Abstract
Study Objectives:
Although respiratory abnormalities occurring during wakefulness are well recognized in patients with Rett syndrome (RS), less has been reported regarding sleep-disordered breathing (SDB) in this population. This study aims to characterize the presenting complaints, types and severity of SDB, and treatment modalities of patients with RS and sleep concerns.
Methods:
Retrospective chart review of pediatric patients with RS referred to our academic tertiary care institution from January 2007 to July 2017.
Results:
Thirteen patients were identified, 11 female (84.6%); mean age at polysomnography (PSG) was 10.3 years (standard deviation 4.94). Eleven were white (84.6%), 2 were black (15.4%). The most common presenting symptoms were snoring (10/13, 77%) and witnessed apnea (7/13, 53.8%). On baseline PSG, all patients (100%) exhibited hyperapneas followed by a central apnea during wake. Nine (69.2%) had obstructive sleep apnea (OSA) (obstructive apnea-hypopnea index (oAHI) > 1); four had severe OSA (oAHI ≥ 10). One had central sleep apnea (central apnea index > 5) and severe OSA. No patients exhibited hypoventilation on baseline PSG. Mean AHI of all patients was 8.77 ± 8.82 (oAHI 6.51 ± 6.91) events/h. Mean oxyhemoglobin nadir was 88.52 ± 5.6%. Treatment modalities included observation: 5 (38%), acetazolamide: 2 (15%), nasal mometasone: 1 (7.7%), adenotonsillectomy: 3 (23.1%), and positive airway pressure: 2 (15%).
Conclusions:
Regarding patients with RS referred to the sleep medicine clinic, snoring and witnessed apneas were the most common presenting complaints. In addition to breathing abnormalities during wake, OSA was very common in our cohort. Further studies are needed to examine the pathogenesis of OSA in RS and relationships between disease genotype and respiratory abnormality phenotype.
Citation:
Sarber KM, Howard JJM, Dye TJ, Pascoe JE, Simakajornboon N. Sleep-disordered breathing in pediatric patients with rett syndrome. J Clin Sleep Med. 2019;15(10):1451–1457.
Keywords: central sleep apnea, obstructive sleep apnea, pediatrics, polysomnography, primary snoring, Rett syndrome, sleep-disordered breathing
BRIEF SUMMARY
Current Knowledge/Study Rationale: Literature describing breathing abnormalities during wakefulness in Rett syndrome (RS) has existed since it was first identified in 1966. Since then, small series have described sleep-disordered breathing (SDB) abnormalities in RS including central and obstructive sleep apnea. The literature describing symptoms of SDB and subsequent treatment are lacking.
Study Impact: Our study reviews our institution’s cases and discusses the presenting symptoms, baseline polysomnography results, and subsequent treatment for patients with RS and SDB treated in the past 10 years. Our findings add to the growing body of literature on the clinical presentation and the types of SDB seen in patients with RS and provide our experience with treatment in this population.
INTRODUCTION
Pediatric obstructive sleep apnea (OSA) is a common condition, affecting approximately 2% to 6% of children.1–3 OSA is characterized by airway obstruction during sleep, despite active respiratory effort, causing blood oxygen desaturation and sleep disruption. In most children, narrowing of the airway due to hypertrophy of the tonsillar and adenoid lymphatic tissue plays a predominant role in OSA pathogenesis. Variations in craniofacial anatomy and neuromuscular tone have also been linked to OSA in children.4,5
Rett syndrome (RS) is a postnatal progressive neurodegenerative disease affecting 1:10,000 live births. After Down syndrome, it is the most prevalent cause of severe cognitive impairment in females.6 Occurring almost exclusively in females, it is characterized by largely normal development until 6 to 18 months of age, followed by a rapid regression of skills and developmental milestones.7 Most cases result from mutations in the gene encoding methyl-CpG binding protein 2 (MeCP2), causing a constellation of various neurologic symptoms. With a median age at diagnosis of 3.5 years, clinical presentations vary widely; however some of the most common symptoms include loss of purposeful hand movements, seizures, spasticity, growth retardation, loss of speech, stereotyped movements (ie hand wringing), balance and coordination difficulties, and breathing problems.8,9
Patients with RS were initially described in the literature as having breathing abnormalities during wakefulness. These daytime respiratory anomalies are the result of autonomic dysfunction and include hyperventilation followed by prolonged apneic spells, air swallowing, and/or Valsalva maneuvers.7,10 Prior studies of children with RS using polysomnography (PSG) have largely focused on electroencephalography (EEG) abnormalities, cardiorespiratory function, and sleep architecture.11–15 Young et al. found that 77% of their large population-based cohort of patients with RS experienced frequent daytime napping. Napping frequency was associated with increased severity of other symptoms.13 Other commonly reported sleep problems include nighttime laughing, bruxism, screaming, and seizures.16 There are currently no large studies evaluating the incidence, prevalence, or treatment of sleep-disordered breathing (SDB) in children with RS, and there is only one case report that describes polysomnographic changes before and after intervention.17
To our knowledge, no studies have assessed presenting sleep complaints with corresponding PSG and treatment in a cohort of patients with RS. The aim of our study was to investigate our population of patients with RS and review their presenting symptoms, polysomnography results, and treatment to better understand the nighttime respiratory abnormalities in this rare disease.
METHODS
A retrospective review of medical records and PSGs was performed in all patients with a diagnosis of RS, Rett disorder, atypical RS, duplication of MeCP2 gene, MeCP2 duplication syndrome, or mutation in MeCP2 gene. Patients who underwent diagnostic PSG at Cincinnati Children's Hospital Medical Center (CCHMC) from January 2007 to July 2017 were included. Additional inclusion criteria were age younger than 18 years at the time of the initial PSG and evaluation at the Sleep Disorders Center or with an inpatient pediatric pulmonary consult within 6 months of the PSG date. The study was approved by the Institutional Review Board at CCHMC.
PSG was performed using the Grass system at CCHMC (Grass Telefactor, West Warwick, Rhode Island, USA). The standard pediatric montage was used and the following parameters were recorded simultaneously: bilateral electrooculogram, EEG (C3A2, C4A1, O1A2, O2A1), chin electromyography (EMG), anterior tibialis EMG, tracheal microphone, electrocardiography, pulse oximetry and pulse waveform (Masimo Corporation, Irvine, California), thoracic and abdominal inductance plethysmography, nasal thermistor, nasal pressure transducer, and end-tidal capnography (ETCO2). PSG was performed in accordance with the American Academy of Sleep Medicine (AASM) guidelines at the time of interpretation (2007–2017).18,19 The record was scored by a registered sleep technologist and reviewed and interpreted by a board-certified sleep physician. Arousals were defined as an abrupt change in EEG frequency for at least 3 seconds, with arousals in stage R sleep also being accompanied by a change in submental or anterior tibialis EMG activity as recommended by the American Sleep Disorders Association Task Force report. Respiratory scoring followed the AASM guidelines that were in place at the time the study was performed. Apneas were defined as a drop in the baseline airflow signal ≥ 90% for the duration dictated by the type of apnea. Obstructive apneas were defined by the absence of nasal airflow in the presence of continued respiratory effort lasting ≥ 2 respiratory cycles. Central apneas were defined by the absence of both nasal airflow and respiratory effort lasting more than 20 seconds or ≥ 2 breaths associated with a ≥ 3% arterial oxygen desaturation, arousal, or awakening. Mixed apneas were scored if the event lasted for at least two breaths and was associated with both absence and presence of respiratory effort during the event. Hypopneas were defined as a decrease in airflow signal of at least 50% (2007–2012) or 30% (2012–2017) for at least two breath cycles, with a corresponding decrease in arterial oxygen saturation of ≥ 3%, an arousal, or both.18,19 Apnea-hypopnea index (AHI) was defined as the number of apneas and hypopneas per hour of sleep time. Obstructive AHI (oAHI) was defined as the number of obstructive and mixed apneas and hypopneas per hour of sleep. OSA in this study was defined as an oAHI of 1 event/h.
Our data collection included patient demographics, medical diagnoses, presenting clinical features, PSG results, and information on patients’ subsequent clinic visits and PSGs. Descriptive statistics were calculated for key demographic and PSG variables. Means and standard deviations (SD) are reported for continuous variables and frequencies and percentages are reported for categorical variables.
RESULTS
Demographics and Symptoms
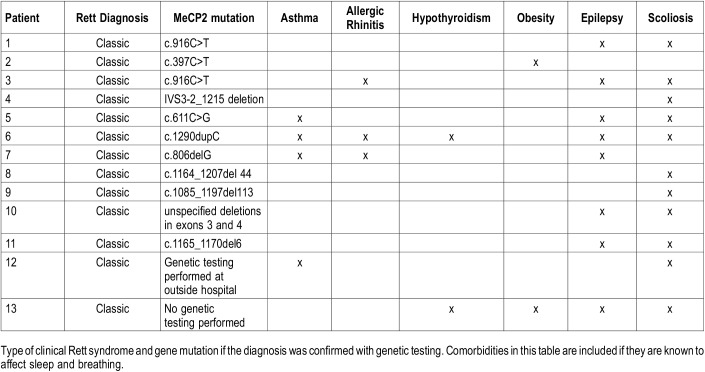
One hundred ninety-two patients with RS were treated at the CCHMC between January 2007 and July 2017. Nineteen patients underwent PSG, but two sleep studies were normal and those patients were not evaluated by a pulmonary or sleep physician. Four patients were adults. Thirteen patients met all inclusion criteria, 11 female (84.6%) and 2 male (15.4%). Age ranged from 2.6 to 17.4 years (mean ± SD 9.09 ± 4.94) at time of initial PSG. Eleven patients were white non-Hispanic (84.6%) and 2 were black non-Hispanic (15.4%). All but one of the patients had confirmation of classic RS diagnosis by genetic evaluation. Nine patients had known mutations and two patients had novel mutations in the MeCP2 gene at the time the screening was performed. Table 1 lists the type of RS and gene mutation for each patient.
Table 1.
Diagnosis, gene mutation, and comorbidities for 13 patients evaluated and treated for sleep complaints.
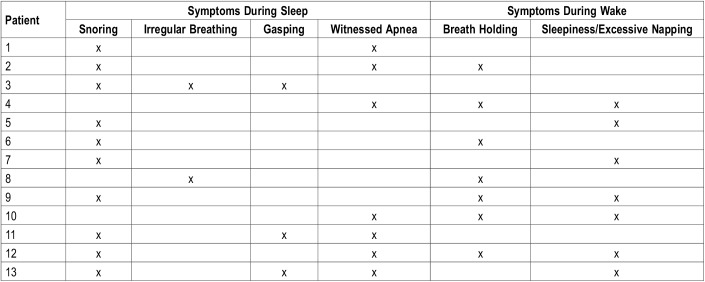
There were several comorbidities that affect sleep and breathing. The most common comorbidity was scoliosis (11/13, 84.6%), followed by epilepsy (8/13, 61.5%), asthma (4/13, 30.8%), seasonal allergic rhinitis (3/13, 23.1%), obesity (2/13, 15.4%) and hypothyroidism (2/13, 15.4%). Table 1 describes the comorbidities for each patient. The most common presenting sleep symptom was snoring (10/13, 76.9%), followed by witnessed apnea (7/13, 53.8%), excessive daytime sleepiness/excessive napping (7/13, 53.8%), irregular breathing (2/13, 15.4%), and gasping (2/13, 15.4%). Three patients also had insomnia (23.1%) and 5/13 patients had sleep-related bruxism (38.5%). The only reported breathing abnormality during wakefulness was breath holding (7/13, 53.8%). Table 2 summarizes each patient and their presenting self-reported complaints.
Table 2.
Presenting complaints of patients with Rett syndrome that were referred to the sleep medicine clinic/pulmonary consult service.
PSG Results
Sleep Architecture
Mean ± SD sleep latency was 29.67 ± 28.08 minutes. Mean sleep efficiency was 70 ± 19% and mean stage R sleep latency was 141.25 ± 70.5 minutes. The mean arousal index was 11.52 ± 7.15 events/h. All patients had a periodic limb movement index of zero. Baseline sleep architecture showed mean stage: N1 4% (range 1% to 8.8%), N2 44% (range 24% to 63%), N3 32% (range 3% to 58%), R 17% (range zero to 34%).
Respiratory Values
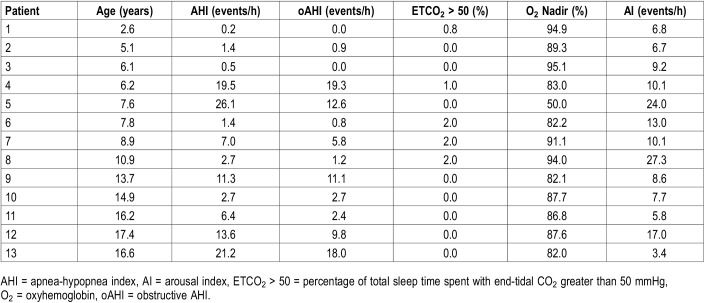
Baseline PSG findings demonstrated irregular breathing during wake with hyperventilation followed by central apnea in all patients. Respiratory findings during sleep showed that 9/13 (69.2% of cohort) had OSA with oAHI > 1 events/h; 3 (23.1%) had mild OSA (oAHI 1 to 4.99 events/h), 2 (15.4%) had moderate OSA (oAHI 5 to 9.9 events/h), and 4 (30.8%) had severe OSA (oAHI ≥ 10 events/h). One patient (7.7%) had central sleep apnea (CSA; central apnea index > 5 events/h) concurrently with severe OSA. The mean AHI was 8.77 ± 8.82 events/h (oAHI 6.51 ± 6.91 events/h). No patients demonstrated alveolar hypoventilation; mean ETCO2 was 41.1 mmHg (range 32–46) with mean maximum ETCO2 48.8 mmHg (range 41.6–53.0). The mean oxyhemoglobin nadir was 88.52 ± 5.6%. Table 3 outlines baseline PSG results by patient.
Table 3.
Baseline polysomnography values for all patients in the cohort.
Evaluation, Treatment, and Follow-Up
Treatment of OSA ranged from observation with clinical follow-up to surgical evaluation depending on the disease severity, age, and consideration of other comorbidities. Patients who also experienced recurrent upper respiratory infections were further evaluated with bronchoscopy to determine the etiology of these infections. To treat central apnea, patients were prescribed acetazolamide, a carbonic anhydrase inhibitor that shifts the hypercapnic ventilatory response and lowers the partial pressure of carbon dioxide apnea threshold to drive ventilation.20 Table 4 summarizes the treatment recommendations and follow-up PSG results for each patient. A discussion of treatments for each patient in detail follows in the next paragraphs.
Table 4.
Treatment recommendations and posttreatment polysomnography data if performed.
Bronchoscopy
Two of the 13 patients underwent bronchoscopy for further evaluation of recurrent upper respiratory infection. Both of these patients had severe OSA with components of both central and obstructive events. Both had normal anatomy with focal bronchomalacia. Bronchoalveolar lavage results showed an inflammatory profile in one and oral flora in one. Neither patient had positive cultures.
Observation
Five patients were followed clinically without specific treatment: three with primary snoring and two with mild OSA. In patients 1, 2, and 6, primary snoring was diagnosed at the first PSG and they were observed. None of these patients displayed excessive sleepiness or napping. Patients 1 and 6 underwent repeat PSG. Patient 1 had primary snoring on PSG that was repeated after 1 year (for continued concern for apnea) and observation was continued. Patient 6 had moderate OSA on PSG 1.9 years later that was repeated for wheezing and frequent nighttime awakenings. It showed an oAHI of 5.3 with significant oxyhemoglobin desaturations. He was subsequently treated with supplemental oxygen to treat both OSA and hypoxia secondary to reactive airway disease and recurrent pneumonias. Patient 2 has followed up clinically with no new respiratory concerns. Mild OSA was diagnosed in patients 8 and 11. Patient 8 underwent repeat PSG 6 years later when she presented to the otolaryngology clinic with loud nighttime breathing, new daytime sleepiness, and worsening apneas. PSG results showed severe OSA with AHI 28.6 and oAHI 20.4; she ultimately underwent adenotonsillectomy. Patient 11 has a scheduled follow-up in the near future.
Acetazolamide
Two patients were treated with oral acetazolamide. For these patients, acetazolamide was started at 5 mg/kg/dose given in the morning. A baseline serum bicarbonate level was obtained and monitored weekly with a goal bicarbonate level close to 15 mEq/L; medication was titrated if necessary until levels and dose were stable. Patient 12 complained of snoring and witnessed apneas, daytime breath holding and excessive napping. Although her oAHI was 18 events/h and her AHI was 21.2 events/h most of her events were scored as hypopneas that were thought to have a significant central component. She did not require further titration of medication. PSG was repeated 17 months later and demonstrated resolution of both OSA and CSA. She was no longer napping at her follow-up sleep study. The family moved away and has not followed up. Patient 3 was treated with acetazolamide due to daytime breath holding spells, nighttime witnessed apnea, and PSG showing periodic breathing with clustered central respiratory pauses that did not qualify for significant central apnea. Her dose was increased to 8 mg/kg/dose (to round to 250-mg dose) after 1 week as her serum bicarbonate did not change significantly (26 to 23 mEq/L). The 250-mg dose decreased bicarbonate to 19 mEq/L and this remained stable on the next blood draw 1 week later. Subjectively, patients’ daytime and nighttime breathing abnormalities resolved. She was followed clinically, and acetazolamide was stopped after 3 years.
Nasal Mometasone
One patient (patient 10) was treated with nasal mometasone spray for mild OSA. A repeat PSG 3 years later revealed no change in AHI although the mometasone was only used for 1 year, after which time the family discontinued treatment due to improvement in snoring and reduction of daytime naps. Because of the patient’s age (almost 18 years) and lack of symptoms, she was subsequently observed.
Adenotonsillectomy
Three patients underwent adenotonsillectomy for treatment of OSA. Patients 4, 7, and 9 underwent adenotonsillectomy for moderate or severe OSA with a mean AHI 12.6 events/h, oAHI 12.07 events/h. Postoperative PSG approximately 3 months following surgery showed mean AHI was 2.9 events/h and oAHI was 0.47 events/h. All patients were symptomatically improved (improvement or resolution of snoring and heavy breathing). A borderline mild degree of OSA persisted for patient 4 who was subsequently observed, whereas the oAHI of patients 7 and 9 normalized.
Continuous Positive Airway Pressure
Continuous positive airway pressure (CPAP) was recommended for treatment in two patients for severe OSA. Patient 5 did not tolerate CPAP titration or mask desensitization and was ultimately treated with supplemental oxygen. The repeat PSG with supplemental oxygen titration showed improvement in AHI to 2.1 events/h. Four months after starting supplemental oxygen, the patient’s Epworth Sleepiness Scale score improved from 24 to 15. Therefore, the patient was managed with 0.25 L/min of oxygen during sleep. Patient 13 was lost to follow-up and never underwent CPAP titration.
DISCUSSION
Our cohort of patients with RS who have undergone PSG for concerns of SDB revealed a high incidence of OSA; only one patient had comorbid CSA. All patients had breath holding noted during wakefulness on PSG. Presenting symptoms observed during sleep were similar to those in other children with SDB, with the addition of commonly noted daytime breath-holding spells.
A reduced sleep efficiency and increased arousal index are consistent with previous reports of patients with RS.11,13,18 Our patients also demonstrated a lower percentage of stage R sleep and a higher percentage of stage N3 sleep, a finding that has also been reported in other studies of RS.19,21 Treatment followed similar pathways as that for patients who are nonsyndromic with the exception of acetazolamide (usually reserved for significantly higher CSA). Adenotonsillectomy was highly successful in three patients who underwent the procedure for moderate to severe OSA. Due to the retrospective nature of the study, it was difficult to identify improvement in OSA-related comorbidities, but the patient did experience improvements in OSA-specific complaints of snoring, witnessed apneas, and heavy breathing.
Compared to healthy control patients, patients with RS have more irregular breathing and increased respiratory rate. Although wakeful breathing abnormalities in patients with RS have been well described and documented in the literature, SDB has only recently been documented. In 1995, a case report described two female patients with RS who had repeated episodes of hyperventilation followed by “respiratory arrest” on polygraphic (sic) sleep recording.22 Marcus et al published a case series of 30 patients with RS but did not find significant sleep breathing abnormalities overall. The speculation was that breathing abnormalities that were seen during wakefulness were generally less severe during sleep and were not clinically significant.19 In 2007 and 2008 two institutions evaluated heart rate and respiratory characterizations during several nights of sleep in the home environment using two different proprietary devices.14,15 Both studies noted recurrent episodes consisting of apnea, hypoventilation, or reduced tidal volume that varied from night to night and between patients. Both studies hypothesized that these episodes were secondary to abnormal central control of breathing.
More recently, OSA has been implicated as the etiology of some of the irregular breathing patterns noted on earlier studies. Hagebeuk et al. reported on 12 patients with RS and sleep complaints and found 10 of the 12 patients had an AHI > 1 event/h, with both central and obstructive events in most patients, and one-fourth of patients with severe OSA.20 Our study results closely resemble these. A study of 13 patients with advanced RS found abnormality of all respiratory parameters on PSG: notably, a mean oAHI of 5.67 events/h and central apnea index of 7.78 events/h.12 There was variability in the degrees of CSA and OSA in these patients and the authors hypothesized that perhaps the abnormalities change with age. In this study, respiratory events were scored according to the 1996 American Thoracic Society scoring guidelines.23 A central apnea was scored when there was a cessation of airflow and effort for two breath cycles; this definition is broader than the AASM scoring rules and likely accounts for the substantially higher central apnea index compared to our study. In both of these studies, the median age of children with RS was 8 years. Our results parallel these studies, with a similar mean age (10 years of age in our study) and a high incidence of OSA with high variability in severity in patients with RS and respiratory complaints.
Animal models may explain the wide spectrum of SDB seen in patients with RS. Mouse models with MeCP2 knockout gene only in embryonic neurons have found erratic breathing and apneas with abnormal postinspiratory bulbar discharge and spontaneous fluctuations in postinspiratory motor output to laryngeal adductor muscles, suggesting a central component that leads to peripheral upper airway instability.24 This phenomenon could also explain other glottic control dysfunction in patients with RS, including the loss of speech and impaired swallowing. However, it should be noted that the respiratory phenotype of RS appears to be varied and extremely complex, which suggests that the cardiorespiratory dysfunction likely does not result from a single mechanism.
Our study has several limitations. First, our cohort represents a referred population of patients with RS. Approximately 10% of patients with RS at our institution were referred to the sleep laboratory. Although it provides practical information on the evaluation and management of SDB encountered by sleep providers, the findings from this study may not be applicable to a general population of RS. Second, due to retrospective design, the data on treatment outcomes (including impact on comorbidities) are not consistently available or not clear from the medical records. Although previous studies have linked improved control of SDB with better outcome on comorbidities such as seizure control,25,26 we are unable to provide this information. Finally, no general paradigm may be drawn from the management of these patients due to the small sample size and variability of type and severity of SDB in our cohort. However, we hope the detailed individual data may add to the growing body of literature for this rare syndrome.
CONCLUSIONS
In our cohort of 13 patients with RS referred for sleep evaluation, snoring and witnessed apneas were the most common presenting complaints noted during sleep. PSG showed that all patients exhibited breathing abnormalities during wakefulness. SDB was very common, most frequently OSA. CSA and hypoventilation were rare in our cohort. Findings of decreased sleep efficiency and increased arousal index are consistent with prior reports. Treatment was similar to that of other patients with SDB.
It is evident from this report and review of the literature that children with RS and sleep and breathing complaints have a high incidence of SDB. It is possible that their breathing abnormalities evolve over time, making continued follow-up of these patients imperative. Alternatively, the heterogeneous genotype of the disease may account for the varied reports of respiratory abnormalities. Our experience has been to tailor treatment individually and repeat evaluation with PSG for changes in the clinical picture. Further studies, likely multi-institutional, are needed to examine how respiratory control abnormalities and anatomical obstruction play a role in pathogenesis of OSA in this population and how the genotype of the disease affects the phenotype of the clinical presentation.
DISCLOSURE STATEMENT
All authors have seen and approved the manuscript. This research was funded by the Cincinnati Children’s Hospital Research Fund. Parts of this manuscript were presented at the 32nd Annual Meeting of the Associated Professional Sleep Societies, Baltimore, Maryland. The authors report no conflicts of interest.
ABBREVIATIONS
- AASM
American Academy of Sleep Medicine
- AHI
apnea-hypopnea index
- CSA
central sleep apnea
- CCHMC
Cincinnati Childrens Hospital Medical Center
- CPAP
continuous positive airway pressure
- EEG
electroencephalography
- EMG
electromyography
- ETCO2
end-tidal carbon dioxide
- MeCP2
methyl-CpG binding protein 2
- oAHI
obstructive apnea-hypopnea index
- OSA
obstructive sleep apnea
- PaCO2
partial pressure of carbon dioxide
- PSG
polysomnography
- RS
Rett syndrome
- SD
standard deviation
- SDB
sleep-disordered breathing
REFERENCES
- 1.Bixler EO, Vgontzas AN, Lin HM, et al. Sleep disordered breathing in children in a general population sample: prevalence and risk factors. Sleep. 2009;32(6):731–736. doi: 10.1093/sleep/32.6.731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gislason T, Benediktsdottir B. Snoring, apneic episodes, and nocturnal hypoxemia among children 6 months to 6 years old. An epidemiologic study of lower limit of prevalence. Chest. 1995;107(4):963–966. doi: 10.1378/chest.107.4.963. [DOI] [PubMed] [Google Scholar]
- 3.Redline S, Tishler PV, Schluchter M, Aylor J, Clark K, Graham G. Risk factors for sleep-disordered breathing in children. Associations with obesity, race, and respiratory problems. Am J Respir Crit Care Med. 1999;159(5 Pt 1):1527–1532. doi: 10.1164/ajrccm.159.5.9809079. [DOI] [PubMed] [Google Scholar]
- 4.Arens R, Marcus CL. Pathophysiology of upper airway obstruction: a developmental perspective. Sleep. 2004;27(5):997–1019. doi: 10.1093/sleep/27.5.997. [DOI] [PubMed] [Google Scholar]
- 5.Katz ES, D’Ambrosio CM. Pathophysiology of pediatric obstructive sleep apnea. Proc Am Thora Soc. 2008;5(2):253–262. doi: 10.1513/pats.200707-111MG. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ellaway C, Christodoulou J. Rett syndrome: clinical characteristics and recent genetic advances. Disabil Rehabil. 2001;23(3-4):98–106. doi: 10.1080/09638280150504171. [DOI] [PubMed] [Google Scholar]
- 7.Chahrour M, Zoghbi HY. The story of Rett syndrome: from clinic to neurobiology. Neuron. 2007;56(3):422–437. doi: 10.1016/j.neuron.2007.10.001. [DOI] [PubMed] [Google Scholar]
- 8.Einspieler C, Kerr AM, Prechtl HF. Is the early development of girls with Rett disorder really normal? Pediatr Res. 2005;57(5 Pt 1):696–700. doi: 10.1203/01.PDR.0000155945.94249.0A. [DOI] [PubMed] [Google Scholar]
- 9.Hagberg B, Hanefeld F, Percy A, Skjeldal O. An update on clinically applicable diagnostic criteria in Rett syndrome. Comments to Rett Syndrome Clinical Criteria Consensus Panel Satellite to European Paediatric Neurology Society Meeting, Baden Baden, Germany, 11 September 2001. Eur J Paediatr Neurol. 2002;6(5):293–297. doi: 10.1053/ejpn.2002.0612. [DOI] [PubMed] [Google Scholar]
- 10.Southall DP, Kerr AM, Tirosh E, Amos P, Lang MH, Stephenson JB. Hyperventilation in the awake state: potentially treatable component of Rett syndrome. Arch Dis Child. 1988;63(9):1039–1048. doi: 10.1136/adc.63.9.1039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.McArthur AJ, Budden SS. Sleep dysfunction in Rett syndrome: a trial of exogenous melatonin treatment. Dev Med Child Neurol. 1998;40(3):186–192. doi: 10.1111/j.1469-8749.1998.tb15445.x. [DOI] [PubMed] [Google Scholar]
- 12.Carotenuto M, Esposito M, D’Aniello A, et al. Polysomnographic findings in Rett syndrome: a case-control study. Sleep Breath. 2013;17(1):93–98. doi: 10.1007/s11325-012-0654-x. [DOI] [PubMed] [Google Scholar]
- 13.Young D, Nagarajan L, de Klerk N, Jacoby P, Ellaway C, Leonard H. Sleep problems in Rett syndrome. Brain Dev. 2007;29(10):609–616. doi: 10.1016/j.braindev.2007.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Weese-Mayer DE, Lieske SP, Boothby CM, Kenny AS, Bennett HL, Ramirez JM. Autonomic dysregulation in young girls with Rett Syndrome during nighttime in-home recordings. Pediatr Pulmonol. 2008;43(11):1045–1060. doi: 10.1002/ppul.20866. [DOI] [PubMed] [Google Scholar]
- 15.Rohdin M, Fernell E, Eriksson M, Albage M, Lagercrantz H, Katz-Salamon M. Disturbances in cardiorespiratory function during day and night in Rett syndrome. Pediatr Neurol. 2007;37(5):338–344. doi: 10.1016/j.pediatrneurol.2007.06.009. [DOI] [PubMed] [Google Scholar]
- 16.Mount RH, Hastings RP, Reilly S, Cass H, Charman T. Behavioural and emotional features in Rett syndrome. Disabil Rehabil. 2001;23(3-4):129–138. doi: 10.1080/09638280150504207. [DOI] [PubMed] [Google Scholar]
- 17.Bassett E, Heinle R, Johnston D. Sleep apnea in patients with rett syndrome: roles for polysomnography and adenotonsillectomy. J Child Neurol. 2016;31(14):1633–1634. doi: 10.1177/0883073816671439. [DOI] [PubMed] [Google Scholar]
- 18.Schluter B, Aguigah G, Buschatz D, Trowitzsch E, Aksu F. Polysomnographic recordings of respiratory disturbances in Rett syndrome. J Sleep Res. 1995;4(S1):203–207. doi: 10.1111/j.1365-2869.1995.tb00216.x. [DOI] [PubMed] [Google Scholar]
- 19.Marcus CL, Carroll JL, McColley SA, et al. Polysomnographic characteristics of patients with Rett syndrome. J Pediatr. 1994;125(2):218–224. doi: 10.1016/s0022-3476(94)70196-2. [DOI] [PubMed] [Google Scholar]
- 20.Hagebeuk EE, Bijlmer RP, Koelman JH, Poll-The BT. Respiratory disturbances in rett syndrome: don’t forget to evaluate upper airway obstruction. J Child Neurol. 2012;27(7):888–892. doi: 10.1177/0883073811429859. [DOI] [PubMed] [Google Scholar]
- 21.Carotenuto M, Esposito M, D'Aniello A, et al. Polysomnographic findings in Rett syndrome: A case-control study. Sleep Breath. 2013;17(1):93–98. doi: 10.1007/s11325-012-0654-x. [DOI] [PubMed] [Google Scholar]
- 22.Iber C, Ancoli-Israel S, Chesson AL, Jr, Quan SF, for the American Academy of Sleep Medicine . The AASM Manual for the Scoring of Sleep and Associated Events: Rules, Terminology, and Technical Specifications. 1st ed. Westchester, IL: American Academy of Sleep Medicine; 2007; [Google Scholar]
- 23.Standards and indications for cardiopulmonary sleep studies in children. American Thoracic Society. Am J Respir Crit Care Med. 1996;153(2):866–878. doi: 10.1164/ajrccm.153.2.8564147. [DOI] [PubMed] [Google Scholar]
- 24.Katz DM, Dutschmann M, Ramirez JM, Hilaire G. Breathing disorders in Rett syndrome: progressive neurochemical dysfunction in the respiratory network after birth. Respir Physiol Neurobiol. 2009;168(1-2):101–108. doi: 10.1016/j.resp.2009.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Vaughn BV, D’Cruz OF, Beach R, Messenheimer JA. Improvement of epileptic seizure control with treatment of obstructive sleep apnoea. Seizure. 1996;5(1):73–78. doi: 10.1016/s1059-1311(96)80066-5. [DOI] [PubMed] [Google Scholar]
- 26.Malow BA, Weatherwax KJ, Chervin RD, et al. Identification and treatment of obstructive sleep apnea in adults and children with epilepsy: a prospective pilot study. Sleep Med. 2003;4(6):509–515. doi: 10.1016/j.sleep.2003.06.004. [DOI] [PubMed] [Google Scholar]