Abstract
Objective:
Up to 60% of patients with bipolar disorder develop a substance use disorder during their lifetime. The purpose of this paper was to assess the impact of substance use disorders on depression recovery among bipolar patients randomly assigned to different psychotropic medications and psychosocial interventions. We hypothesized that patients with a comorbid substance use disorder would benefit less from psychotherapy regardless of treatment intensity/length compared to patients without a comorbid substance use disorder.
Method:
We conducted post hoc analyses among bipolar disorder patients (n = 270) with and without comorbid substance use disorders enrolled in the Systematic Treatment Enhancement Program for Bipolar Disorder randomized psychosocial intervention trial. All patients entered during or shortly after the onset of a bipolar depressive episode. Logistic regression and Cox proportional hazard models were used to assess whether current or past substance use disorders moderated the response of patients to intensive psychosocial intervention or brief psychoeducation with collaborative care, operationalized as full recovery from an episode of bipolar depression.
Results:
Current comorbid substance use disorders significantly predicted likelihood of recovery (odds ratio = 2.25, p = 0.025) and time to recovery (odds ratio = 1.71, p = 0.006) from bipolar depression. We found that 74.5% of patients with a current substance use disorder, compared to 56.5% without a current substance use disorder, recovered from bipolar depression. Past substance use disorders did not predict likelihood of recovery or time to recovery. Current substance use disorders did not significantly moderate response to intensive psychotherapy versus collaborative care.
Conclusion:
Contrary to our hypotheses, bipolar disorder participants with a current comorbid substance use disorder were more likely to recover from psychosocial treatment for bipolar depression than patients without a current comorbid substance use disorder. If this finding is replicated, it has implications for the ordering of treatment for patients with comorbid bipolar disorder and substance use disorders.
Keywords: Bipolar disorder, substance use disorders, alcohol use disorders, drug use disorders, psychotherapy
Introduction
Comorbid psychiatric conditions are a common feature of bipolar disorder (BD) with estimated rates of the presence of at least one comorbid psychiatric disorder ranging between 65% and 95% (Kessler, 1999; McElroy et al., 2001; Sagman and Tohen, 2009). Substance use disorders (SUDs), including drug and alcohol use disorders, represent one of the most common psychiatric comorbidities in BD with data suggesting that up to 60% of BD patients will develop an SUD during their lifetime (Tolliver, 2010). Relative to patients with BD who do not have comorbid SUDs, BD patients with a comorbid SUD experience earlier onset of mood symptoms, increased frequency of mood episodes, greater severity of illness course, more suicide attempts, greater number of hospitalizations, increased treatment non-adherence and greater financial expenses (Jaffee et al., 2009; McElroy et al., 2001; Mitchell et al., 2007; Nery and Soares, 2011; Salloum and Thase, 2000; Tolliver, 2010). Given the particularly high illness burden associated with comorbid SUDs in BD, increased understanding of the role of comorbid SUDs on BD treatment response is of considerable clinical importance. However, diagnosis of a current SUD is an exclusion criterion in many BD clinical trials, making it difficult to study these comorbid conditions on a broad scale (Cerullo and Strakowski, 2007).
In the Systematic Treatment Enhancement Program for Bipolar Disorder (STEP-BD), one of the largest multi-center studies of treatment effectiveness, participants had the option to participate in naturalistic follow-up studies and/or a trial that evaluated the efficacy of intensive psychotherapy versus a brief psychoeducation-based intervention (collaborative care) for treating bipolar patients with current depression (Miklowitz et al., 2007; Sachs et al., 2003). Overall, study outcomes revealed that intensive psychotherapy was superior to a collaborative care condition in yielding higher rates of recovery and a faster time to recovery. Secondary analyses revealed moderators of this effect: presence of a current anxiety disorder and number of prior episodes (Deckersbach et al., 2014; Peters et al., 2014).
Concerning the predictive significance of the presence of SUDs in BD, to date, two STEP papers have evaluated comorbid SUDs in a sample of STEP-BD participants followed naturalistically. Participants were evaluated by a clinician at each visit (the timing of which was determined based on clinical necessity given the naturalistic design of the study) who noted current mood status through use of a Clinical Monitoring Form (CMF) that assessed for Diagnostic and Statistical Manual of Mental Disorders (4th ed.; DSM-IV) depressive, manic, hypomanic or mixed states in the prior 14 days. Weiss et al. (2005) evaluated SUD recovery among the first 1000 participants enrolled in the naturalistic STEP-BD clinical monitoring study and found that participants with either a current or past SUD were less likely to achieve recovery over an ~1-year period than participants without an SUD. Recovery was defined by two or fewer depressive, manic, or hypomanic symptoms for at least 8 weeks (Sachs et al., 2002, 2003). In a subsequent STEP-BD analysis of a larger sample of participants (N = 3750), Ostacher et al. (2010) found that bipolar participants with a current or past SUD did not differ in time to recovery relative to participants without a comorbid SUD but that the presence of a SUD was associated with an increased likelihood of switching into manic, hypomanic or mixed episode states.
To our knowledge, this is one of the first studies to examine SUDs as an independent predictor of psychosocial treatment response in a randomized controlled trial of patients with BD. The STEP-BD program allowed for the participation of individuals with comorbid SUDs in randomized psychosocial and pharmacological treatment. This design enabled us to examine, in a post hoc manner, the impact of SUDs on recovery from depression among participants. We examined (1) whether comorbid SUDs predict the likelihood of recovery or time until recovery from bipolar depression and (2) whether comorbid SUDs moderate participants’ response to psychotherapy for bipolar depression (e.g. intensive psychotherapy vs collaborative care). Although several review articles have cited naturalistic data suggesting that patients with comorbid BD and SUDs have worsened outcomes and illness course relative to patients with BD alone (Frye and Salloum, 2006; Levin and Hennessy, 2004; Nery and Soares, 2011; Salloum and Thase, 2000; Tolliver, 2010), the STEP-BD trial is unique in terms of allowing entry of participants with comorbid SUDs into a BD randomized treatment trial. As such, analysis of the STEP-BD data gives a unique perspective on how well bipolar patients can respond to a specialized treatment for BD. To our knowledge, few prior studies have examined the predictive significance of SUDs in a randomized trial of BD that involved examining BD outcomes in those with and without a comorbid SUD. Manwani et al. (2006) examined historical characteristics of rapid-cycling patients with BD on antidepressant medication who did or did not have past SUDs but they did not complete a prospective trial or examine the influence of current SUDs. Kilbourne et al. (2009) explored SUDs as a moderator of the effects of a collaborative care treatment incorporating pharmacotherapy and psychoeducation versus usual care (e.g. providers informed of general practice guidelines) on treatment outcomes but did not explore current SUDs as an independent predictor of treatment response. In a rare example of a prospective study examining the influence of current SUDs on treatment outcome, Inder and colleagues evaluated both current and past comorbid SUDs as an independent predictor of depression outcomes in patients with BD who were randomly assigned to interpersonal and social rhythm therapy or a control psychosocial treatment that incorporated supportive psychotherapy elements and was based on the American Psychiatric Association (APA) guidelines for the clinical care of BD. They found no main effect of either current or past comorbid SUDs on depression outcomes although there was an interaction with treatment group such that patients with a current comorbid SUD who received the control treatment improved more than those patients who received interpersonal and social rhythm therapy (Inder et al., 2015). Given data from previous naturalistic studies (e.g. observations outside the confines of a randomized trial of a targeted treatment), we hypothesized that comorbid SUDs would impair response to targeted psychotherapy for bipolar depression relative to patients without comorbid SUDs, regardless of the therapy’s intensity or length.
Methods
Study design
The STEP-BD psychosocial trial was a nested clinical trial within the larger STEP-BD study program. STEP-BD was a 22-site, 7-year longitudinal study program with 4361 participants that was aimed at discovering the most effective treatments or combination of treatments for preventing the onset and recurrence of mood episodes. These treatments were evaluated through several clinical trials embedded within the STEP-BD study program that assessed the effectiveness of various pharmacological treatments (e.g. mood stabilizers, antidepressants) and psychosocial interventions for BD (Sachs et al., 2003). The randomized, controlled STEP-BD psychosocial trial compared a 30-session, 9-month intensive psychotherapy treatment to a 3-session, 6-week psychoeducation-based treatment (collaborative care). Participants receiving intensive psychotherapy were randomly assigned to receive intensive psychotherapy (cognitive-behavioral therapy, interpersonal and social rhythm therapy, or family-focused treatment) or collaborative care (a brief intervention combining a variety of psychosocial approaches that had demonstrated effectiveness in treating BD) (Miklowitz and Otto, 2007). As the psychosocial trial revealed no statistically significant differences in responder rates among the three intensive psychotherapies, and these had many common elements (Miklowitz et al., 2007), these intensive psychotherapies were combined into a single ‘intensive psychotherapy’ metric for our moderator analyses.
Participants
Eligible participants were 18 years or older, met DSM-IV criteria for bipolar I or II disorder and a current major depressive episode, and were not currently receiving psychotherapy outside of the study. All diagnoses were assessed via the DSM-IV Mini International Neuropsychiatric Interview (MINI; Sheehan et al., 1998) and confirmed with information from the Affective Disorders Evaluation (Sachs et al., 2003). Exclusion criteria included a current DSM-IV mixed or depressive episode not otherwise specified, current pregnancy or a planned pregnancy within the following year, acute DSM-IV substance abuse or dependence disorder (excluding nicotine) requiring immediate treatment, and history of medical contraindication, nonresponse, or intolerance to bupropion or paroxetine. Patients with SUDs who did not require immediate treatment were eligible for this study. For a full description of study methodology, please refer to Miklowitz et al. (2007). Of the 293 participants enrolled in the STEP-BD psychosocial trial, 270 individuals provided data at their baseline session on comorbid alcohol use disorders and drug use disorders.
Measures
Primary outcome measure.
The CMF (Sachs et al., 2002) was used at all study sessions for participants receiving both intensive psychotherapy and collaborative care to assess symptoms of depression or mania. Intraclass inter-rater reliability coefficients (referenced to gold standard ratings for CMF depression and mania items) were in the range of 0.83–0.99. Designations of ‘recovery’ or ‘non-recovery’ were based on the presence or absence of DSMIV criteria for depression or mania/hypomania. Participants were designated as ‘recovered’ if they had two or fewer moderate mood symptoms for 8 or more consecutive weeks. Participants were designated as ‘non-recovered’ if they did not meet these criteria. The number of days from randomization until achievement of ‘recovered’ or ‘non-recovered’ status was recorded for each participant, with a maximum number of 365 days in the study.
Comorbid SUDs.
Information on current and past comorbid alcohol use disorders and drug use disorders was obtained from the MINI (Sheehan et al., 1998). Participants were noted as having a ‘past’ diagnosis if they met criteria for a past diagnosis of an alcohol use disorder or drug use disorder. Current alcohol use disorder, past alcohol use disorder, current drug use disorder and past drug use disorder were each coded as binary variables. Two additional, binary-coded SUD variables—current SUD and past SUD—were created based on these MINI data. Participants were recorded as having a current SUD if they met criteria for a current alcohol use disorder or drug use disorder. Participants were noted as having a past SUD if they met criteria for a past alcohol use disorder or drug use disorder.
Data analyses
To examine whether comorbid SUDs (combined metric of alcohol use disorders and drug use disorders) and alcohol use disorders or drug use disorders alone predicted likelihood of recovery or time until recovery from bipolar depression, we conducted logistic regression and Cox proportional hazard (survival) models. All analyses were by intention to treat. Participants were included until their final assessment point with a maximum of 365 days in the study (mean = 174.27, SD = 103.67; Sachs et al., 2003). The proportionality of risk assumption was met for all survival analyses. Current SUDs, past SUDs, current alcohol use disorders, past alcohol use disorders, current drug use disorders and past drug use disorders were independently evaluated in six exploratory regression models. In a second step for each of these models, we then examined whether comorbid alcohol use disorders, drug use disorders or SUDs moderated likelihood of recovery or time until recovery from bipolar depression; thus, we added an interaction term with treatment condition to our logistic regression and Cox survival models. For significant findings, we conducted post hoc logistic and Cox regression analyses to assess whether findings still held after controlling for multiple covariates. Selected covariates were clinical variables that had demonstrated an impact on treatment response in previous analyses of the STEP-BD psychosocial trial sample—these variables were comorbid anxiety, illness duration, age of BD onset, number of prior depressive episodes, number of prior manic episodes, current depression severity, current mania severity and medical burden.
Results
Study sample
Table 1 displays demographic and clinical characteristics of the STEP-BD randomized psychosocial treatment trial (n = 270). There were 23 participants who did not have current or past SUD scores. The mean age of the sample was 40.2 (SD = 11.6). Consistent with findings from the full sample of participants in the psychosocial trial (n = 293), in this subsample, intensive psychotherapy yielded a significantly greater likelihood of recovery (B = 0.51, Wald = 4.11, odds ratio [OR] = 1.66, p = 0.04, 95% confidence interval [CI] = [1.02, 2.71]) and a significantly faster time to recovery (B = 0.35, Wald = 4.77, OR = 1.42, p = 0.03, 95% CI = [1.04, 1.96]) than collaborative care. Baseline depression severity, as assessed via the CMF which assesses symptoms such as decreased interest and pleasure in most activities and presence of depressed mood to capture the severity of the depressive episode, did not significantly differ between participants with (n = 46, M = 2.36, SD = 2.21) and without (n = 219, M = 3.02, SD = 2.70) current SUDs or between participants with (n = 144, M = 2.91, SD = 2.59) and without (n = 121, M = 2.91, SD = 2.70) past SUDs.
Table 1.
Demographic characteristics of subset of patients in the STEP-BD psychosocial trial (n = 270).
| Overall (n = 270) | Intensive psychotherapya (n = 149) | Collaborative carea (n = 121) | |
|---|---|---|---|
| Age(M ± SD, n = 266) | 40.24 ± 11.62 | 41.21 ± 11.26 | 39.04 ± 12.00 |
| Female sex (N%, n = 266) | 59.8% | 61.2% | 58% |
| Race (N%, n = 266) | |||
| Caucasian/White | 94.4% | 93.2% | 95.8% |
| African American/Black | 3.4% | 3.4% | 3.4% |
| Asian/Pacific Islander | 0.8% | 1.4% | 0% |
| Native American | 0.4% | 0.68% | 0% |
| Other | 0.4% | 0.68% | 0% |
| Hispanic ethnicity (N%, n = 266) | 3.8% | 4.8% | 2.5% |
| Education > 1 year of college (N%, n = 262) | 80.5% | 81.9% | 78.9% |
| Marital status (N%, n = 265) | |||
| Married | 29.4% | 31.5% | 26.9% |
| Living as married | 3.0% | 2.1% | 4.2% |
| Never married | 36.2% | 32.9% | 40.3% |
| Separated | 7.2% | 8.2% | 5.9% |
| Divorced | 22.6% | 23.3% | 21.8% |
| Widowed | 1.5% | 2.1% | 0.84% |
| Diagnosis (N%, n = 269) | |||
| Bipolar I | 62.5% | 66% | 57.5% |
| Bipolar II or NOS | 37.5% | 34% | 42.5% |
| Depression severityb (M ± SD, n = 265) | 2.91 ± 2.63 | 2.83 ± 2.46 | 3.00 ± 2.84 |
| Mania severityb (M ± SD, n = 265) | 0.80 ± 1.15 | 0.77 ± 1.09 | 0.82 ± 1.22 |
| Substance use comorbidity (N%) | |||
| Any lifetime alcohol use disorder (AUD) | 49.6% | 53.7% | 43.8% |
| Any current AUD | 13.3% | 13.6% | 13.2% |
| Any lifetime drug use disorder (DUD) | 32.6% | 32.9% | 32.2% |
| Any current DUD | 7.8% | 8.1% | 7.4% |
| Any lifetime substance use disorder (SUD) | 54.8% | 59.1% | 49.6% |
| Any current SUD | 17.4% | 17.4% | 17.4% |
| Baseline medications (N%) | |||
| Antidepressants (n = 269) | 44.6% | 44.9% | 45% |
| Atypical antipsychotics (n = 269) | 28.3% | 30.9% | 25% |
| Benzodiazepines (n = 269) | 24.9% | 24.2% | 25.8% |
| Anticonvulsants (n = 269) | 54.3% | 51.7% | 57.5% |
| Lithium (n = 268) | 33.6% | 32.2% | 35.3% |
| Valproate (n = 269) | 32.3% | 29.5% | 35.8% |
| Other mood stabilizers (n = 269) | 28.6% | 28.2% | 29.2% |
| Stimulants (n = 269) | 1.7% | 2.0% | 1.7% |
STEP-BD: Systematic Treatment Enhancement Program for Bipolar Disorder; NOS: not otherwise specified; IP: intensive psychotherapy; CC: collaborative care.
The randomization formula to psychosocial treatment was 60:40 (IP:CC).
Depressive and mania severity rated via the depression (SUM-D) and mania (SUM-M) subscales of the Clinical Monitoring Form. Scores in this sample ranged from 0 to 13.5 on the SUM-D and 0 to 7.5 on the SUM-M with higher scores reflecting greater symptom severity.
Predictor analyses (SUDs as predictor of recovery and time to recovery from bipolar depression)
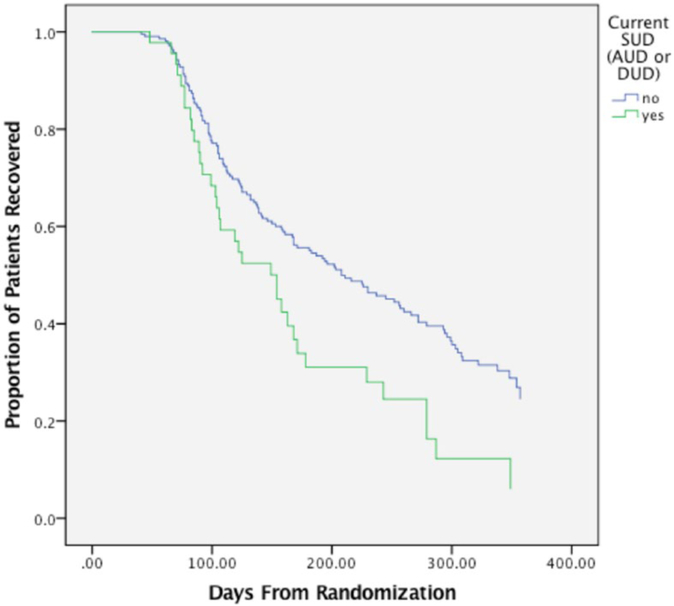
Results of the modeling sequence, including beta coefficients, Wald statistics, ORs, p values and 95% CIs, are shown in Table 2. When alcohol use disorders and drug use disorders were combined into a single metric of SUDs, current SUDs significantly predicted recovery likelihood (OR = 2.25, p = 0.025) and time to recovery (OR = 1.71, p = 0.006; Figure 1). Among participants with a current SUD, 74.5% recovered from bipolar depression over the study duration and 25.5% did not recover or dropped out of the study. For those participants without a current SUD, 56.5% recovered from bipolar depression and 43.5% did not recover or dropped out of the study. When alcohol use disorders and drug use disorders were evaluated separately, current comorbid alcohol use disorders and drug use disorders were a significant predictor of likelihood of recovery (alcohol use disorders: OR = 2.24, p = 0.048; drug use disorders: OR = 3.10, p = 0.047) and time to recovery (alcohol use disorders: OR = 1.70, p = 0.01, drug use disorders: OR = 1.81, p = 0.02) such that participants with a current comorbid alcohol use disorder or drug use disorder were more likely to recover and recovered faster than patients without a current comorbid alcohol use disorder or drug use disorder. There were no differences in likelihood of recovery or time until recovery among participants with or without a past comorbid SUD; this finding was maintained when alcohol use disorders and drug use disorders were evaluated in separate models. The predictor effect for current SUDs also remained robust after controlling for several clinical variables that have previously shown to impact treatment response in this sample (e.g. medical burden, comorbid anxiety, illness duration, age of BD onset, number of prior depressive episodes, number of prior manic episodes, baseline mania severity; Deckersbach et al., 2014; Peters et al., 2014, 2016) though the significance of current SUDs and current drug use disorders on time to recovery was not maintained after controlling for baseline depression severity. Post hoc analysis revealed that there was not a significant difference in baseline depression between SUD groups (p = 0.12, d = 0.27 for current SUD vs not; p = 0.11, d = 0.40 for current drug use vs not). Please refer to Table 3 (online data supplement) for covariate analyses findings.
Table 2.
Predictor analyses evaluating role of SUDs on likelihood of recovery and time until recovery.a
| b | Wald | OR | p | 95% CI | |
|---|---|---|---|---|---|
| Past AUD | |||||
| Logistic regression: predicting recovery | 0.29 | 1.35 | 1.34 | 0.25 | [0.82, 2.18] |
| Cox regression: predicting time until recovery | 0.17 | 1.19 | 1.19 | 0.28 | [0.87, 1.62] |
| Current AUD | |||||
| Logistic regression: predicting recovery | 0.81 | 3.92 | 2.24 | 0.05 | [1.01, 4.97] |
| Cox regression: predicting time until recovery | 0.53 | 6.31 | 1.70 | 0.01 | [1.12, 2.58] |
| Past DUD | |||||
| Logistic regression: predicting recovery | −0.03 | 0.02 | 0.97 | 0.90 | [0.58, 1.62] |
| Cox regression: predicting time until recovery | −0.02 | 0.009 | 0.98 | 0.92 | [0.70, 1.37] |
| Current DUD | |||||
| Logistic regression: predicting recovery | 1.13 | 3.93 | 3.10 | 0.05 | [1.01, 9.48] |
| Cox regression: predicting time until recovery | 0.59 | 5.26 | 1.81 | 0.02 | [1.09, 2.99] |
| Past SUDb | |||||
| Logistic regression: predicting recoveryb | 0.27 | 1.17 | 1.31 | 0.28 | [0.80, 2.13] |
| Cox regression: predicting time until recoveryb | 0.19 | 1.36 | 1.20 | 0.24 | [0.89, 1.65] |
| Current SUDb | |||||
| Logistic regression: predicting recoveryb | 0.81 | 5.03 | 2.25 | 0.03 | [1.11, 4.55] |
| Cox regression: predicting time until recoveryb | 0.54 | 7.70 | 1.71 | 0.01 | [1.17, 2.49] |
Note. The bolded values are statistically significant (p= .05 or p < .05).
OR: odds ratio; CI: confidence interval.
Predictor analyses evaluating role of SUDs on likelihood of recovery and time until recovery.
SUD includes AUD and DUD.
Figure 1.
Time to recovery from bipolar depression for patients with and without current comorbid SUDs.
Moderator analyses (SUDs as moderator of effects of intensive psychotherapy vs collaborative care on likelihood of recovery and time to recovery from bipolar depression)
Results of the modeling sequence are shown in Table 4 (online data supplement). In order to evaluate whether comorbid SUDs, alcohol use disorders alone, or drug use disorders alone moderated treatment response in this sample, we added an interaction term with treatment group (e.g. intensive psychotherapy vs collaborative care) to the models predicting likelihood of recovery and time until recovery. Neither current nor past SUDs, comorbid alcohol use disorders alone or drug use disorders alone significantly moderated the difference between the intensive psychotherapy and collaborative care conditions as assessed by likelihood of recovery (current SUDs by treatment condition: OR = 1.30, p = 0.72; past SUDs by treatment condition: OR = 1.25, p = 0.66) or time until recovery (current SUDs by treatment condition: OR = 0.97, p = 0.94; past SUDs by treatment condition: OR = 0.94, p = 0.85) from bipolar depression.
Discussion
This is among the first studies to investigate the predictive influence of SUDs on patients with BD in specialty psychosocial treatment. Contrary to our hypothesis, patients with BD and a current comorbid SUD were more likely to recover from a bipolar depressive episode and took less time to recover than patients without a current comorbid SUD. These findings were largely maintained (e.g. significant and/or showing a similar effect size/OR) after controlling for various possible clinical correlates of treatment response. Of note, when controlling for baseline depression severity, current SUDs did not predict time to recovery from bipolar depression. This was also true when separately evaluating current drug use disorders; of note, current alcohol use disorders still predicted time to recovery after controlling for baseline depression. Patients with or without a comorbid SUD were not differentially likely to recover in intensive psychotherapy versus collaborative care. Recovery rates and time to recovery did not differ among patients with or without a past comorbid SUD. Thus, our findings suggest that past SUDs are not associated with bipolar depression treatment response unless the patient is currently abusing substances.
Potential explanations for the better treatment response among patients with current comorbid SUDs include the following. First, the STEP-BD trial excluded any participants who required immediate treatment for a comorbid SUD. If this study had sampled BD participants with acute SUDs, it is possible that we would have found a lower rate of recovery among currently substance-abusing participants. Although baseline depression severity did not significantly differ between participants with and without current comorbid SUDs, trends reflecting small to moderate effect sizes toward lower depression severity at baseline were observed for those with comorbid SUDs. Notably, the significance of the predictive influence of current SUDs on time to recovery was attenuated after controlling for baseline depression, indicating that the results are at least partially dependent on lower depression severity, even though there were no significant differences in baseline depression severity between patients with and without co-occurring SUDs. This observation is at odds with the higher severity of mood episodes over time and more severe illness course found for patients with BD in naturalistic study (McElroy et al., 2001; Tolliver, 2010). Detailed analysis of potential confounding influences from other illness course, comorbidity and severity effects did not explain the association between the presence of comorbid SUDs and depression recovery. In sum, although these findings may be explained by differences in depression severity between patients with and without current comorbid SUDs, the inability of several other potentially confounding factors to account for these findings suggests that a consideration of other possible explanations is warranted.
These findings are in clear need of replication and speak to the importance of including comorbidity in randomized treatment trials. Given our results, future studies should consider stratifying for comorbid SUDs in prospective trials of BD treatment. If our findings are confirmed—specifically, that comorbid SUDs do not predict worse outcome for focused BD treatment and may even be a marker for individuals who are especially responsive to this treatment—then ordering of BD and SUD treatment should be carefully considered. Specifically, randomized trials of the most well-studied integrated treatment for comorbid BD and SUDs, for example, Weiss et al.’s integrated group therapy (Weiss et al., 1999, 2007, 2009), reveal that integrated treatment for both disorders is likely to reduce substance use but not benefit mood symptoms (Gold et al., 2018; Weiss et al., 2007, 2009). Accordingly, if depression symptoms are readily modifiable in those with comorbid BD and SUDs, as potentially evidenced in our study through the greater improvements in depressive symptoms among participants with current comorbid SUDs, clinicians will have additional options for how to stage psychosocial treatment with greater confidence that bipolar depression can be targeted first.
This study has limitations. First, we were not able to complete analyses on the full sample of participants in the psychosocial trial as we were limited to participants who had data on the presence or absence of comorbid SUDs. Second, the sample of participants with current SUDs was small (47 participants), limiting power for subsequent analyses. Third, we did not have data on the severity of substance use, and as such, our clinical outcomes were limited to depression recovery. Moreover, this study was designed to reflect ‘real world’ clinical care and thus participants in this study may have been on a varied number and combination of psychiatric medications, making it difficult to evaluate whether patients with current SUDs were independently receiving more effective drugs for bipolar depression relative to patients without current SUDs. Baseline differences in classes of medications among patients with and without current SUDs were not significant with the exception of mood stabilizers; however, patients without current SUDs were more likely to be receiving some type of mood stabilizer (not including lithium or valproate) than patients with current SUDs (p = 0.05). This finding is likely spurious but, in any case, may provide further evidence that our findings on current SUDs yielding superior bipolar depressive episode recovery were unrelated to group differences in medications (please refer to Table 1 for the description of classes of medications evaluated in this study). A final limitation of this study is that we were not able to evaluate manic symptomatology during the follow-up period and thus could not investigate in this sample whether patients with current SUDs were more likely to experience a switch into a manic or hypomanic episode at follow-up, a hypothesis supported by the Ostacher et al. (2010) STEP-BD naturalistic study described previously.
Our study provides a novel perspective on the role of current SUDs on recovery from bipolar depression, and, pending replication of our findings, encourages the efficacy of psychosocial interventions targeting mood symptoms in patients with BD. Our data indicate that psychosocial treatment of the mood disorder can be achieved with bipolar patients with ongoing, current substance abuse. Thus, our findings enhance options for the ordering of treatment of comorbid BD and SUDs, suggesting that psychosocial treatment can intervene effectively with bipolar depression prior to treating the SUD. Further investigation of brief psychosocial interventions as a cost-effective option for the treatment of comorbid BD and SUDs is warranted.
Supplementary Material
Declaration of Conflicting Interests
A.K.G. has no competing interests to report. A.T.P. has no competing interests to report. M.W.O. receives speaker fees from Big Health. L.G.S. has served as a consultant for United Biosource Corporation, Clintara, Bracket and Clinical Trials Network and Institute. She receives royalties from New Harbinger. She has received grant/research support from NIMH, PCORI, AFSP and Takeda. P.V.d.S.M. has no competing interests to report. M.B. is supported by a NHMRC Senior Principal Research Fellowship (APP1059660). D.D.D. receives research support and consulting/honoraria from Medtronic, research support from Eli Lilly, research support and travel support from Roche and research support from Cyberonics. D.J.M. reports research funding from the National Institute of Mental Health and from several private foundations, and book royalties from Guilford Press and John Wiley & Sons. E.F. reports the following disclosures: Honoraria—Servier; Equity—Psychiatric Assessments, Inc.; HealthRhythms, Inc. A.A.N. reports the following disclosures: Consultant—Abbott Laboratories, Alkermes, American Psychiatric Association, Appliance Computing Inc. (Mindsite), Basliea, BrainCells Inc., Brandeis University, Bristol-Myers Squibb, Clintara, Corcept, Dey Pharmaceuticals, Dainippon Sumitomo (now Sunovion), Eli Lilly and Company, EpiQ, L.P./Mylan Inc., Forest, Genaissance, Genentech, GlaxoSmithKline, Healthcare Global Village, Hoffman LaRoche, Infomedic, Intra-Cellular Therapies, Lundbeck, Janssen Pharmaceutica, Jazz Pharmaceuticals, Medavante, Merck, Methylation Sciences, NeuroRx, Naurex, Novartis, PamLabs, Parexel, Pfizer, PGx Health, Otsuka, Ridge Diagnostics Shire, Schering-Plough, Somerset, Sunovion, Takeda Pharmaceuticals, Targacept, and Teva; consulted through the MGH Clinical Trials Network and Institute (CTNI) for Astra Zeneca, BrainCells Inc., Dainippon Sumitomo/Sepracor, Johnson and Johnson, Labopharm, Merck, Methylation Science, Novartis, PGx Health, Shire, Schering-Plough, Targacept and Takeda/Lundbeck Pharmaceuticals, NeuroRx Pharma, Pfizer, Physician’s Postgraduate Press, Inc. Grants/Research support—American Foundation for Suicide Prevention, AHRQ, Brain and Behavior Research Foundation, Bristol-Myers Squibb, Cederroth, Cephalon, Cyberonics, Elan, Eli Lilly & Company, Forest, GlaxoSmithKline, Intra-Cellular Therapies, Janssen Pharmaceuticals, Lichtwer Pharma, Marriott Foundation, Mylan, NIMH, PamLabs, Patient Centered Outcomes Research Institute (PCORI), Pfizer Pharmaceuticals, Shire, Stanley Foundation, Takeda/Lundbeck and Wyeth-Ayerst. Honoraria—Belvoir Publishing, University of Texas Southwestern Dallas, Brandeis University, Bristol-Myers Squibb, Hillside Hospital, American Drug Utilization Review, American Society for Clinical Psychopharmacology, Baystate Medical Center, Columbia University, CRICO, Dartmouth Medical School, Health New England, Harold Grinspoon Charitable Foundation, IMEDEX, International Society for Bipolar Disorder, Israel Society for Biological Psychiatry, Johns Hopkins University, MJ Consulting, New York State, Medscape, MBL Publishing, MGH Psychiatry Academy, National Association of Continuing Education, Physicians Postgraduate Press, SUNY Buffalo, University of Wisconsin, University of Pisa, University of Michigan, University of Miami, University of Wisconsin at Madison, APSARD, ISBD, SciMed, Slack Publishing and Wolters Klower Publishing, ASCP, NCDEU, Rush Medical College, Yale University School of Medicine, NNDC, Nova Southeastern University, NAMI, Institute of Medicine, CME Institute, ISCTM, World Congress on Brain Behavior and Emotion, Congress of the Hellenic Society for Basic and Clinical Pharmacology, ADAA. Stock—Appliance Computing, Inc. (MindSite); BrainCells Inc., Medavante. Copyrights—Clinical Positive Affect Scale and the MGH Structured Clinical Interview for the Montgomery Asberg Depression Scale exclusively licensed to the MGH Clinical Trials Network and Institute (CTNI). Speaker Bureaus—none since 2003. T.D.’s research has been funded by NIH, NIMH, NARSAD, TSA, IOCDF, Tufts University, DBDAT, Otsuka Pharmaceuticals, Cogito Inc. and Sunovion. He has received honoraria, consultation fees and/or royalties from the MGH Psychiatry Academy, BrainCells Inc., Clintara, LLC., Systems Research and Applications Corporation, Boston University, the Catalan Agency for Health Technology Assessment and Research, the National Association of Social Workers Massachusetts, the Massachusetts Medical Society, Tufts University, NIDA, NIMH and Oxford University Press. He has also participated in research funded by DARPA, NIH, NIMH, NIA, AHRQ, PCORI, Janssen Pharmaceuticals, The Forest Research Institute, Shire Development Inc., Medtronic, Cyberonics, Northstar, Takeda and Sunovion.
Funding
Funding for STEP-BD was provided through the National Institute of Mental Health (N01MH80001). This study was supported in part by the Dauten Family Center for Bipolar Treatment Innovation.
Footnotes
Supplementary material
Supplementary material is available at: journals.sagepub.com/doi/suppl/10.1177/0004867418788172
References
- Cerullo MA and Strakowski SM (2007) The prevalence and significance of substance use disorders in bipolar type I and II disorder. Substance Abuse Treatment, Prevention, and Policy 2: 29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deckersbach T, Peters AT, Sylvia L, et al. (2014) Do comorbid anxiety disorders moderate the effects of psychotherapy for bipolar disorder? Results from STEP-BD. American Journal of Psychiatry 171: 178–186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frye MA and Salloum IM (2006) Bipolar disorder and comorbid alcoholism: Prevalence rate and treatment considerations. Bipolar Disorders 8: 677–685. [DOI] [PubMed] [Google Scholar]
- Gold AK, Otto MW, Deckersbach T, et al. (2018) Substance use comorbidity in bipolar disorder: A qualitative review of treatment strategies and outcomes. American Journal on Addictions 27: 188–201. [DOI] [PubMed] [Google Scholar]
- Inder ML, Crowe MT, Luty SE, et al. (2015) Randomized, controlled trial of interpersonal and social rhythm therapy for young people with bipolar disorder. Bipolar Disord 17: 128–138. [DOI] [PubMed] [Google Scholar]
- Jaffee WB, Griffin ML, Gallop R, et al. (2009) Depression precipitated by alcohol use in patients with co-occurring bipolar and substance use disorders. Journal of Clinical Psychiatry 70: 171–176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kessler R (1999) Comorbidity of unipolar and bipolar depression with other psychiatric disorders in a general population survey In: Tohen M (ed.) Comorbidity in Affective Disorders. New York: Marcel Dekker, pp. 1–27. [Google Scholar]
- Kilbourne AM, Biswas K, Pirraglia PA, et al. (2009) Is the collaborative chronic care model effective for patients with bipolar disorder and co-occurring conditions? Journal of Affective Disorders 112: 256–261. [DOI] [PubMed] [Google Scholar]
- Levin FR and Hennessy G (2004) Bipolar disorder and substance abuse. Biological Psychiatry 56: 738–748. [DOI] [PubMed] [Google Scholar]
- McElroy SL, Altshuler LL, Suppes T, et al. (2001) Axis I psychiatric comorbidity and its relationship to historical illness variables in 288 patients with bipolar disorder. American Journal of Psychiatry 158: 420–426. [DOI] [PubMed] [Google Scholar]
- Manwani SG, Pardo TB, Albanese MJ, et al. (2006) Substance use disorder and other predictors of antidepressant-induced mania: A retrospective chart review. Journal of Clinical Psychiatry 67: 1341–1345. [DOI] [PubMed] [Google Scholar]
- Miklowitz DJ and Otto MW (2007) Psychosocial interventions for bipolar disorder: A review of literature and introduction of the systematic treatment enhancement program. Psychopharmacology Bulletin 40: 116–131. [PubMed] [Google Scholar]
- Miklowitz DJ, Otto MW, Frank E, et al. (2007) Psychosocial treatments for bipolar depression: A 1-year randomized trial from the systematic treatment enhancement program. Archives of General Psychiatry 64: 419–426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell JD, Brown ES and Rush AJ (2007) Comorbid disorders in patients with bipolar disorder and concomitant substance dependence. Journal of Affective Disorders 102: 281–287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nery FG and Soares JC (2011) Comorbid bipolar disorder and substance abuse: Evidence-based options: Medication selection May very based on which substance patients abuse. Current Psychiatry 10: 57. [Google Scholar]
- Ostacher MJ, Perlis RH, Nierenberg AA, et al. (2010) Impact of substance use disorders on recovery from episodes of depression in bipolar disorder patients: Prospective data from the systematic treatment enhancement program for bipolar disorder (STEP-BD). American Journal of Psychiatry 167: 289–297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters AT, Shesler LW, Sylvia L, et al. (2016) Medical burden, body mass index and the outcome of psychosocial interventions for bipolar depression. Australian and New Zealand Journal of Psychiatry 50: 667–677. [DOI] [PubMed] [Google Scholar]
- Peters AT, Sylvia LG, Magalhaes PV, et al. (2014) Age at onset, course of illness and response to psychotherapy in bipolar disorder: Results from the systematic treatment enhancement program for bipolar disorder (STEP-BD). Psychological Medicine 44: 3455–3467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sachs GS, Guille C and McMurrich SL (2002) A clinical monitoring form for mood disorders. Bipolar Disorders 4: 323–327. [DOI] [PubMed] [Google Scholar]
- Sachs GS, Thase ME, Otto MW, et al. (2003) Rationale, design, and methods of the systematic treatment enhancement program for bipolar disorder (STEP-BD). Biological Psychiatry 53: 1028–1042. [DOI] [PubMed] [Google Scholar]
- Sagman D and Tohen M (2009) Comorbidity in bipolar disorder. Psychiatric Times, 24 May, pp. 1–5. [Google Scholar]
- Salloum IM and Thase ME (2000) Impact of substance abuse on the course and treatment of bipolar disorder. Bipolar Disord 2: 269–280. [DOI] [PubMed] [Google Scholar]
- Sheehan DV, Lecrubier Y, Sheehan KH, et al. (1998) The mini-international neuropsychiatric interview (MINI): The development and validation of a structured diagnostic psychiatric interview for DSM-IV and ICD-10. Journal of Clinical Psychiatry 59: 22–23. [PubMed] [Google Scholar]
- Tolliver BK (2010) Bipolar disorder and substance abuse: Overcome the challenges of ‘dual diagnosis’ patients. Current Psychiatry 9: 33–38. [Google Scholar]
- Weiss RD, Griffin ML, Jaffee WB, et al. (2009) A ‘community-friendly’ version of integrated group therapy for patients with bipolar disorder and substance dependence: A randomized controlled trial. Drug and Alcohol Dependence 104: 212–219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss RD, Griffin ML, Kolodziej ME, et al. (2007) A randomized trial of integrated group therapy versus group drug counseling for patients with bipolar disorder and substance dependence. American Journal of Psychiatry 164: 100–107. [DOI] [PubMed] [Google Scholar]
- Weiss RD, Najavits LM and Greenfield SF (1999) A relapse prevention group for patients with bipolar and substance use disorders. Journal of Substance Abuse Treatment 16: 47–54. [DOI] [PubMed] [Google Scholar]
- Weiss RD, Ostacher MJ, Otto MW, et al. (2005) Does recovery from substance use disorder matter in patients with bipolar disorder? Journal of Clinical Psychiatry 66: 730–735. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.