Abstract
Objective
The use of some antiretroviral drugs has been associated with a higher risk of diabetes mellitus (DM) in HIV-infected patients, but the risk associated with antiretroviral drug combinations remains unclear. We investigated the association between first-line antiretroviral therapy (ART) regimens, recommended by the World Health Organization (WHO) in 2016, and the risk of DM in adults.
Method
We selected all HIV-infected adults within the Thai National AIDS Program who started a first-line ART regimen consisting the following between October 2006 and September 2013: zidovudine+lamivudine+nevirapine; tenofovir disoproxil fumarate (TDF)+lamivudine+nevirapine; zidovudine+lamivudine+efavirenz; TDF+lamivudine/emtricitabine+efavirenz; zidovudine+lamivudine+ritonavir-boosted lopinavir (LPV/r); or TDF+lamivudine+LPV/r. Diagnosis of DM was defined as having at least 2 of the following characteristics: fasting plasma glucose ≥126 mg/dl, 2010 WHO ICD-10 codes E11-E14, or prescription of antidiabetic drugs. To identify ART regimens associated with DM, we used competing risks regression models that considered mortality without DM as a competing event and adjusted for sex, age, pancreas disease, and stratified by groups defined by a score summarizing the propensity to receive a specific first-line ART regimen.
Results
Data from 35 710 adults (49.1% male; median age, 35.0 years; median follow-up, 2.0 years) were included. In the multivariable analysis with zidovudine+lamivudine+nevirapine as the reference group, a higher risk of DM was observed with TDF+lamivudine/emtricitabine+efavirenz (adjusted sub-distribution hazard ratio [aSHR], 1.6; 95% confidence interval [CI], 1.3–1.9), zidovudine+lamivudine+efavirenz (aSHR, 2.0; 95% CI, 1.7–2.3), and TDF+lamivudine+LPV/r (aSHR, 2.7; 95% CI, 1.9–3.9).
Conclusions
Several of the WHO recommended ART regimens, particularly tenofovir + lamivudine +LPV/r and regimens containing efavirenz, may be associated with an increased risk of DM.
Keywords: antiretroviral treatment regimen, diabetes mellitus, efavirenz, HIV, ritonavir-boosted lopinavir
Introduction
The burden of diabetes is rising especially in low and middle-income countries [1]. In 2017, the International Diabetes Federation estimated that 425 million people worldwide, or 8.8% of adults aged 20–79 years (8.3% in Thailand), were affected by diabetes, of whom half were unaware of their disease [2]. Known risk factors of Type 2 diabetes mellitus (DM) include age, male sex, family history of diabetes, alcohol use, adiposity, and hyperlipidemia [3]. Several studies have suggested that virological, immunological, or clinical failure, as well as antiretroviral (ARV) drug use, may increase the risk of DM in HIV-infected adults [4–6].
The Thailand National AIDS Program (NAP), under the National Health Security Office (NHSO), has provided free health services and antiretroviral therapy (ART) for HIV-infected patients since 2004. Three studies in Thailand have reported high DM incidence rates of 5.0 to 11.0 per 1000 person-years of follow-up (PYFU) in HIV-infected patients on ART [7–9]. Individual ARV drugs, such as stavudine and didanosine, have been associated with a higher risk of DM in HIV-infected patients, but the risk of DM associated with currently recommended ARV drug combinations remains unclear [5, 7, 9–12]. Thus, we investigated the risk of DM based on nationwide data and its association with first-line ART regimens currently recommended by the World Health Organization (WHO) and the Thai Ministry of Public Health.
METHODS
This was an analysis based on the data collected within the national cohort of HIV-infected adults (≥18 years old), who started ART in the NAP within the Universal Health Coverage (UHC) scheme between fiscal year (FY) 2006 and FY2013 (ie, from October 1, 2005 to September 30, 2013). We censored data after the end of FY2014. We restricted our analysis to patients receiving 1 of the 6 first-line combinations currently recommended by either the WHO 2016 Consolidated guidelines on the use of antiretroviral drugs for treating and preventing HIV infection [13] or by the Thailand National Guideline on HIV/AIDS Treatment and Prevention 2017 [14]: (1) zidovudine+lamivudine+nevirapine; (2) tenofovir disoproxil fumarate (TDF)+lamivudine+nevirapine; (3) zidovudine+lamivudine+efavirenz; (4) TDF+lamivudine/emtricitabine+efavirenz; (5) zidovudine+lamivudine+ritonavir-boosted lopinavir (LPV/r); or (6) TDF+lamivudine+LPV/r.
We used the following patient characteristics from the NHSO patient database: sex; age; body mass index (BMI); history of comorbidity (pancreas disease, hepatitis B and hepatitis C infection); fasting plasma glucose (FPG); triglycerides, total cholesterol and absolute CD4 cell count at the time of ART initiation (baseline); and time-updated triglycerides, total cholesterol, absolute CD4 cell count, and HIV-1 RNA viral load during follow-up (HIV-1 RNA viral load was not routinely measured at baseline in this program). Evidence of DM diagnosis was defined by at least 2 of the following records: FPG ≥126 mg/dl following the 2013 American Diabetes Association criteria [15], the 2010 WHO International Classification of Diseases (ICD-10) criteria codes E11–E14 (excluding Type-1 DM) [16], or prescription of antidiabetic drugs [15]. DM diagnosis date was defined as the first date of those records. Hyperlipidemia was defined as either total cholesterol ≥ 240 mg/dl or triglycerides ≥ 200 mg/dl [3]. Pancreas disease (codes C25, K85–K86, D017, D136, D137, K871, Q450, Q451, Q452, and Q453), hepatitis B (codes B16, B170, B180, and B181), and hepatitis C (codes B171 and B182) were identified based on the 2010 WHO ICD-10 [16].
The number of person-years of follow-up (PYFU) was calculated from the date of ART initiation (baseline) to censoring date (ie, date of switching to second-line ART regimen), date of loss to follow-up (7 months after last visit date), or September 30, 2014, whichever occurred first. For descriptive purposes, the overall DM incidence rate was estimated by the number of new diagnoses divided by the total number of PYFU, and the 95% confidence interval (95% CI) was calculated using the quadratic approximation to the Poisson distribution [17].
Study population characteristics were presented as medians and interquartile ranges (IQR) for continuous variables and as counts and percentages for categorical variables. The following factors were analyzed: sex; baseline age (18–34, 35–44, 45–59 or ≥60 years [18]); BMI using the Asia-Pacific classification (<18.5, 18.5–22.9, 23.0–24.9 or ≥25 kg/m2 [19]); history of comorbidities (yes or no); baseline and time-updated hyperlipidemia (yes or no) and absolute CD4 cell count (≤200 or >200 cells/mm3); and time-updated HIV-1 RNA viral load (<1000 or ≥1000 copies/mL) [3, 18].
We estimated the cumulative incidence of DM and corresponding 95% CI [20] using a cumulative incidence function accounting for deaths without DM diagnosis as competing events [17]. We analyzed the association between first-line ART regimens and the risk of DM using Fine and Gray’s competing risks regression [21–23]. We conducted multivariable analyses using a backward elimination approach, starting with factors associated with DM occurrence in the univariable analysis (P value ≤ .20). P values were derived from the Huber-White (robust) sandwich estimator of the variance [24].
We imputed missing time-updated absolute CD4 values (the only variable with < 20% missing data) by multiple imputation with chained equations (MICE; StataCorp, College Station, TX) based on logistic regression [25]. Variables with ≥ 20% missing values (ie, baseline BMI, baseline and time-updated hyperlipidemia, baseline CD4 cell count and time-updated HIV-1 RNA viral load) were excluded from the main analysis, but they were included without imputation in sensitivity analyses comparing results of the final multivariable model when including or excluding each of these variables.
To minimize the possible bias effect of the choice of first-line ART regimen by the physician, all models were stratified by groups defined by a score summarizing the propensity to receive a specific first-line ART regimen. This score was generated using multinomial (polytomous) logistic regression adjusting for FY of ART initiation, sex, age group, and history of hepatitis infection or pancreas disease at baseline (Supplementary Figure 1) [26].
Analyses were performed using Stata software, version 15.1 (StataCorp, College Station, TX). NHSO authorized the use of anonymized records. The study plan was approved by the Ethical Committee of the Faculty of Medicine, Chiang Mai University, Thailand, on March 18, 2014 (114/2014, Research ID: COM-2557-02140).
RESULTS
Study Population
A total of 35 710 adults were included, of whom 17 528 (49.1%) were male. At baseline, median age was 35.0 years (IQR, 28.7–41.7), BMI 20.6 kg/m2 (IQR, 18.8–23.0), absolute CD4 cell count 159 cells/mm3 (IQR, 49–281), 0.4% had history of pancreas disease, 1.9% hepatitis B infection, and 1.5% hepatitis C infection. All patients received 1 of the following first-line ART regimens: 40.4% zidovudine+lamivudine+nevirapine, 2.8% TDF+lamivudine+nevirapine, 20.7% zidovudine+lamivudine+efavirenz, 21.7% TDF +lamivudine/emtricitabine+efavirenz, 13.0% zidovudine+lamivudine+ LPV/r, and 1.4% TDF+lamivudine+ LPV/r (Table 1). Of note, the baseline median CD4 cell count in the group of patients on AZT+3TC+LPV/r was higher than in the other groups, because it included 57.0% of women starting ART during pregnancy at any level of CD4.
Table 1.
Baseline Characteristics of HIV-Infected Adults Who Received Recommended First-Line Antiretroviral Therapy Regimens
| Baseline Characteristics | Antiretroviral Therapy Regimens | Total | |||||
|---|---|---|---|---|---|---|---|
| AZT+3TC+NVPa | TDF+3TC+NVPa | AZT+3TC+EFVa | TDF+3TC (or FTC)+EFVa | AZT+3TC+LPV/rb | TDF+3TC+LPV/rb | ||
| (n = 14 424) | (n = 1007) | (n = 7401) | (n = 7754) | (n = 4631) | (n = 493) | (N = 35 710) | |
| Fiscal year of antiretroviral initiation | |||||||
| 2007 | 1665 (11.54%) | 72 (7.15%) | 878 (11.86%) | 300 (3.87%) | 129 (2.79%) | 26 (5.27%) | 3070 (8.60%) |
| 2008 | 1548 (10.73%) | 87 (8.64%) | 791 (10.69%) | 468 (6.04%) | 203 (4.38%) | 38 (7.71%) | 3135 (8.78%) |
| 2009 | 1576 (10.93%) | 89 (8.84%) | 806 (10.89%) | 596 (7.69%) | 235 (5.07%) | 71 (14.40%) | 3373 (9.45%) |
| 2010 | 1895 (13.14%) | 144 (14.30%) | 1005 (13.58%) | 871 (11.23%) | 450 (9.72%) | 60 (12.17%) | 4425 (12.39%) |
| 2011 | 2715 (18.82%) | 203 (20.16%) | 1359 (18.36%) | 1603 (20.67%) | 1270 (27.42%) | 84 (17.04%) | 7234 (20.26%) |
| 2012 | 2746 (19.04%) | 220 (21.85%) | 1426 (19.27%) | 1896 (24.45%) | 1252 (27.04%) | 101 (20.49%) | 7641 (21.40%) |
| 2013 | 2279 (15.80%) | 192 (19.07%) | 1136 (15.35%) | 2020 (26.05%) | 1092 (23.58%) | 113 (22.92%) | 6832 (19.13%) |
| Sex, n (%) | |||||||
| Female | 7256 (50.31%) | 484 (48.06%) | 2875 (38.85%) | 2798 (36.08%) | 4523 (97.67%) | 246 (49.90%) | 18 182 (50.92%) |
| Male | 7168 (49.69%) | 523 (51.94%) | 4526 (61.15%) | 4956 (63.92%) | 108 (2.33%) | 247 (50.10%) | 17 528 (49.08%) |
| Age, y | |||||||
| median (IQR) | 36.10 (30.08–42.65) | 36.90 (31.03–43.87) | 36.41 (30.71–42.57) | 35.85 (29.62–42.68) | 26.80 (22.11–32.09) | 36.05 (30.51–42.59) | 34.99 (28.63–41.67) |
| n (%) | |||||||
| 18–34 | 3704 (25.68%) | 237 (23.54%) | 1760 (23.78%) | 2163 (27.90%) | 3144 (67.89%) | 131 (26.57%) | 11 139 (31.19%) |
| 35–44 | 7803 (54.10%) | 552 (54.82%) | 4203 (56.79%) | 4041 (52.12%) | 1438 (31.05%) | 273 (55.38%) | 18 310 (51.27%) |
| 45–59 | 2664 (18.47%) | 194 (19.27%) | 1308 (17.67%) | 1379 (17.78%) | 45 (0.97%) | 80 (16.23%) | 5670 (15.88%) |
| ≥60 | 253 (1.75%) | 24 (2.38%) | 130 (1.76%) | 171 (2.21%) | 4 (0.09%) | 9 (1.83%) | 591 (1.65%) |
| History of comorbidity at baseline, n (%) | |||||||
| Pancreas disease | 46 (0.32%) | 3 (0.30%) | 40 (0.54%) | 40 (0.52%) | 3 (0.06%) | 2 (0.41%) | 134 (0.38%) |
| Hepatitis B infection | 54 (0.37%) | 48 (4.77%) | 37 (0.50%) | 501 (6.46%) | 12 (0.26%) | 21 (4.26%) | 673 (1.88%) |
| Hepatitis C infection | 89 (0.62%) | 18 (1.79%) | 152 (2.05%) | 269 (3.47%) | 7 (0.15%) | 16 (3.25%) | 551 (1.54%) |
| Variables with ≥ 20% missing values | |||||||
| Body mass index, kg/m2 (n = 12 619) | |||||||
| median (IQR) | 20.60 (18.80–22.60) | 20.60 (18.40–23.20) | 20.00 (18.00–22.00) | 20.00 (18.20–22.20) | 22.40 (20.20–25.00) | 20.40 (18.80–22.80) | 20.60 (18.8–23.0) |
| n (%) | |||||||
| <18.5 | 1209 (22.48%) | 105 (25.18%) | 652 (30.06%) | 717 (29.19%) | 196 (9.47%) | 26 (19.85%) | 2905 (23.02%) |
| 18.5–22.9 | 2940 (54.68%) | 198 (47.48%) | 1130 (52.10%) | 1253 (51.02%) | 960 (46.40%) | 76 (58.02%) | 6557 (51.96%) |
| 23.0–24.9 | 650 (12.09%) | 57 (13.67%) | 213 (9.82%) | 253 (10.30%) | 380 (18.37%) | 17 (12.98%) | 1570 (12.44%) |
| ≥25.0 | 578 (10.75%) | 57 (13.67%) | 174 (8.02%) | 233 (9.49%) | 533 (25.76%) | 12 (9.16%) | 1587 (12.58%) |
| Absolute CD4 cell count, cells/mm3 (n = 23 681) | |||||||
| median (IQR) | 142 (47–242) | 92 (33–239) | 115 (37–244) | 113 (35–251) | 372 (256–518) | 219 (63–381) | 159 (49–281) |
| n (%) | |||||||
| <200 | 6740 (64.87%) | 369 (69.49%) | 3212 (66.50%) | 2980 (65.75%) | 493 (15.44%) | 98 (47.57%) | 13 892 (58.66%) |
| Hyperlipidemia (n = 6017), n (%) | 570 (20.81%) | 26 (17.57%) | 251 (19.97%) | 198 (15.84%) | 194 (34.64%) | 22 (34.92%) | 1261 (20.96%) |
Abbreviations: 3TC, lamivudine; AZT, zidovudine; CI, confidence interval; EFV, efavirenz; FTC, emtricitabine; IQR, interquartile range; LPV/r, ritonavir-boosted lopinavir; n, number of patients with available data; NVP, nevirapine; TDF, tenofovir.
aRecommended by the World Health Organization 2016 Consolidated Guidelines (http://www.ncbi.nlm.nih.gov/books/NBK374294/) on the use of antiretroviral drugs for treating and preventing HIV infection.
bRecommended by the Thailand National Guidelines on HIV/AIDS Treatment and Prevention 2017 (http://www.thaiaidssociety.org/images/PDF/hiv_thai_guideline_2560.pdf).
Antiretroviral Regimens During Pregnancy
Of the 18 182 women in this cohort, 4680 (25.7%) received ART during pregnancy (77.6% zidovudine+lamivudine+LPV/r, 18.6% zidovudine+lamivudine+nevirapine; 2.4% zidovudine+lamivudine+efavirenz, 1.0% TDF+lamivudine+LPV/r, 0.3% TDF+lamivudine/emtricitabine+efavirenz, and 0.1% TDF+lamivudine+nevirapine). Among these 4680 pregnant women, 3558 (76.0%) received ART for the first-time during pregnancy. Of note, 102 (2.2%) experienced new onset of diabetes (gestational diabetes mellitus [GDM]) and 90 (1.9%) developed DM thereafter. Of 102 women with GDM, 9 (8.8%) developed DM thereafter.
Patient Follow-Up
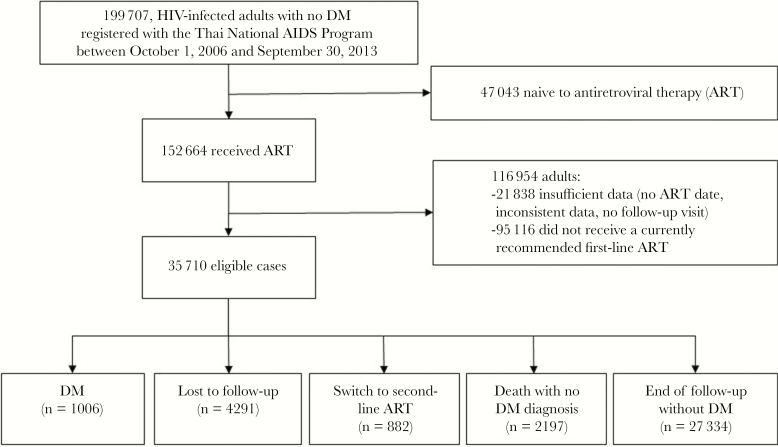
There was a total of 84 238 PYFU on first-line ART regimen (median duration of follow-up 2.0 years; IQR, 1.1–3.3). Over the study period, 1006 (2.8%) were diagnosed with DM. A total of 5173 (14.5%) were censored: 4291 (12.0%) of patients were lost to follow-up and 882 (2.5%) switched to second-line ART regimen. A total of 2197 (6.2%) died without DM diagnosis (Figure 1). In terms of documentation of the DM onset, 360 (35.8%) had records of ICD-10 diagnosis of DM and antidiabetic drug prescription and elevated FPG, 368 (36.6%) had only ICD-10 diagnosis of DM and antidiabetic drug prescription, and 278 (27.6%) had only elevated FPG.
Figure 1.
Flow Chart of Study Population
The estimated overall DM incidence rate was 11.9 per 1000 PYFU (95% CI, 11.2–12.7), ranging from 6.3 per 1000 PYFU (95% CI, 4.7–8.4) for zidovudine+lamivudine+LPV/r to 22.3 per 1000 PYFU (95% CI, 15.8–31.6) for TDF+lamivudine+LPV/r (Supplementary Table 1).
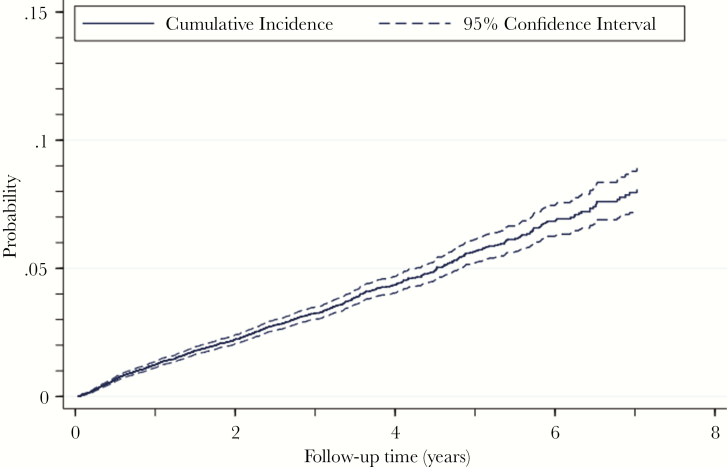
The estimated cumulative incidence of DM (accounting for deaths without DM diagnosis as competing events) was 2.2% (95% CI, 2.1%–2.4%) at 2 years after ART initiation (Figure 2).
Figure 2.
Estimated Cumulative Incidence Function of Diabetes Mellitus in HIV-Infected Adults
Association Between the Use of Some Antiretroviral Regimens and Risk of Diabetes Mellitus
In the univariable analyses, male sex, older age at baseline, higher BMI at baseline, history of pancreas disease at baseline, and time-updated hyperlipidemia were associated with an increased risk of DM diagnosis (all P ≤ .05, see Table 2). In the multivariable analysis adjusting for sex, age, history of pancreas disease at baseline, previous FPG measurement, and propensity score stratification, where zidovudine+lamivudine+nevirapine was the reference regimen, the following 3 regimens were associated with a higher risk of DM: (1) TDF+lamivudine/emtricitabine+efavirenz (adjusted sub-distribution hazard ratio [aSHR], 1.6; 95% CI, 1.3–1.9), zidovudine+lamivudine+efavirenz (aSHR, 2.0; 95% CI, 1.7–2.3), and TDF+lamivudine+LPV/r (aSHR, 2.7; 95% CI, 1.9–3.9).
Table 2.
Factors Associated With the Risk of Diabetes Mellitus in HIV-Infected Adults Who Received Recommended First-Line Antiretroviral Therapy Regimens
| Variables (n = 35 710) | Univariablec | Multivariablec | ||
|---|---|---|---|---|
| SHR (95% CI) | P value | aSHR (95% CI) | P value | |
| Male sex | 1.70 (1.46, 1.98) | <.001 | 1.40 (1.19, 1.64) | <.001 |
| Baseline age, y | ||||
| 18–34 | 1 | 1 | ||
| 35–44 | 2.11 (1.71, 2.61) | <.001 | 2.02 (1.64, 2.49) | <.001 |
| 45–59 | 4.31 (3.43, 5.41) | <.001 | 3.89 (3.11, 4.88) | <.001 |
| ≥60 | 5.79 (4.07, 8.23) | <.001 | 5.46 (3.85, 7.73) | <.001 |
| Baseline history of comorbidity | ||||
| Pancreas disease | 2.78 (1.48, 5.24) | .002 | 2.22 (1.16, 4.24) | .016 |
| Hepatitis B infection | 1.61 (0.79, 3.30) | .191 | ||
| Hepatitis C infection | 1.37 (0.72, 2.62) | .334 | ||
| First-line antiretroviral regimens | ||||
| AZT+3TC+NVPa | 1 | 1 | ||
| TDF+3TC+NVPa | 0.84 (0.53, 1.34) | .457 | 0.78 (0.49, 1.25) | .300 |
| AZT+3TC+EFVa | 2.09 (1.79, 2.44) | <.001 | 1.98 (1.70, 2.32) | <.001 |
| TDF+3TC (or FTC)+EFVa | 1.67 (1.41, 1.97) | <.001 | 1.57 (1.33, 1.86) | <.001 |
| AZT+3TC+LPV/rb | 0.96 (0.69, 1.33) | .800 | 1.27 (0.90, 1.78) | .173 |
| TDF+3TC+LPV/rb | 2.80 (1.94, 4.04) | <.001 | 2.69 (1.87, 3.87) | <.001 |
| Time-updated absolute CD4 cell count <200 cells/mm3 (n = 30 789) | 1.13 (0.96, 1.34) | .146 | ||
| Variables with ≥20% missing values | ||||
| Baseline body mass index, kg/m2 (n = 12 619) | ||||
| <18.5 | 0.90 (0.66, 1.23) | .506 | ||
| 18.5–22.9 | 1 | |||
| 23.0–24.9 | 1.29 (0.93, 1.81) | .132 | ||
| ≥25.0 | 1.71 (1.25, 2.34) | .001 | ||
| Baseline absolute CD4 cell count <200 cells/mm3 (n = 23 681) | 0.85 (0.72, 1.01) | .064 | ||
| Baseline hyperlipidemia (n = 6017) | 1.00 (0.66, 1.51) | .995 | ||
| Time-updated HIV-1 RNA viral load ≥ 1000 copies/mL (n = 26 556) | 1.30 (0.94, 1.82) | .116 | ||
| Time-updated hyperlipidemia (n = 20 768) | 4.19 (3.27, 5.37) | <.001 | ||
Abbreviations: 3TC, lamivudine; aSHR, adjusted sub-hazard ratio; AZT, zidovudine; CI, confidence interval; EFV, efavirenz; FTC, emtricitabine; LPV/r, ritonavir-boosted lopinavir; n, number of patients with available data; NVP, nevirapine; SHR, sub-hazard ratio; TDF, tenofovir.
a Recommended by the WHO 2016 Consolidated guidelines (http://www.ncbi.nlm.nih.gov/books/NBK374294/) on the use of antiretroviral drugs for treating and preventing HIV infection.
b Recommended by the Thailand National Guideline on HIV/AIDS Treatment and Prevention 2017 (http://www.thaiaidssociety.org/images/PDF/hiv_thai_guideline_2560.pdf).
c Adjusted for previous fasting plasma glucose measurement and propensity score stratification.
Sensitivity Analyses
We ran the analyses using the same multivariable model, but we included variables for which the percentage of missing data was ≥ 20%: baseline BMI, absolute CD4 cell count and hyperlipidemia, and 2 time-updated variables that were not consistently recorded (time-updated hyperlipidemia and time-updated HIV-1 viral load). Our conclusions were not modified when the analyses included these variables. (Supplementary Table 2).
Discussion
The overall incidence rate of DM was 11.9 per 1000 PYFU (95% CI, 11.2–12.7). Efavirenz and LPV/r-containing ART regimens (except zidovudine+lamivudine+LPV/r) were associated with a higher risk of DM than zidovudine+lamivudine+nevirapine.
In univariable analyses, male sex, older age ≥35 years old, obesity, hyperlipidemia, and a history of hypertension and pancreas disease were associated with a higher risk of DM. This observation is consistent with previous reports [5, 9–12, 27].
The incidence of DM in HIV-infected adults aged 35 to 59 years in our study was 14.1 per 1000 PYFU (95% CI, 13.2–15.1), higher than that reported in the Thai general adult population (7.8–11.4 per 1000 PYFU) [28, 29], suggesting a contribution of HIV disease or ARV drug use, or both, on the risk of DM. However, there was no report on the incidence DM in general Thai adult population during the period of the study.
The DM incidence was higher than that found in the Data Collection on Adverse Events of Anti-HIV Drugs (D:A:D) study (4.2 per 1000 PYFU) [30], the SWISS HIV cohort (4.4 per 1000 PYFU) [31] and the Program for HIV Prevention and Treatment cohort study in Thailand (5.0 per 1000 PYFU) [7]. However, it was lower than that observed in studies from the US Multicenter AIDS Cohort (47 per 1000 PYFU) [32] and the US Women’s Interagency HIV Study (25–28.9 per 1000 PYFU) [33]. It was similar to that found in the French APROCO-COPILOTE cohort (14.1 per 1000 PYFU) [6], a case-control cohort from Taiwan (13.1 per 1000 PYFU) [34], and a recent meta-analysis study (pooled incidence rate of 13.7 per 1000 PYFU) [35]. The patients in our study received only currently recommended first-line ART regimens, which may have explained differences in the incidence rate of DM. Other differences in the risk of DM between the studies may include host genetics, proportion of males:females, age, BMI, stage of HIV disease, nutritional and behavioral factors, coinfections, comorbidity, and ART adherence [3–6, 15].
Patients receiving efavirenz-containing regimens or LPV/r-containing regimens had a higher risk of DM compared to those receiving zidovudine+lamivudine+nevirapine. Of note, zidovudine+lamivudine+ LPV/r was not associated with a higher risk of DM, most likely because participants receiving this regimen were primarily young women.
We found that ART containing efavirenz increased the risk of developing DM compared to those containing nevirapine. Similar findings were observed in a large multi-country cohort in Africa [12].
A strength of our study was that the analysis of the association between first-line regimens and the risk of DM was performed on a large, nationwide dataset. Another strength was that we used death as a competing event of DM diagnosis, missing data imputation, and propensity scores in order to use as accurate models as possible.
A limitation of our study is that the NAP database, primarily designed for overall monitoring of the program, has missing data or some possibly associated with other outcomes, such as BMI or lipid plasma concentration. However, the NAP database represents a unique source of information that likely reflects the actual DM burden in the HIV-infected population. Also, the lack of systematic FPG assessments in some patients may have led to an underestimation of DM incidence, even though some diagnoses were made because of diabetes-related complications (16.6% of diabetic patients) [8].
In summary, several of the ART regimens recommended by WHO, particularly tenofovir+lamivudine+LPV/r and regimens containing efavirenz, may be associated with an increased risk of DM. All patients who receive these regimens need to be closely monitored for DM.
Supplementary Data
Supplementary materials are available at Open Forum Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Acknowledgments
The authors wish to thank the National Health Security Office of Thailand for providing the data, including Winai Sawasdivorn, Jadej Thammatacharee, Yolsilp Suchonwanich, Sinchai Tawwuttanakidgul, Suchada Chaivooth, Chirod Narkpaichit, Artit Pusamang, Pornpimol Sirimai, Sumitra Daengprasert, Sumalee Hiranmongkholkul, Traithep Fongthong, Kanjana Sirigomon, and Jutatip Thungthong.
Authors’ contributions. N.P., N.K., R.C., and G.J. originally designed the study, which was discussed and reviewed by all co-authors. N.P., N.S., J.M., G.J., and A.T. analyzed the data. N.P., G.J., N.K., S.B., C.B., and R.C. interpreted the analysis results. N.P., N.K., N.S., and G.J. contributed to review the literature. N.P. wrote the first draft of the manuscript, which was commented and edited by all co-authors. G.J. supervised the whole study process. All authors approved the final manuscript of this article prior to submission.
Financial support. None reported.
Potential conflicts of interest. All authors: No reported conflicts of interest. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. World Health Organization Global Report on Diabetes. http://apps.who.int/iris/bitstream/10665/204871/1/9789241565257_eng.pdf. Published 2016. Accessed March 10, 2017. [Google Scholar]
- 2. International Diabetes Federation. IDF Diabetes Atlas—8th Edition. https://diabetesatlas.org/resources/2017-atlas.html. Published 2017. Accessed June 29, 2019. [Google Scholar]
- 3. Jellinger PS, Smith DA, Mehta AE, et al. ; AACE Task Force for Management of Dyslipidemia and Prevention of Atherosclerosis American Association of Clinical Endocrinologists’ guidelines for management of dyslipidemia and prevention of atherosclerosis. Endocr Pract 2012; 18(Suppl 1):1–78. [DOI] [PubMed] [Google Scholar]
- 4. Tripathi A, Liese AD, Jerrell JM, et al. Incidence of diabetes mellitus in a population-based cohort of HIV-infected and non-HIV-infected persons: the impact of clinical and therapeutic factors over time. Diabet Med 2014; 31:1185–93. [DOI] [PubMed] [Google Scholar]
- 5. De Wit S, Sabin CA, Weber R, et al. ; Data Collection on Adverse Events of Anti-HIV Drugs (D:A:D) study Incidence and risk factors for new-onset diabetes in HIV-infected patients: the Data Collection on Adverse Events of Anti-HIV Drugs (D:A:D) study. Diabetes Care 2008; 31:1224–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Capeau J, Bouteloup V, Katlama C, et al. ; ANRS CO8 APROCO-COPILOTE Cohort Study Group Ten-year diabetes incidence in 1046 HIV-infected patients started on a combination antiretroviral treatment. AIDS 2012; 26:303–14. [DOI] [PubMed] [Google Scholar]
- 7. Riyaten P, Salvadori N, Traisathit P, et al. New-onset diabetes and antiretroviral treatments in HIV-infected adults in Thailand. J Acquir Immune Defic Syndr 2015; 69:453–9. [DOI] [PubMed] [Google Scholar]
- 8. Paengsai N, Jourdain G, Chaiwarith R, et al. Incidence and clinical outcomes of diabetes mellitus in HIV-infected adults in Thailand: a retrospective cohort study. BMC Public Health 2018; 18:1079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Putcharoen O, Wattanachanya L, Sophonphan J, et al. ; HIV-NAT 006 team New-onset diabetes in HIV-treated adults: predictors, long-term renal and cardiovascular outcomes. AIDS 2017; 31:1535–43. [DOI] [PubMed] [Google Scholar]
- 10. Spagnuolo V, Galli L, Poli A, et al. Associations of statins and antiretroviral drugs with the onset of type 2 diabetes among HIV-1-infected patients. BMC Infect Dis 2017; 17:43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Santiprabhob J, Tanchaweng S, Maturapat S, et al. Metabolic disorders in HIV-infected adolescents receiving protease inhibitors. Biomed Res Int 2017; 2017:7481597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Karamchand S, Leisegang R, Schomaker M, et al. Risk factors for incident diabetes in a cohort taking first-line nonnucleoside reverse transcriptase inhibitor-based antiretroviral therapy. Medicine (Baltimore) 2016; 95:e2844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. World Health Organization. Consolidated Guidelines on the Use of Antiretroviral Drugs for Treating and Preventing HIV Infection: Recommendations for a Public Health Approach http://www.ncbi.nlm.nih.gov/books/NBK374294/. Published June 2016. Accessed June 24, 2017. [PubMed]
- 14. Bureau of AIDS, TB, and STIs, Department of Disease Control, Ministry of Public Health. Thailand National Guideline on HIV/AIDS Treatment and Prevention July 2017 http://www.thaiaidssociety.org/images/PDF/hiv_thai_guideline_2560.pdf. Accessed March 25, 2017.
- 15. American Diabetes Association. Standards of medical care in diabetes--2013. Diabetes Care 2013; 36(Suppl 1):S11–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. World Health Organization. International Statistical Classification of Diseases and Related Health Problems, 10th revision. 2010 ed.https://www.who.int/classifications/icd/ICD10Volume2_en_2010.pdf. Published 2011. Accessed June 29, 2019.
- 17. Loader C. Local Regression and Likelihood. New York: Springer; 1999. Available at: http://web.ipac.caltech.edu/staff/fmasci/home/astro_refs/LocalRegressionBook_1999.pdf. Accessed April 11, 2017. [Google Scholar]
- 18. Diabetes Association of Thailand, Department of Medical Services Ministry of Public Health, The Endocrine Society of Thailand, National Health Security Office. Clinical Practice Guideline for Diabetes 2014. 1st ed. Bangkok, Thailand: Aroonkarnpim, Ltd; 2014. [Google Scholar]
- 19. Pan WH, Yeh WT. How to define obesity? Evidence-based multiple action points for public awareness, screening, and treatment: an extension of Asian-Pacific recommendations. Asia Pac J Clin Nutr 2008; 17:370–4. [PubMed] [Google Scholar]
- 20. Cefalu M. Pointwise confidence intervals for the covariate-adjusted survivor function in the Cox model. Stata J 2011; 11:64–81. [Google Scholar]
- 21. Barnett A, Graves N. Competing risks models and time-dependent covariates. Crit Care 2008; 12:134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Lim HJ, Zhang X, Dyck R, Osgood N. Methods of competing risks analysis of end-stage renal disease and mortality among people with diabetes. BMC Med Res Methodol 2010; 10:97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Fine JP, Gray RJ. A proportional hazards model for the subdistribution of a competing risk. J Am Stat Assoc 1999; 94:496–509. [Google Scholar]
- 24. Freedman DA. On the so-called “Huber Sandwich Estimator” and “Robust Standard Errors.” Am Stat 2006; 60:299–302. [Google Scholar]
- 25. Azur MJ, Stuart EA, Frangakis C, Leaf PJ. Multiple imputation by chained equations: what is it and how does it work? Int J Methods Psychiatr Res 2011; 20:40–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Kuss O, Blettner M, Börgermann J. Propensity score: an alternative method of analyzing treatment effects. Dtsch Arztebl Int 2016; 113:597–603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Jha AK, Goenka MK, Goenka U. Chronic pancreatitis in Eastern India: Experience from a tertiary care center. Indian J Gastroenterol 2017; 36:131–6. [DOI] [PubMed] [Google Scholar]
- 28. Jiamjarasrangsi W, Aekplakorn W. Incidence and predictors of type 2 diabetes among professional and office workers in Bangkok, Thailand. J Med Assoc Thai 2005; 88:1896–904. [PubMed] [Google Scholar]
- 29. Jiamjarasrangsi W, Lohsoonthorn V, Lertmaharit S, Sangwatanaroj S. Incidence and predictors of abnormal fasting plasma glucose among the university hospital employees in Thailand. Diabetes Res Clin Pract 2008; 79:343–9. [DOI] [PubMed] [Google Scholar]
- 30. Worm SW, De Wit S, Weber R, et al. Diabetes mellitus, preexisting coronary heart disease, and the risk of subsequent coronary heart disease events in patients infected with human immunodeficiency virus: the Data Collection on Adverse Events of Anti-HIV Drugs (D:A:D Study). Circulation 2009; 119:805–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Ledergerber B, Furrer H, Rickenbach M, et al. ; Swiss HIV Cohort Study Factors associated with the incidence of type 2 diabetes mellitus in HIV-infected participants in the Swiss HIV Cohort Study. Clin Infect Dis 2007; 45:111–9. [DOI] [PubMed] [Google Scholar]
- 32. Brown TT, Cole SR, Li X, et al. Antiretroviral therapy and the prevalence and incidence of diabetes mellitus in the multicenter AIDS cohort study. Arch Intern Med 2005; 165:1179–84. [DOI] [PubMed] [Google Scholar]
- 33. Tien PC, Schneider MF, Cole SR, et al. Antiretroviral therapy exposure and incidence of diabetes mellitus in the Women’s Interagency HIV Study. AIDS 2007; 21:1739–45. [DOI] [PubMed] [Google Scholar]
- 34. Lo YC, Chen MY, Sheng WH, et al. Risk factors for incident diabetes mellitus among HIV-infected patients receiving combination antiretroviral therapy in Taiwan: a case-control study. HIV Med 2009; 10:302–9. [DOI] [PubMed] [Google Scholar]
- 35. Nansseu JR, Bigna JJ, Kaze AD, Noubiap JJ. Incidence and risk factors for prediabetes and diabetes mellitus among HIV-infected adults on antiretroviral therapy: a systematic review and meta-analysis. Epidemiology 2018; 29: 431–41. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.