Abstract
We present the case of a 12-year-old girl with medically refractory epilepsy and a vagal nerve stimulator (VNS), who experienced severe obstructive sleep apnea (OSA) with respiratory events closely matching her VNS settings. We demonstrated a real-time decrease in OSA through an in-laboratory VNS titration study, decreasing her VNS frequency from 20 Hz to 10 Hz. We were able to demonstrate a baseline level of OSA by turning off the VNS. We then effectively treated her residual OSA with continuous positive airway pressure (CPAP). Novel to our case is that this in-laboratory VNS titration did not result in any subsequent increase in seizure frequency. After 5 months, her seizure frequency had decreased. Our case demonstrates that in-laboratory VNS titration can be an efficient tool for optimizing treatment of VNS-induced OSA and assert that polysomnography before VNS placement is important for guiding future care.
Citation:
Chan J, Owens JW, Wrede JE. Case of an in-laboratory vagal nerve stimulator titration for vagal nerve stimulator-induced pediatric obstructive sleep apnea. J Clin Sleep Med. 2019;15(10):1539–1542.
Keywords: obstructive sleep apnea, pediatric, polysomnography, titration, vagal nerve stimulator, VNS
INTRODUCTION
Vagal nerve stimulators (VNS) have proved to be helpful in reducing seizure frequency in some children with medically refractory epilepsy.1 Side effects include voice alteration, hoarseness, sore throat, cough, dyspnea, nausea, vomiting, headache, and paresthesias.1 One of the lesser-discussed side effects is sleep-disordered breathing, including both obstructive sleep apnea (OSA) and central sleep apnea.2–4 We report a case of a pediatric patient with VNS-related OSA who underwent a successful in-laboratory polysomnography (PSG) titration of VNS and continuous positive airway pressure (CPAP) without subsequent increase in seizure frequency.
REPORT OF CASE
A 6-year old girl presented to the sleep medicine clinic for evaluation for possible sleep disordered breathing. Her pertinent past medical history included tuberous sclerosis complex with developmental delay and pharmacoresistant epilepsy requiring three seizure medications (lamotrigine, zonisamide, and clobazam at moderate to high therapeutic doses), as well as VNS implanted 1.5 years prior to presentation. Her current VNS settings included an output current of 1.75 mA, frequency of 20 Hz, on-time of 14 seconds, and off-time of 0.5 minutes. She was referred to the clinic because of soft snoring that started about 6 months prior to presentation as well as witnessed breathing pauses, and mouth breathing during sleep. She mostly slept in the supine and the lateral positions, but sometimes slept cross-legged and folded forward. She had periods of insomnia as well as days where she would sleep an entire 24-hour period. Physical examination was notable for body mass index of 13.3 kg/m2 (third percentile), and oxygen saturation was 97% while awake on room air. The patient was nonverbal and nonambulatory. She had no facial deformities or nasal congestion. Tonsils were small at 1+. Chest was clear to auscultation. No scoliosis was noted.
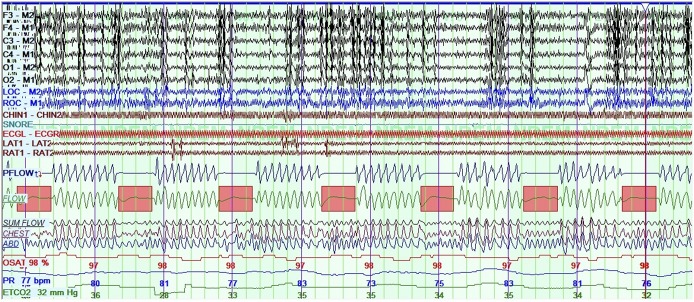
The patient underwent diagnostic PSG using standard pediatric scoring criteria, which showed severe OSA with a baseline apnea-hypopnea index (AHI) of 59.8 events/h, obstructive AHI of 59.8 events/h, central apnea index of 0 events/h, a normal 3% oxygen desaturation index of just 3.5 events/h, and minimum saturation of 91.7%. Total sleep time was 429.5 minutes, sleep efficiency was 81.2%, arousal index 4.3 events/h (with caveats discussed in the next paragraphs), periodic limb movement index 0 events/h, and end-tidal and transcutaneous carbon dioxide levels generally ranged from 34 mmHg to 41 mmHg, with a maximum of 45 mmHg. Most scored respiratory events were obstructive apneas. There were many more episodes highly suspicious for obstructive hypopneas; however, electroencephalography could not consistently be used to score arousals because of abnormal background, recurrent epileptiform discharges, and paucity of normal sleep architecture. There were pulse rate fluctuations with those suspicious events, suggesting some level of physiologic arousal. Scored events were more frequent in supine sleep. Upon detailed review of raw data, the patient had a recurrent pattern of airflow decrement lasting about 14 seconds and recurring after 30 seconds, which was seen throughout the entire study (Figure 1). These episodes corresponded well with her VNS settings, which had a 14-second on-time followed by a 0.5-minute off-time. There was no change in chin tone or electroencephalography results with these events. There was no significant flow limitation or additional respiratory events between the presumed VNS-triggered events. Initial treatment considerations included first determining whether sleep apnea could be improved with changing VNS settings, as approved by the patient’s primary epileptologist. We arranged for PSG with real-time VNS titration. The plan was to titrate CPAP as well if there were any residual OSA, to determine optimal pressures. Surgical options were not yet considered because tonsils were small and it was unclear whether baseline OSA was present.
Figure 1. Baseline polysomnogram with VNS at 20 Hz.
Baseline polysomnogram while at initial VNS frequency of 20 Hz, shown at 5 minutes per screen. A dramatic time-locked obstructive apnea pattern is seen matching the patient’s VNS settings with associated changes in pulse rate, little to no desaturations, and no detectable change in chin tone. Scored obstructive apneas are indicated with pink rectangles. F3, F4, C3, C4, O1, O2, M1, M2 = electroencephalography leads, LOC and ROC = ocular leads, Chin = chin electromyography LAT and RAT = left and right anterior tibialis EMG, Pflow = nasal airflow pressure, Flow = thermistor, Chest and abdomen = inductance plethysmography bands, OSAT = percent oxygen saturation, PR = pulse rate, VNS = vagal nerve stimulator.
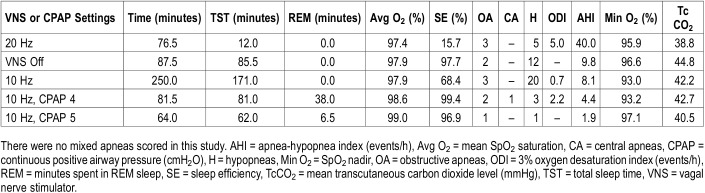
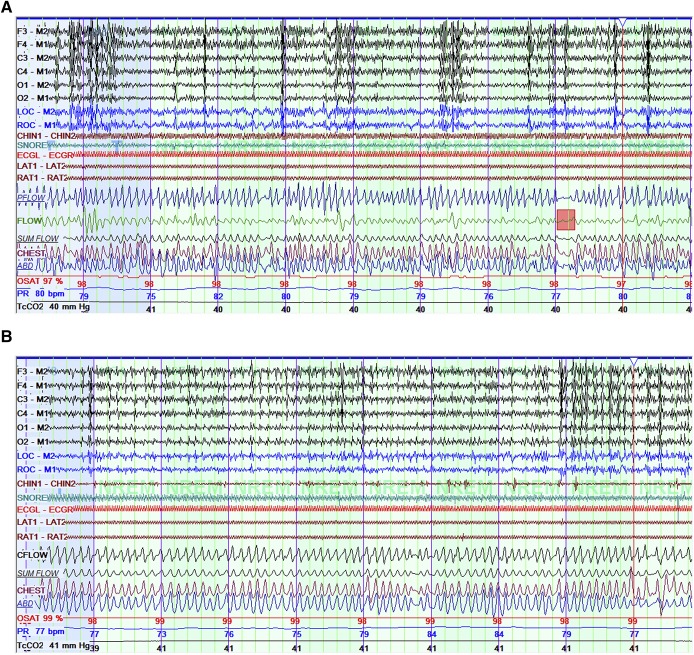
A repeat overnight sleep study was performed 3 weeks later (Table 1), with the patient’s epileptologist present for titration of her VNS settings. For each change, her epileptologist entered the room and placed the wand over the VNS to interrogate it. Changes were made with a tablet to reprogram the VNS during the interrogation. A baseline portion at current VNS settings included limited sleep time, but again demonstrated the previously observed pattern, with an extrapolated AHI of 40 events/h and additional unscorable episodes of airflow decrement as previously described. The frequency of the patient’s VNS was then decreased from 20 Hz to 10 Hz, which showed a decrease in AHI to 8.1 events/h, and resolution of apparent VNS-dependent pattern (Figure 2). VNS was turned off for 86 minutes, with a resultant AHI of 9.8 events/h showing that she had residual OSA at baseline similar to that seen at 10 Hz, implying VNS effect at 10 Hz was negligible. VNS was turned on again at 10 Hz. To manage the residual OSA, CPAP was added at 4 cmH2O, with residual AHI 4.4 events/h. CPAP was increased to 5 cmH2O with reduction of AHI to 1.9 events/h.
Table 1.
Vagal nerve stimulator and continuous positive airway pressure titration study.
Figure 2. Titration polysomnogram with VNS at 10 Hz.
(A) Titration polysomnogram at VNS 10 Hz, no continuous positive airway pressure (CPAP), shown at 5 minutes per screen. This shows resolution of the prior pattern of time-locked respiratory events, but with ongoing evidence of sleep-disordered breathing, including a brief obstructive apnea (pink rectangle). (B) Improvement of obstructive respiratory patterns on CPAP of 5 cmH2O. VNS = vagal nerve stimulator.
The patient was subsequently treated as an outpatient with CPAP with an auto-titrating pressure range of 5 to 7 cmH2O and a changed VNS frequency of 10 Hz. At follow-up 4 months after the titration, she was compliant with CPAP, having used it 100% of the prior 30 days with average usage on days used of 9 hours 20 minutes, median pressure 6.0 cm H2O. Her mother reported that with CPAP use, the patient awoke less often during the night, more often awoke independently in the morning, and had improved daytime energy. Her seizure frequency had significantly improved from three to four seizures daily as documented 1 month prior to titration study, to no seizures since 3 weeks after the titration. This could be because of improvement of her OSA5 or perhaps simply the natural history of her epilepsy or delayed effects of medication changes (although she had no change in seizure medications in the prior 12 months, she had initiated fluoxetine 2 months prior to titration study, which can increase clobazam levels). Her decrease in seizures would not likely be attributable to the change in her VNS settings as a decrease in frequency by itself is expected to increase the risk of seizures. Although the source of improvement could be multifactorial, it is important to note that seizures certainly did not worsen with the decreased VNS settings.
DISCUSSION
VNS is a useful tool for medically refractory epilepsy, but its side effects can include worsening of sleep-disordered breathing, which has been shown in adults2 as well as children.3,4 How VNS causes worsening of OSA is not clearly understood, though various theories have been proposed involving action of VNS on central respiratory centers and peripheral stimulation of vagus nerve afferents to the laryngeal and pharyngeal muscles, with measured effects including increased respiratory rate, decreased respiratory amplitude, decreased tidal volume, and decreased oxygen saturation.2,6,7 Our case study is novel because we were able to perform an in-laboratory VNS titration on a pediatric patient to eliminate any detectable VNS-induced OSA, and then optimize treatment of residual baseline OSA with a CPAP titration, all without increasing long-term seizure frequency. We chose to change the output frequency rather than the duty cycle based in part on the rationale that spreading out the timing of VNS discharges would likely result in spreading out respiratory events, but the events would likely still occur. In contrast, it has been shown that output frequency affects the severity of airflow obstruction, such that decreasing frequency decreases the likelihood that any given VNS discharge would result in an obstructive event.6 Two other adult studies have reported in-laboratory VNS titrations, which decreased the frequency of sleep apnea events by reducing the stimulus frequency6 or decreasing the VNS output current from 1 milliamp to 0.5 milliamps.8 Only one report of an in-laboratory VNS titration in a pediatric patient was found. This report demonstrated the ability to decrease VNS output current during PSG in a 12-year-old patient who did not tolerate CPAP, but the VNS had to be increased in a subsequent outpatient clinic because of worsening seizure frequency.9
Other studies have shown that different VNS settings can have differing effects on OSA. Gschliesser et al10 demonstrated that rapid cycling mode was associated with increased respiratory effort-related arousals in comparison with standard method in two patients. Interestingly, in a case series involving a 9-year-old boy who had apneic episodes due to vocal cord adduction caused by VNS stimulation, increasing the stimulus frequency from 20 Hz to 30 Hz actually diminished the apneic episodes.7
One limitation to our study is that we were unable to time-lock the apneic events with VNS discharges. This was inferred, however, as frequency of apneic events precisely correlated with duty cycle of our patient’s VNS. In the future, we would consider the use of an additional surface electromyography electrode on the left side of the neck in order to visualize the VNS discharge.
Our experience with this patient showed that it is possible to reduce a patient’s AHI by decreasing the VNS frequency during an in-laboratory titration without increasing long-term seizure burden. Other options include increasing the off-time to decrease the number of stimulation events. Treatment of OSA is considered especially important in those with epilepsy, because it has been associated with decreased seizure frequency and severity.5 However, the need for treatment of OSA must be also balanced with other modes of seizure control as some patients have worsening of the epilepsy with VNS setting changes.9 This requires close coordination and availability of a trained epileptologist and sleep medicine physician. In many cases, this may require outpatient serial adjustments of VNS settings, confirmed in turn on PSG. When VNS titration can be performed in real time in the laboratory environment, this can be an efficient and resource conserving method of optimizing treatment. We assert that it would also be helpful to routinely perform PSG prior to VNS placement in children with medically refractory epilepsy, to establish baseline OSA burden and better determine what later component of OSA may be attributable to VNS.
DISCLOSURE STATEMENT
All authors have seen and approved the manuscript. This work was performed through Seattle Children’s Hospital, Seattle, Washington. The authors report no conflicts of interest.
ABBREVIATIONS
- AHI
apnea-hypopnea index
- CPAP
continuous positive airway pressure
- OSA
obstructive sleep apnea
- PSG
polysomnography
- VNS
vagal nerve stimulator
REFERENCES
- 1.Milby AH, Halpern CH, Baltuch GH. Vagus nerve stimulation in the treatment of refractory epilepsy. Neurotherapeutics. 2009;6(2):228–237. doi: 10.1016/j.nurt.2009.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Parhizgar F, Nugent K, Raj R. Obstructive sleep apnea and respiratory complications associated with vagus nerve stimulators. J Clin Sleep Med. 2011;7(4):401–407. doi: 10.5664/JCSM.1204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hsieh T, Chen M, McAfee A, Kifle Y. Sleep-related breathing disorder in children with vagal nerve stimulators. Pediatr Neurol. 2008;38(2):99–103. doi: 10.1016/j.pediatrneurol.2007.09.014. [DOI] [PubMed] [Google Scholar]
- 4.Khurana DS, Reumann M, Hobdell EF, et al. Vagus nerve stimulation in children with refractory epilepsy: unusual complications and relationship to sleep-disordered breathing. Childs Nerv Syst. 2007;23(11):1309–1312. doi: 10.1007/s00381-007-0404-8. [DOI] [PubMed] [Google Scholar]
- 5.Malow BA, Weatherwax KJ, Chervin RD, et al. Identification and treatment of obstructive sleep apnea in adults and children with epilepsy: a prospective pilot study. Sleep Med. 2003;4(6):509–515. doi: 10.1016/j.sleep.2003.06.004. [DOI] [PubMed] [Google Scholar]
- 6.Malow BA, Edwards J, Marzec M, Sagher O, Fromes G. Effects of vagus nerve stimulation on respiration during sleep: a pilot study. Neurology. 2000;55(10):1450–1454. doi: 10.1212/wnl.55.10.1450. [DOI] [PubMed] [Google Scholar]
- 7.Aron M, Vlachos-Mayer H, Dorion D. Vocal cord adduction causing obstructive sleep apnea from vagal nerve stimulation: case report. J Pediatr. 2012;160(5):868–870. doi: 10.1016/j.jpeds.2012.01.045. [DOI] [PubMed] [Google Scholar]
- 8.St Louis EK, Faber K. Reversible sleep-related stridor during vagus nerve stimulation. Epileptic Disord. 2010;12(1):76–80. doi: 10.1684/epd.2010.0294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Upadhyay H, Bhat S, Gupta D, Mulvey M, Ming S. The therapeutic dilemma of vagus nerve stimulator-induced sleep disordered breathing. Ann Thorac Med. 2016;11(2):151–154. doi: 10.4103/1817-1737.180025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gschliesser V, Högl B, Frauscher B, Brandauer E, Poewe W, Luef G. Mode of vagus nerve stimulation differentially affects sleep related breathing in patients with epilepsy. Seizure. 2009;18(5):339–342. doi: 10.1016/j.seizure.2008.12.003. [DOI] [PubMed] [Google Scholar]