Abstract
Epithelial-mesenchymal transition (EMT) is a fundamental process which governs invasiveness. E-cadherin plays a major role in development, organogenesis and tissue formation, but also in tumor progression. Snail is a transcription factor described as a direct repressor of E-cadherin during development and in carcinogenesis. In this study we analyzed E-cadherin and Snail immunoexpression in 47 cases of colorectal adenocarcinoma (CRC) in comparison with some histopathological prognostic factors. The majority of cases were G2 tumors in stages II and III, with vascular and perineural invasion. All cases showed positive cytoplasmic signal for E-cadherin and Snail. E-cadherin reactions were intense with the highest composite score (CS) values in CRC G1. CS values of E-cadherin decreased with the advancement in tumor stage and the association with vascular and perineural invasion was statistically significant. Snail immunoreaction was intense with the highest values of CS in CRC G3, being more evident with the increase of tumor staging, aspect which was statistically significant. CS and Snail association demonstrated a statistically significant aspect related to vascular invasion. We found a negative linear correlation of E-cadherin and Snail expression. The obtained results indicate the implication of Snail and E-cadherin in EMT of CRC, aspect which is useful in the evaluation and stratification of patients with CRC for the targeted specific therapy.
Keywords: CRC, Snail, E-cadherin, histopathological parameters
Introduction
Colorectal carcinoma (CRC) is the third most frequent type of cancer worldwide and one of the most common cancer related death causes [1,2]. Cancer cells which undergo epithelial-mesenchymal transition (EMT) can acquire invasive properties, by entering in the adjacent stroma, resulting in the creation of a favorable micro-medium for progression and tumor metastasis [3]. Tumor invasion represents the first step in the cascade of events which lead to the development of metastasis, and EMT is considered one of the central mechanisms which induces this process [4,5].
One of the characteristics of EMT is loss of cell adhesion with the decline of E-cadherin expression. Loss of E-cadherin may lead to tumor progression, metastasis and being an unfavorable prognostic factor of cancer with different localizations, especially CRC [5]. The main mechanism by which EMT is induced is represented by inhibition of E-cadherin, through the binding of Snail and Twist in E-cadherin’s promotor e-boxes [7,8].
The present study followed the correlations of E-cadherin and Snail immunoexpression, in relation with the prognostic histopathological parameters for CRC.
Material and Method
The study included 47 cases of CRC obtained from the Clinics of Surgery of Emergency County Hospital of Craiova, Romania, during 2017 and diagnosed in the Pathology Department of the same hospital. Biological material consisted of specimens of colectomy and hemicolectomy, fixed in 10% buffered formalin and then processed by the classic histhopathological technique consisting on paraffin embedding and Haematoxylin-Eosin staining (HE). Tumor staging was made in concordance with the WHO 2010 guidelines [9]. Histopathological parameters (grading, tumor staging, vascular and perineural invasion) were analyzed in comparison with immunoexpression of the rabbit-antihuman polyclonal antibodies, Snail (NBP1-822) and rabbit-antihuman monoclonal E-cadherin (36B5), both of IgG isotype. For the immunohistochemical (IHC) analysis, serial sections were used, which were processed by Labeled Streptavidin-Biotin (LSAB)+System-Horseradish peroxidase (HRP) (Dako, Redox, Romania, code K0675), signal developing being made with the chromogen 3,3’-Diaminobenzidine tetrahydrochloride (Dako, Redox, Romania, code K3468). We used 1:100 dilution for Snail and E-cadherin (ready to use) and antigen recovery pretreatment was done with microwaving in citrate buffer pH6.
The quantification of IHC reactions was realized by using a composite score (CS) resulted by multiplying the percentage of marked cells with the immunostaining intensity. Depending on the number of marked tumor cells, cases were divided into several categories, respectively 0 (absence of marked cells), 1 (less then 10% marked cells), 2 (11-25% marked cells), 3 (26-50% marked cells), 4 (more then 50% marked cells). The immunostaining intensity was divided into 4 categories, respectively 0 (absent), 1 (weak), 2 (moderate) and 3 (intense). The images were acquired with the Nikon Eclipse E600 microscope and imaging software Lucia 5.
Statistical analysis was made by X2 (chi-square test) and Pearson’s comparison tests, within Statistical Package for the Social Sciences (SPSS) 10 software. Mean values were reported as standard deviation (SD). The results were considered significant for values of p<0.05. For the statistical analysis the resulted scores were considered low for values between 1 and 4 and high for values between 6 and 12.
The study was approved by the Local Ethical Committee (no. 42/27.03.2018).
Results
Analysis of the 47 cases indicated that the majority were moderately differentiated tumors (G2-53,2%) and corresponded to stage II and III (29,8% each), 59,6% presenting vascular invasion and 70,2% perineural invasion (Table 1).
Table 1.
Relation between histopathological parameters and E-cadherin and Snail immunoexpression *G1: well differentiated CRC, G2: moderately differentiated CRC, G3: low differentiated CRC
|
Parameters |
Variables and number of cases (%) |
E-cadherin |
Snail |
||
|
% |
Score |
% |
Score |
||
|
Tumor stage |
I-6 (12.8%) II-14 (29.8%) III-14 (29.8%) IV-13 (27.7%) |
94.16+/-8.01 |
10 |
38.33+/-6.83 |
6.5 |
|
85.57+/-22.31 |
8.42 |
68.92+/-20.20 |
11 |
||
|
77.14+/-15.89 |
7.85 |
78.57+/-11.40 |
11.78 |
||
|
58.84+/-26.23 |
4.69 |
90.38+/-8.77 |
11.38 |
||
|
p=0.009 |
p=0.001 |
||||
|
Grading |
G1-8 (17.0%) G2-25 (53.2%) G3-14 (29.8%) |
88.75+/-20.83 |
8.25 |
40+/-20.87 |
7.5 |
|
80.2+/-19.22 |
8.04 |
74.6+/-19.36 |
11.4 |
||
|
61.78+/-25.54 |
5.85 |
87.5+/-9.14 |
11.5 |
||
|
p=0.170 |
p=0.005 |
||||
|
Vascular invasion |
Present-28 (59.6%) Absence-19 (40.4%) |
70.17+/-24.01 |
7.39 |
81.60+/-14.59 |
11.59 |
|
85+/- 19.57 |
7.47 |
59.21+/-27.29 |
9.68 |
||
|
p=0.038 |
p=0.047 |
||||
|
Perineural invasion |
Present-33 (70.2%) Absence-14 (29.8%) |
71.36+/-23.66 |
6.45 |
78.33+/-18.56 |
11.24 |
|
87.5+/-18.57 |
9.71 |
58.92+/-27.88 |
9.64 |
||
|
p=0.049 |
p=0.109 |
||||
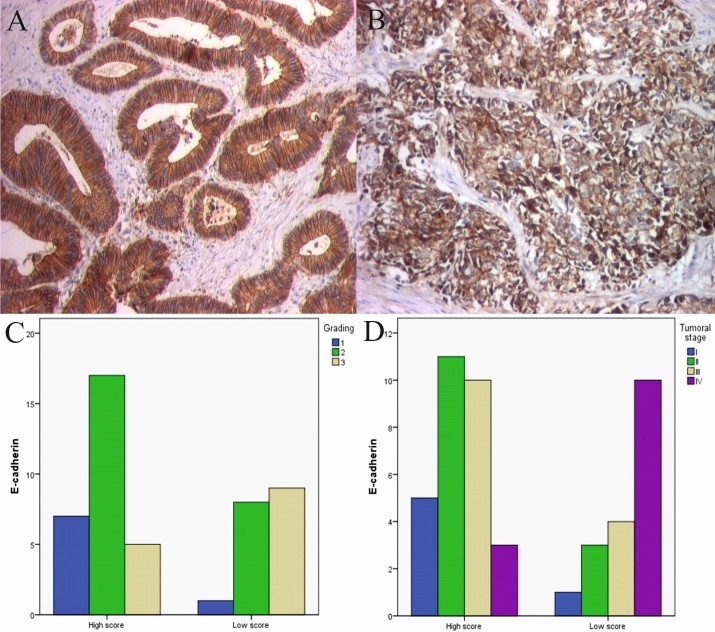
E-cadherin reaction was identified with a membranar and cytoplasmic pattern in 76,17% of cases (Fig.1A-B). E-cadherin intensity and immunostained cell percentage were different depending on the differentiation grade. Well differentiated cancers (G1) had an intense reaction, with a mean CS value of 8.25, moderately differentiated cancers (G2) had a intense reaction with a mean CS value of 8.04, while low differentiated cancers (G3) had a low reaction with a mean CS value of 5.85, aspect which is statistically insignificant (Fig.1C).
Figure 1.
A. Well differentiated CRC, E-cadherin immunostaining, x100; B. Low differentiated CRC, E-cadherin immunostaining, x100; C. Cases distribution depending on E-cadherin scores and grading; D. Cases distribution depending on E-cadherin scores and tumor stage
In the same time, the mean CS values decreased with advancement of tumor stage, respectively 10 (stage I), 8,42(stage II), 7,85 (stage III) and 4,69 (stage IV), which was statistically significant (Fig.1D).
Referring to the relation of CS and vascular invasion, we observed a tendency towards rising in the presence of vascular invasion. Mean CS values were higher in the presence of vascular invasion (7,39 vs. 7,47) which was statistically significant. Similarly, the analysis of the relation between CS and perineural invasion, indicated a statistically significant aspect (p=0,049) in the presence of perineural invasion (6,45 vs. 9,71).
Snail immunoreaction was identified with a cytoplasmic pattern in 72.55% of cases (Fig.1A-B). Snail intensity and percentage of stained cells were different depending on grade of differentiation. Well differentiated cancers (G1) indicated intense reactions and high CS with a mean value of 7.5. Also moderately and low differentiated cancers revealed an intense reaction with mean CS values of 11.4 for G2 and 11.5 in G3 (Fig.2C).
Figure 2.
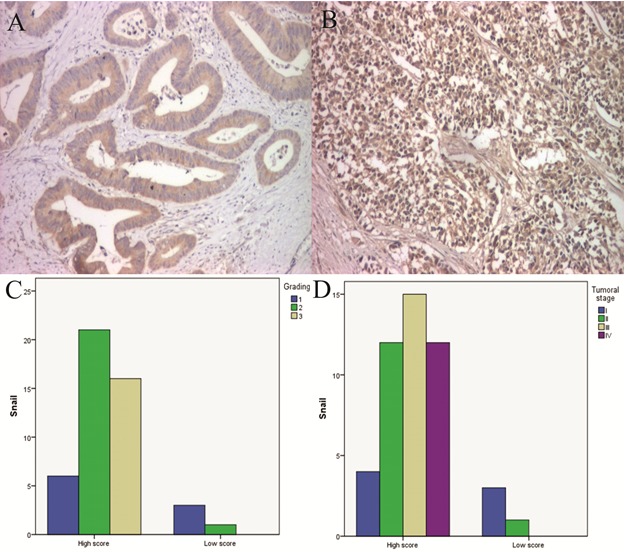
A. Well differentiated CRC, Snail immunostaining, x100; B. Low differentiated CRC, Snail immunostaining, x100; C. Cases distribution depending on Snail scores and grading; D. Cases distribution depending on Snail scores and tumor stage
Referring to tumor stage, the marked cells presented an increase of mean CS values with the advancement of tumor stage, respectively 6.5 (stage I), 11 (stage II), 11.87 (stage III) and 11.38 (stage IV) (Fig.2D)
Regarding the relation between CS and vascular invasion, we observed an increase of CS values in the presence of vascular invasion, compared with cases without vascular invasion (11,59 vs. 9,68), aspect which was statistically significant. Similarly, the analysis of the relation between CS with perineural invasion indicated an increase of CS values in the presence of perineural invasion (11,24 vs. 9,64), but without a statistic significance.
Statistical analysis revealed a negative linear correlation of E-cadherin expression and Snail (p<0.05, Pearson) (Fig.3).
Figure 3.
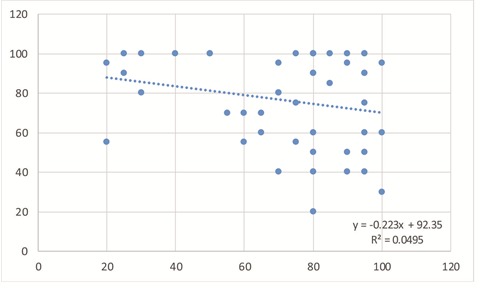
E-cadherin and Snail percentage values distribution
Discussion
CRC is the third most frequently diagnosed type of cancer worldwide. Tumor stage represents the most important prognostic factor for patients with CRC, being associated with a low grade of differentiation, perineural and vascular invasion [10]. Most cases that were analyzed were moderately differentiated tumors, which corresponded to stage III and presented frequent perineural and vascular invasion, with statistically significant results across various parameters (p<0.05, χ2 test). Related to this aspect, the literature data is extensive [11,12]. Hashmi et al. demonstrated that the majority of tumors are diagnosed in late stages III/IV, being associated with vascular and perineural invasion [12]. Furthermore, with the increase in tumor stage the grade of differentiation also modifies in parallel, most tumors being moderately differentiated [13].
EMT is implicated in tumor progression and metastasis being thus considered one of the essential mechanisms involved in aggressive cancers. Epithelial tumor cells, loose their polarity and are converted to the mesenchymal phenotype, receiving migratory capabilities. They receive invasive properties and enter the surrounding stroma, thus resulting in the formation of a favorable micro-medium for tumor progression and metastasis. This process is accompanied by different modifications, such as inhibition of epithelial markers and appearance of mesenchymal markers [14]. The definitory role of EMT is represented by the direct link to invasion, metastasis and with an unfavorable prognostic of patients with CRC [15].
E-cadherin is a transmembranary calcium dependent glycoprotein, expressed in most epithelial tissues, which forms a tight junction which connects adjacent cells. One of the primary discoveries in EMT is loss of cell adhesion with the lowering of E-cadherin. Loss of E-cadherin leads to tumor progression, metastasis and an unfavorable prognostic in different cancers [6,16].
E-cadherin expression analysis showed its presence in 76,17% of studied cases, being evidentiated in membranary and cytoplasmic levels. Similar to our study, Brabletz et al. showed E-cadherin expression in the membrane and cytoplasm level [17]. Elzaghied et al. concluded that aberrant cytoplasmic expression of E-cadherin may predict a recurrent disease, which would be of clinical and therapeutic interest [18]. E-cadherin markings were intense, with the highest values of mean CS in CRC G1 (8,25) and G2 (8,04), in comparison with G3 (5,85) being statistically insignificant. Palaghia et al. evidenced E-cadherin membranary and cytoplasmic immunoreaction but also the association of it with tumor differentiation. Thus they concluded that well and moderately differentiated tumors have a higher E-cadherin expression in comparison with low differentiated, the association being statistically significant [19].
In our study the tumor cells intensity decreased in advanced tumor stage, aspect which was statistically significant. Similarly, Kwak et al., demonstrated that low expression of E-cadherin was significantly correlated with grade of tumor differentiation and advanced tumor stage [20]. Association of E-cadherin immunoreaction with perineural and vascular invasion was statistically significant, being demonstrated in many studies [21,22]. Wang et al., in a study on signet cell CRC, demonstrated that vascular invasion and perineural invasion correlated with overexpression of E-cadherin may predict patient prognostic with this type of cancer [23].
Transcriptional repressors of E-cadherin, of which the family of zinc finger proteins (Snail, Slug) are associated with EMT, being markers useful for establishing a prognostic for different cancers [24].
Snail is one of the best promoters of EMT, being considered with a prognostic role in CRC. Additionally, it mediates invasiveness and metastasis in many types of malignant tumors [25,26].
Snail highlighting CRC was identified in 73% of cases which presented overexpression of this protein, with a cytoplasmic pattern. In literature many studies showed Snail overexpression in CRC [27,28]. For example, Fan et al. showed that 78% of analyzed cases showed Snail overexpression [8]. Many studies claim that intense Snail reaction of tumor cells is responsible for a decrease in E-cadherin signal in many types of cancers [29,30].
In our study, we identified a statistically significant association between Snail immunoreaction and vascular invasion. We didn’t find any relation between tumor cell immunostaining and perineural invasion. The invasiveness ability and migratory ability of Snail and an unfavorable prognostic, were cited in literature and is demonstrated the overexpression of this biomarker with vascular invasion and perineural invasion [31,32,33].
Yung et al. showed that overexpression of Snail and EMT correlates with aggressive CRC [27]. In this study the Snail reactions were intense, with the highest mean values of CS in CRC G2 (11.4) and G3 (11.5), aspect which was statistically significant (p<0,05, χ2 test), the results being similar to literature data [34]. Kwoon et al., demonstrated that overexpression of Snail in 75% of cases, being correlated with low differentiated CRC and in advanced stages of disease. Furthermore, the association with perinerual invasion and vascular invasion was shown [35].
Furthermore, we found significant linear negative correlation of E-cadherin expression and Snail (p<0.05, Pearson), which suggests that association of the two markers demonstrates EMT presence and a low survival rate, similar to data found in literature [24].
Conclusions
In this study E-cadherin expression was significant superior in low stage well differentiated CRCs, while immunoexpression of Snail was superior in advanced and low differentiated lesions. This aspect suggests the implication of the two biomarkers in EMT of CRC, and results obtained can be used in the stratification of patients and therapeutic attitude.
Conflicts of interests
The authors declare the have no conflicts.
References
- 1.Cronin KA, Lake AJ, Scott S, Sherman RL, Noone AM, Howlader N, Henley SJ, Anderson RN, Firth AU, Ma J, Kohler BA, Jemal A. Annual Report to the Nation on the Status of Cancer, part I: National cancer statistics. Cancer. 2018;124(13):2785–2800. doi: 10.1002/cncr.31551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Siegel R, Ward E, Brawley O, Jemal A. Cancer statistics, 2011: the impact of eliminating socioeconomic and racial disparities on premature cancer deaths. CA Cancer J Clin. 2011;61:212–236. doi: 10.3322/caac.20121. [DOI] [PubMed] [Google Scholar]
- 3.Voulgari A, Pintzas A. Epithelial-mesenchymal transition in cancer metastasis: mechanisms, markers and strategies to overcome drug resistance in the clinic. Biochim Biophys Acta. 2009;17969(2):75–90. doi: 10.1016/j.bbcan.2009.03.002. [DOI] [PubMed] [Google Scholar]
- 4.Kalluri R. The basics of epithelial-mesenchymal transition. J Clin Invest. 2009;119(6):1420–1428. doi: 10.1172/JCI39104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Thiery JP, Acloque H, Huang RY. Epithelial-mesenchymal transitions in development and disease. Cell. 2009;139(5):871–890. doi: 10.1016/j.cell.2009.11.007. [DOI] [PubMed] [Google Scholar]
- 6.Dorudi S, Sheffield JP, Poulsom R, Northover JM, Hart IR. E‐cadherin expression in colorectal cancer. An immunocytochemical and in situ hybridization study. Am J Pathol. 1993;142:981–6. [PMC free article] [PubMed] [Google Scholar]
- 7.Valdés-Mora F, Gómez del Pulgar T, Bandrés E, Cejas P, Ramírez de Molina A, Pérez-Palacios R, Gallego-Ortega D, García-Cabezas MA, Casado E, Larrauri J, Nistal M, González-Barón M, García-Foncillas J, Lacal JC. TWIST1 overexpression is associated with nodal invasion and male sex in primary colorectal cancer. Ann Surg Oncol. 2009;16(1):78–87. doi: 10.1245/s10434-008-0166-x. [DOI] [PubMed] [Google Scholar]
- 8.Fan F, Samuel S, Evans KW, Lu J, Xia L, Zhou Y, Sceusi E, Tozzi F, Ye XC, Mani SA. Overexpression of Snail induces epithelial-mesenchymal transition and a cancer stem cell-like phenotype in human colorectal cancer cells. Cancer Med. 2012;1(1):5–16. doi: 10.1002/cam4.4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bosman FT, Carneiro F, Hruban RH, Theise ND. WHO Classification of Tumours of the digestive system. World Healt Organization, International Agency for research on Cancer . Lyon: IARC Press; 2010. pp. 132–146. [Google Scholar]
- 10.Fleming M, Ravula S, Tatishchev SF, Wang HL. Colorectal carcinoma: Pathologic aspects. Journal of gastrointestinal oncology. 2012;3(3):153–73. doi: 10.3978/j.issn.2078-6891.2012.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fujii T, Sutoh T, Morita H, Yajima R, Yamaguchi S, Tsutsumi S, Asao T, Kuwano H. Vascular invasion, but not lymphatic invasion, of the primary tumor is a strong prognostic factor in patients with colorectal cancer. Anticancer Res. 2014;34(6):3147–3151. [PubMed] [Google Scholar]
- 12.Hashmi AA, Hashmi SK, Ali N, Thara K, Ali R, Edhi MM, Faridi N, Khan A. Clinicopathologic features of colorectal carcinoma: features predicting higher T-stage and nodal metastasis. BMC Res Notes. 2018;11(1):52–52. doi: 10.1186/s13104-018-3183-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Barresi V, Reggiani Bonetti L, Ieni A, Caruso RA, Tuccari G. Histological grading in colorectal cancer: new insights and perspectives. Histol Histopathol. 2015;30(9):1059–1067. doi: 10.14670/HH-11-633. [DOI] [PubMed] [Google Scholar]
- 14.Kim YH, Kim G, Kwon CI, Kim JW, Park PW, Hahm KB. TWIST1 and SNAI1 as markers of poor prognosis in human colorectal cancer are associated with the expression of ALDH1 and TGF-β1. Oncology Reports. 2014;31(3):1380–1388. doi: 10.3892/or.2014.2970. [DOI] [PubMed] [Google Scholar]
- 15.Zlobec I, Lugli A. Epithelial mesenchymal transition and tumor budding in aggressive colorectal cancer: tumor budding as oncotarget. Oncotarget. 2010;1(7):651–661. doi: 10.18632/oncotarget.199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chan AO, Chu KM, Lam SK. Soluble E‐cadherin is an independent pretherapeutic factor for long‐term survival in gastric cancer. J Clin Oncol. 2003;21:2288–2293. doi: 10.1200/JCO.2003.08.078. [DOI] [PubMed] [Google Scholar]
- 17.Brabletz T, Jung A, Reu S, Porzner M, Hlubek F, Leoni A, Schughart K, Knuechel R, Kirchner T. Variable β-catenin expression in colorectal cancers indicates tumor progression driven by the tumor environment. Proceedings of the National Academy of Sciences. 2001;98(18):10356–10361. doi: 10.1073/pnas.171610498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Elzagheid A, Algars A, Bendardaf R, Lamlum H, Ristamaki R, Collan Y, Syrjanen K, Pyrhonen S. E-cadherin expression pattern in primary colorectal carcinomas and their metastases reflects disease outcome. World J Gastroenterol. 2006;12(27):4304–4309. doi: 10.3748/wjg.v12.i27.4304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Palaghia M, Mihai C, Lozneanu L, Ciobanu D, Trofin AM, Rotariu A, Târcoveanu F, Cijevschi Prelipcean C. E-cadherin expression in primary colorectal cancer and metastatic lymph nodes. Rom J Morphol Embryol. 2016;57(1):205–209. [PubMed] [Google Scholar]
- 20.Kwak JM, Min BW, Lee JH, Choi JS, Lee SI, Park SS, Kim J, Um JW, Kim SH, Moon HY. The prognostic significance of E-cadherin and Liver Intestine-cadherin expression in colorectel cancer. Dis Colon Rectum. 2007;50:1873–1873. doi: 10.1007/s10350-007-9034-1. [DOI] [PubMed] [Google Scholar]
- 21.Yun JA, Kim SH, Hong HK, Yun SH, Kim HC, Chun HK, Cho YB, Lee WY. Loss of E-Cadherin expression is associated with a poor prognosis in stage III colorectal cancer. Oncology. 2014;86(5-6):318–328. doi: 10.1159/000360794. [DOI] [PubMed] [Google Scholar]
- 22.Elzagheid A, Buhmeida A, Laato M, El-Faitori O, Syrjänen K, Collan Y, PyrhönenS S. Loss of E-cadherin expression predicts disease recurrence and shorter survivalin colorectal carcinoma. APMIS. 2012;120(7):539–548. doi: 10.1111/j.1600-0463.2011.02863.x. [DOI] [PubMed] [Google Scholar]
- 23.Wang R, Ma X, Li Y, He Y, Huang D, Cai S, Peng J. The Characteristics and Prognostic Effect of E-Cadherin Expression in Colorectal Signet Ring Cell Carcinoma. PLoS One. 2016;11(8):e0160527–e0160527. doi: 10.1371/journal.pone.0160527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Batlle E, Sancho E, Franci C. The transcription factor snail is a repressor of E‐cadherin gene expression in epithelial tumour cells. Nat Cell Biol 2000; 2: 84-9. 37 Comijn J, Berx G, Vermassen P et al.The two‐handed E box binding zinc finger protein SIP1 downregulates E‐cadherin and induces invasion. Mol Cell. 2001;7:1267–1278. [Google Scholar]
- 25.Thorpe H, Asiri A, Akhlaq M, Ilyas M. Cten promotes epithelial-mesenchymal transition through the post-transcriptional stabilization of Snail. Mol Carcinog. 2017;56(12):2601–2609. doi: 10.1002/mc.22704. [DOI] [PubMed] [Google Scholar]
- 26.Yang YJ, Li ZB, Zhang GR, Wu LJ, Yu JY, Hu LJ, Zhou YL, Wang HD, Liang D. Snail-induced epithelial-mesenchymal transition in gastric carcinoma cells and generation of cancer stem cell characteristics. Genet Mol Res. 2016;15(3) doi: 10.4238/gmr.15038510. [DOI] [PubMed] [Google Scholar]
- 27.Yang J, Zhang K, Song H, Wu M, Li J, Yong Z, Jiang S, Kuang X, Zhang T. Visfatin is involved in promotion of colorectal carcinoma malignancy through an inducing EMT mechanism. Oncotarget. 2016;7(22):32306–32317. doi: 10.18632/oncotarget.8615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hwang WL, Yang MH, Tsai ML, Lan HY, Su SH, Chang SC, Teng HW, Yang SH, Lan YT, Chiou SH, Wang HW. SNAIL regulates interleukin-8 expression, stem cell-like activity, and tumorigenicity of human colorectal carcinoma cells. Gastroenterology. 2011;141(1):279–291. doi: 10.1053/j.gastro.2011.04.008. [DOI] [PubMed] [Google Scholar]
- 29.Wells A, Yates C, Shepard CR. E-cadherin as an indicator of mesenchymal to epithelial reverting transitions during the metastatic seeding of disseminated carcinomas. Clin Exp Metastasis. 2008;25(6):621–628. doi: 10.1007/s10585-008-9167-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Iwatsuki M, Mimori K, Yokobori T, Ishi H, Beppu T, Nakamori S, Baba H, Mori M. Epithelial-mesenchymal transition in cancer development and its clinical significance. Cancer Sci. 2010;101(2):293–299. doi: 10.1111/j.1349-7006.2009.01419.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wang H, Wang HS, Zhou BH, Li CL, Zhang F, Wang XF, Zhang G, Bu XZ, Cai SH, Du J. Epithelial-mesenchymal transition (EMT) induced by TNF-α requires AKT/GSK-3β-mediated stabilization of snail in colorectal cancer. PLoS One. 2013;8(2):e56664–e56664. doi: 10.1371/journal.pone.0056664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Xiang Z, Jiang DP, Xia GG, Wei ZW, Chen W, He Y, Zhang CH. CXCL1 expression is correlated with Snail expression and affects the prognosis of patients with gastric cancer. Oncol Lett. 2015;10(4):2458–2464. doi: 10.3892/ol.2015.3614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ganesan R, Mallets E, Gomez-Cambronero J. The transcription factors Slug (SNAI2) and Snail (SNAI1) regulate phospholipase D (PLD) promoter in opposite ways towards cancer cell invasion. Mol Oncol. 2015;10(5):663–676. doi: 10.1016/j.molonc.2015.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Luo WR, Li SY, Cai LM, Yao KT. High expression of nuclear Snail, but not cytoplasmic staining, predicts poor survival in nasopharyngeal carcinoma. Ann Surg Oncol. 2012;19(9):2971–2979. doi: 10.1245/s10434-012-2347-x. [DOI] [PubMed] [Google Scholar]
- 35.Kwon CH, Park HJ, Choi JH, Lee JR, Kim HK, Jo HJ, Kim HS, Oh N, Song GA, Park DY. Snail and serpinA1 promote tumor progression and predict prognosis in colorectal cancer. Oncotarget. 2015;6(24):20312–20326. doi: 10.18632/oncotarget.3964. [DOI] [PMC free article] [PubMed] [Google Scholar]