Abstract
Introduction: Menopause is the transition from the reproductive phase of a woman to the non-reproductive. It may impair quality of life. The study aims to determine the effectiveness of mixed herbal medicines on menopause symptoms
Methods: A randomized, triple-blind, clinical trial and placebo-controlled study on 120 peri-menopausal women aged 45-65 years for 12 weeks. All participants took herbal extracts drops orally daily and randomly allocated into four groups: placebo (C), A (250 mg chamomile, 30 mg fennel, 15 mg saffron), B (1000 mg, 120 mg, 60 mg), and D (500 mg, 60 mg, 30 mg). Primary outcome was the mean change in scores of the menopause rating scale that evaluates 11 symptoms.
Results: The median (IQR) physical score significantly reduced from 8.5(4) to 2(3), in psych score reduced from 12(4) to 2 (2) and in urogenital score reduced from 6.5(3) to 3(2) in group B. In group D physical score decreased from 12(6) to 8(4), in psychological score reduced from12 (3) to 8(4) and urogenital score reduced from 7.5 (3) to 8(3) at week 12. No significant differences in group A and C. With comparison the scores of physical, psych and urogenital domain of MRS questionnaire in 1th ,6th and 12th, no significant difference within group A and C were seen, but statistically significant difference was within group B (p<0.001) and D (p<0.001) in all weeks. The effect size was 0.92.
Conclusion: A 12 weeks extracts treatment, there were significant improvement in physical, psychological and urogenital domains in group B.
Keywords: Menopause, Herbal Medicines, Alternative Medicines, Menopause Rating Scale
Introduction
One of matters that threatened the quality of life a woman is menopause. Menopause disturbs all aspects of a woman's life and annually affecting 25 million women worldwide. WHO (World Health Organization) estimates that 1.2 billion women will be postmenopausal by 2030.1
Menopause is described as the transition from the reproductive phase to the non-reproductive. It occurs in many industrial countries on average at age 51. However, in Iran, according to a meta-analysis study (2011) the mean age of menopause was 48.18 years.2 Hence, menopause starts between the age of 40 and 50 and is marked by the reduction of estrogen levels.3 Main complaints in menopause are vasomotor and vaginal symptoms, cardiovascular, mood changes, dysphoria, hot flashes, night sweats, vaginal dryness, decreased libido, imbalanced cognitive function, osteoporosis, osteoarthritis, depression, dementia, and frailty.4,5 Most of these problems can be effectively treated with HRT (hormone replacement therapy). However, HRT may cause serious risk hazards to one's health, and is therefore only recommended for temporary, limited therapy for severe vasomotor symptoms.3 The possible adverse effects of long-term HRT have lead women to seek out alternative therapies.6 However, the role of complementary and alternative therapies in the management of menopause, both for the relief of symptoms and prevention of long-term complications, is a hotly debated issue.
Nowadays, complementary and alternative therapies to treat menopausal symptoms in place of HRT are pharmaceutical and botanical.7 About 15 to 17% of postmenopausal women with menopausal symptoms, who are influenced by one's ethnicity, education, and medical and herbal consultation, use alternative treatment methods and phytotherapeutics.3 In U.S.A and Australia, about half of woman used Complementary therapies. These are included herbal remedies, homeopathy, nutritional supplements, acupuncture, reflexology, relaxation and so on.8 however these therapies cannot improve all the menopause symptoms. Mirzaii reported that exercise might be effective in relieving the somatic and psychological symptoms of menopause, including depression and anxiety. This could be hopeful news for women who are interested in using non-pharmacological interventions for menopausal symptoms.9 In the recent years, using herbal medicines is one of the most common solution to decrease the menopause symptoms. On the other hand because of variety of menopause symptoms, the use of one plant is not effective in treating all the symptoms, and a combination of several plants with the least side effects for reducing most symptoms, is often required. Yakkoot (2011) by reviewing the effects of the herbal combination of Lady 4 (combination of four medicinal plants), reported helpful consequences on menopause symptoms.10 Change (2012) studied the effect of medicinal plant Estrog 100 (combination of three medicinal plants) and reported this drug significantly improved some menopausal symptoms without weight gain or any serious side effects.11 By the way, many studies have been conducted to reduce the symptoms of menopause by the use of medicinal plants, which has been somewhat effectiveness. But according to their results, it is clear that most of them, especially non-Combined plants, have only been able to improve a few symptoms not all of them. According to the literature, many medicinal plants can be effective in improving menopausal symptoms including indigenous plants such as Anise, Dill, Marigold, Fumaria, Mary, Fennel, Chamomile, Saffron and so forth. Doubtlessly they have benefits and complications. It has been reported that many menopause symptoms have been reduced due to the effects of these plants. For example, chamomile and fennel as phytoestrogens alleviate hot flashes strengthen the reproductive and urinary system, and to some extent increase sexual desires. Fennel has a positive effect on osteoporosis.12-14
Chamomile is also effective in digestive system dysfunction and it can be used as a sedative during this period.15,16 Saffron is effective in relieving symptoms such as depression because it is a mood modifier, as well as improving heart and vascular problems and sexual dysfunction in menopausal women.17-19 We need to find suitable treatments with better results and fewer side effects. Regarding the effects of these plants, it is expected that the combined extract of plants can reduce all symptoms of menopause that such effect has not been seen in previous domestic and foreign studies. Therefore we decided to combine three of the plants (fennel, chamomile, and saffron) and investigate the effect of this extract on menopause symptoms.
Materials and methods
We conducted a randomized clinical trial and placebo controlled study at the Medical University of Mashhad, Iran. This research was registered and approved by Ethics Committee approval of the Mashhad University.(IR.MUMS.REC1394.499) The RCT number was IRCT 2016092429950N1.
Also fortunately these herbal medicines extract got patent with number 95729 at 24//02/97. The study was triple blind and was conducting on women with menopausal symptoms. The study participants were selected from all segments of the society and enrolled in the study from May to November 2016. All the eligible participants were pre-menopause, menopause and post -menopausal women. Inclusion criteria included: cease of menstruation within 12 months of our study or presently experiencing menopausal symptoms and age range between 45-65 years old. Exclusion criteria: using any drugs and hormones, suffering any disease, unable to use the trial extracts properly, and experiencing allergies, skin irritation, and sensitivity to these herbal medicines.
The primary selection of samples was available. An independent data manager carried out randomization by using blocking random allocation sequence. Block randomization is a commonly used technique in clinical trial design to decrease bias and achieve balance in the allocation of participants to treatment arms, especially when the sample size is small.20 It is used to ensure that comparison groups will be generated according to a predetermined ratio.21 In this study we had four stratification due to age range (45-50, 50-55, 55-60, and 60-65). We had 7 participants in age range 45- 50 years and 60 -65 years and 8 participants in age range 50- 55 years and 60 -65 years. Then the treatment groups will be equal in size and will tend to be equally distributed by main outcome-related characteristics. Allocation concealment was achieved through sequentially numbered, sealed envelopes. After the baseline questionnaire was completed, eligible participants were assigned to one of the four study groups by consecutively opening the envelopes as they entered the study. All the herbal medicines bottles was labeled with A, B, C, or D. The probability of being allocated to the placebo group (C) and medicinal groups (A, B, D) was equal (1:1:1:1).
Participants were unaware of their assignment to the treatment or placebo. The complete randomization list was placed in a sealed envelope and kept there until the study was completed. Aside from the subjects, the research staff and analysts were also unaware of the treatment assignment (Figure 1).
Figure 1.
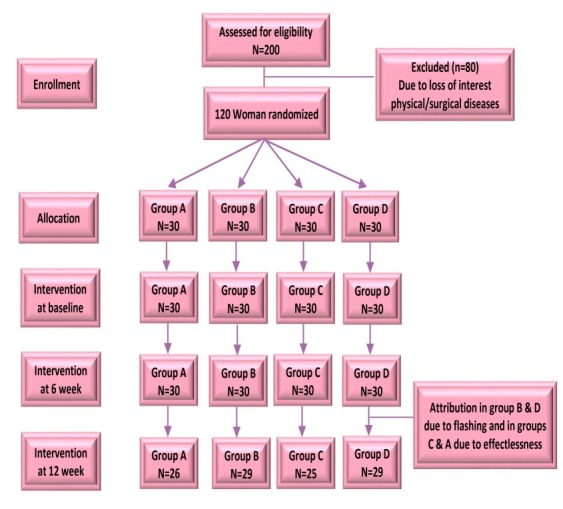
Flow chart of the trail
Data from previous studies were used to calculate sample size. The sample size was according to a study on the impact of chamomile and Angelica on menopause symptoms with the following formula.22
Figure 2.
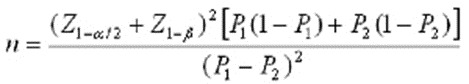
P1=93, P2=20
Equal sample size for each group was anticipated at about 27 women; therefore, a total sample size of 108 participants was required, but we enrolled 120 participants considering the 10% attrition rate. The number of participants in 45-50 years old were 8 women, in 50-55 years old were 8 women , in 50-55 years old and in 60 -65 years old were 7 women. For more confidence, at the beginning of the research, a pilot study was carried out and the effect size in MRS (Menopause Rating Scale) obtained was 0.92. The term effect size can refer to the value of a statistic calculated from a sample of data23 that refers to odd ratio. For data collection, a demographic and MRS questionnaire were used. The Persian version of the MRS was validated and determined reliable in assessing the Iranian population.24-26 Demographic data included: age, BMI, and education level of the study subject and her spouse and their occupations. The standardized MRS questionnaire is a health-related scale that collects comprehensive data. It measures changes in the subjects across cultures and time, and factor analysis is based on three dimensions: somatic symptoms of menopause (vasomotor), psychological, and urogenital. This questionnaire has been used in many clinical studies and epidemiology research to determine the frequency and Severity of menopausal symptoms in middle-aged women and according to many studies; it has a high reliability and validity.25,27,28
Scoring increases based on the intensity of the subjects' complaints in each of the 11 items (subsets): Somato-vegetative symptoms (sweating/flush, cardiac complaints, sleeping disorders, joint and muscle complaint; psychological symptoms: (depression, irritability, anxiousness, exhaustion; and urogenital symptoms: (sexual problems, urinary complaints, vaginal dryness. Answers of the questions are set in form of a rating scale from Zero to Four .Zero is with no symptoms, One is little, Two: average, Three: severe and Four is very severe symptoms. The higher the overall scores of MRS in each area, indicating worse menopause symptoms and lower scores indicate better improvement. This questionnaire has internal and external validity and reliability as established by previous studies.24,29 Simbar et al., determined the reliability by Cronbach's alpha (ra= 0.933).26 In this study, the reliability of the questionnaire was examined by the Cronbach's alpha (ra=0.9). The plant extracts and placebo agents were supplied by Exir Golesorkh Pharmaceutical Co., Ltd. The process included: Plant entry, Plant identification, Extraction, Formulation, pasteurization, In-process tests, Packaging, Final Test and microbiologic and final endorsement. This extract was alcohol based. The main plant extract components were seed of fennel, flower of chamomile, and stigma of saffron. We also did HPLC test (High-performance Liquid Chromatography) for more configuration of this extract. In the similar studies, the fennel dose was 120 mg a day, for saffron it was 60 mg daily, and for chamomile it was 1000 mg a day (30-32). In our study, we examined the effect of these three herbs in a high-dose concentration (as other studies) and two lower concentrations. The high dose (group B) (n= 30) contained a combination of 120 mg of fennel extract, 60 mg of saffron extract, and 1000 mg of chamomile extract.
In medium dose (group D) (n= 30) was 60 mg fennel extract, 30 mg saffron extract, and 500 mg chamomile extract. In low dose (group A) (n = 30) was 30 mg fennel extract, 15 mg saffron extract and 250 mg chamomile extract and placebo (group C) (n= 30) (sterile dH2O).Women who were interested in participating in the study underwent screening to determine initial eligibility. Selected women received a complete written and oral description of the study before signing an informed consent form. Next, the women were randomized into one of four study groups. All study participants completed demographic and MRS questionnaires under the same circumstances at baseline, 6th and 12th week. All participants were instructed not to take hormonal medication or to initiate other treatments for their symptoms for the duration of the study. Then for 12 weeks one group received placebo, 3 other groups received different doses of the extract. A daily dose (2 ML) of the extract or placebo was administered as oral drops. All the bottles were dark. Because of the saffron, the color of extracts were yellow, then the color of the placebo were yellow too. Since this was a triple-blinded trial and only the pharmacist was aware of the contents of the bottle, each participant was given a bottle that was labeled with A, B, C, or D. The women were also requested to report if they experienced any adverse effects.
Physical, psych and urogenital domains score of menopause symptoms after intervention, The MRS questioner is a validated and established instrument that can easily be completed by women. Data analysis was conducted using SPSS Version 13 (IBM, Armonk, NY, USA) statistical software. The level of significance was P<0.05. Differences in group characteristics and baseline values were analyzed using the following tests: Kolmogorov-Smirnov test to detect the normality of variables, Kruskal-Wallis test for continuous variables with abnormal distributions, ANOVA for normally distributed Quantitative variables. To compare treatment effects on the outcome measures within and between group changes, the Wilcoxon and Friedman test were used. Also prism 6 software was used to create the graphs.
Results
Of the 120 participants who participated in the research, 108 of them fulfilled the eligibility criteria and successfully completed the baseline assessment with complete data for the entire 12-weeks clinical trial. Study retention was excellent, with 108 women (90%) remaining in the study. Intention-to-treat (ITT) analysis was done and every subject who is randomized according to randomized treatment assignment and ignores noncompliance, protocol deviations, withdrawal, and anything that happens after randomization. The subjects were randomly assigned to one of the four study groups. One participant in group B and one participant in group D withdrew because of flushing and one participant in group D, four participants in group A and five participants in group C withdrew because they did not experience any change in their menopausal symptoms (Figure 1). Confidence interval was 95%. Test results for data normalization that were obtained by the Kolmogorov-Smirnoff indicated that age and BMI in all the subjects were not normal (P = 0.01, 0.04). Mean scores of all domains of MRS questionnaire were not normal (P <0.001).There were no differences between groups regarding demographic data (Table 1).
Table 1. Background data on in four study groups .
| Characteristic |
A
N=30 |
B
N=30 |
C
N=30 |
D
N=30 |
P |
| Age Mean (SD) | 51.53 (4.72) | 51.63 (4.83) | 51.50 (4.14) | 51.20 (4.34) | 0.98 |
| BMI Mean (SD) | 22.53 (1.91) | 22.66 (1.97) | 22.73 (1/98) | 22.64 (1.98) | 0.98 |
| Job Number median (IQR) | 0.82 | ||||
| Housewife | 17 (33.33) | 16 (53/33) | 16 (53.33) | 19 (33/63) | |
| Employee | 13 (43.3) | 13 (43.43) | 12 (40) | 10 (30.33) | |
| Worker | 0(0) | 1 (3.33) | 2 (6.66) | 1 (1.33) | |
| Spouse Job Number median (IQR) | 0.45 | ||||
| Unemployed | 0 | 0 | 0 | 0 | |
| Employee | 26 (86.66) | 20 (66.66) | 24 (80) | 23 (66.76) | |
| Free job | 4 (13.33) | 9 (30) | 4 (13.33) | 6 (20) | |
| Worker | 0 (0) | 1 (3.33) | 2 (6.66) | 1 (1.33) | |
| Education Number median (IQR) | 0.84 | ||||
| illiterate | 1 (3.33) | 4 (13.33) | 4 (13.33) | 3 (10) | |
| Diploma | 1 (53.33)6 | 13 (43.33) | 12 (40) | 14 (46.66) | |
| Collage education | 13 (10) | 13 (10) | 14 (46.66) | 13 (10) | |
| Spouse Education Number median (IQR) | 0.84 | ||||
| illiterate | 1 (3.33) | 3 (10) | 2 (6.66) | 2 (6.66) | |
| Diploma | 12 (40) | 9 (30) | 10 (30.33) | 13 (10) | |
| Collage education | 17 (56.66) | 18 (60) | 18 (60) | 15 (50) |
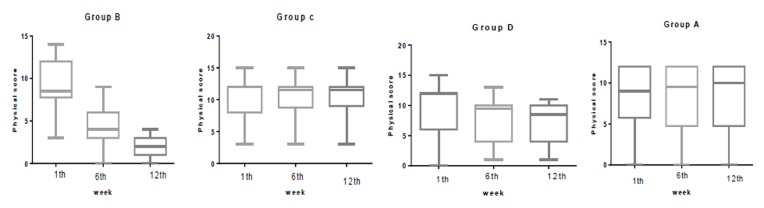
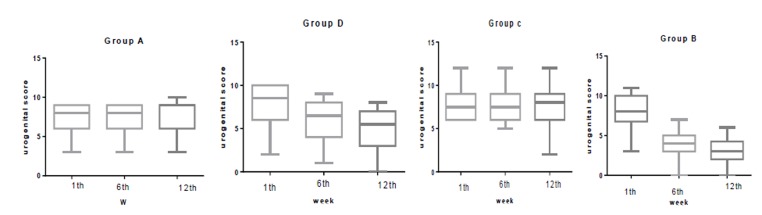
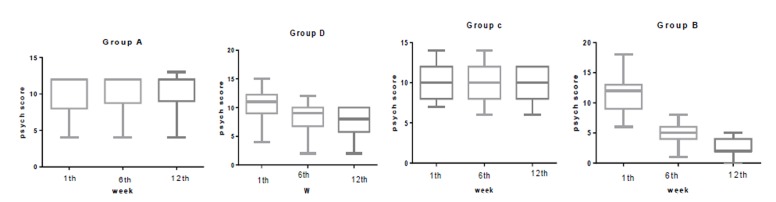
The median (IQR) physical score was significantly reduced in group B from 8.5(4) at baseline to 4 (3) at week 6 and to 2 (2) at week 12. In group A, this was 9(6) at baseline to 9.5 (7) at week 6 and to 12 (3) at week 12. In group C, this was 12 (4) at baseline to 11.5 (3) at week 6 and to 10 (4) at week 12. In group D this was 12 (6) at baseline to 9.5 (6) at week 6 and to 8 (4) at week 12. The median (IQR) psychological score was significantly reduced in group B from 12 (4) at baseline to 5 (2) at week 6 and to 2 (2) at week 12. In group A, this was 12(4) at baseline to 9.5 (7) at week 6 and to 12 (3) at week 12. In group C, this was 10 (4) at baseline to 10 (4) at week 6 and to 10 (4) at week 12. In group D this was 12 (3) at baseline to 9 (3) at week 6 and to 8 (4) at week 12. The median (IQR) urogenital score was significantly reduced in group B from 6.5 (3) at baseline to 4 (2) at week 6 and to 3 (2) at week 12. In group A, this was 8 (3) at baseline to 8 (3) at week 6 and to 9 (3) at week 12. In group C, this was 8(4) at baseline to 6.5(4) at week 6 and to 5.5 (4) at week 12. In group D, this was 7.5(3) at baseline to 7.5 (3) at week 6 and to 8(3) at week 12.Statistically significant difference was not observed in any of the physical, psychological, and urogenital MRS dimensions, in the first week (Table 2). However, significant differences in all the domains (Physical, Psychological and Urogenital) were seen in the sixth (p<0.001) and twelfth weeks (P<0.001).
Table 2. Comparison the median and inter quartile range in all MRS domains at week 1th, 6th and 12th .
| Groups | A/ Low dose | B/ High dose | C/ Placebo | D/ Median dose | Koroscal -wallis | ||||||||
| Domains | M. R | IQR | M | M. R | I QR | M | M. R | IQR | M | M. R | IQR | M | |
| Physical 1th | 9 | 2.98 | 6 | 12 | 2.07 | 4 | 12 | 3 | 4 | 8.5 | 2.07 | 6 | Chi-square:4.46 P=0.2 |
| Physical 6th | 9.5 | 1.75 | 6 | 9.5 | 1.95 | 3 | 11.5 | 1.97 | 3 | 4 | 1.92 | 7 | Chi-square:34.6 P<0.001 |
| Physical 12th | 12 | 1.27 | 6 | 9.5 | 1.98 | 10 | 3 | 1.03 | 2 | 2 | 2.02 | 3 | Chi-square:60.7 P<0.001 |
| Psych 1th | 12 | 2.93 | 3 | 12 | 2.10 | 4 | 10 | 3 | 4 | 12 | 1.95 | 4 | Chi-square:5.786 P=0.123 |
| Psych 6th | 12 | 1.73 | 3 | 9 | 1.90 | 4 | 10 | 2 | 2 | 5 | 2 | 3 | Chi-square:61.5 P<0.001 |
| Psych 12th | 12 | 1.33 | 4 | 8 | 2 | 4 | 10 | 1 | 2 | 2 | 2.05 | 3 | Chi-square:77.83 P<0.001 |
| Urogenital 1th | 8 | 2.83 | 4 | 8 | 1.88 | 3 | 7.5 | 3 | 3 | 6.5 | 2 | 3 | Chi-square:2.84 P=0.41 |
| Urogenital 6th | 8 | 1.97 | 4 | 6.5 | 1.78 | 3 | 7.5 | 1.82 | 2 | 4 | 1.95 | 3 | Chi-square:43.33 P<0.001 |
| Urogenital 12th | 9 | 1.20 | 4 | 5.5 | 2.33 | 3 | 8 | 1.18 | 2 | 3 | 2.05 | 3 | Chi-square:62.87 P<0.001 |
M: median, IQR: inter quartile range, M.R: mean rank
With comparison the scores of physical, psych and urogenital domain of MRS questionnaire in 1th ,6th and 12th, no statistical significant difference within group A and C were seen, but statistical significant difference was within group B (P<0.001) and D (P<0.001) in all weeks.
In comparing groups in the first week, there were no significant differences between groups in all domains of MRS questionnaire. In the sixth week there were significant differences between group B (P<0.001) and all other groups in all domains of MRS. A significant statistical difference was between groups D and C in physical domain (P=0.01 and a significant statistical difference was between groups D and A in psych domain (P=0.02) in week 6. There was a significant statistical difference between group B and all other groups in week 12 in all domains of MRS. (P<0.001) .There was a significant statistical difference between group D in all domains of MRS except physical domain with all other groups in 12th week. (P=0.01) (Figure 2-4)
Figure 2.
Comparison the physical score in group B, C, D and A at 1th, 6th and 12th week
Figure 4.
Comparison the urogenital score in group B, C, D and A at 1th, 6th and 12th week
In comparison the difference MRS scores and its domains, there were significant difference between group B with all other groups. (Table 3) Adverse events: No adverse events were observed or reported by participants who received the herbal extracts in the present study.
Table 3. Comparison the median and inter quartile range in all MRS domains differences at week 1th, 6th and 12th .
| Groups | A/ Low dose | B/ High dose | C/ Placebo | D/ Median dose | Koroscal -wallis | ||||||||
| Domains | M. R | IQR | M | M. R | I QR | M | M. R | IQR | M | M. R | IQR | M | |
| diff physical 6th week from 1th | 0 | 0 | 82.5 | -4.5 | 1.25 | 17.22 | 0 | 0 | 89.82 | -2 | 0 | 45.38 | Chi-square:78.43 P<0.001 |
| Diff physical 12th week from 1th | 0 | 0 | 90.53 | -7 | 3.25 | 16.5 | 0 | 0 | 89.76 | -2.5 | 1 | 45.30 | Chi-square:102.601 P<0.001 |
| diff physical 12th week from 6th | 0 | 0 | 83 | -3 | 1 | 21.18 | 0 | 0 | 80.33 | 0 | 1.25 | 57.48 | Chi-square:101.554 P<0.001 |
| Diff psych 6thweek from 12th week | 0 | 0 | 92.42 | -7 | 3 | 15.92 | 0 | 0 | 87.3 | -2 | 1.25 | 46.63 | Chi-square:106.795 P<0.001 |
| diff psych 12th week from 1th | 0 | 0 | 91.73 | -7 | 3.25 | 15.53 | 0 | 0 | 87.78 | -2.5 | 1 | 46.95 | Chi-square:104.677 P<0.001 |
| diff psych 12th from 6th | 0 | 0 | 80.65 | -2 | 1 | -2 | 0 | 0 | 81.5 | 0 | 1 | 62.78 | Chi-square:87.200 P<0.001 |
| diff urogenital 6th from 1th | 0 | 0 | 89.75 | -4 | 2 | 16.75 | 0 | 0 | 88.5 | -1.5 | 1 | 46.80 | Chi-square:105.541 P<0.001 |
| diff urogenital 12th from 1th | 0 | 0 | 86.15 | -5 | 3 | 20.50 | 0 | 0 | 91.88 | -2 | 1.25 | 43.47 | Chi-square:92.370 P<0.001 |
| diff urogenital 12th from 6th | 0 | 0 | 85 |
-1 | 1 | 40.83 | 0 | 1.25 | 87 | -1 | 1 | 37.92 | Chi-square:56.135 P<0.001 |
M: median, IQR: inter quartile range, M.R: mean rank, DIFF: difference
Figure 3.
Comparison the psyche score in group B, C, D and A at 1th, 6thand 12th week
Discussion
In this study, we evaluated the effects of a compound herbal medicine on menopause symptoms. This randomized, triple-blind, placebo-controlled 12-weeks trial showed that our herbal extract effectively improved various climacteric symptoms. We could not find any previous study that assessed the effects of fennel, chamomile or saffron on menopause symptoms.
However, because an active biological compound in fennel and chamomile is phytoestrogen, we debated to studies that assessed the effects of phytoestrogens. Phytoestrogens are plant compounds with estrogen-like properties. The two major classes of phytoestrogen are isoflavones and lignans; soybeans are rich in isoflavones, and lignans are found in flaxseed, whole grains, legumes, fruits, and vegetables. The chemical structures of isoflavones and lignans are similar to those of estradiol.33
Although, the effect of phytoestrogen on menopausal complications are discussed in some studies, but their effects have been not confirmed. In the physical, psychological and Urogenital MRS dimension, group B and D showed significant improvement within the groups in the sixth and twelfth week. Comparing the groups in the sixth week showed that group B had the most improvement and group D showed improvement in physical domain in comparison with group A, but it did not differ with Group C. Given that groups A and C did not differ with each other and there was no change within the groups, it could be concluded that no significant differences existed in Group D in the sixth week. In the twelfth week, we observed improvement in the psychological dimension in groups B and D, with significant improvement in group B in comparison with group D. In the sixth week compared to the first week in all dimension of MRS, statistical differences were observed. In group B and D in the twelfth week compared to the first week, significant differences were observed with other groups and with each other in improving physical, psychological and urogenital. In the twelfth week compared to the sixth week, significant difference was only observed in group B, which indicated significant improvement. Groups A and C in any weeks did not show any significant improvement. Group B, when compared to the six weeks ago showed significant improvement. In group D, significant improvement observed between the twelfth week with the sixth and first week, but there was no difference between the twelfth week and the sixth. A meta-analysis was conducted by Chen on effect of phytoestrogen on menopausal symptoms. Results showed, seven studies about Kupperman index, data showed no significant effect in compare to the placebo. However the results of 10 studies about hot flashes showed the significant reduction in frequency of hot flashes compared to the control.34 Kashani (2012) reported that saffron was particularly effective in improving arousal, vaginal dryness, and pain dimensions.35 Hosseinzadeh studied the effect of saffron in male rats. By intraperitoneal injection of Crocus sativus stigma aqueous extract, significant improvement was observed in several sexual behavior-related factors including rising, intromission, and erection frequencies, and ejaculation latency.36 one of our herbal extract in this study was saffron and a reason that sexual behavior was improved in women in this study may be saffron. In addition, fennel and chamomile because of phytostrogenic effects might have the same effect too.
The MRS score difference in groups B and D in the sixth week compared to the first week, the twelfth week compared to the first and sixth week, showed statistical differences with the other groups and with each other in terms of lower scores and improved menopausal symptoms. Groups A and C in any week did not show any significant improvement. Groups B and D, when compared to the six weeks ago, showed significant differences in terms of lower score, but group B showed significant improvement in comparison with group D.A long with the results of our study, Nedeljkovic, reported significant improvements in the severity and frequency of hot flushes and other menopausal symptoms with traditional Chinese herbal medicine (Zhi Mu 14).37
Akbari Torkestani reported that fenugreek and flaxseed did not influence the severity and frequency of hot flushes before eight weeks, but after eight weeks a decrease in severity and frequency was observed in subjects who used fenugreek.38 Our trial was 12 weeks, but from 6th week, significant improvement was seen. In another study on the effectiveness of an herbal extract (prim rose oil, Damiyana, Gensing and Royall jelly) statistically significant improvement was observed in the MRS-II score in both groups(placebo and treatment) after two and four weeks of treatment; but improvement was significantly better in the Lady 4 group.10 In another study on the effects of the natural plant extracts, for the treatment of flushes was reported significant difference between the study and control group. A decrease only in number and intensity of hot flushes from baseline to the completion of treatment was observed.22 In most studies, by using the herbal extract that most of them are phytoestrogens, could improve a few menopause symptoms but as we expected, most of the menopause symptoms in this research got better. Chang reported KMI (the mean Kupperman Menopause Index (score was significantly reduced in the EstroG-100(Cinanchem, Flomis and Angelica) when compared to that of the placebo group. For each constituting symptom of KMI, the mean scores for vasomotor, insomnia, nervousness, melancholia, vertigo, and fatigue were significantly lower in the EstroG-100 group at weeks 6 and 12 compared to the baseline (P<0.01). Moreover, vaginal dryness was also significantly improved, along with most of the individual symptoms in the KMI questionnaire.11 Green (2007) reported treatment by qualified herbal practitioners was able to reduce the total scores of the GCS (Greene Climacteric Scale). All subscales showed greater improvement in the treated group compared to the control group, in regards to only vasomotor symptoms (hot flushes and night sweats) and libido scores.39 But Plotnikoff reported treatment failure and TU-025 did not demonstrate efficacy beyond the placebo for reducing the severity and frequency of hot flash symptoms, climacteric symptoms, or disrupted sleep symptoms in post-menopausal American women.40
This was due to TU-025 was not phytoestrogens. Although the mechanism underlying menopause symptoms has not been fully clarified, the results of various basic and clinical studies have indicated that dramatic changes in the hormonal environment, especially the sharp decline of estrogen, during menopause play key role. We though most of these herbal medicines, alleviate the menopausal symptoms because of phytoestrogenic effects. In our study we examined two phytoestrogen herbal medicine plus Saffron that might have been synergism effects on menopausal symptoms. This is the first time that a study has assessed the effects of fennel, chamomile and saffron on vaginal atrophy in menopausal women. Many menopausal women believe menopause symptoms are normal, and therefore, do not seek treatment. The results of this study can be useful for many women who suffer from menopause symptoms but are not willing to use hormone therapy. The other strengths of this study are that it was a randomized controlled trial of a complex medical intervention of quality complementary and alternative medicine. The length of the trial was adequate to measure change and although symptoms may fluctuate during menopause, the treatment was particularly effective for the troublesome symptoms commonly associated with menopause (e.g., hot flashes, night sweats, and insomnia).The study limitation was the somewhat small size. A larger population is needed to confirm the results and to increase the statistical power of the study.
Conclusion
The 12-week treatment with the herbal compound has shown a statistically significant improvement in the various menopausal symptoms in group B.
Acknowledgments
This research was supported by faculty of nursing and midwifery of Mashhad university of medical sciences.
Ethical issues
None to be declared.
Conflict of interest
The authors declare no conflict of interest in this study
Citation: Mahdavian M, Mirzaii Najmabadi KH, Hosseinzade H, Mirzaeian S, Badiee SH, Esmaeeli H. Effect of the mixed herbal medicines extract (fennel, chamomile, and saffron) on menopause syndrome: a randomized controlled clinical trial.j Caring Sci 2019; 8 (3): 181-89. doi:10.15171/jcs.2019.026.
References
- 1.Stephenson K, Neuenschwander PF, Kurdowska AK. The effects of compounded bioidentical transdermal hormone therapy on hemostatic, inflammatory, immune factors; cardiovascular biomarkers; quality-of-life measures; and health outcomes in perimenopausal and postmenopausal women. Int J Pharm Compd. 2012;17(1):74–85. [PubMed] [Google Scholar]
- 2.Rajaeefard A, Mohammad-Beigi A, Mohammad-Salehi N. Estimation of natural age of menopause in Iranian women: a meta-analysis study. Koomesh. 2011;13(1):1–7. (Persian) [Google Scholar]
- 3. Aidelsburger P, Schauer S, Grabein K, Wasem J. Alternative methods for the treatment of post-menopausal troubles. GMS Health Technol Assess 2012; 8. [DOI] [PMC free article] [PubMed]
- 4.Stephenson K, Neuenschwander PF, Kurdowska AK. The effects of compounded bioidentical transdermal hormone therapy on hemostatic, inflammatory, immune factors; cardiovascular biomarkers; quality-of-life measures; and health outcomes in perimenopausal and postmenopausal women. Int J Pharm Compd. 2012;17(1):74–85. [PubMed] [Google Scholar]
- 5.Maki PM, Freeman EW, Greendale GA, Henderson VW, Newhouse PA, Schmidt PJ. et al. Summary of the NIA-sponsored conference on depressive symptoms and cognitive complaints in the menopausal transition. Menopause. 2010;17(4):815–822. doi: 10.1097/gme.0b013e3181d763d2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kim M-S, Lim H-J, Yang HJ, Lee MS, Shin B-C, Ernst E. Ginseng for managing menopause symptoms: a systematic review of randomized clinical trials. J Ginseng Res. 2013;37(1):30–6. doi: 10.5142/jgr.2013.37.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Ernst E, Pittler MH, Wider B, Boddy K. Oxford handbook of complementary medicine: OUP Oxford; 2008.
- 8.Rees M. Alternative treatments for the menopause. Best Pract Res Clin Obstet Gynaecol. 2009;23(1):151–61. doi: 10.1016/j.bpobgyn.2008.10.006. [DOI] [PubMed] [Google Scholar]
- 9.Mirzaiinjmabadi K, Anderson D, Barnes M. The relationship between exercise, Body Mass Index and menopausal symptoms in midlife Australian women. Int J Nurs Pract. 2006;12(1):28–34. doi: 10.1111/j.1440-172X.2006.00547.x. [DOI] [PubMed] [Google Scholar]
- 10.Yakoot M, Salem A, Omar A-M. Effectiveness of a herbal formula in women with menopausal syndrome. Complementary Medicine Research. 2011;18(5):264–8. doi: 10.1159/000333430. [DOI] [PubMed] [Google Scholar]
- 11.Chang A, Kwak BY, Yi K, Kim JS. The Effect of Herbal Extract (EstroG-100) on Pre-, Peri-and Post-Menopausal Women: A Randomized Double-blind, Placebo-controlled Study. Phytotherapy Research. 2012;26(4):510–6. doi: 10.1002/ptr.3597. [DOI] [PubMed] [Google Scholar]
- 12.pourabbas S, kesmati M, rasekh A. Study of the the anxiolytic effects of fennel and possible roles of bothGABAergic system and estrogen receptors in these effects in adult female rat. Physiology and Pharmacology. 2011;15(1):134–43. (Persian) [Google Scholar]
- 13.Kim T-H KH-J, Lee S-H, Kim S-Y. Potent inhibitory effect of Foeniculum vulgare Miller extract on osteoclast differentiation and ovariectomy-induced bone loss. Int J Mol Med. 2012;29(6):1053–9. doi: 10.3892/ijmm.2012.950. [DOI] [PubMed] [Google Scholar]
- 14.Malini T VG, Megala N, Anusya S, Devi K, Elango V. Effect of Foeniculum vulgare mill seed extract on the genital organs ofmale and female rats. Indian J Physiol Pharmacol. 1985;29(1):21–6. [PubMed] [Google Scholar]
- 15.Barene I, Daberte I, Zvirgzdina L, Iriste V. The complex technology on products of German chamomile. Medicina. 2003;39(2):127–31. [PubMed] [Google Scholar]
- 16.Zeggwagh NA, Michel JB, Eddouks M. Vascular Effects of Aqueous Extract of Chamaemelum nobile: In Vitro Pharmacological Studies in Rats. Clin Exp Hypertens. 2013;35(3):200–6. doi: 10.3109/10641963.2012.712179. [DOI] [PubMed] [Google Scholar]
- 17.Emamghoreishi M, Ghasemi F. The Effect of Subchronic Administration of the Aqueous and Hydro-alcoholic Extracts of Crocus sativus from Estahbanat, Fars Province, on Mice. Armaghane-danesh. Yasuj University of Original Article Medical Sciences Journal. 2011;16(6):527–36. (Persian) [Google Scholar]
- 18.Kashani L RF, Saroukhani S, Sohrabi H, Modabbernia A, Nasehi AA. et al. Saffron for treatment of fluoxetine-induced sexual dysfunction in women: randomized double-blind placebo-controlled study. Hum Psychopharmacol. 2013;28(1):54–60. doi: 10.1002/hup.2282. [DOI] [PubMed] [Google Scholar]
- 19.Rajput MS, Sinha S, Mathur V, Agrawal P. Herbal antidepressants. IJPFR. 2011;1(1):159–69. doi: 10.20959/wjpps20194-13399. [DOI] [Google Scholar]
- 20.Efird J. Blocked Randomization with Randomly Selected Block Sizes. Int J Environ Res Public Health. 2011;8(1):15–20. doi: 10.3390/ijerph8010015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Altman DG, Bland JM. How to randomise. BMJ. 1999;319(7211):703–4. doi: 10.1136/bmj.319.7211.703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kupfersztain C, Rotem C, Fagot R, Kaplan B. The immediate effect of natural plant extract, Angelica sinensis and Matricaria chamomilla (Climex) for the treatment of hot flushes during menopause. A preliminary report. Clin Exp Obstet Gynecol. 2002;30(4):203–6. [PubMed] [Google Scholar]
- 23.Kelley KP, Kristopher J. On effect size. Psychological Methods. 2012;17(2):137–52. doi: 10.1037/a0028086. [DOI] [PubMed] [Google Scholar]
- 24.al msae p. The frequency and severity of menopausal symptoms and its relationship with personal factorsThe young women before and after menopause in Ahvaz. IJOGI. 2013;16:7–15. [Google Scholar]
- 25.Heinemann LA, Potthoff P, Schneider HP. International versions of the menopause rating scale (MRS) Health and quality of life outcomes. 2003;1(1):28. doi: 10.1186/1477-7525-1-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nazarpour S, Simbar M, Tehrani FR. Effects of the severity of menopausal symptoms on sexual function in postmenopausal women. The Journal of Qazvin University of Medical Sciences. 2015;19(4):54–63. (Persian) [Google Scholar]
- 27.Schneider HP, Heinemann LA, Thiele K. The menopause rating scale (mrs): cultural and linguistic translation into English. Life and Medical Science Online. 2003;1:28. doi: 10.1186/1477-7525-1-28. [DOI] [Google Scholar]
- 28.Heinemann K, Ruebig A, Potthoff P, Schneider HP, Strelow F, Heinemann LA. The Menopause Rating Scale (MRS) scale: a methodological review. Health and Quality of life Outcomes. 2004;2(1):45. doi: 10.1186/1477-7525-2-45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jalili L H N DPA, Najar EAMS. The relationship between physical activity and intensityMenopausal symptoms in postmenopausal women in Ahvaz. IJOGI. 2014;17(98):15–23. [Google Scholar]
- 30.Agha-Hosseini M, Kashani L, Aleyaseen A, Ghoreishi A, Rahmanpour HA, Zarrinara AR. et al. Crocus sativus L.(saffron) in the treatment of premenstrual syndrome: a double‐blind, randomised and placebo‐controlled trial. BJOG. 2008;115(4):515–9. doi: 10.1111/j.1471-0528.2007.01652.x. [DOI] [PubMed] [Google Scholar]
- 31.Amsterdam JD, Li Y, Soeller I, Rockwell K, Mao JJ, Shults J. A Randomized, double blind, placebo-controlled trial of oral Matricara recutita (chamomile) extract therapy for generalized anxiety disorder. J Clin Psychopharmacol. 2009;29(4):378–82. doi: 10.1097/JCP.0b013e3181ac935c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Omidvar S, Esmailzadeh S, Baradaran M, Basirat Z. Effect of fennel on pain intensity in dysmenorrhoea: A placebo-controlled trial. Ayu. 2012;33(2):311–3. doi: 10.4103/0974-8520.105259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bedell S, Nachtigall M, Naftolin F. The pros and cons of plant estrogens for menopausa. J Steroid Biochem Mol Biol. 2014;139:225–36. doi: 10.1016/j.jsbmb.2012.12.004. [DOI] [PubMed] [Google Scholar]
- 34.Chen MN, Lin CC, Liu CF. Efficacy of phytoestrogens for menopausalsymptoms: a meta-analysis and systematic review. Climacteric. 2015;18(2):260–9. doi: 10.3109/13697137.2014.966241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kashani L, Raisi F, Saroukhani S, Sohrabi H, Modabbernia A, Nasehi AA. et al. Saffron for treatment of fluoxetine‐induced sexual dysfunction in women: randomized double‐blind placebo‐controlled study. Hum Psychopharmacol. 2013;28(1):54–60. doi: 10.1002/hup.2282. [DOI] [PubMed] [Google Scholar]
- 36.Hosseinzadeh H, Ziaee T, Sadeghi A. The effect of saffron,Crocus sativusstigma, extract and its constituents, safranal and crocin on sexual behaviors in normal male rats. Phytomedicine. 2008;15(6–7):491–5. doi: 10.1016/j.phymed.2007.09.020. [DOI] [PubMed] [Google Scholar]
- 37.Nedeljkovic M, Tian L, Ji P, Déglon-Fischer A, Stute P, Ocon E. et al. Effects of acupuncture and Chinese herbal medicine (Zhi Mu 14) on hot flushes and quality of life in postmenopausal women: results of a four-arm randomized controlled pilot trial. Menopause. 2014;21(1):15–24. doi: 10.1097/GME.0b013e31829374e8. [DOI] [PubMed] [Google Scholar]
- 38.Akbari Torkestani N, Attarha M, Heidari T, Narenji F. The Effect of Fenugreek and Flaxseed on Menopausal Hot Flash. Complementary Medicine Journal of Faculty of Nursing and Midwifery. 2011;1(1):68–74. (Persian) [Google Scholar]
- 39.Green J, Denham A, Ingram J, Hawkey S, Greenwood R. Treatment of menopausal symptoms by qualified herbal practitioners: a prospective, randomized controlled trial. Family Practice. 2007;24(5):468–74. doi: 10.1093/fampra/cmm048. [DOI] [PubMed] [Google Scholar]
- 40.Plotnikoff GA, Watanabe K, Torkelson C, La Valleur J, Radosevich DM. The tu-025 keishibukuryogan for hot flash management in postmenopausal women: results and lessons for future Research. Menopause. 2011;18(8):886–92. doi: 10.1097/gme.0b013e31821643d9. [DOI] [PMC free article] [PubMed] [Google Scholar]