Abstract
Study Objectives:
A strong association between sleep-disordered breathing (SDB) and atrial fibrillation and/or atrial flutter (AF) has consistently been observed in epidemiologic and interventional studies. The effect of positive airway pressure (PAP) on AF recurrence is inconclusive. This study sought to evaluate the effectiveness of PAP therapy for SDB on AF recurrence.
Methods:
This was a single-center, retrospective study conducted at a tertiary referral center. All adult patients who had SDB on polysomnography and underwent AF intervention (ablation or cardioversion) following polysomnography from January 1992-December 2014 were analyzed. Primary outcome was time to first-documented recurrence of AF after AF intervention by Kaplan–Meier estimates.
Results:
Among 30,188 patients with obstructive and central SDB, 429 had this diagnosis before AF intervention; 269 were “PAP-adherent users,” the remaining 160 were “PAP-nonusers.” Patients in both groups had similar age, sex, body mass index (BMI), ejection fraction, left atrial volume index (LAVI), antiarrhythmic medications, diabetes mellitus, systemic hypertension, and heart failure diagnoses. Time to recurrence of AF postintervention was no different in PAP-adherent users and nonusers (4.8 and 4.1 months respectively, P = .7). Cardioversion (compared to catheter ablation) was the strongest independent predictor of recurrent AF (hazard ratio [HR] 2.02, 95% confidence interval [CI] 1.39–2.94, P < .001). BMI and LAVI were also significant predictors of recurrence in adjusted analyses (HR 1.01, 95% CI 1.003–1.023, P = .10 and HR 1.01, 95% CI 1.001–1.019, P = .024 respectively).
Conclusions:
Our study found no effect of PAP treatment of SDB on time to recurrence of AF post-AF intervention. Increased risk of recurrent AF was associated with high BMI and LAVI. These findings may affect the clinical management of AF.
Citation:
Srivali N, Chahal AC, Mansukhani MP, Mandrekar J, Somers VK, Caples SM. The effect of positive airway pressure treatment of obstructive and central sleep apnea on the recurrence of atrial fibrillation/flutter postintervention. J Clin Sleep Med. 2019;15(10):1459–1468.
Keywords: atrial fibrillation, positive airway pressure, recurrence, sleep apnea, treatment
BRIEF SUMMARY
Current Knowledge/Study Rationale: The literature on the impact of positive airway pressure (PAP) treatment on the recurrence of atrial fibrillation/atrial flutter (AF) is inconclusive.
Study Impact: This retrospective study is the largest to date examining the effect of PAP treatment of sleep disordered breathing and did not find an effect of adequate PAP use on time to first recurrence of AF post-catheter ablation or cardioversion. Further large prospective randomized-controlled trials are required to guide clinical decisions.
INTRODUCTION
Atrial fibrillation and/or atrial flutter (AF) are the most common cardiac arrhythmias, are increasing in prevalence, and are associated with heightened mortality, morbidity, and cost burden.1 Similarly, obstructive sleep apnea (OSA) is increasing in prevalence, due in part to better recognition and diagnosis.2 OSA and AF share multiple risk factors including advancing age, male sex, obesity, hypertension, and heart failure.3 An independent and consistent association between OSA and AF has been reported.4,5 Additionally, there is a strong relationship between AF and OSA in heart failure, although central sleep apnea (CSA) is seen frequently in this population as well.6,7
There is great interest in the effect of treatment of sleep-disordered breathing (SDB) on cardiac arrhythmias.8 Many potential pathophysiologic OSA triggers for AF such as hypoxemia, autonomic remodeling, left atrial stretch, and inflammation are attenuated by positive airway pressure (PAP) therapy.9 Yet, in the absence of rigorous studies, the role of PAP treatment of SDB in AF outcomes remains unproven.10 This study examines the effect of PAP therapy for SDB on time to first documented recurrence of AF following interventions to restore sinus rhythm, including cardioversion (chemical and direct current) and catheter ablation.
METHODS
Study Population
We performed a retrospective study using a prospectively maintained registry. Eligibility criteria included adult (age older than 18 years) male and female patients who underwent standard, attended, in-laboratory polysomnography (PSG) at Mayo Clinic, Rochester, Minnesota, from 1992 to 2014. Each patient must have experienced an episode of electrocardiography (ECG)-documented atrial fibrillation and/or flutter (referred to collectively as AF for this analysis) at any time point before PSG. International Classification of Diseases, Ninth Revision codes for AF (427.31 and 427.32) were used to identify potential patients. Consecutive chart review was manually conducted to ensure inclusion criteria were met and to abstract data on medical history, medication use, ECG, hospital visits, and cardiac imaging.
Patients with AF who were treated with a rate-control strategy and did not undergo pulmonary vein ablation or chemical or direct current cardioversion were excluded, as were those in whom the intervention occurred before PSG. Patients were also excluded if the date of AF intervention could not be accurately ascertained.
After determination of AF, PSG data were evaluated. Patients with a diagnosis of SDB (obstructive, central, or mixed) prescribed any form of PAP therapy who were adherent to the device was classified as the treatment group. Those with a diagnosis of SDB who were not on any treatment (including non-PAP alternatives such as an oral appliance) or were nonadherent to PAP served as the control group. We identified the endpoint of date of recurrence of AF by the first ECG-documented episode of AF in the inpatient or outpatient setting (with or without symptoms).
All study participants provided authorization for records to be used for purposes of medical research. This study was approved by the Mayo Clinic Institutional Review Board.
Polysomnography
All patients underwent attended, in-laboratory PSG at the Mayo Clinic Center for Sleep Medicine. The laboratory is accredited by the American Academy of Sleep Medicine. PSG data were scored in a standard fashion by registered polysomnographic technologists and interpreted by board-certified sleep medicine specialists in compliance with rules, terminology, and technical specifications as specified by the American Academy of Sleep Medicine. Every study was dual reported by a consultant, and every study was rereported in blinded fashion to ensure interreporter and intrareporter reliability and validity. Standard recording parameters included electroencephalogram, electromyogram of the limbs and submental area, electrooculogram, nasal pressure transducer and oronasal thermistor airflow measurements, thoracic and abdominal inductance plethysmography, ECG, digit pulse oximetry, body position sensor, upper airway sound recording, and video monitoring. Apneas were defined as a decrease in oronasal thermistor or alternate sensor amplitude by ≥ 90% from baseline for ≥ 10 seconds; hypopneas were defined as a decrease in nasal pressure transducer signal by ≥ 30% for 10 seconds or more with a ≥ 4% oxygen desaturation from preevent baseline. Respiratory events with associated inspiratory effort were scored as obstructive, whereas events with absent effort were scored as central. CSA was defined as a central apnea-hypopnea index more than 5 per hour on the diagnostic study, with more than 50% of the apneas plus hypopneas being central in nature. OSA was defined as an obstructive apnea-hypopnea index more than 5 per hour on the diagnostic study, with more than 50% of the apneas plus hypopneas being obstructive in nature. Patients with mixed sleep apnea had an equal number of apneas plus hypopneas that were deemed to be obstructive and central in nature on the diagnostic study. Hypopneas were noted to be obstructive appearing or central appearing by the interpreting sleep medicine physician in the study report. Similarly, Cheyne-Stokes breathing was scored on the sleep study applying the scoring rules in use at the time and mentioned in the study interpretation by the sleep medicine physician if applicable; this finding also contributed to the overall diagnostic classification as OSA, CSA, or mixed sleep apnea made by the physician.
Electrocardiography
The presence or absence of AF was determined through a manual review of the electronic medical record for each case and control. All patients had ECGs performed with scheduled routine follow-up or as clinically indicated. AF was defined as a diagnosis of atrial fibrillation and/or atrial flutter with confirmation by ECG.
Determination of PAP Adherence and Efficacy
Adherence to treatment was defined as equal to or greater than 4 hours of nightly use on at least 70% of days in the past 30 days or more.11,12 Information on PAP adherence and efficacy was determined based on review of objective download data from the electronic medical record.
Statistical Analysis
Continuous variables were reported as means, standard deviation and median, interquartile range. All categorical variables were reported as counts with percentages. For patients with multiple AF interventions, only the first AF intervention during the study period was included in the analysis. A time-to-event model was initiated at the time of AF intervention and followed until recurrence of AF or date of last follow-up. Survival was presented using the Kaplan–Meier plot and tested using the log-rank test.
For the analyses, the groups were divided into four based on their type of SDB and treatment: (1) all types of SDB with treatment; (2) OSA with treatment; (3) CSA with treatment; and (4) mixed sleep apnea with treatment. All groups were compared against the group that did not receive PAP therapy similarly divided into four groups: (1) all types of SDB without treatment; (2) OSA with nontreatment; (3) CSA with nontreatment; and (4) mixed sleep apnea with nontreatment.
Univariate analyses for the outcome of AF recurrence were performed using two-tailed Fisher exact test for age, sex, body mass index (BMI), neck circumference, Epworth Sleepiness Scale score, thyroid disease, systemic hypertension (or the use of antihypertensive medications), diabetes mellitus (or the use of diabetic medications) coronary artery disease and heart failure. Univariate analyses were also performed using echocardiography data including ejection fraction, left atrial volume, left atrial volume index (LAVI), grading of diastolic dysfunction and medications at dismissal including amiodarone, beta blockers, dihydropyridines, nondihydropyridines, disopyramide, dofetilide, flecainide, procainamide, propafenone, sotatol, digoxin, diuretics and angiotensin-converting enzyme inhibitors. Last, univariate analyses were performed using polysomnographic variables including apnea-hypopnea index, mean oxyhemoglobin saturation, minimum oxyhemoglobin saturation, arousal index as well as any PAP usage and PAP adherence. Age, sex, BMI, thyroid disease, other medical comorbidities, echocardiographic data, and medication use at the time of AF intervention were identified by reviewing the detailed medical record. These covariates were chosen as they were considered to be important risk factors for recurrence of AF as shown in non-OSA studies and are potential confounders or effect-modifiers. Following univariate analyses, multiple logistic regression analyses were performed with all the covariates and separately with only covariates where the association with AF was significant or borderline significant (P < .05 a priori) in the univariate analysis, that is, BMI, left atrial size, LAVI, type of intervention, and use of diuretics and digoxin. Further analyses were conducted using PAP adherence as an additional covariate in the latter model as well as with any PAP usage instead of PAP adherence. All analyses were performed with JMP statistical software version 14 (SAS Institute, Cary, North Carolina, United States).
RESULTS
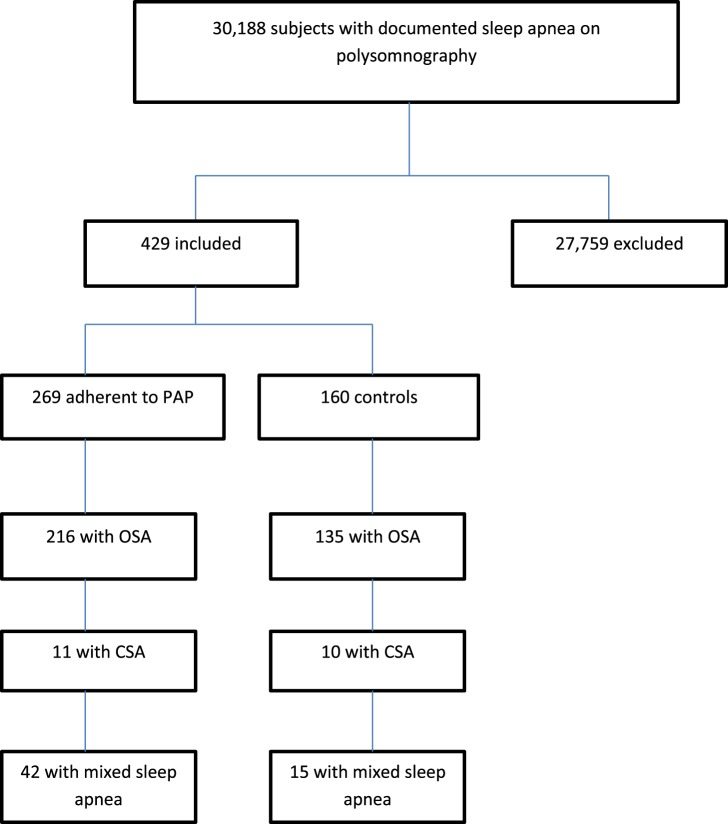
An initial database query yielded 30,188 possible individuals with documented SDB on PSG. After consecutive chart review 29,759 individuals were excluded because they did not have AF with an attempt at restoration of sinus rhythm by catheter ablation or cardioversion after PSG. Individuals in whom the AF intervention occurred before PSG were excluded. Individuals were also excluded if the date of AF intervention was indeterminable, leaving 429 individuals eligible for inclusion in the analysis (Figure 1).
Figure 1. Enrollment.
CSA = central sleep apnea, OSA = obstructive sleep apnea, PAP = positive airway pressure.
Characteristics of the Study Population
After categorization of AF interventions, patients were classified into groups by type of SDB with adherence to PAP treatment or controls (patients who elected not to use therapy or were nonadherent to PAP therapy).
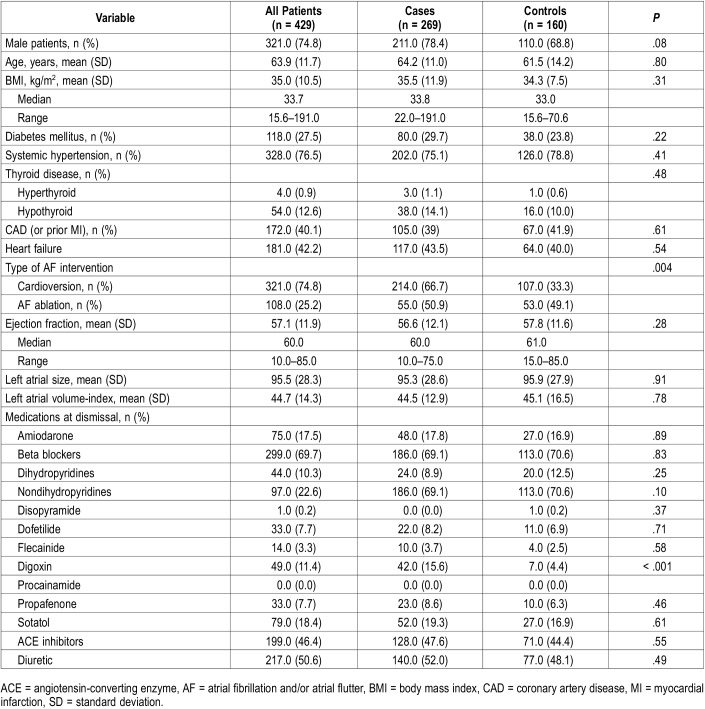
Baseline characteristics for the 269 cases and 160 control patients are outlined in Table 1. A total of 56 patients (13.1%) demonstrated some PAP use but were not adherent with the device as defined previously. The treatment and control groups had similar sex distribution, age, BMI, and rates of diabetes, hypertension, thyroid disease, coronary artery disease, and congestive heart failure. Baseline echocardiography parameters including ejection fraction, left atrial size and LAVI were also similar. Medications at dismissal were comparable between groups, except the treatment group had a higher usage of digoxin (15.6% versus 4.4%, P = .0003). Cardioversion as an AF intervention was significantly more common in the treatment group compared to the control group (66.7% versus 33.3%, P = .004).
Table 1.
Patient characteristics.
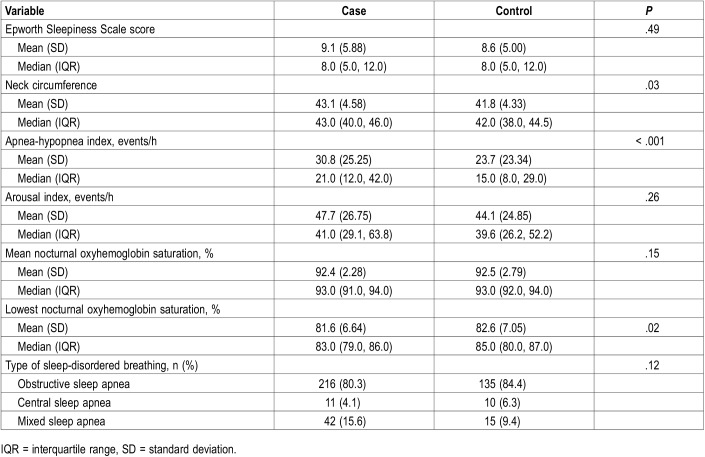
Table 2 describes the characteristics of the PSG. The treatment and control groups had similar Epworth Sleepiness Scale scores, arousal index, mean nocturnal oxyhemoglobin saturation, and type of SDB, with no statistically significant differences. The treatment group had slightly larger neck circumference (43 versus 42 cm, P = .026), a higher apnea-hypopnea index (30.8 versus 23.7 events/h, P = .0002), and slightly lower nadir nocturnal oxyhemoglobin saturation (81.6 versus 82.6%, P = .02). Review of the clinical follow-up notes including objective PAP download data did not suggest that SDB was undertreated in the PAP-adherent patients and none of these patients underwent repeat sleep study under the direction of the treating sleep medicine provider.
Table 2.
Baseline polysomnography.
Duration of Recurrence of AF
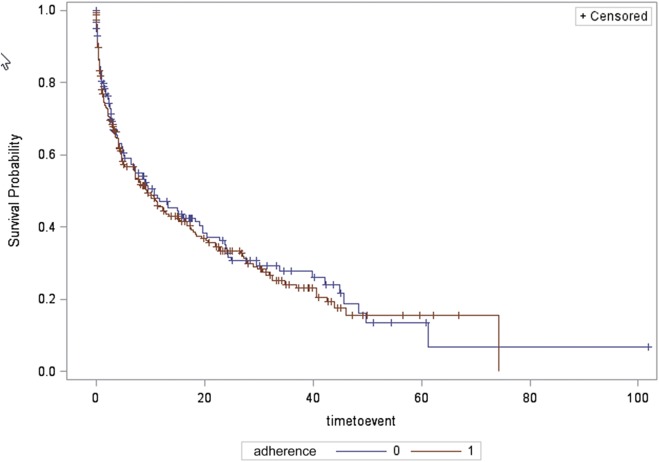
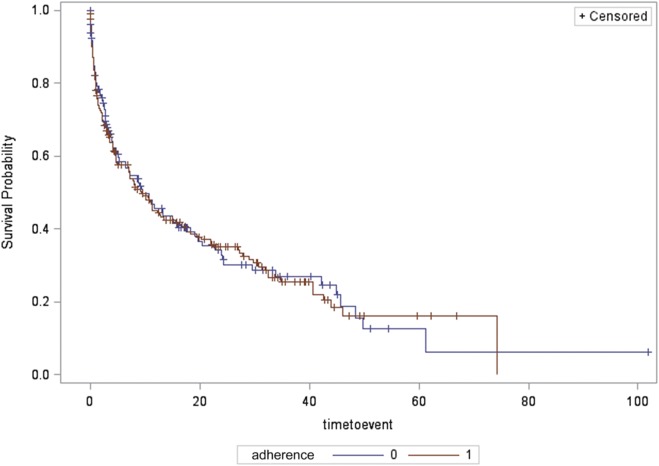
Median duration of follow-up was 4.6 months (interquartile range 17.5) for the entire sample. Overall, in all types of patients with SDB, time to first-documented recurrence of AF was not significantly different between groups (median 4.8 [0.9, 18.5] months in the treatment group versus 4.1 [1.2, 18.0] months in the control group) by Kaplan–Meier analysis (log-rank P = .70, Figure 2).
Figure 2. Time to recurrence of AF for all sleep apnea types, in those adherent and nonadherent to treatment.
Survival probability (depicted on the y axis) is the probability of survival free of AF. Time to event (depicted on the x axis) is the time to first documented ECG recurrence of AF. Compliance refers to adherence to positive airway pressure treatment with 0 representing nonadherent to treatment group and 1 representing adherent to treatment group. AF = atrial fibrillation and/or atrial flutter, ECG = electrocardiography.
We performed a subgroup analysis for the same outcome, with patients classified by the type of SDB (OSA, CSA, and mixed sleep apnea). In 321 patients with OSA (216 individuals in the PAP-treatment group and 135 in the control group), time to recurrence of AF was not significantly different between groups (median 9.4 [1.5, 40.7] months in the treatment group versus 9.5 [2.3, 43.2] months in the control group) by Kaplan–Meier analysis (log-rank P = .94, Figure 3).
Figure 3. Time to recurrence of AF for the obstructive sleep apnea group, in those adherent and nonadherent to treatment.
Survival probability (depicted on the y axis) is the probability of survival free of AF. Time to event (depicted on the x axis) is the time to first documented ECG recurrence of AF. Compliance refers to adherence to positive airway pressure treatment with 0 representing nonadherent to treatment group and 1 representing adherent to treatment group. AF = atrial fibrillation and/or atrial flutter, ECG = electrocardiography.
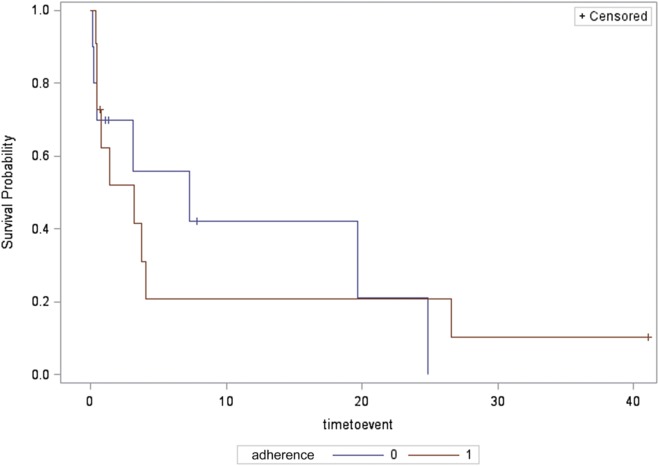
In 21 patients with CSA (11 in PAP-treatment group and 10 in the control group), time to recurrence of AF was not significantly different between groups (median 3.2 [0.5, 4.1] months in PAP treatment group versus 7.3 [0.5, 19.7] months in the control group by Kaplan–Meier analysis (log-rank P = .96, Figure 4).
Figure 4. Time to recurrence of AF for the central sleep apnea group, in those adherent and nonadherent to treatment.
Survival probability (depicted on the y axis) is the probability of survival free of AF. Time to event (depicted on the x axis) is the time to first documented ECG recurrence of AF. Compliance refers to adherence to positive airway pressure treatment with 0 representing nonadherent to treatment group and 1 representing adherent to treatment group. AF = atrial fibrillation and/or atrial flutter, ECG = electrocardiography.
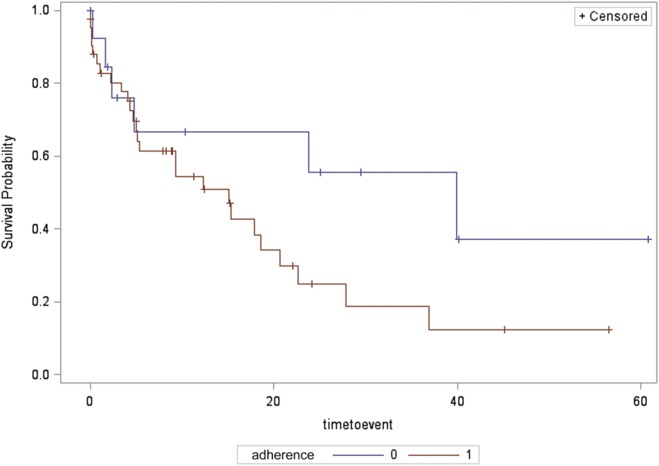
In 57 patients with mixed sleep apnea (42 in PAP-treatment group and 15 in control group), time to recurrence of AF was not significantly different between groups (median 15.1 [4.3, 22.6] months in PAP-treatment group versus 39.9 [4.8, 60.8] months in the control group) by Kaplan–Meier analysis (log-rank P = .12, Figure 5).
Figure 5. Time to recurrence of AF for the mixed sleep apnea group, in those adherent and nonadherent to treatment.
Survival probability (depicted on the y axis) is the probability of survival free of AF. Time to event (depicted on the x axis) is the time to first documented ECG recurrence of AF. Compliance refers to adherence to positive airway pressure treatment with 0 representing nonadherent to treatment group and 1 representing adherent to treatment group. AF = atrial fibrillation and/or atrial flutter, ECG = electrocardiography.
Analysis by sex, including all types of SDB (log-rank P = .69), patients with OSA (log-rank P = .57), patients with CSA (log-rank P = .81), and mixed sleep apnea (log-rank P = .12), showed that time to recurrence of AF was not significantly different between groups.
Univariate Analysis
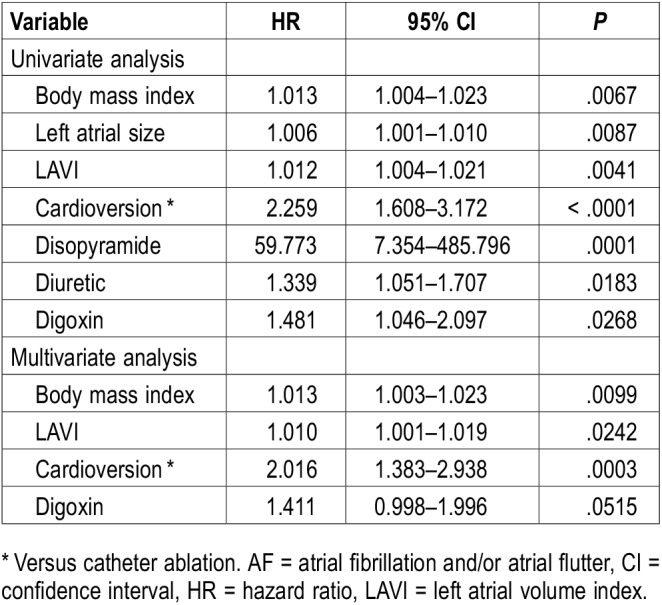
Table 3 summarizes the statistically significant univariate associations between the outcome variables and recurrent AF (P < .05). Patients with higher BMI, left atrial size, and LAVI, and those treated with disopyramide, diuretic, or digoxin had a higher risk of AF recurrence during follow-up. From a procedural perspective, patients who underwent cardioversion had higher risk of recurrence of AF compared with catheter ablation (hazard ratio [HR] 2.26, 95% confidence interval [CI] 1.61–3.17; P < .0001). None of the other demographic, clinical, or polysomnographic variables in Table 1 and Table 2 were found to be significantly associated with AF recurrence in univariate analyses.
Table 3.
Univariate and multivariate analyses of predictors of first-time recurrence of AF after AF intervention.
Multivariate Analysis
Table 3 summarizes borderline and statistically significant associations on multivariate analyses including covariates of BMI, left atrial size, LAVI, type of intervention (cardioversion compared with catheter ablation), and use of diuretics and digoxin. We did not include disopyramide in the analysis because of a limited number of study participants. Type of intervention (cardioversion compared to catheter ablation) was the strongest independent predictor of recurrent AF (HR 2.016, 95% CI 1.38–2.94, P = .0003). Higher BMI (P = .010) and LAVI (P = .024) were also significant predictors of recurrent AF in adjusted analyses; there was a borderline significant association between the use of digoxin and increased risk of recurrence of AF (P = .052). In multiple regression analyses additionally accounting for PAP adherence as a covariate and any PAP usage instead of PAP adherence, results were similar (not shown).
In female participants, among the variables of BMI, LAVI, type of intervention (cardioversion compared with catheter ablation), and use of digoxin, only BMI was an independent predictor of recurrent AF after intervention (HR 1.027, 95% CI 1.001–1.053, P = .0412) (Table S1 in the supplemental material).
In male participants, among the variables of BMI, LAVI, type of intervention (cardioversion compared with catheter ablation), and use of digoxin, only type of intervention was an independent predictor of recurrent AF after intervention (HR 1.926, 95% CI 1.25–2.97, P = .003) (Table S2 in the supplemental material).
DISCUSSION
Main Findings
In the largest sample studied to date of a rigorously abstracted sample of patients with SDB and AF, we showed no effect of PAP treatment of SDB in those adherent to the use of the device on time to first ECG-documented AF recurrence following cardioversion or catheter ablation. This finding is important because, in the absence of supporting high-level evidence, many patients with AF are referred for SDB testing and treatment, at significant expense and resource utilization, in the hopes of altering AF outcomes.
As our findings contradict other published studies,13–15 including one from our own institution,16 which show SDB treatment effects on important AF outcomes, we should draw attention to methodologic distinctions. Our sample size of patients proven to have both AF and SDB by attended PSG is larger and more detailed than any other. Our study is the first to determine the effects of PAP therapy in terms of duration of recurrence of AF after intervention.10 Prior studies that analyzed the effect of PAP therapy in recurrence of AF in patients with OSA focused on risk factors and were inconsistent.16–19 Because of the nature of the disease and contemporary standard treatments, a randomized trial focused on AF in patients with OSA alone is a logistical challenge. A recent small randomized trial failed to show a difference in time to recurrence of AF in treated versus untreated SDB.20 Our study excluded patients who did not undergo intervention for their atrial fibrillation. It is conceivable that the effects of treatment of SDB may have been more pronounced in patients whose AF was managed using a rate-control strategy rather than cardioversion or catheter ablation. Finally, the patients in our study were generally not sleepy and a previous study also showed that most patients with SDB and AF do not report sleepiness; it is unknown whether sleepiness can affect the natural history of AF or modify the effects of treatment of SDB.21
Left Atrial Volume Index
Our study demonstrated that increase in LAVI was independently associated with an increase in the probability of recurrent AF intervention, but the strength of association was small. OSA can contribute to left atrial enlargement by sudden negative intrathoracic pressures that lead to repetitive atrial stretch and gradually to left atrial enlargement.22 As a result of left atrial enlargement, OSA can contribute to remodeling at the pulmonary vein ostia, a site known to initiate and propagate AF.23 There is sparse literature on the effects of PAP therapy on cardiac structure, in particular, LAVI.24
Medications
In our study, any concomitant treatment was left to the treating clinicians’ discretion. As a consequence, any apparent effect of drugs such as diuretics, antiarrhythmics, beta blockers, and angiotensin-converting enzyme inhibitors on AF recurrence could be biased by the indications for their use. There have been changes in the recommendations regarding the treatment of AF over the years.25–27 Additionally, it is possible that there were changes in the dosage and adherence to antiarrhythmic medications during the follow-up period in the two groups. Nevertheless, we observed a few interesting findings. Disopyramide was associated with a significant increase in the recurrence rate of AF but there were too few participants on this medication to be able to draw any meaningful conclusions. The relationship between diuretic use and increased AF recurrence was unexpected. The two main indications for diuretics are heart failure and hypertension. It is well known that diuretics can cause hypokalemia but there is limited information on whether it can increase the risk of arrhythmias.28–31 The use of diuretics could result in alterations in intravascular and atrial volumes which in turn could affect the development of arrhythmias.32,33 There are no studies reported in the literature that have investigated the potentially proarrhythmogenic effects of these medications in patients with a history of AF. In adjusted analyses, the hazard ratio for diuretic use was not significant; future studies on the effect of diuretic use on risk of recurrence of AF are needed to clarify this issue.
Our study showed a trend toward an increased risk of AF recurrence in those on digoxin. However, digoxin has poor, if any, effects for cardioversion or maintenance of sinus rhythm, with use being restricted for rate control in nonambulatory patients or patients with heart failure, because of its positive inotropic effects. Recently, digoxin use was found to be associated with increased risk of cardiovascular death, arrhythmic death, and stroke.34 Digoxin has a narrow therapeutic index and the heightened mortality risk may be related to dangerously high serum drug levels and/or lack of efficacy at subtherapeutic drug levels.
Body Mass Index and Age
There is a strong association between BMI and left atrial size35; the effect of obesity may thus be mediated by left atrial enlargement.36 Our study found each one-unit increase in BMI in patients with SDB was associated with a 13% independent increase in the probability of recurrent AF, after AF intervention; however, the strength of the association was relatively small. Our findings extend previous knowledge regarding the negative influence of obesity on AF.37–40 Weight reduction was also associated with a reduced risk for the development of new-onset AF in a previous study.38 Increased risk of AF recurrence in patients with a high BMI may affect the clinical management of AF. It should be noted that increasing age can affect recurrence of AF, but an association between age and recurrent AF was not noted in univariate analyses in our study.41
Limitations
There are some limitations to our study. This study has all the biases inherent in a retrospective investigation and may not be generalizable to all populations with AF. Second, the duration of AF before AF intervention was not considered; furthermore, recurrence of AF developed in many patients while on PAP therapy. Hence continuing PAP therapy may not have had a mitigating effect on AF recurrence. Third, a higher percentage of PAP-adherent users versus control patients underwent cardioversion; although we accounted for PAP adherence and type of intervention in multiple regression analyses looking at the outcome of AF recurrence in the entire sample, it is possible that type of intervention may have confounded the results. It should also be noted that PAP adherence was not obtained in nightly hours of usage and used as a dichotomous variable in analyses. Fourth, the control patients were a heterogeneous group and may have included those with less severe disease, fewer symptoms and/or insufficient understanding of their disease or its consequences and perhaps more likely to be nonadherent with treatment recommendations in general. Last, the definitions of hypopneas have changed over time, which was not accounted for and this may have affected results.
In conclusion, our retrospective study did not demonstrate an effect of PAP treatment of SDB in those who were adherent to the device on first-documented electrocardiography recurrence of AF postintervention. It remains to be determined whether aggressive management of SDB will result in improvement in clinical efficacy of AF intervention in terms of time to recurrence. BMI is linked to OSA and other variables, such as left atrial enlargement, which promote AF. Our study demonstrated increased risk of recurrence of AF postintervention in patients with high BMI. This finding may affect the clinical management of AF.
DISCLOSURE STATEMENT
All authors have seen and approved the manuscript. Work for this study was performed at Mayo Clinic, Rochester, Minnesota, USA. VKS is supported by research grants from the National Institutes of Health (HL 65176, HL 134808, HL 134885). MPM is the principal investigator on a research grant funded by ResMed Foundation that is unrelated to the current study and the recipient of the Paul and Ruby Tsai and Family Fund Career Development Award at Mayo Clinic, Rochester, Minnesota. VKS is a Consultant for Respicardia, ResMed, U-Health, GlaxoSmithKline, Roche, and Bayer. He is an investigator on the SERVE-HF Steering Committee and is working with Mayo Health Solutions and their industry partners on intellectual property related to sleep and cardiovascular disease. The Philips Respironics Foundation has provided a gift to the Mayo Foundation.
ABBREVIATIONS
- AF
atrial fibrillation and/or atrial flutter
- BMI
body mass index
- CSA
central sleep apnea
- ECG
electrocardiography
- LAVI
left atrial volume index
- OSA
obstructive sleep apnea
- PAP
positive airway pressure
- PSG
polysomnography
- SDB
sleep-disordered breathing
REFERENCES
- 1.Wolf PA, Benjamin EJ, Belanger AJ, Kannel WB, Levy D, D’Agostino RB. Secular trends in the prevalence of atrial fibrillation: The Framingham Study. Am Heart J. 1996;131(4):790–795. doi: 10.1016/s0002-8703(96)90288-4. [DOI] [PubMed] [Google Scholar]
- 2.Bixler EO, Vgontzas AN, Lin HM, et al. Prevalence of sleep-disordered breathing in women: effects of gender. Am J Respir Crit Care Med. 2001;163(3 Pt 1):608–613. doi: 10.1164/ajrccm.163.3.9911064. [DOI] [PubMed] [Google Scholar]
- 3.Shamsuzzaman AS, Gersh BJ, Somers VK. Obstructive sleep apnea: implications for cardiac and vascular disease. JAMA. 2003;290(14):1906–1914. doi: 10.1001/jama.290.14.1906. [DOI] [PubMed] [Google Scholar]
- 4.Gami AS, Pressman G, Caples SM, et al. Association of atrial fibrillation and obstructive sleep apnea. Circulation. 2004;110(4):364–367. doi: 10.1161/01.CIR.0000136587.68725.8E. [DOI] [PubMed] [Google Scholar]
- 5.Braga B, Poyares D, Cintra F, et al. Sleep-disordered breathing and chronic atrial fibrillation. Sleep Med. 2009;10(2):212–216. doi: 10.1016/j.sleep.2007.12.007. [DOI] [PubMed] [Google Scholar]
- 6.Javaheri S, Parker TJ, Liming JD, et al. Sleep apnea in 81 ambulatory male patients with stable heart failure. Types and their prevalences, consequences, and presentations. Circulation. 1998;97(21):2154–2159. doi: 10.1161/01.cir.97.21.2154. [DOI] [PubMed] [Google Scholar]
- 7.Sin DD, Fitzgerald F, Parker JD, Newton G, Floras JS, Bradley TD. Risk factors for central and obstructive sleep apnea in 450 men and women with congestive heart failure. Am J Respir Crit Care Med. 1999;160(4):1101–1106. doi: 10.1164/ajrccm.160.4.9903020. [DOI] [PubMed] [Google Scholar]
- 8.Guilleminault C, Connolly SJ, Winkle RA. Cardiac arrhythmia and conduction disturbances during sleep in 400 patients with sleep apnea syndrome. Am J Cardiol. 1983;52(5):490–494. doi: 10.1016/0002-9149(83)90013-9. [DOI] [PubMed] [Google Scholar]
- 9.Nalliah CJ, Sanders P, Kalman JM. Obstructive sleep apnea treatment and atrial fibrillation: a need for definitive evidence. J Cardiovasc Electrophysiol. 2016;27(8):1001–1010. doi: 10.1111/jce.12981. [DOI] [PubMed] [Google Scholar]
- 10.Linz D, McEvoy RD, Cowie MR, et al. Associations of obstructive sleep apnea with atrial fibrillation and continuous positive airway pressure treatment: a review. JAMA Cardiol. 2018;3(6):532–540. doi: 10.1001/jamacardio.2018.0095. [DOI] [PubMed] [Google Scholar]
- 11.Weaver TE, Grunstein RR. Adherence to continuous positive airway pressure therapy: the challenge to effective treatment. Proc Am Thorac Soc. 2008;5(2):173–178. doi: 10.1513/pats.200708-119MG. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gay P, Weaver T, Loube D, et al. Evaluation of positive airway pressure treatment for sleep related breathing disorders in adults. Sleep. 2006;29(3):381–401. doi: 10.1093/sleep/29.3.381. [DOI] [PubMed] [Google Scholar]
- 13.Li L, Wang ZW, Li J, et al. Efficacy of catheter ablation of atrial fibrillation in patients with obstructive sleep apnoea with and without continuous positive airway pressure treatment: a meta-analysis of observational studies. Europace. 2014;16(9):1309–1314. doi: 10.1093/europace/euu066. [DOI] [PubMed] [Google Scholar]
- 14.Qureshi WT, Nasir UB, Alqalyoobi S, et al. Meta-analysis of continuous positive airway pressure as a therapy of atrial fibrillation in obstructive sleep apnea. Am J Cardiol. 2015;116(11):1767–1773. doi: 10.1016/j.amjcard.2015.08.046. [DOI] [PubMed] [Google Scholar]
- 15.Holmqvist F, Guan N, Zhu Z, et al. Impact of obstructive sleep apnea and continuous positive airway pressure therapy on outcomes in patients with atrial fibrillation-Results from the Outcomes Registry for Better Informed Treatment of Atrial Fibrillation (ORBIT-AF) Am Heart J. 2015;169(5):647–654.e2. doi: 10.1016/j.ahj.2014.12.024. [DOI] [PubMed] [Google Scholar]
- 16.Bitter T, Nolker G, Vogt J, Prinz C, Horstkotte D, Oldenburg O. Predictors of recurrence in patients undergoing cryoballoon ablation for treatment of atrial fibrillation: the independent role of sleep-disordered breathing. J Cardiovasc Electrophysiol. 2012;23(1):18–25. doi: 10.1111/j.1540-8167.2011.02148.x. [DOI] [PubMed] [Google Scholar]
- 17.Matiello M, Nadal M, Tamborero D, et al. Low efficacy of atrial fibrillation ablation in severe obstructive sleep apnoea patients. Europace. 2010;12(8):1084–1089. doi: 10.1093/europace/euq128. [DOI] [PubMed] [Google Scholar]
- 18.Kanagala R, Murali NS, Friedman PA, et al. Obstructive sleep apnea and the recurrence of atrial fibrillation. Circulation. 2003;107(20):2589–2594. doi: 10.1161/01.CIR.0000068337.25994.21. [DOI] [PubMed] [Google Scholar]
- 19.Abe H, Takahashi M, Yaegashi H, et al. Efficacy of continuous positive airway pressure on arrhythmias in obstructive sleep apnea patients. Heart Vessels. 2010;25(1):63–69. doi: 10.1007/s00380-009-1164-z. [DOI] [PubMed] [Google Scholar]
- 20.Caples SM, Mansukhani MP, Friedman PA, Somers VK. The impact of continuous positive airway pressure treatment on the recurrence of atrial fibrillation post cardioversion: a randomized controlled trial. Int J Cardiol. 2019;278:133–136. doi: 10.1016/j.ijcard.2018.11.100. [DOI] [PubMed] [Google Scholar]
- 21.Albuquerque FN, Calvin AD, Sert Kuniyoshi FH, et al. Sleep-disordered breathing and excessive daytime sleepiness in patients with atrial fibrillation. Chest. 2012;141(4):967–973. doi: 10.1378/chest.11-0975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Otto ME, Belohlavek M, Romero-Corral A, et al. Comparison of cardiac structural and functional changes in obese otherwise healthy adults with versus without obstructive sleep apnea. Am J Cardiol. 2007;99(9):1298–1302. doi: 10.1016/j.amjcard.2006.12.052. [DOI] [PubMed] [Google Scholar]
- 23.Haïssaguerre M, Jais P, Shah DC, et al. Spontaneous initiation of atrial fibrillation by ectopic beats originating in the pulmonary veins. N Engl J Med. 1998;339(10):659–666. doi: 10.1056/NEJM199809033391003. [DOI] [PubMed] [Google Scholar]
- 24.Neilan TG, Farhad H, Dodson JA, et al. Effect of sleep apnea and continuous positive airway pressure on cardiac structure and recurrence of atrial fibrillation. J Am Heart Assoc. 2013;2(6):e000421. doi: 10.1161/JAHA.113.000421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jacobs AK, Anderson JL, Halperin JL. The evolution and future of ACC/AHA clinical practice guidelines: a 30-year journey: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2014;64(13):1373–1384. doi: 10.1016/j.jacc.2014.06.001. [DOI] [PubMed] [Google Scholar]
- 26.January CT, Wann LS, Alpert JS, et al. 2014 AHA/ACC/HRS guideline for the management of patients with atrial fibrillation: a report of the American College of Cardiology/American Heart Association Task Force on practice guidelines and the Heart Rhythm Society. Circulation. 2014;130(23):e199–e267. doi: 10.1161/CIR.0000000000000041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kirchhof P, Benussi S, Kotecha D, et al. 2016 ESC Guidelines for the management of atrial fibrillation developed in collaboration with EACTS. Eur Heart J. 2016;37(38):2893–2962. doi: 10.1093/eurheartj/ehw210. [DOI] [PubMed] [Google Scholar]
- 28.ALLHAT Officers and Coordinators for the ALLHAT Collaborative Research Group Major outcomes in moderately hypercholesterolemic, hypertensive patients randomized to pravastatin vs usual care: the Antihypertensive and Lipid-Lowering Treatment to Prevent Heart Attack Trial (ALLHAT-LLT) JAMA. 2002;288(23):2998–3007. doi: 10.1001/jama.288.23.2998. [DOI] [PubMed] [Google Scholar]
- 29.Emara MK, Saadet AM. Transient atrial fibrillation in hypertensive patients with thiazide induced hypokalaemia. Postgrad Med J. 1986;62(734):1125–1127. doi: 10.1136/pgmj.62.734.1125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Papademetriou V, Burris JF, Notargiacomo A, Fletcher RD, Freis ED. Thiazide therapy is not a cause of arrhythmia in patients with systemic hypertension. Arch Intern Med. 1988;148(6):1272–1276. [PubMed] [Google Scholar]
- 31.Law MR, Wald NJ, Morris JK, Jordan RE. Value of low dose combination treatment with blood pressure lowering drugs: analysis of 354 randomised trials. BMJ. 2003;326(7404):1427. doi: 10.1136/bmj.326.7404.1427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Strobeck JE, Feldschuh J, Miller WL. Heart failure outcomes with volume-guided management. JACC Heart Fail. 2018;6(11):940–948. doi: 10.1016/j.jchf.2018.06.017. [DOI] [PubMed] [Google Scholar]
- 33.Takemoto Y, Ramirez RJ, Kaur K, et al. Eplerenone reduces atrial fibrillation burden without preventing atrial electrical remodeling. J Am Coll Cardiol. 2017;70(23):2893–2905. doi: 10.1016/j.jacc.2017.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Qureshi W, O’Neal WT, Soliman EZ, Al-Mallah MH. Systematic review and meta-analysis of mortality and digoxin use in atrial fibrillation. Cardiol J. 2016;23(3):333–343. doi: 10.5603/CJ.a2016.0016. [DOI] [PubMed] [Google Scholar]
- 35.Pritchett AM, Jacobsen SJ, Mahoney DW, Rodeheffer RJ, Bailey KR, Redfield MM. Left atrial volume as an index of left atrial size: a population-based study. J Am Coll Cardiol. 2003;41(6):1036–1043. doi: 10.1016/s0735-1097(02)02981-9. [DOI] [PubMed] [Google Scholar]
- 36.Vaziri SM, Larson MG, Benjamin EJ, Levy D. Echocardiographic predictors of nonrheumatic atrial fibrillation. The Framingham Heart Study. Circulation. 1994;89(2):724–730. doi: 10.1161/01.cir.89.2.724. [DOI] [PubMed] [Google Scholar]
- 37.Jongnarangsin K, Chugh A, Good E, et al. Body mass index, obstructive sleep apnea, and outcomes of catheter ablation of atrial fibrillation. J Cardiovasc Electrophysiol. 2008;19(7):668–672. doi: 10.1111/j.1540-8167.2008.01118.x. [DOI] [PubMed] [Google Scholar]
- 38.Berkovitch A, Kivity S, Klempfner R, et al. Body mass index and the risk of new-onset atrial fibrillation in middle-aged adults. Am Heart J. 2016;173:41–48. doi: 10.1016/j.ahj.2015.11.016. [DOI] [PubMed] [Google Scholar]
- 39.Gami AS, Hodge DO, Herges RM, et al. Obstructive sleep apnea, obesity, and the risk of incident atrial fibrillation. J Am Coll Cardiol. 2007;49(5):565–571. doi: 10.1016/j.jacc.2006.08.060. [DOI] [PubMed] [Google Scholar]
- 40.Wanahita N, Messerli FH, Bangalore S, Gami AS, Somers VK, Steinberg JS. Atrial fibrillation and obesity–results of a meta-analysis. Am Heart J. 2008;155(2):310–315. doi: 10.1016/j.ahj.2007.10.004. [DOI] [PubMed] [Google Scholar]
- 41.Go AS, Hylek EM, Phillips KA, et al. Prevalence of diagnosed atrial fibrillation in adults: national implications for rhythm management and stroke prevention: the AnTicoagulation and Risk Factors in Atrial Fibrillation (ATRIA) study. JAMA. 2001;285(18):2370–2375. doi: 10.1001/jama.285.18.2370. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.