Abstract
The effect of hormone therapy on sleep-disordered breathing in transgender patients has not been described. We present three cases of patients undergoing gender reassignment and treated with hormone replacement. The first case was a transgender woman (assigned male at birth) with a prolonged history of severe obstructive sleep apnea (OSA) that resolved following initiation of female sex hormones. The second and third cases both address transgender males (assigned female at birth) in whom OSA developed following initiation of male sex hormones (with pretreatment polysomnography documenting absence of OSA). The growing interest in transgender health warrants further evaluation of the effects of related therapies on sleep and sleep-disordered breathing.
Citation:
Robertson BD, Lerner BS, Collen JF, Smith PR. The effects of transgender hormone therapy on sleep and breathing: a case series. J Clin Sleep Med. 2019;15(10):1529–1533.
INTRODUCTION
Obstructive sleep apnea (OSA) is a common sleep disorder characterized by repetitive collapse of the upper airway, disrupting airflow with resultant hypoxemia and arousals from sleep. Several studies have assessed whether sex hormones in usual doses for testosterone replacement (men) or menopause (women) affect sleep-disordered breathing,1–3 but the results have been inconclusive. Concerns have arisen that testosterone replacement can cause or worsen OSA based primarily on observational data. Although progesterone is a respiratory stimulant, it has not proven effective as a medical therapy for OSA.4 When exogenous sex hormones are administered for gender reassignment, there is no published evidence about possible side effects on sleep-disordered breathing. We present three cases of transgender patients evaluated for sleep-disordered breathing with polysomnography (PSG) pre and post initiation of hormone therapy.
REPORT OF CASES
Case 1
A 47-year-old transgender woman (assigned male at birth) with a prolonged history of OSA was referred to our center for treatment. Moderate OSA was originally diagnosed in this patient in 2008 via in-laboratory, attended, level 1 polysomnography (PSG,) with an apnea-hypopnea index (AHI) of 18 events/h. She was subsequently treated with uvulopalatopharyngoplasty with initial improvement in sleep-disordered breathing symptoms. Over time her sleep worsened and repeat PSG in February 2014 showed an AHI of 46.8 events/h. Her neck circumference was 44.5 cm and the patient’s body mass index (BMI) was 30 kg/m2 at that time. Unfortunately, she was lost to follow-up until December 2015. The patient was once again evaluated for excessive daytime somnolence and snoring, with repeat level 1 PSG once again confirming her diagnosis (AHI 52 events/h, oxygen saturation nadir 84%). On this study, the rapid eye movement (REM) AHI was 33 events/h, and the non-rapid eye movement (NREM) AHI was 60.9 events/h. Her neck circumference was unchanged at 44.5 cm and the BMI was decreased slightly to 28.7 kg/m2. Treatment with continuous positive airway pressure (CPAP) therapy was prescribed, but poorly tolerated. In September 2016, the patient continued to be nonadherent with CPAP therapy. Digital tracking demonstrated that she used her CPAP device on 57% of all nights in the prior 30 days, averaging 2.75 hours of use when the device was worn. During that encounter, the patient disclosed that she had been placed on transdermal estradiol and spironolactone 5 months earlier, as part of the gender transition process. The patient was not treated with progesterone at any time. Her serum testosterone had responded as expected with treatment, decreasing from 482 to 3.4 ng/dL over the previous 5 months. At the time of the encounter, the patient had lost a modest amount of body weight (BMI reduced from 28.1 to 25.4 kg/m2, or less than 10%). At that time, she denied snoring or daytime sleepiness, and repeat level 1 PSG demonstrated complete resolution of her OSA, with an AHI of 0.4 events/h and an oxygen saturation nadir of 95%. The REM AHI was 2.4 events/h and the NREM AHI was 0.0 events/h. Given the patient’s intolerance to CPAP and lack of daytime sleepiness and snoring, she was relieved to hear that she no longer needed treatment for her sleep-related breathing disorder.
Case 2
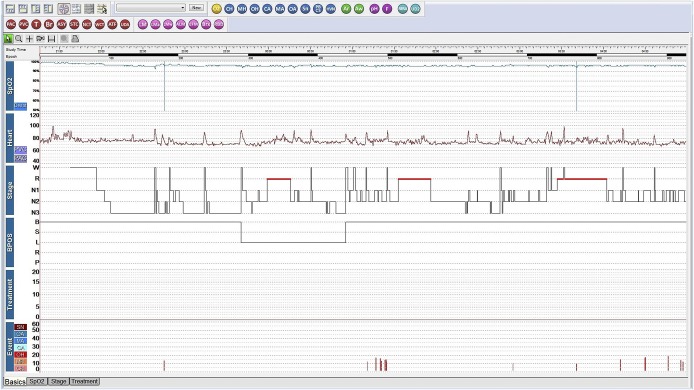
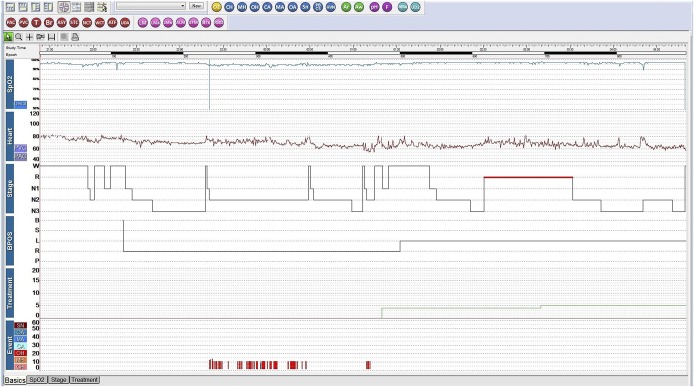
A 17-year-old transgender man (assigned female at birth) presented for snoring and daytime sleepiness (Epworth Sleepiness Scale [ESS] score 13). His past medical history included hypothyroidism, polycystic ovary syndrome (PCOS), obesity, anxiety, depression, and attention deficit hyperactivity disorder. He was taking fluoxetine, methylphenidate, levothyroxine, oral contraceptives, and aripiprazole, but was not on testosterone at the time of our initial evaluation. The initial level 1 PSG was unremarkable with an AHI of 2 events/h, with a REM AHI of 1.0 and NREM AHI of 4.3 events/h, and an oxygen saturation nadir of 92% (Figure 1). One year later, he began testosterone therapy as part of the gender transition process. His serum testosterone increased as expected with treatment, from 71 to 514 ng/dL. Six months after initiating testosterone therapy, the patient experienced disruptive, habitual snoring and witnessed apneas. A repeat level 1 split PSG demonstrated moderate OSA with an AHI of 25 events/h and an oxygen saturation nadir of 89%. There was no REM sleep noted during the diagnostic portion of this PSG (Figure 2). The patient reported immediately improved restorative sleep with the use of CPAP on the night of the PSG (during the therapeutic portion). The BMI of 33 kg/m2 had not changed with hormone treatment. One month after starting CPAP, the patient’s ESS decreased to 8 and his self-reported sleep quality improved with resolution of snoring on CPAP.
Figure 1. Case 2 hypnogram of polysomnogram prior to testosterone treatment.
3% hypopnea criteria was used. Apnea-hypopnea index was 2 events/h. BPOS = body position, CA = central apnea, CH = central hypopnea, Heart = heart rate, MA = mixed apnea, MH = mixed hypopnea, OA = obstructive apnea, OH = obstructive hypopnea, SN = snoring, SpO2 = pulse oximetry, Stage = sleep stage, Treatment = CPAP treatment pressure in cmH2O.
Figure 2. Case 2 hypnogram of polysomnogram after testosterone began.
This is a split-night study, and 3% hypopnea criteria was used for scoring. Apnea-hypopnea index of 25 events/h was calculated using only the pre-CPAP titration portion of the study. The green line in the treatment area indicates when CPAP was used and at what pressure. BPOS = body position, CA = central apnea, CH = central hypopnea, Heart = heart rate, MA = mixed apnea, MH = mixed hypopnea, OA = obstructive apnea, OH = obstructive hypopnea, SN = snoring, SpO2 = pulse oximetry, Stage = sleep stage, Treatment = CPAP treatment pressure in cmH2O.
Case 3
A 35-year-old transgender man (assigned female at birth) presented for snoring and excessive daytime sleepiness (ESS 11) after starting testosterone. His past medical history included posttraumatic stress disorder, depression, and anxiety disorder. The patient had been using transdermal testosterone 4 mg daily for 2 years. His serum testosterone level was appropriately elevated with treatment, in the 500–600 ng/dL range. Five years previously, he underwent a level 1 PSG for concerns about excessive daytime sleepiness, nocturnal arousals, and snoring, which demonstrated AHI of 1.4 events/h, REM AHI of 3.1 events/h, NREM AHI of 1 events/h, and oxygen saturation nadir of 93%. His surgical history was significant for a total hysterectomy and bilateral salpingo-oophorectomy for pelvic pain approximately 1 month prior to the repeat sleep evaluation for persistent snoring and excessive daytime sleepiness. A repeat PSG demonstrated an AHI of 14.4 events/h (REM AHI of 37.8 events/h, NREM AHI of 7.2 events/h), and oxygen saturation nadir of 91%. The patient’s BMI of 31 kg/m2 and neck size of 38.5 cm had not changed in the interval between the PSG tests. The patient was started on CPAP therapy to treat his OSA with resolution of his excessive daytime sleepiness and snoring. The patient continued on testosterone therapy.
Measurements
For all patients, progesterone medications were not used and progesterone serum levels were not measured. None of the patients were using any other medications suspected of influencing sleep-disordered breathing. The contemporaneous version (version 2.5) of The AASM Manual for the Scoring of Sleep and Associated Events: Rules, Terminology and Technical Specifications was used for PSG scoring. Hypopneas in all the studies were scored using the 3% desaturation rule.5 The scoring of respiratory events across these cases were consistent and the AHIs comparable.
DISCUSSION
Transgender medicine is a growing field with complex patients and limited evidence-based guidance. As the medical community engages further in the care of these patients, it remains to be seen what the physiologic effect of treatment-specific hormonal therapies will be. Androgens and estrogens influence sleep and respiratory stability regardless of sex, and the effect of doses and formulations used in gender transition is unclear. In the case of testosterone, existing data on treatment effects are conflicting.3,6–8
Case 1 had a higher NREM AHI than REM AHI prior to estradiol and spironolactone, which is expected for a male patient. After taking estradiol and spironolactone, her NREM sleep-disordered breathing was completely resolved and the REM AHI was still present, although much reduced compared to the previous PSG results. In Case 2, our patient had a post-testosterone NREM AHI of 25 events/h, but there was no diagnostic REM sleep with which to compare. However, the high NREM AHI is more consistent with a male pattern of OSA. In Case 3, post-testosterone PSG results demonstrated a relatively high REM AHI/low NREM AHI pattern that has been seen in females. Gender differences in the mechanisms by which OSA develops are important to the understanding of how hormone therapy affects OSA in this population; these differences include obesity and the distribution of adipose tissue, upper airway anatomy, upper airway function, ventilator control, and the effect of sex hormones.9 There are previously reported gender differences in the ratios of the REM AHI and NREM AHI, as well as several other PSG parameters.10,11 In general, women have a lower NREM AHI compared to men, and a higher REM AHI. There was no clear pattern of changes in the REM/NREM AHI ratios noted in our patients. It is unclear how these gender differences will change with the use of gender-affirming hormone therapy. Further study of transgender patients is needed to answer these questions.
Some gender differences in OSA are unlikely to be affected by hormone therapy. The longer upper airway and larger soft palate in men predisposes them to OSA and can worsen severity.12–14 Hormone therapy is unlikely to change the anatomy of the airway, so airway structure is unlikely to be the cause of the changes in OSA status for our patients. Future studies of the sleep effects of gender-affirming therapy should include the effects on these subtypes of sleep- disordered breathing, as well as other sleep parameters, to determine how the hormone treatments affect these aspects of the PSG.
Although there is some lower-quality evidence that estrogen might have an effect on OSA, practice guidelines recommend against use of estrogen for the pharmacologic treatment of OSA.4 Estrogen is believed to be protective of sleep-disordered breathing given the low prevalence of OSA in premenopausal women. The Wisconsin Sleep Cohort demonstrated an odds ratio of 3.5 for having moderate to severe OSA in postmenopausal women, compared to only 1.1 in those who were perimenopausal, compared to the premenopausal group.3 Additionally, a low estrogen level is believed to explain the increased prevalence of OSA in patients with PCOS.15 The mechanism of estrogen’s role in preventing OSA is unclear. It has been suggested that estrogen raises the apneic threshold, thereby stabilizing the upper airway during sleep.16 Another theory is that estrogen reduces the oxidative stress in the upper airway muscles, reducing the risk of oropharyngeal collapse in the setting of chronic intermittent hypoxia.17 Supporting this notion, a study of the effects of estrogen on the genioglossus muscle fibers in rats demonstrate that the adverse effects of chronic intermittent hypoxia on contractility can be partially reversed with estrogen.18 When administered to postmenopausal women, estrogen replacement therapy has improved the AHI in several small studies. One study demonstrated an improvement of the AHI by 75% after treatment with estrogen in four postmenopausal women.19
There is some evidence that the effects of estrogen and/or progesterone on OSA are overstated. Polo-Kantola et al reported no significant effect on partial airway obstruction in a randomized crossover trial of estrogen in postmenopausal women.20 A study looking at data from the Sleep in Midlife Women Study before and after July 2002 (when the Women’s Health Initiative Study reported that estrogen/progestin increased heart disease risk) reported that the effect on OSA from these hormones may have been due to the healthy user effect.21 A short-term estrogen replacement study in 15 postmenopausal women showed no improvement in the AHI, but an improvement in the hypoxic ventilatory response was observed.22
In addition to postmenopausal women, two other relevant cohorts of patients to consider are men with prostate cancer treated with castration and/or antiandrogen medications, and women with breast cancer treated with estrogen-lowering medications. The effect of these treatments on OSA has not been studied well, although there is a case report of enzalutamide, an antiandrogen medication, being associated with OSA.23 The study of these patients may prove enlightening in understanding the effect of sex hormones on OSA.
The association between body weight and OSA is well known. Obesity is a common underlying risk factor in the development of sleep apnea.24 However, weight loss is rarely associated with a drastic reduction in AHI.25 The Wisconsin Sleep Cohort demonstrated that a 10% reduction in body weight would only result in a reduction in AHI by 26%.26 Even after significant weight loss following bariatric surgery, a reduction in AHI to normal levels (less than 5 events/h) infrequently occurs.27
Another potential cofounder is that our transgender patient assigned male at birth had also been taking spironolactone, frequently used as an antiandrogen in the nonsurgical treatment of transgender women.28 Spironolactone reduces serum testosterone levels, which allows for lower dosages of estradiol during the gender transition process. In addition, spironolactone has also been shown to reduce the AHI in patients with resistant hypertension and OSA.29 The mechanism was thought to be due to diuretic effect reducing oropharyngeal edema and subsequent upper airway resistance. Because hypertension had not been diagnosed in our patient, she was not considered to be in a volume overloaded state, making it unlikely that spironolactone had any effect on our patient’s “cure” of OSA.
The marked reduction in AHI in that patient is believed to be secondary to the initiation of transdermal estrogen therapy. Treatment with estrogen should be investigated further to determine its role as a potential management option of OSA in the transgender population. The effects of testosterone on upper airway patency during sleep in men are not clearly understood.6–8,30 Testosterone deficiency in men is associated with OSA, and testosterone replacement may cause or worsen OSA.30 If testosterone is a major cause of OSA in the patients we report, it is possible that the effect of testosterone therapy is different between the genders, which further complicates our understanding of its role in OSA. The phenomenon of testosterone causing OSA in women has not been well described; only two relevant case reports have been published. The first involved a 54-year-old woman receiving androgen medications as treatment for anemia due to chronic renal failure. OSA symptoms appeared after beginning treatment, resolved when treatment was discontinued, and recurred with a repeated trial of the androgen medications.31 The second report was of a 70-year-old woman who had a testosterone-producing lipoid tumor of the ovary. OSA symptoms developed along with virilization, both of which resolved with excision of this benign tumor.32 Women with PCOS are more likely to have OSA; this has been attributed to elevated testosterone in these patients.15 Interestingly, in our patient with PCOS (Case 2) OSA did not develop until exogenous testosterone was given. The other transgender male patient (Case 3) had a bilateral oophorectomy about 1 month before his second PSG. A lack of estrogen and progesterone may have contributed to his OSA, in addition to the use of exogenous testosterone. Additionally, the sudden surgical menopause experienced by this patient may have increased his risk of OSA, as opposed to natural menopause.33 The relative contribution of these hormonal influences is unknown, but all of these hormones seem to play a role in contributing to or protecting patients from OSA.
To our knowledge, these are the first reports of OSA coinciding with testosterone use in transgender men. Given the common use of testosterone in gender-reaffirming therapy, the risk of developing OSA in these patients should be considered, and the patients counseled on this possible adverse reaction. Further research is needed to evaluate the effect of sex hormone replacement on sleep-disordered breathing at doses used for gender transition.
The effects of estrogens on sleep-disordered breathing have been studied in cisgender patients, but not in transgender patients. The apparent beneficial effect of estradiol in our transgender woman patient, and the adverse effect of testosterone in our transgender patients assigned female at birth have not been reported in the past. As the use of hormone treatment for gender dysphoria becomes more common, the effects of these treatments should be considered when weighing the benefits and risks of the treatments. This is especially true of testosterone, as these patients may be unknowingly exposing themselves to an adverse effect. That said, it is unclear from the literature whether the increase in AHI in transgender men is due to the addition of testosterone or the suppression of estrogen and/or progesterone. Gender-affirming therapy adds an additional layer of complexity to the physiology of sex hormones and sleep-disordered breathing. We believe that systematic study of the respiratory effects of hormone treatment for gender dysphoria should be pursued, and that this patient population should be screened for sleep-disordered breathing as clinically indicated.
DISCLOSURE STATEMENT
All authors have seen and approved the final manuscript. Work for this study was performed at Walter Reed National Military Medical Center-Bethesda, Bethesda, Maryland. The authors report no conflicts of interest.
ABBREVIATIONS
- AHI
apnea-hypopnea index
- BMI
body mass index
- CPAP
continuous positive airway pressure
- ESS
Epworth Sleepiness Scale
- NREM
non-rapid eye movement
- OSA
obstructive sleep apnea
- PCOS
polycystic ovary syndrome
- PSG
polysomnography
- REM
rapid eye movement
REFERENCES
- 1.Barrett-Connor E, Dam TT, Stone K, et al. The association of testosterone levels with overall sleep quality, sleep architecture, and sleep-disordered breathing. J Clin Endocrinol Metab. 2008;93(7):2602–2609. doi: 10.1210/jc.2007-2622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Behan M, Wenninger JM. Sex steroidal hormones and respiratory control. Respir Physiol Neurobiol. 2008;164(1-2):213–221. doi: 10.1016/j.resp.2008.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Young T, Finn L, Austin D, Peterson A. Menopausal status and sleep-disordered breathing in the Wisconsin Sleep Cohort Study. Am J Respir Crit Care Med. 2003;167(9):1181–1185. doi: 10.1164/rccm.200209-1055OC. [DOI] [PubMed] [Google Scholar]
- 4.Morgenthaler TI, Kapen S, Lee-Chiong T, et al. Practice parameters for the medical therapy of obstructive sleep apnea. Sleep. 2006;29(8):1031–1035. [PubMed] [Google Scholar]
- 5.Berry RB, Albertario CL, Harding SM, et al. for the American Academy of Sleep Medicine. The AASM Manual for the Scoring of Sleep and Associated Events: Rules, Terminology and Technical Specifications. Version 2.5. Darien, IL: American Academy of Sleep Medicine; 2018. [Google Scholar]
- 6.Burschtin O, Wang J. Testosterone deficiency and sleep apnea. Sleep Med Clin. 2016;11(4):525–529. doi: 10.1016/j.jsmc.2016.08.003. [DOI] [PubMed] [Google Scholar]
- 7.Hanafy HM. Testosterone therapy and obstructive sleep apnea: is there a real connection? J Sex Med. 2007;4(5):1241–1246. doi: 10.1111/j.1743-6109.2007.00553.x. [DOI] [PubMed] [Google Scholar]
- 8.Liu PY, Yee B, Wishart SM, et al. The short-term effects of high-dose testosterone on sleep, breathing, and function in older men. J Clin Endocrinol Metab. 2003;88(8):3605–3613. doi: 10.1210/jc.2003-030236. [DOI] [PubMed] [Google Scholar]
- 9.Kapsimalis F, Kryger MH. Gender and obstructive sleep apnea syndrome, part 2: mechanisms. Sleep. 2002;25(5):497–504. [PubMed] [Google Scholar]
- 10.Basoglu OK, Tasbakn MS. Gender differences in clinical and polysomnographic features of obstructive sleep apnea: a clinical study of 2827 patients. Sleep Breath. 2018;22(1):241–249. doi: 10.1007/s11325-017-1482-9. [DOI] [PubMed] [Google Scholar]
- 11.O’Connor C, Thornley KS, Hanly PJ. Gender differences in the polysomnographic features of obstructive sleep apnea. Am J Respir Crit Care Med. 2000;161(5):1465–1472. doi: 10.1164/ajrccm.161.5.9904121. [DOI] [PubMed] [Google Scholar]
- 12.Malhotra A, Huang Y, Fogel RB, et al. The male predisposition to pharyngeal collapse: importance of airway length. Am J Respir Crit Care Med. 2002;166(10):1388–1395. doi: 10.1164/rccm.2112072. [DOI] [PubMed] [Google Scholar]
- 13.Segal Y, Malhotra A, Pillar G. Upper airway length may be associated with the severity of obstructive sleep apnea syndrome. Sleep Breath. 2008;12(4):311–316. doi: 10.1007/s11325-008-0191-9. [DOI] [PubMed] [Google Scholar]
- 14.Ronen O, Malhotra A, Pillar G. Influence of gender and age on upper-airway length during development. Pediatrics. 2007;120(4):e1028–e1034. doi: 10.1542/peds.2006-3433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tasali E, Van Cauter E, Ehrmann DA. Polycystic ovary syndrome and obstructive sleep apnea. Sleep Med Clin. 2008;3(1):37–46. doi: 10.1016/j.jsmc.2007.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rowley JA, Zhou XS, Diamond MP, Badr MS. The determinants of the apnea threshold during NREM sleep in normal subjects. Sleep. 2006;29(1):95–103. doi: 10.1093/sleep/29.1.95. [DOI] [PubMed] [Google Scholar]
- 17.O’Halloran KD, Lewis P, McDonald F. Sex, stress and sleep apnoea: Decreased susceptibility to upper airway muscle dysfunction following intermittent hypoxia in females. Respir Physiol Neurobiol. 2017;245:76–82. doi: 10.1016/j.resp.2016.11.009. [DOI] [PubMed] [Google Scholar]
- 18.Liu Y, Huang Y, Shao X. Effects of estrogen on genioglossal muscle contractile properties and fiber‐type distribution in chronic intermittent hypoxia rats. Eur J Oral Sci. 2009;117(6):685–690. doi: 10.1111/j.1600-0722.2009.00681.x. [DOI] [PubMed] [Google Scholar]
- 19.Wesstrom J, Ulfberg J, Nilsson S. Sleep apnea and hormone replacement therapy: a pilot study and a literature review. Acta Obstet Gynecol Scand. 2005;84(1):54–57. doi: 10.1111/j.0001-6349.2005.00575.x. [DOI] [PubMed] [Google Scholar]
- 20.Polo-Kantola P, Rauhala E, Helenius H, et al. Breathing during sleep in menopause: a randomized, controlled, crossover trial with estrogen therapy. Obstet Gynecol. 2003;102(1):68–75. doi: 10.1016/s0029-7844(03)00374-0. [DOI] [PubMed] [Google Scholar]
- 21.Mirer AG, Peppard PE, Palta M, Benca RM, Rasmuson A, Young T. Menopause hormone therapy and sleep-disordered breathing: evidence for a healthy user bias. Ann Epidemiol. 2015;25(10):779–784.e1. doi: 10.1016/j.annepidem.2015.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cistulli PA, Barnes DJ, Grunstein RR, Sullivan CE. Effect of short-term hormone replacement in the treatment of obstructive sleep apnoea in postmenopausal women. Thorax. 1994;49(7):699–702. doi: 10.1136/thx.49.7.699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Labrize F, Cany L, Massard C, et al. Enzalutamide and sleep apnea: an emerging central nervous system side-effect? Ann Oncol. 2016;27(1):206. doi: 10.1093/annonc/mdv481. [DOI] [PubMed] [Google Scholar]
- 24.Newman AB, Foster G, Givelber R, Nieto FJ, Redline S, Young T. Progression and regression of sleep-disordered breathing with changes in weight: the Sleep Heart Health Study. Arch Intern Med. 2005;165(20):2408–2413. doi: 10.1001/archinte.165.20.2408. [DOI] [PubMed] [Google Scholar]
- 25.Joosten SA, Hamilton GS, Naughton MT. Impact of weight loss management in OSA. Chest. 2017;152(1):194–203. doi: 10.1016/j.chest.2017.01.027. [DOI] [PubMed] [Google Scholar]
- 26.Peppard PE, Young T, Palta M, Dempsey J, Skatrud J. Longitudinal study of moderate weight change and sleep-disordered breathing. JAMA. 2000;284(23):3015–3021. doi: 10.1001/jama.284.23.3015. [DOI] [PubMed] [Google Scholar]
- 27.Greenburg DL, Lettieri CJ, Eliasson AH. Effects of surgical weight loss on measures of obstructive sleep apnea: a meta-analysis. Am J Med. 2009;122(6):535–542. doi: 10.1016/j.amjmed.2008.10.037. [DOI] [PubMed] [Google Scholar]
- 28.Moore E, Wisniewski A, Dobs A. Endocrine treatment of transsexual people: a review of treatment regimens, outcomes, and adverse effects. J Clin Endocrinol Metab. 2003;88(8):3467–3473. doi: 10.1210/jc.2002-021967. [DOI] [PubMed] [Google Scholar]
- 29.Gaddam K, Pimenta E, Thomas SJ, et al. Spironolactone reduces severity of obstructive sleep apnoea in patients with resistant hypertension: a preliminary report. J Hum Hypertens. 2010;24(8):532–537. doi: 10.1038/jhh.2009.96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Grech A, Breck J, Heidelbaugh J. Adverse effects of testosterone replacement therapy: an update on the evidence and controversy. Ther Adv Drug Saf. 2014;5(5):190–200. doi: 10.1177/2042098614548680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Johnson MW, Anch AM, Remmers JE. Induction of the obstructive sleep apnea syndrome in a woman by exogenous androgen administration. Am Rev Respir Dis. 1984;129(6):1023–1025. doi: 10.1164/arrd.1984.129.6.1023. [DOI] [PubMed] [Google Scholar]
- 32.Dexter DD, Dovre EJ. Obstructive sleep apnea due to endogenous testosterone production in a woman. Mayo Clin Proc. 1998;73(3):246–248. doi: 10.4065/73.3.246. [DOI] [PubMed] [Google Scholar]
- 33.Huang T, Lin BM, Redline S, et al. Type of menopause, age at menopause, and risk of developing obstructive sleep apnea in postmenopausal women. Am J Epidemiol. 2018;187(7):1370–1379. doi: 10.1093/aje/kwy011. [DOI] [PMC free article] [PubMed] [Google Scholar]