used to stop the growth or killing the p53 mutated cells [38].
Thus, many studies have attempted to demonstrate the relationship between p53 and target genes to be the subject of study for new therapeutic strategies. Making the efforts to interconnect p53 and miR-29c-3p, the miR-29c-3p gene has been shown to contain a p53 response which is driven by p53 transcription factor activity, demonstrating that miR-29c-3p is the p53 target gene directly. This study also demonstrated that the reduction the migration and invasion of colon cancer cell by miR-29c-3p was significantly reduced in the presence of PHLDB2, demonstrating that PHLDB2 is a critical target of miR-29c-3p, being associated with a significant reduction of global survival and progression-free survival of patients with colon cancer [39].
Another study drew attention to the HNF1A-AS1 gene demonstrating the implication of this in the metastatic progression of colon cancer by means of the miR-34a/p53 signaling axis, thus establishing its application as a new prognostic biomarker and a potential new target therapeutic [40].
The study led by Nakayama M revealed that missense mutations of p53 combined with wild type p53 deficiency accelerated progression of the final stage of colorectal cancer by activating two pathways: oncogenic and inflammatory. Therefore, medical world is hoping that the suppression of mutant p53 function is going to be a great and effective strategy against progression of colorectal cancer [36].
The expression of the p53 protein was also evaluated in the present study, including 20 patients with colorectal cancer, revealing varied response to anti-p53 antibodies, with both negative and highly positive results, demonstrating the variability of the Tp53 gene mutation in colorectal cancer.
Figure 1.
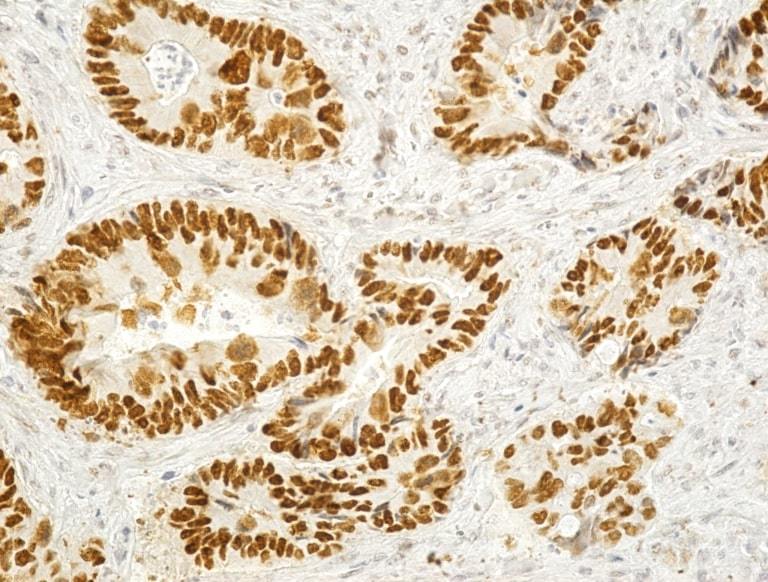
Image of adenocarcinoma with intense positive reaction to p53 (Anti-p53 antibody immunostaining, x200)
Figure 2.
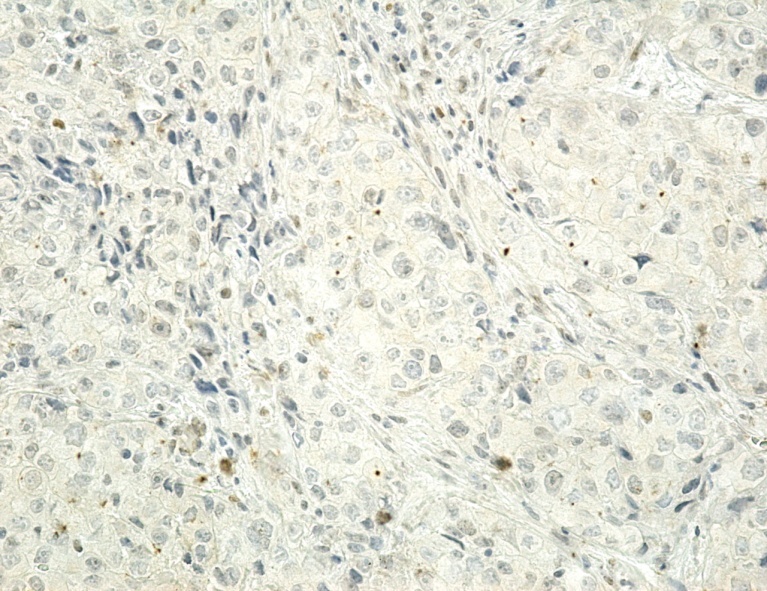
Image of adenocarcinoma with negative reaction to p53, x200
Vascular Endothelial Growth Factor (VEGF)
Vascular Endothelial Growth Factor is known to be the most important promoter of angiogenesis which is the formation of new blood and lymph vessels, therefore playing an important nutritional support for malignant tumors growth and metastasis. Nowadays, due to the numerous trials that demonstrated improved outcomes in terms of overall survival at patients treated with a combination of chemotherapy and anti VEGF, bevacizumab is now considered the standard of care in metastatic left colon and rectal cancer with mutant RAS gene [41,42].
Recently, a new study introduced a new drug regimen, thalidomid, demonstrating that thalidomid inhibits the expression of VEGF and hypoxia-inducible factor 1, the latter being directly involved in angiogenesis stimulation in hypoxic conditions, promoting VEGF transcription [43,44]. It has also stated that the combination of thalidomid and oxaliplatin can become the new therapy strategy for advanced colon and rectal cancer [44].
In our study, we tried to analyze the ability of tumor cells to stimulate angiogenesis by investigating specific biomarkers such as VEGF A and VEGF C, with intense positive reaction in well and moderate differentiated tumors, and negative response in poorly differentiated ones.
Figure 3.
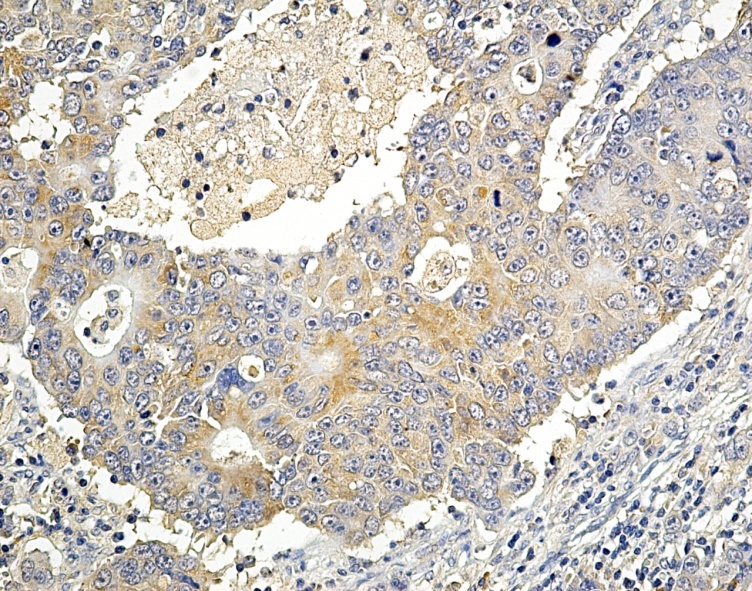
Moderate differentiated adenocarcinoma with positive VEGF-A expression, x200
Figure 4.
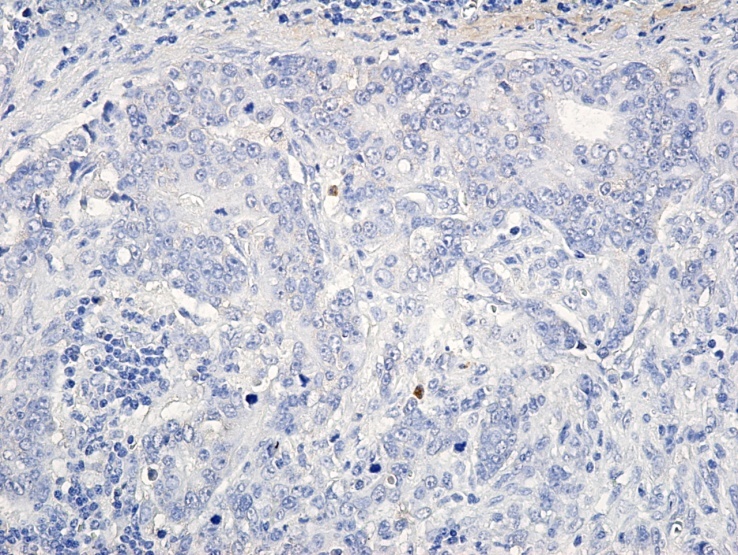
Image of adenocarcinoma with negative VEGF-A expression, x200
Cytokeratines 7 and 20
The most conclusive cytokeratines used in immunohistochemistry for colorectal cancer are cytokeratines 7 and 20. Numerous studies have concluded that the typical profile of colorectal cancer is CK7 negative and CK20 positive. A recent study supports this information, publishing data of only 95% CK7 negative and 100% CK 20 positive in colorectal cancer patients, being the most useful markers in differential diagnosis in metastatic colon or ovarian adenocarcinoma [45].
In many cases, distinguishing metastatic from primary tumors can be very difficult, but immunohistochemistry markers come in rescue and decide the correct diagnosis and therefore help oncologists to implement the right treatment option. This was the case presented by Kojima S with ovarian and rectal metastasis of colon cancer, a diagnosis sustained by the immunopositivity for cytokeratines CK7-and CK 20 [46]. Also, the rare case of a patient with transverse colon cancer with metastasis of both ovaries and left breast was presented by Luo XY, highlighting the role of cytokeratines in the diagnosis, with the same pattern of negative CK7 and positive CK20 [47].
Our study demonstrated the same pattern of immunohistochemical staining for colorectal adenocarcinoma with negative expression of CK7 and positive expression of CK20, demonstrating the role of these biomarkers in establishing the diagnosis of certainty necessary for the initiation of the proper treatment regime.
Figure 5.
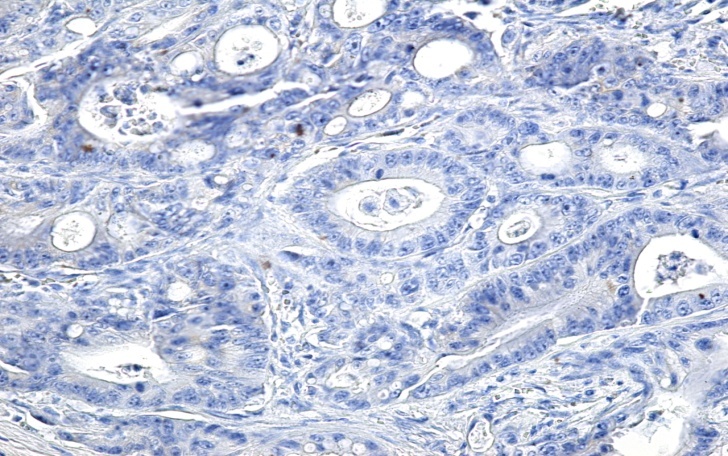
Image of adenocarcinoma with a negative reaction to CK7 (Anti-CK7 antibody immunolabeling, x200)
Figure 6.
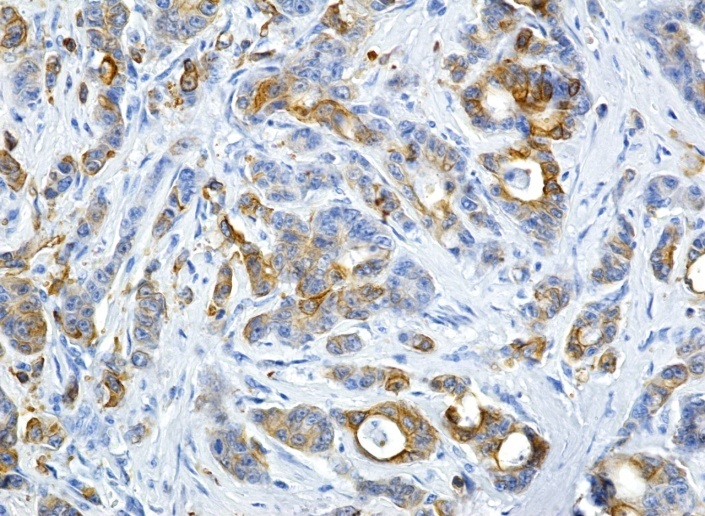
Image of colon adenocarcinoma with a positive reaction to CK20 (Anti-CK20 antibody immunolabeling, x200)
Conclusions
As CRC continues to prevail as one of the leading causes of cancer diagnosis and death in the world, the pressure to improve the prognosis and QoL for the patients suffering from this disease through the discovery of new diagnostic tools and therapeutic agents is ever increasing.
The evolution of the genomic landscape through novel sequencing techniques has uncovered major clues about the key mechanisms behind the development of CRC. More importantly, this knowledge was used to develop and tailor state of the art therapies in accordance with specific genetic key points. However, despite these discoveries, survival for patients suffering from mCRC has remained quite grim, with some biomarkers, such as the RAS mutation playing a major role in limiting the potential of therapeutic options. Other treatment options, such as immunotherapy or anti-BRAF agents have proven effective only for a very small percentage of patients, having a very limited or even a non-existent contribution for the vast majority of patients.
Constant refinement of current techniques and the discovery of new ones are required to better understand the molecular intricacies behind the evolution of CRC. However, a change in the mentality is begging to prevail, with more and more professionals embracing the idea that individual biomarkers are as important to the diagnosis and treatment as the localization and histological aspect of the tumor.
References
- 1.Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68(6):394–424. doi: 10.3322/caac.21492. [DOI] [PubMed] [Google Scholar]
- 2.Vatandoust S, Price TJ, Karapetis CS. Colorectal cancer: Metastases to a single organ. World journal of gastroenterology. 2015;21(41):11767–11776. doi: 10.3748/wjg.v21.i41.11767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Overman MJ, McDermott R, Leach JL, Lonardi S, Lenz HJ, Morse MA, Desai J, Hill A, Axelson M, Moss RA, Goldberg MV, Cao ZA, Ledeine JM, Maglinte GA, Kopetz S, André T. Nivolumab in patients with metastatic DNA mismatch repair-deficient or microsatellite instability-high colorectal cancer (CheckMate 142): an open-label, multicentre, phase 2 study. The Lancet Oncology. 2017;18(9):1182–1191. doi: 10.1016/S1470-2045(17)30422-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Diaz L, Marabelle A, Kim TW, Geva R, Van Cutsem E, André T, Ascierto PA, Maio M, Delord J, Gottfried M, Guimbaud R, Jaeger D, Elez E, Yoshino T, Joe A, Lam B, Ding J, Pruitt S, Kang SP, Le DT. Efficacy of pembrolizumab in phase 2 KEYNOTE-164 and KEYNOTE-158 studies of microsatellite instability high cancers. Annals of Oncology. 2017;28(Supp l5):v122–v141. [Google Scholar]
- 5.Overman MJ, Lonardi S, Wong KYM, Lenz HJ, Gelsomino F, Aglietta M, Morse MA, Van Cutsem E, McDermott R, Hill A, Sawyer MB, Hendlisz A, Neyns B, Svrcek M, Moss RA, Ledeine JM, Cao ZA, Kamble S, Kopetz S, André T. Durable Clinical Benefit With Nivolumab Plus Ipilimumab in DNA Mismatch Repair-Deficient/Microsatellite Instability-High Metastatic Colorectal Cancer. J Clin Oncol. 2018;36(8):773–779. doi: 10.1200/JCO.2017.76.9901. [DOI] [PubMed] [Google Scholar]
- 6.Bendell J, Ciardiello F, Tabernero J, Tebbutt N, Eng C, Di Bartolomeo A, Falcone A, Fakih M, Kozloff M, Segal N, Sobrero A, Shi Y, Roberts L, Yan Y, Chang I, Uyei A, Kim T. LBA-004 efficacy and safety results from IMblaze370, a randomised Phase III study comparing atezolizumab +cobimetinib and atezolizumab monotherapy vs regorafenib in chemotherapy-refractory metastatic colorectal cancer. Annals of Oncology. 2018;29(Supp l5):v123–v123. [Google Scholar]
- 7.Hochster HS, Bendell JC, Cleary JM, Foster P, Zhang W, He X, Hernandez G, Iizuka K, Eckhardt S. G. Efficacy and safety of atezolizumab (atezo) and bevacizumab (bev) in a phase Ib study of microsatellite instability (MSI)-high metastatic colorectal cancer (mCRC) American Society of Clinical Oncology. 2017;35(Supp 4):673–673. [Google Scholar]
- 8.Morse MA, Chaudhry A, Gabitzsch ES, Hobeika AC, Osada T, Clay TM, Amalfitano A, Burnett BK, Devi GR, Hsu DS, Xu Y, Balcaitis S, Dua R, Nguyen S, Balint JP, Jones FR, Lyerly k. Novel adenoviral vector induces T-cell responses despite anti-adenoviral neutralizing antibodies in colorectal cancer patients. Cancer immunology immunotherapy - CII. 2013;62(8):1293–1301. doi: 10.1007/s00262-013-1400-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Conry RM, Khazaeli MB, Saleh MN, Allen KO, Barlow DL, Moore SE, Craig D, Arani RB, Schlom J, LoBuglio AF. Phase I trial of a recombinant vaccinia virus encoding carcinoembryonic antigen in metastatic adenocarcinoma: comparison of intradermal versus subcutaneous administration. Clinical cancer research. 1999;5(9):2330–2337. [PubMed] [Google Scholar]
- 10.Sobol RE, Shawler DL, Carson C, Van Beveren C, Mercola D, Fakhrai H, Garrett MA, Barone R, Goldfarb P, Bartholomew RM, Brostoff S, Carlo DJ, Royston I, Gold DP. Interleukin 2 gene therapy of colorectal carcinoma with autologous irradiated tumor cells and genetically engineered fibroblasts: a Phase I study. Clinical cancer research. 1999;5(9):2359–2365. [PubMed] [Google Scholar]
- 11.von Mehren M, Arlen P, Tsang KY, Rogatko A, Meropol N, Cooper HS, Davey M, McLaughlin S, Schlom J, Weiner LM. Pilot study of a dual gene recombinant avipox vaccine containing both carcinoembryonic antigen (CEA) and B7.1 transgenes in patients with recurrent CEA-expressing adenocarcinomas. Clinical cancer research. 2000;6(6):2219–2228. [PubMed] [Google Scholar]
- 12.Marshall JL, Hoyer RJ, Toomey MA, Faraguna K, Chang P, Richmond E, Pedicano JE, Gehan E, Peck RA, Arlen P, Tsang KY, Schlom J. Phase I study in advanced cancer patients of a diversified prime-and-boost vaccination protocol using recombinant vaccinia virus and recombinant nonreplicating avipox virus to elicit anti-carcinoembryonic antigen immune responses. J Clin Oncol. 2000;18(23):3964–3973. doi: 10.1200/JCO.2000.18.23.3964. [DOI] [PubMed] [Google Scholar]
- 13.Atreya CE, Van Cutsem, Bendell JC, Andre T, Schellens JH, Gordon MS, McRee AJ, O'Dwyer PJ, Muro K, Tabernero undefined, van Geel, Sidhu R, Greger GJ, Rangwala FA, Motwani M, Wu Y, Orford KW, Corcoran RB. Updated efficacy of the MEK inhibitor trametinib (T), BRAF inhibitor dabrafenib (D), and anti-EGFR antibody panitumumab (P) in patients (pts) with BRAF V600E mutated (BRAFm) metastatic colorectal cancer (mCRC) American Society of Clinical Oncology. 2015;33(Supp 15):103–103. [Google Scholar]
- 14.Kopetz S, Desai J, Chan E, Hecht JR, O'Dwyer PJ, Maru D, Morris V, Janku F, Dasari A, Chung W, Issa JP, Gibbs P, James B, Powis G, Nolop KB, Bhattacharya S, Saltz L. Phase II Pilot Study of Vemurafenib in Patients With Metastatic BRAF-Mutated Colorectal Cancer. J Clin Oncol. 2015;33(34):4032–4038. doi: 10.1200/JCO.2015.63.2497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kopetz S, Grothey A, Yaeger R, Cuyle P-JA, Huijberts S, Schellens JHM, Elez E, Fakih M, Montagut Viladot, Peeters M, Desai J, Yoshino T, Ciardiello F, Wasan HS, Maharry K, Christy-Bittel J, Gollerkeri A, Van Cutsem, Tabernero J. Updated results of the BEACON CRC safety lead-in: Encorafenib (ENCO)+binimetinib (BINI) +cetuximab (CETUX) for BRAFV600E-mutant metastatic colorectal cancer (mCRC) Journal of Clinical Oncology. 2019;37(Supp 4):688–688. doi: 10.1200/JCO.18.02459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Adjei AA. Blocking oncogenic Ras signaling for cancer therapy. Journal of the National Cancer Institute. 2001;93(14):1062–1074. doi: 10.1093/jnci/93.14.1062. [DOI] [PubMed] [Google Scholar]
- 17.Bennouna J, Lang I, Valladares-Ayerbes M, Boer K, Adenis A, Escudero P, Kim TY, Pover GM, Morris CD, Douillard JY. A Phase II, open-label, randomised study to assess the efficacy and safety of the MEK1/2 inhibitor AZD6244 (ARRY-142886) versus capecitabine monotherapy in patients with colorectal cancer who have failed one or two prior chemotherapeutic regimens. Investigational new drugs. 2011;29(5):1021–1028. doi: 10.1007/s10637-010-9392-8. [DOI] [PubMed] [Google Scholar]
- 18.Pek M, Yatim S, Chen Y, Li J, Gong M, Jiang X, Zhang F, Zheng J, Wu X, Yu Q. Oncogenic KRAS-associated gene signature defines co-targeting of CDK4/6 and MEK as a viable therapeutic strategy in colorectal cancer. Oncogene. 2017;36(35):4975–4986. doi: 10.1038/onc.2017.120. [DOI] [PubMed] [Google Scholar]
- 19.Malumbres M, Barbacid M. Cell cycle, CDKs and cancer: a changing paradigm. Nature reviews Cancer. 2009;9(3):153–166. doi: 10.1038/nrc2602. [DOI] [PubMed] [Google Scholar]
- 20.Choi YJ, Anders L. Signaling through cyclin D-dependent kinases. Oncogene. 2014;33(15):1890–1903. doi: 10.1038/onc.2013.137. [DOI] [PubMed] [Google Scholar]
- 21.Anders L, Ke N, Hydbring P, Choi YJ, Widlund HR, Chick JM, Zhai H, Vidal M, Gygi SP, Braun P, Sicinski P. A systematic screen for CDK4/6 substrates links FOXM1 phosphorylation to senescence suppression in cancer cells. Cancer cell. 2011;20(5):620–634. doi: 10.1016/j.ccr.2011.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.O'Hara MH, Edmonds C, Farwell M, Perini RF, Pryma DA, Teitelbaum UR, Giantonio BJ, Damjanov N, Lal P, Feldman MD, Zhang PJ, Mankoff DA, Gallagher M, DeMichele A, Vaughn DJ, O'Dwyer PJ. Phase II pharmacodynamic trial of palbociclib in patients with KRAS mutant colorectal cancer. American Society of Clinical Oncology. 2015;33(Supp 3):626–626. [Google Scholar]
- 23.Bencardino K, Lonardi S, Bergamo F, Zagonel V, Leone F, Depetris I, Martinelli E, Troiani T, Ciardiello F, Racca P, Bertotti A, Siravegna G, Torri V, Amatu A, Ghezzi S, Marrapese G, Palmeri L, Valtorta E, Cassingena A, Lauricella C, Vanzulli A, Regge D, Veronese S, Comoglio PM, Bardelli A, Marsoni S, Siena S. Dual-targeted therapy with trastuzumab and lapatinib in treatment-refractory, KRAS codon 12/13 wild-type, HER2-positive metastatic colorectal cancer (HERACLES): a proof-of-concept, multicentre, open-label, phase 2 trial. The Lancet Oncology. 2016;17(6):738–746. doi: 10.1016/S1470-2045(16)00150-9. [DOI] [PubMed] [Google Scholar]
- 24.Giovannucci E, Willett WC. Dietary factors and risk of colon cancer. Annals of medicine. 1994;26(6):443–52. doi: 10.3109/07853899409148367. [DOI] [PubMed] [Google Scholar]
- 25.Aggarwal BB, Sundaram C, Malani N, Ichikawa H. Curcumin: the Indian solid gold. Advances in experimental medicine and biology. 2007;595:1–75. doi: 10.1007/978-0-387-46401-5_1. [DOI] [PubMed] [Google Scholar]
- 26.Hatcher H, Planalp R, Cho J, Torti FM, Torti SV. Curcumin: from ancient medicine to current clinical trials. Cellular and molecular life sciences: CMLS. 2008;65(11):1631–1652. doi: 10.1007/s00018-008-7452-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Moon HJ, Ko WK, Han SW, Kim DS, Hwang YS, Park HK, Kwon IK. Antioxidants, like coenzyme Q10, selenite, and curcumin, inhibited osteoclast differentiation by suppressing reactive oxygen species generation. Biochemical and biophysical research communications. 2012;418(2):247–253. doi: 10.1016/j.bbrc.2012.01.005. [DOI] [PubMed] [Google Scholar]
- 28.Chen WT, Yang TS, Chen HC, Chen HH, Chiang HC, Lin TC, Yeh CH, Ke TW, Chen JS, Hsiao KH, Kuo ML. Effectiveness of a novel herbal agent MB-6 as a potential adjunct to 5-fluoracil-based chemotherapy in colorectal cancer. Nutrition research. 2014;34(7):585–594. doi: 10.1016/j.nutres.2014.06.010. [DOI] [PubMed] [Google Scholar]
- 29.He ZY, Shi CB, Wen H, Li FL, Wang BL, Wang J. Upregulation of p53 expression in patients with colorectal cancer by administration of curcumin. Cancer investigation. 2011;29(3):208–213. doi: 10.3109/07357907.2010.550592. [DOI] [PubMed] [Google Scholar]
- 30.Avila MA, Cansado J, Harter KW, Velasco JA, Notario V. Quercetin as a modulator of the cellular neoplastic phenotype. Effects on the expression of mutated H-ras and p53 in rodent and human cells. Advances in experimental medicine and biology. 1996;401:101–110. [PubMed] [Google Scholar]
- 31.Yoshida M, Yamamoto M, Nikaido T. Quercetin arrests human leukemic T-cells in late G1 phase of the cell cycle. Cancer research. 1992;52(23):6676–6681. [PubMed] [Google Scholar]
- 32.Ranelletti FO, Maggiano N, Serra FG, Ricci R, Larocca LM, Lanza P, Scambia G, Fattorossi A, Capelli A, Piantelli M. Quercetin inhibits p21-RAS expression in human colon cancer cell lines and in primary colorectal tumors. International journal of cancer. 2000;85(3):438–445. [PubMed] [Google Scholar]
- 33.Matter WF, Brown RF, Vlahos CJ. The inhibition of phosphatidylinositol 3-kinase by quercetin and analogs. Biochemical and biophysical research communications. 1992;186(2):624–631. doi: 10.1016/0006-291x(92)90792-j. [DOI] [PubMed] [Google Scholar]
- 34.Theodoratou E, Kyle J, Cetnarskyj R, Farrington SM, Tenesa A, Barnetson R, Porteous M, Dunlop M, Campbell H. Dietary flavonoids and the risk of colorectal cancer. Cancer epidemiology biomarkers and prevention. 2007;16(4):684–693. doi: 10.1158/1055-9965.EPI-06-0785. [DOI] [PubMed] [Google Scholar]
- 35.Djuric Z, Severson RK, Kato I. Association of dietary quercetin with reduced risk of proximal colon cancer. Nutrition and cancer. 2012;64(3):351–360. doi: 10.1080/01635581.2012.658950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Nakayama M, Oshima M. Mutant p53 in colon cancer. J Mol Cell Biol. 2019;11(4):267–276. doi: 10.1093/jmcb/mjy075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sabapathy K, Lane DP. Therapeutic targeting of p53: all mutants are equal, but some mutants are more equal than others. Nat Rev Clin Oncol. 2018;15(1):13–30. doi: 10.1038/nrclinonc.2017.151. [DOI] [PubMed] [Google Scholar]
- 38.Gurpinar E, Vousden KH2. Hitting cancers' weak spots: vulnerabilities imposed by p53 mutation. Trends Cell Biol. 2015;25(8):486–495. doi: 10.1016/j.tcb.2015.04.001. [DOI] [PubMed] [Google Scholar]
- 39.Chen G, Zhou T, Li Y, Yu Z, Sun L. p53 target miR-29c-3p suppresses colon cancer cell invasion and migration through inhibition of PHLDB2. Biochem Biophys Res Commun. 2017;487(1):90–95. doi: 10.1016/j.bbrc.2017.04.023. [DOI] [PubMed] [Google Scholar]
- 40.Fang C, Qiu S, Sun F, Li W, Wang Z, Yue B, Wu X, Yan D. Long non-coding RNA HNF1A-AS1 mediated repression of miR-34a/SIRT1/p53feedback loop promotes the metastatic progression of colon cancer by functioning as a competing endogenous RNA. Cancer Lett. 2017;410:50–62. doi: 10.1016/j.canlet.2017.09.012. [DOI] [PubMed] [Google Scholar]
- 41.Hurwitz H, Fehrenbacher L, Novotny W, Cartwright T, Hainsworth J, Heim W, Berlin J, Baron A, Griffing S, Holmgren E, Ferrara N, Fyfe G, Rogers B, Ross R, Kabbinavar F. Bevacizumab plus irinotecan, fluorouracil, and leucovorin for metastatic colorectalcancer. N Engl J Med. 2004;350:2335–2342. doi: 10.1056/NEJMoa032691. [DOI] [PubMed] [Google Scholar]
- 42.Rosen LS, Jacobs IA, Burkes RL. Bevacizumab in Colorectal Cancer: Current Role in Treatment and the Potential of Biosimilars. Target Oncol. 2017;12(5):599–610. doi: 10.1007/s11523-017-0518-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ahluwalia A, Tarnawski AS. Critical role of hypoxia sensor-HIF-1α in VEGF gene activation. Implications for angiogenesis and tissue injury healing. Curr Med Chem. 2012;19:90–97. doi: 10.2174/092986712803413944. [DOI] [PubMed] [Google Scholar]
- 44.Zhang X, Luo H. Effects of thalidomide on growth and VEGF-A expression in SW480 colon cancer cells. Oncol Lett. 2018;15(3):3313–3320. doi: 10.3892/ol.2017.7645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Chu P, Wu E, Weiss LM. Cytokeratin 7 and cytokeratin 20 expression in epithelial neoplasms: a survey of 435 cases. Mod Pathol. 2000;13(9):962–972. doi: 10.1038/modpathol.3880175. [DOI] [PubMed] [Google Scholar]
- 46.Kojima S, Sakamoto T, Nagai Y, Honda M, Ogawa F. Metachronous rectal metastasis from primary transverse colon cancer: a case report. Surg Case Rep. 2018;9;4(1):90–90. doi: 10.1186/s40792-018-0498-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Luo XY, Wang J, Zhao J, Chen R, Zha XM. Metastases of transverse colon cancer to bilateral ovaries (Krukenberg tumor) and the left breast: A case report. Oncol Lett. 2017;14(1):31–34. doi: 10.3892/ol.2017.6158. [DOI] [PMC free article] [PubMed] [Google Scholar]


