Abstract
Malignant pleural mesothelioma remains difficult to treat, with high failure rates despite optimal therapy. We present a novel prospective trial combining proton therapy (PT) and photodynamic therapy (PDT) and the largest-ever mesothelioma PT experience (n = 10). PDT photosensitizers included porfimer sodium (2 mg-kg−1; 24 h drug-light interval) or 2-[1-hexylox-yethyl]-2-devinyl pyropheophorbide-a (HPPH) (4 mg m−2; 48 h) with wavelengths of 630 nm to 60Jcm−2 and 665 nm to 15–45Jcm−2, respectively. With a median age of 69 years, patients were predominantly male (90%) with epithelioid histology (100%) and stage III-IV disease (100%). PT was delivered to a median of 55.0 CGE/1.8–2.0 CGE (range 50–75 CGE) adjuvantly (n = 8) or as salvage therapy (n = 2) following extended pleurectomy/decortication (ePD)/PDT. Two-year local control was 90%, with distant and regional failure rates of 50% and 30%, respectively. All patients received chemotherapy, and four received immunotherapy. Surgical complications included atrial fibrillation (n = 3), pneumonia (n = 2), and deep vein thrombosis (n = 2). Median survival from PT completion was 19.5 months (30.3 months from diagnosis), and 1-and 2-year survival rates were 58% and 29%. No patient experienced CTCAEv4 grade ≥2 acute or late toxicity. Our prolonged survival in very advanced-stage patients compares favorably to survival for PT without PDT and photon therapy with PDT, suggesting possible spatial or systemic cooperativity and immune effect.
INTRODUCTION
Malignant pleural mesothelioma (MPM) is a tumor that arises from the mesothelial surfaces of the lung pleura that is more often seen in men and diagnosed most typically in the 5th to 7th decade of life (1). Approximately 2500 cases occur per year in the United States, and MPM is linked with asbestos exposure (2,3). Survival remains poor, with stage III-IV patients having estimated median overall survivals ranging from 10 to 14 months (4). Multiple studies have shown that patients with epithelial subtype have a more favorable prognosis than biphasic and sarcomatous subtypes of mesothelioma across treatment approaches (5–14).
Combined chemotherapy, most commonly with cisplatin or carboplatin and pemetrexed, remains a mainstay of treatment (15–18). In well-selected patients, surgical resection can improve survival and is recommended on the basis of consensus guidelines by the International Mesothelioma Interest Group Congress (19,20). For patients who are surgically resectable, two options of surgical excision exist: extrapleural pneumonectomy (EPP) in which the lung, pleura, pericardium and diaphragm are removed en bloc, or extended pleurectomy/decortication (ePD), a lung-sparing surgery that removes macroscopic disease with the parietal/visceral pleura but that generally allows for preservation of the diaphragm and phrenic nerve (21). EPP can leave less residual tumor cells compared with ePD; however, it often results in high morbidity and even mortality rates, severe depression of cardiorespiratory function, and poor quality of life (22). In a systematic review and meta-analysis of seven studies with comparative data on EPP and ePD, perioperative mortality and morbidity was found to be 2.9% and 27.9% in the ePD group compared to 6.8% and 62.0% in the EPP arm, respectively (22).
Photodynamic therapy (PDT) can be utilized for the treatment of malignant and non-malignant causes (23–27). It is composed of three components that include a photosensitizing chemical, a light source with a wavelength appropriate to cause excitation, and oxygen within a tissue that is exposed. The wavelength of light is specific to the photosensitizer and is responsible for producing free radical and/or reactive oxygen species. Energy is transferred mainly from the triplet sensitizer to ground state oxygen to produce a highly reactive form of oxygen known as singlet oxygen. Singlet oxygen is highly cytotoxic with a very short duration in tissue on the order of microseconds (28,29). PDT has been used in conjunction with both EPP and ePD (30–32).
An older phase III trial performed from 1993 to 1996 to compare maximal debulking surgery with postoperative cisplatin, interferon alpha-2b and tamoxifen ± intraoperative PDT failed to influence the patterns of recurrence or improve median survival in 63 patients with malignant pleural mesothelioma (14.1 vs 14.4 months) (33). All underwent maximum debulking surgery and were randomized to either PDT or no PDT intra-operatively with photophrin II and 630-nm laser light. While there were no differences between surgical or immunochemotherapeutic toxicities in the PDT and no PDT arms, the toxicity was notable with 11 postoperative complications in 10 patients, most commonly medically reversible arrhythmia (n = 4), bronchopleural fistula (n = 4), and cardian herniation requiring pericardial patching (n = 1). The bronchopleural fistulas were evenly split between the PDT and no PDT arm. One intraoperative death occurred in the PDT arm due to avulsion of the IVC after a right-sided pleuropneumonectomy. While this trial did not employ real-time PDT dosimetry and included patients with gross residual disease for which PDT would not be expected to be treated effectively with the limited penetration of PDT, a number of additional studies including some that employed real-time dosimetry (34), showed that PDT is well-tolerated, improves local control (35) and may offer a survival advantage in the Phase I and Phase II settings (32,36–38).
Mesothelioma has intrinsic sensitivity to radiation as evidenced by in vitro analysis of mesothelioma and non-small cell lung cancer cells (39,40). Radiation has been used for palliation with a notable dose-response to higher doses and dose-per-fraction, as well as prophylaxis for biopsy sites and drain sites (41–45). Phase II data for preoperative radiation from Princess Margaret Hospital delivering 25 Gy in 5 fractions to the entire affected lung and pleura with a simultaneous integrated boost to 30 Gy to gross disease followed by EPP within 7 days and adjuvant chemotherapy for lymph node positive patients showed excellent results with a median overall survival of 36 months (46). The use of external beam radiotherapy given adjuvantly for mesothelioma has historically been limited by significant morbidities and risk of fatal pneumonitis when treating large pleural volumes (47–49). A Dana-Farber Cancer Institute-Brigham and Women’s Hospital study showed fatal pneumonitis in 46% when delivering radiotherapy after EPP (48). Factors associated with development of pneumonitis in this series included the dosimetric parameters of mean lung dose (MLD), volume of lung receiving at least 5 Gy (V5) and volume of lung receiving at least 20 Gy (V20).
While there has been a trend toward increased utilization of intensity-modulated radiation therapy (IMRT) for malignant pleural mesothelioma (50), proton therapy (PT) may more optimally treat this challenging disease than IMRT (51–53). PT is an emerging modality that reduces irradiation to normal tissues compared with photon therapy, which has been shown to improve outcomes and/or reduce toxicities in other thoracic tumors like non-small cell lung cancer (54,55), small cell lung cancer (56), thymic tumors (57,58) and thoracic reirradiation (59). As such, it may be more safely delivered to large pleural surfaces and more safely combined with chemotherapy and surgery/PDT.
The abscopal effect has been described dating back to 1953 and refers to the effects of ionizing radiation at a distance from the irradiated volume but within the same organism (60,61). Immune modulation and recognition is responsible for this event, and an in vitro study of multiple cancer cell lines evaluating the up-regulation of surface molecules involved in immune recognition showed that there is upregulation of these molecules and also increased sensitivity to cytotoxic T-lymphocyte killing of tumor cells (62). Additionally, the use of PDT has been shown to be immunogenic in multiple studies (63–65). We, therefore, hypothesized that the use of ePD with PDT and adjuvant PT would offer immunogenic stimulation through multiple mechanisms and enhance tumor kill in mesothelioma patients.
We present a novel prospective experience of patients treated with chemotherapy, ePD/PDT and PT, as well as the largest PT mesothelioma experience reported to date. As there currently are no clinical data combining PDT and PT, we analyze our prospective cohort receiving both modalities to assess for toxicities, local control, and overall survival.
MATERIALS AND METHODS
Patient characteristics and workup.
After IRB approval and patient consent, patients were prospectively enrolled on one of two trials in the Department of Radiation Oncology at the Hospital of University of Pennsylvania between 2011 and 2015. A mesothelioma multidisciplinary team evaluated all patients before enrollment. Criteria for enrollment included epithelial subtype, being deemed medically “fit” for surgery, disease confined to one hemithorax, and informed consent. Patients were selected for this study if they received PT as adjuvant therapy following lung-sparing ePD/PDT or as salvage therapy after ePD/PDT as a part of multi-modality therapy. Ten consecutive patients treated with both PDT and PT were evaluated for this analysis.
Patient characteristics.
A total of 10 patients were enrolled in this trial. Table 1 depicts the patient and treatment characteristics. Patients were predominantly male (90%), and all patients were Caucasian with epithelial histologic subtype and AJCC 7th Edition stage III-IV disease.
Table 1.
Patient and tumor characteristics.
| Characteristic | N (%) | |
|---|---|---|
| Median age at diagnosis in year (range, years) | 69 years (51–80 years) | |
| Sex | Male | 9 (90) |
| Female | 1 (10) | |
| Tumor location | Right | 9 (90) |
| Left | 1 (10) | |
| Median Radiation dose Gy (range, CGE) | 55 CGE (50–75 CGE) | |
| Surgery | ePD | 10 (100) |
| Race | Caucasian | 10 (100) |
| Smoking history | Yes | 2 (20) |
| No | 8 (80) | |
| Known asbestos | Yes | 8 (80) |
| exposure | No | 2 (20) |
| Histologic Subtype | Epithelioid | 10 (100) |
| Biphasic | 0 (0) | |
| Sarcomatoid | 0 (0) | |
| ECOG Performance | 0 | 4 (40) |
| Status | 1 | 4 (40) |
| 2 | 2 (20) | |
| T stage | T1 | 0 (0) |
| T2 | 1 (10) | |
| T3 | 5 (50) | |
| T4 | 4 (40) | |
| N Stage | N0 | 1 (10) |
| N1 | 0 (0) | |
| N2 | 9 (90) | |
| Overall Clinical | I | 0 (0) |
| Stage | II | 0 (0) |
| III | 7 (70) | |
| IV | 3 (30) | |
| Photosensitizer | Photofrin | 7 (70) |
| HPPH | 3 (30) | |
| Chemotherapy timing | Before PT | 7 (70) |
| Concurrent with PT | 1 (10) | |
| After PT | 4 (40) | |
| Chemotherapy | Cisplatin/pemetrexed | 4 (40) |
| Type Pre-PT | Carboplatin/pemetrexed | 3 (30) |
| Concurrent | Pemetrexed | 1 (10) |
| Chemotherapy | ||
| Type | ||
| Chemotherapy | Gemcitabine | 1 (10) |
| Type Post-PT | Gemcitabine/carboplatin | 1 (10) |
| Carboplatin/pemetrexed | 1 (10) | |
| Cisplatin/pemetrexed | 1 (10) | |
| Immunotherapy | Yes | 3 (30) |
| No | 7 (70) | |
| Immunotherapy | Interferon alpha | 3 (30) |
| Type | ||
ePD, extended pleurectomy/decortication; HPPH, 2-[1-hexyloxyethyl]-2-devinyl pyropheophorbide-a.
Radiotherapy.
PT was delivered to a median dose of 5500 centi-Cobalt Grey Equivalent (cCGE) in 180–200 cCGE daily fractions adjuvantly following lung-sparing ePD/PDT (n = 8) or as salvage therapy for progression after surgery/PDT (n = 2) at a median of 7.5 months (range 3.5–68.3 months) after ePD/PDT and 10.9 months (range 3.5–69.3 months) after mesothelioma diagnosis. All patients previously underwent ePD and all achieved a macroscopically complete resection of all gross disease. Definitive PT was delivered using 70–235 MV Proton Uniform or Double Scattering therapy. Target volumes for patients receiving adjuvant PT included any areas of surgical concern, any areas of chest wall invasion, and all high risk hilar and mediastinal lymph node stations, including the ipsilateral lower paratracheal, upper and lower hilar, and subcarinal lymph nodes, as well as any other lymph nodes that were sampled preoperatively or resected intraoperatively and pathologically positive for metastatic disease, inclusive of posterior intercostal lymph nodes when applicable. Target volumes for patients receiving salvage PT included all areas of gross disease as assessed on CT and PET/CT imaging. Using 4D-CT, an internal gross tumor volume (iGTV) was created and expanded 5–8 mm to create a clinical target volume that accounts for microscopic disease spread. A 5 mm expansion was added for a planning target volume (PTV) to account for daily setup errors and proton beam uncertainties. Owing to the already large irradiation volumes and potential risks of additional elective treatment, elective whole pleural irradiation was not administered. Daily image guidance, inclusive of cone-beam CT scans as previously reported (66), was conducted for verification prior to treatment delivery.
Photodynamic therapy (PDT).
Each patient received intravenous porfimer sodium (Photofrin) 2 mg∙kg−1 with a 24-hour drug-light interval or 2-[1-hexyloxyethyl]-2-devinyl pyropheophorbide-a (HPPH) 4 mg∙m−2 with a 48 h drug-light interval based on which of two prospective studies they enrolled on. Laser light at a wavelength of 630 nm for porfimer sodium or 665 nm for HPPH was then delivered to a measured dose of 60 and 15 to 45 J∙cm−2 (per the dose escalation HPPH trial), respectively, as registered on eight strategically placed isotropic light detectors using a custom-built dosimetry system (67–71). The chest was filled with 0.01% dilute intralipid solution to facilitate light dispersion. Light delivery was administered generally over 60–100 min intraoperatively through optical fibers through spinal needles. This lengthened the operating room time 90–120 min per case for diode placement and PDT delivery.
Statistics and follow-up outcomes.
Patients were followed with a PET/CT or chest CT every 3 months following PT completion. Patient demographics and treatment variables were described by frequencies and percentages. Response evaluation criteria in solid tumors (RECIST) v1.1 criteria were used to define disease progression or stability. Local control was defined as resolution or arrested progression of intrathoracic disease, time to progression was defined as the time interval from the end of PT to any failure (local, regional [nodal] or distant), and overall survival was defined as the time interval from PT completion to death from any cause or last follow-up. Biopsies were performed to confirm disease prior to initiating salvage therapy in the recurrent setting. Acute and late toxicities was recorded and graded according to the Common Terminology Criteria for Adverse Events (CTCAE) V4.0. Statistical analysis was performed using the software package SPSS (SPSS Inc., Chicago, IL).
RESULTS
Systemic therapy
Systemic therapy was given before PT in 70% of patients, consisting of cisplatin and pemetrexed (n = 4) or carboplatin and pemetrexed (n = 3). One patient received concurrent pemetrexed with PT. Adjuvant and/or salvage chemotherapy was delivered in four patients consisting of gemcitabine alone (n = 1), gemcitabine with carboplatin (n = 1), carboplatin and pemetrexed (n = 1), and cisplatin and pemetrexed (n = 1). Two patients received neoadjuvant immunotherapy and one received adjuvant therapy with interferon alpha, while an additional patient underwent PD1 treatment adjuvantly. The details of systemic therapy are depicted in Table 2.
Table 2.
Treatments delivered for each patient.
| Patient # | Sex | Surgery | Photosensitizer | Neoadjuvant chemotherapy | Radiation fraction size/total dose (cCGE) |
Adjuvant chemotherapy and/or immunotherapy |
|---|---|---|---|---|---|---|
| 1 | Male | ePD | HPPH | Cisplatin and alimta | 235/7500 | Carboplatin and Alimta |
| 2 | Male | ePD | Photofrin | Cisplatin and alimta | 180/5040 | Gemcitabine |
| 3 | Female | ePD | Photofrin | None | 200/7000 | PD1 |
| 4 | Male | ePD | Photofrin | None | 180/5940 | Carboplatin and Alimta |
| 5 | Male | ePD | Photofrin | Carboplatin and Alimta | 200/5400 | Gemcitabine and Carboplatin |
| 6 | Male | ePD | Photofrin | Cisplatin and Alimta | 180/5040 | None |
| 7 | Male | ePD | Photofrin | Carboplatin and Alimta | 180/5040 | None |
| 8 | Male | ePD | HPPH | Cisplatin and Alimta | 200/6000 | Interferon alpha |
| 9 | Male | ePD | Photofrin | Carboplatin and Alimta | 200/5000 | Interferon alpha |
| 10 | Male | ePD | HPPH | None | 200/7000 | Cisplatin and Alimta, Interferon alpha |
ePD, extended pleurectomy/decortication; HPPH, 2-(1-hexyloxytheyl)-2-devinyl pyropheophorbide-a.
Failure patterns
One patient suffered a local recurrence 3.2 months after the completion of PT. Local control at 1 and 2 years was 90% and 90%, respectively. Three patients had regional recurrences at a median of 2.5 months (range 1.8–6.3 months) after PT. Half of the patients (n = 5) suffered a distant failure, with the initial site of metastases in the liver (n = 2), peritoneum (n = 1), contralateral lung (n = 1), and bone (n = 1) at a median of 2.2 months (range 0.5–8.8 months) after the completion of PT. Individual patient data and failure patterns are depicted in Table 3.
Table 3.
Failure type by patient.
| Patient # | Failure (Y/N) | Type | Location |
|---|---|---|---|
| 1 | Y | Distant | Peritoneum |
| 2 | Y | Distant | Contralateral lung |
| 3 | Y | Distant | Peritoneum |
| 4 | Y | Local | Distant pleura |
| 5 | Y | Regional, Distant | Mediastinal nodes/bone/liver/distant nodes |
| 6 | N | None | None |
| 7 | Y | Distant | Liver |
| 8 | Y | Regional | Level 4R nodes |
| 9 | N | None | None |
| 10 | Y | Regional | Distant pleura |
Survival
At a median follow-up of 7.1 months (range 2.0–33.2 months) from completion of PT and 20.2 months (range 12.4–71.6 months) from diagnosis, the median survival from the end of PT was 19.5 months (Fig. 1), and 1-year and 2-year survival rates were 58% and 29%, respectively. When measured from diagnosis, median survival was 30.3 months.
Figure 1.
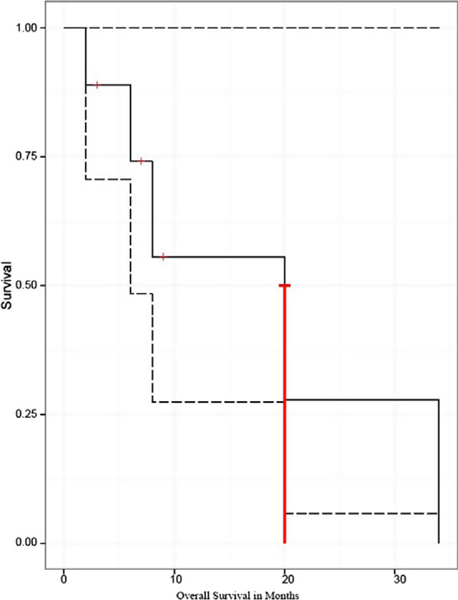
Overall survival (OS). OS of all malignant pleural mesothelioma (MPM) patients who were treated with a combination of extended pleurectomy/decortication with photodynamic therapy (PDT) followed by adjuvant (n = 8) or salvage (n = 2) proton therapy (PT). PT was given to a median dose of 55 Cobalt Grey Equivalents (CGE) in 1.8–2.0 CGE per fraction. Survival was measured as time from completion of PT until date of death or last follow-up (censored). Median survival was 19.5 months, with 1-and 2-year OS of 58% and 29%, respectively.
Toxicity
In general, treatment was well tolerated amongst patients. There was no diaphragmatic rupture, pericardial effusion requiring drainage or prolonged hospitalizations as the result of surgery. Three patients developed transient atrial fibrillation, while two patients developed a deep vein thrombosis and two developed pneumonia. No external phototoxicities were seen from PDT. The most common adverse effects definitely, probably, or possibly related to PT were grade 1 fatigue, experienced by all patients who underwent treatment. Seven patients (70%) developed grade 1 cough, while one patient (10%) had a grade 2 cough. Dyspnea during treatment was grade 1 in 6 (60%) and grade 2 in 2 patients (20%), respectively. One patient developed grade 2 radiation pneumonitis (10%), and no grade ≥3 pneumonitis was observed. Radiation dermatitis was grade 1 in 50% of patients (n = 5) and grade 2 in an additional 50% (n = 5). Half of the patients (50%) developed grade 1 pruritis during their PT treatment course. Two patients (20%) each had grade 1 and grade 2 dysphagia. No patient experienced CTCAEv4 grade ≥3 acute or late toxicities.
DISCUSSION
In the largest report of proton therapy for MPM to date, and the only study to combine PT with PDT, we have shown excellent local control (90%) in a disease typically hard to achieve long-term local control, as well as excellent overall survival values of 56% at 1 year and 29% at 2 years for patients undergoing ePD with PDT followed by PT. With a median survival of 19.5 months from PT completion and 30.3 months from diagnosis, this far exceeds prior median survival reports for patients with Stage III and IV MPM, which per the updated staging guidelines range from 10 to 14 months (4). The authors do note that our histology of epithelial subtype may have contributed, in part, to the prolonged survival achieved in this study, although the present results far exceed what would be expected for stage-matched epithelial patients reported in other surgical series. Survival in this series exceeds the median survival in a phase II study evaluating outcomes in MPM in patients undergoing surgery and adjuvant radiation therapy despite our current study having generally more advanced-stage patients and including salvage patients progressing after pre-PT treatment (72). When measured from the time of diagnosis, the median survival in this cohort exceeded 30 months, in line with a prior study of RP and PDT from our institution despite our current study having no stage I-II patients (31). Furthermore, this survival time far exceeds the median survival of our institutional experience of PT without PDT for malignant pleural mesothelioma (73). This may suggest potential synergistic effects with the combination of PDT and PT.
There are theoretical reasons to think that PDT and PT may be synergistic. PDT promotes the microenvironment to be more conducive to immunologic effect, can lead to an inflammatory reaction that can evoke a host anti-tumor immune response, and can lead to immunogenic cell death (74–76). Recent reports have suggested that proton and heavy ion therapy may stimulate the immune system to a greater degree than photon therapy (62,77). As such, we hypothesized that priming the immune system with intraoperative PDT at the time of lung-sparing surgery for mesothelioma may have allowed for more indolent recurrences and a preferential potentiation of a radiation vaccination effect on the immune system. This may be more prominent for proton therapy than photon therapy and may allow for especially prolonged overall survival in patients sequentially treated with PDT and proton therapy. Additionally, PDT may be synergistic with RT, and it may do so with non-overlapping toxicity.
When microscopic disease remains after surgical resection, even when intraoperative PDT is delivered, pleural and nodal recurrence rates are high for mesothelioma. Fortunately, mesothelioma is known to be radioresponsive tumor (39,40). Since the majority of mesothelioma patients have significant symptoms related to local disease, quality of life, in additional to local control and potentially even overall survival, can be improved if radiation is able to be safely delivered. As it is known that lung irradiation dose correlates with the risk of pulmonary or non-cancer related death (47), PT may be the most ideal radiotherapy delivery modality for mesothelioma, as studies have shown significant reductions in dose of radiation to organs at risk like lung (51,52). As rates of radiation pneumonitis are compounded in patients undergoing surgery and chemotherapy due to the added inflammation to lung parenchyma in these multi-modality patients (78), concerns for toxicity are heightened when adding another inducer of acute inflammation like PDT. PT, therefore, can mitigate these compounding risks and allow for the safe sequential delivery of PDT and radiotherapy.
In fact, the toxicities related to treatment observed in this study were notably low, and the acute toxicities noted were attributable to what would typically be seen in a patient undergoing PT alone without the addition of PDT. The fact that no Grade 3 or higher acute or chronic toxicities occurred speaks to the safety of this multi-modality regimen, and while studies with larger patient numbers would be helpful to confirm our findings, our study should serve as a baseline to encourage further studies of PDT-PT combinatory therapy in mesothelioma.
This work is hypothesis generating, and future prospective trial design at mesothelioma centers of excellence and high-volume mesothelioma centers (79) should consider incorporation of PDT with lung-sparing surgery along with PT to reduce the volume of contralateral lung and other critical organs that are damaged, as this contributes to non-cancer related toxicities and death. It would be a mistake not to mention the need for continued improvements and evaluation of chemotherapy and immunotherapy, as 50% of our patients did fail distantly in this series, and therapies after failure of first-line chemotherapy are limited with low response rates. As the efficacy of both PT (80) and PDT (81) may be impacted by the immune system and immunotherapy, outcomes of this study might have been impacted if more of the current patient cohort received immunotherapy. As such, we look forward to the mature results of the Phase II MAPS-2 trial of immunotherapy consisting of nivolumab monotherapy or nivolumab combined with ipilimumab in the second or third line treatment of mesothelioma. Initial results in the combination arm are promising, with the median survival not yet reached at 15-month follow-up (82). Thus, these results strengthen the argument for an aggressive, multi-modality approach to the treatment of well selected operable MPM patients.
CONCLUSION
This is the first reported study combining chemotherapy, ePD/PDT and PT. It is also the largest ever study of PT for malignant pleural mesothelioma. This study shows the sequential combination to be well tolerated and also shows that PT may reduce toxicities of radiotherapy when delivered after ePD/PDT. Our median survival of over 30 months from mesothelioma diagnosis in a very advanced-stage population compares favorably to previously published reports and to our own mesothelioma institutional survival for PT without PDT and for photon therapy with PDT. This may suggest the possibility of spatial or systemic cooperativity and immune effect with the combination of these two immunogenic therapies of PDT and PT.
Footnotes
This article is part of a Special Issue celebrating Photochemistry and Photobiology’s 55th Anniversary.
REFERENCES
- 1.Scott B, Mukherjee S, Lake R and Robinson BWS (2000). Malignant mesothelioma. In: Textbook of Lung Cancer (1st Edition) (Edited by Hanson H), pp. 273–293. Martin Dunitz, London, UK. [Google Scholar]
- 2.Robinson BWS and Lake RA (2005) Advances in Malignant Mesothelioma. N. Engl. J. Med. 353(15), 1591–1603. [DOI] [PubMed] [Google Scholar]
- 3.Price B and Ware A (2004) Mesothelioma Trends in the United States: An Update Based on Surveillance, Epidemiology, and End Results Program Data for 1973 through 2003. Am. J. Epidemiol. 159 (2), 107–112. [DOI] [PubMed] [Google Scholar]
- 4.Rusch VW, Chansky K, Kindler HL, Nowak AK, Pass HI, Rice DC, Shemanski L, Galateau-Sallé F, McCaughan BC, Nakano T, Ruffini E, van Meerbeeck JP and Yoshimura M; IASLC Staging and Prognostic Factors Committee, advisory boards, and participating institutions (2016) The IASLC Mesothelioma Staging Project: Proposals for the M Descriptors and for Revision of the TNM Stage Groupings in the Forthcoming (Eighth) Edition of the TNM Classification for Mesothelioma. J. Thorac. Oncol. 11(12), 2112–2119. [DOI] [PubMed] [Google Scholar]
- 5.Balduyck B, Trousse D, Nakas A, Martin-Ucar AE, Edwards J and Waller DA (2010) Therapeutic Surgery for Nonepithelioid Malignant Pleural Mesothelioma: Is it Really Worthwhile? Ann. Thorac. Surg. 89(3), 907–911. [DOI] [PubMed] [Google Scholar]
- 6.Bovolato P, Casadio C, Billè A, Ardissone F, Santambrogio L, Ratto GB, Garofalo G, Bedini AV, Garassino M, Porcu L, Torri V and Pastorino U (2014) Does surgery improve survival of patients with malignant pleural mesothelioma?: A multicenter retro-spective analysis of 1365 consecutive patients. J. Thorac. Oncol. 9 (3), 390–396. [DOI] [PubMed] [Google Scholar]
- 7.Van Gelder T, Damhuis RA and Hoogsteden HC (1994) Prognostic factors and survival in malignant pleural mesothelioma. Eur. Respir. J. 7(6), 1035–1038. [PubMed] [Google Scholar]
- 8.Verma V, Ahern CA, G Berlind C, D Lindsay W, Shabason J, Sharma S, Culligan MJ, Grover S, Friedberg JS, and Simone CB 2nd (2018). Survival by histologic subtype of malignant pleural mesothelioma and the impact of surgical resection on overall survival. Clin. Lung Cancer 19(6), e901–e912. [DOI] [PubMed] [Google Scholar]
- 9.Curran D, Sahmoud T, Therasse P, van Meerbeeck J, Postmus PE and Giaccone G (1998) Prognostic factors in patients with pleural mesothelioma: The European organization for research and treatment of cancer experience. J. Clin. Oncol. 16(1), 145–152. [DOI] [PubMed] [Google Scholar]
- 10.Flores RM, Zakowski M, Venkatraman E, Krug L, Rosenzweig K, Dycoco J, Lee C, Yeoh C, Bains M and Rusch V (2007) Prognostic factors in the treatment of malignant pleural mesothelioma at a large tertiary referral center. J. Thorac. Oncol. 2(10), 957–965. [DOI] [PubMed] [Google Scholar]
- 11.Gorini G, De Gregorio G, Silvestri S, Chellini E, Cupelli V and Costantini AS (2005) Survival of malignant pleural mesothelioma cases in the Tuscan Mesothelioma Register, 1988–2000: A population-based study. Eur. J. Cancer Prev. 14(3), 195–199. [DOI] [PubMed] [Google Scholar]
- 12.Magnani C, Viscomi S, Dalmasso P, Ivaldi C, Mirabelli D and Terracini B (2002) Survival after pleural malignant mesothelioma: A population-based study in Italy. Tumori 88(4), 266–269. [DOI] [PubMed] [Google Scholar]
- 13.Milano MT and Zhang H (2010) Malignant pleural mesothelioma: A population-based study of survival. J. Thorac. Oncol. 5 (11), 1841–1848. [DOI] [PubMed] [Google Scholar]
- 14.Ruffie P, Feld R, Minkin S, Cormier Y, Boutan-Laroze A, Ginsberg R, Ayoub J, Shepherd FA, Evans WK and Figueredo A, (1989) Diffuse malignant mesothelioma of the pleura in Ontario and Quebec: A retrospective study of 332 patients. J. Clin. Oncol. 7(8), 1157–1168. [DOI] [PubMed] [Google Scholar]
- 15.Spaggiari L, Marulli G, Bovolato P, Alloisio M, Pagan V, Oliaro A, Ratto GB, Facciolo F, Sacco R, Brambilla D, Maison-neuve P, Mucilli F, Alessandrini G, Leoncini G, Ruffini E, Fontana P, Infante M, Pariscenti GL, Casiraghi M and Rea F (2014) Extrapleural pneumonectomy for malignant mesothelioma: An Italian multicenter retrospective study. Ann. Thorac. Surg. 97(6), 1859–1865. [DOI] [PubMed] [Google Scholar]
- 16.van Zandwijk N, Clarke C, Henderson D, Musk AW, Fong K, Nowak A, Loneragan R, McCaughan B, Boyer M, Feigen M, Currow D, Schofield P, Nick Pavlakis BI, McLean J, Marshall H, Leong S, Keena V and Penman A (2013) Guidelines for the diagnosis and treatment of malignant pleural mesothelioma. J. Thorac. Dis. 5(6), E254–E307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Vogelzang NJ, Rusthoven JJ, Symanowski J, Denham C, Kaukel E, Ruffie P, Gatzemeier U, Boyer M, Emri S, Mane-gold C, Niyikiza C and Paoletti P (2003) Phase III study of peme-trexed in combination with cisplatin versus cisplatin alone in patients with malignant pleural mesothelioma. J. Clin. Oncol. 21 (14), 2636–2644. [DOI] [PubMed] [Google Scholar]
- 18.Van Meerbeeck JP, Gaafar R, Manegold C, Van Klaveren RJ, Van Marck EA, Vincent M, Legrand C, Bottomley A, Deb-ruyne C and Giaccone G; European Organisation for Research and Treatment of Cancer Lung Cancer Group,; National Cancer Institute of Canada (2005) Randomized phase III study of cisplatin with or without raltitrexed in patients with malignant pleural mesothelioma: An intergroup study of the European organisation for research and treatment of cancer lung cancer group and the National Cancer Institute. J. Clin. Oncol. 23(28), 6881–6889. [DOI] [PubMed] [Google Scholar]
- 19.Rusch VW, Giroux D, Kennedy C, Ruffini E, Cangir AK, Rice D, Pass H, Asamura H, Waller D, Edwards J, Weder W, Hoffmann H and van Meerbeeck JP; IASLC Staging Committee (2012) Initial analysis of the international association for the study of lung cancer mesothelioma database. J. Thorac. Oncol. 7(11), 1631–1639. [DOI] [PubMed] [Google Scholar]
- 20.Rusch V, Baldini EH, Bueno R, De Perrot M, Flores R, Hasegawa S, Klepetko W, Krug L, Lang-Lazdunski L, Pass H, Weder W and Sugarbaker DJ; participants in the 2012 International Mesothelioma Interest Group Congress (2013) The role of surgical cytoreduction in the treatment of malignant pleural mesothelioma: Meeting summary of the International Mesothelioma Interest Group Congress, September 11–14, 2012, Boston, Mass. J. Thorac. Cardio-vasc. Surg. 145, 909–910. [DOI] [PubMed] [Google Scholar]
- 21.Verma V, Ahern CA, Berlind CG, Lindsay WD, Sharma S, Shabason J, Culligan MJ, Grover S, Friedberg JS and Simone CB 2nd (2017) National cancer database report on pneumonectomy versus lung-sparing surgery for malignant pleural mesothelioma. J. Thorac. Oncol. 12(11), 1704–1714. [DOI] [PubMed] [Google Scholar]
- 22.Cao C, Tian D, Park J, Allan J, Pataky KA and Yan TD (2014) A systematic review and meta-analysis of surgical treatments for malignant pleural mesothelioma. Lung Cancer 83(2), 240–245. [DOI] [PubMed] [Google Scholar]
- 23.Chen J, Keltner L, Christophersen J, Zheng F, Krouse M, Singhal A and Wang SS (2002) New technology for deep light distribution in tissue for phototherapy. Cancer J. 8(2), 154–163. [DOI] [PubMed] [Google Scholar]
- 24.Chen B, Pogue BW, Hoopes PJ and Hasan T (2006) Vascular and cellular targeting for photodynamic therapy. Crit. Rev. Eukaryot. Gene Expr. 16(4), 279–305. [DOI] [PubMed] [Google Scholar]
- 25.Hamblin MR and Hasan T (2004) Photodynamic therapy: A new antimicrobial approach to infectious disease? Photochem. Photobiol. Sci. 3(5), 436–450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Swartling J, Axelsson J, Ahlgren G, Kalkner KM, Nilsson S, Svanberg S, Svanberg K and Andersson-Engels S (2010) System for interstitial photodynamic therapy with online dosimetry: First clinical experiences of prostate cancer. J. Biomed. Opt. 15(5), 058003. [DOI] [PubMed] [Google Scholar]
- 27.Saini R, Lee N, Liu K and Poh C (2016) Prospects in the application of photodynamic therapy in oral cancer and premalignant lesions. Cancers (Basel) 8(9), 83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Simone CB 2nd and Cengel KA (2014) Photodynamic therapy for lung cancer and malignant pleural mesothelioma. Semin. Oncol. 41(6), 820–830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Skovsen E, Snyder JW, Lambert JDC and Ogilby PR (2005) Lifetime and diffusion of singlet oxygen in a cell. J Phys Chem B. 109(18), 8570–8573. [DOI] [PubMed] [Google Scholar]
- 30.Du K, Both S and Friedberg J (2010) Extrapleural pneumonectomy, photodynamic therapy and intensity modulated radiation therapy for the treatment of malignant pleural mesothelioma. Biol Ther. 10(5), 425–429. [DOI] [PubMed] [Google Scholar]
- 31.Friedberg JS, Culligan MJ, Mick R, Stevenson J, Hahn SM, Sterman D, Punekar S, Glatstein E and Cengel K (2012) Ann. Thorac. Surg. 93(5), 1658–1668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Friedberg JS, Simone CB 2nd, Culligan MJ, Barsky AR, Doucette A, McNulty S, Hahn SM, Alley E, Sterman DH, Glatstein E and Cengel KA (2017) Extended pleurectomy-decortication–based treatment for advanced stage epithelial mesothelioma yielding a median survival of nearly three years. Ann. Thorac. Surg. 103, 912–919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pass HI, Temeck BK, Kranda K, Thomas G, Russo A, Smith P, Friauf W and Steinberg SM (1997) Phase III randomized trial of surgery with or without intraoperative photodynamic therapy and postoperative immunochemotherapy for malignant pleural mesothelioma. Ann. Surg. Oncol. 4(8), 628–633. [DOI] [PubMed] [Google Scholar]
- 34.Zhu TC, Liang X, Kim MM, Finlay JC, Dimofte A, Rodriguez C, Simone CB 2nd, Friedberg JS and Cengel KA (2015a) An IR navigation system for pleural PDT. Front Phys. 3, 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Schouwink H, Rutgers ET, Van der Sijp J, Oppelaar H, van Zandwijk N, van Veen R, Burgers S, Stewart FA, Zoet-mulder F and Baas P (2001) Intraoperative photodynamic therapy after pleuropneumonectomy in patients with malignant pleural mesothelioma: Dose finding and toxicity results. Chest 120(4), 1167–1174. [DOI] [PubMed] [Google Scholar]
- 36.Matzi V, Maier A, Woltsche M and Smolle-Juttner FM (2004) Polyhematoporphyrin-mediated photodynamic therapy and decortication in palliation of malignant pleural mesothelioma: A clinical pilot study. Interact. Cardiovasc. Thorac. Surg. 3, 52–56. [DOI] [PubMed] [Google Scholar]
- 37.Friedberg JS, Mick R, Stevenson J, Metz J, Zhu T, Buyske J, Sterman DH, Pass HI, Glatstein E and Hahn SM (2003) A phase I study of Foscan-mediated photodynamic therapy and surgery in patients with mesothelioma. Ann. Thorac. Surg. 75(3), 952–959. [DOI] [PubMed] [Google Scholar]
- 38.Friedberg JS, Mick R, Culligan M, Stevenson J, Fernandes A, Smith D, Glatstein E, Hahn SM and Cengel K (2011) Photodynamic therapy and the evolution of a lung-sparing surgical treatment for mesothelioma. Ann. Thorac. Surg. 91(6), 1738–1745. [DOI] [PubMed] [Google Scholar]
- 39.Häkkinen AM, Laasonen A, Linnainmaa K, Mattson K and Pyrhönen S (1996) Radiosensitivity of mesothelioma cell lines. Acta Oncol. 35(4), 451–456. [DOI] [PubMed] [Google Scholar]
- 40.Carmichael J, Degraff WG, Gamson J, Russo D, Gazdar AF, Levitt ML, Minna JD, Mitchell JB (1989) Radiation sensitivity of human lung cancer cell lines. Eur J Cancer Clin Oncol. 25(3), 527–534. [DOI] [PubMed] [Google Scholar]
- 41.Gordon W Jr, Antman KH, Greenberger JS, Weichselbaum RR and Chaffey JT (1982) Radiation therapy in the management of patients with mesothelioma. Int. J. Radiat. Oncol. Biol. Phys. 8 (1), 19–25. [DOI] [PubMed] [Google Scholar]
- 42.De Graaf-Strukowska L, Van Der Zee J, Van Putten W and Senan S (1999) Factors influencing the outcome of radiotherapy in malignant mesothelioma of the pleura—A single-institution experience with 189 patients. Int. J. Radiat. Oncol. Biol. Phys. 43(3), 511–516. [DOI] [PubMed] [Google Scholar]
- 43.Boutin C, Rey F and Viallat JR (1995) Prevention of malignant seeding after invasive diagnostic procedures in patients with pleural mesothelioma: A randomized trial of local radiotherapy. Chest 108 (3), 754–758. [DOI] [PubMed] [Google Scholar]
- 44.O’Rourke N, Garcia JC, Paul J, Lawless C, McMenemin R and Hill J (2007) A randomised controlled trial of intervention site radiotherapy in malignant pleural mesothelioma. Radiother. Oncol. 84(1), 18–22. [DOI] [PubMed] [Google Scholar]
- 45.Bydder S, Phillips M, Joseph DJ, Cameron F, Spry NA, DeMelker Y and Musk AW (2004) A randomised trial of single-dose radiotherapy to prevent procedure tract metastasis by malignant mesothelioma. Br. J. Cancer 91(1), 9–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.De Perrot M, Feld R, Leighl NB, Hope A, Waddell TK, Keshavjee S and Cho BC (2016) Accelerated hemithoracic radiation followed by extrapleural pneumonectomy for malignant pleural mesothelioma. J. Thorac. Cardiovasc. Surg. 151, 468–473. [DOI] [PubMed] [Google Scholar]
- 47.Rice DC, Smythe WR, Liao Z, Guerrero T, Chang JY, McAleer MF, Jeter MD, Correa A, Vaporciyan AA, Liu HH, Komaki R, Forster KM and Stevens CW (2007) Dose-dependent pulmonary toxicity after postoperative intensity-modulated radiotherapy for malignant pleural mesothelioma. Int. J. Radiat. Oncol. Biol. Phys. 69(2), 350–357. [DOI] [PubMed] [Google Scholar]
- 48.Allen AM, Czerminska M, Jänne PA, Sugarbaker DJ, Bueno R, Harris JR, Court L and Baldini EH (2006) Fatal pneumonitis associated with intensity-modulated radiation therapy for mesothelioma. Int. J. Radiat. Oncol. Biol. Phys. 65(3), 640–645. [DOI] [PubMed] [Google Scholar]
- 49.Cramer G, Simone CB 2nd, Busch TM and Cengel KA (2018) Adjuvant, neoadjuvant, and definitive radiation therapy for malignant pleural mesothelioma. J. Thorac. Dis. 10(Suppl 21), S2565–S2573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Shaaban SG, Verma V, Choi JI, Shabason J, Sharma S, Glass E, Grover S, Badiyan SN and Simone CB 2nd (2018) Utilization of intensity-modulated radiation therapy for malignant pleural mesothelioma in the United States. Clin. Lung Cancer 19(5), e685–S692. [DOI] [PubMed] [Google Scholar]
- 51.Lorentini S, Amichetti M, Spiazzi L, Tonoli S, Magrini SM, Fellin F, Schwarz M (2012) Adjuvant intensity-modulated proton theapy in malignant pleural mesothelioma: A comparison with intensity-modulated radiotherapy and a spot size variation assessment. Strahlentherapie und Onkol. 188(3), 216–225. [DOI] [PubMed] [Google Scholar]
- 52.Krayenbuehl J, Hartmann M, Lomax AJ, Kloeck S, Hug EB and Ciernik IF (2010) Proton therapy for malignant pleural mesothelioma after extrapleural pleuropneumonectomy. Int. J. Radiat. Oncol. Biol. Phys. 78(2), 628–634. [DOI] [PubMed] [Google Scholar]
- 53.Badiyan SN, Molitoris JK, Zhu M, Glass E, Diwanji T and Simone CB 2nd (2018) Proton beam therapy for malignant pleural mesothelioma. Transl Lung Cancer Res. 7(2), 189–198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Chang JY, Verma V, Li M, Zhang W, Komaki R, Lu C, Allen PK, Liao Z, Welsh J, Lin SH, Gomez D, Jeter M, O’Reilly M, Zhu RX, Zhang X, Li H, Mohan R, Heymach JV, Vaporciyan AA, Hahn S and Cox JD (2017) Proton beam radio-therapy and concurrent chemotherapy for unresectable stage III non-small cell lung cancer: Final results of a phase 2 study. JAMA Oncol. 3(8), e172032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Higgins KA, O’Connell K, Liu Y, Gillespie TW, McDonald MW, Pillai RN, Patel KR, Patel PR, Robinson CG, Simone CB 2nd, Owonikoko TK, Belani CP, Khuri FR, Curran WJ, Ramalingam SS and Behera M (2017) National cancer database analysis of proton versus photon radiation therapy in non-small cell lung cancer. Int. J. Radiat. Oncol. Biol. Phys. 97(1), 128–137. [DOI] [PubMed] [Google Scholar]
- 56.Rwigema J-CM, Verma V, Lin L, Berman AT, Levin WP, Evans TL, Aggarwal C, Rengan R, Langer C, Cohen RB and Simone CB 2nd (2017) Prospective study of proton-beam radiation therapy for limited-stage small cell lung cancer. Cancer 123, 4244–4251. [DOI] [PubMed] [Google Scholar]
- 57.Vogel J, Lin L, Litzky LA, Berman AT and Simone CB 2nd (2017) Predicted rate of secondary malignancies following adjuvant proton versus photon radiation therapy for thymoma. Int. J. Radiat. Oncol. Biol. Phys. 99(2), 427–433. [DOI] [PubMed] [Google Scholar]
- 58.Vogel J, Berman AT, Lin L, Pechet TT, Levin WP, Gabriel P, Khella SL, Singhal S, Kucharczuk JK and Simone CB 2nd (2016) Prospective study of proton beam radiation therapy for adjuvant and definitive treatment of thymoma and thymic carcinoma: Early response and toxicity assessment. Radiother. Oncol. 118(3), 504–509. [DOI] [PubMed] [Google Scholar]
- 59.Chao HH, Berman AT, Simone CB 2nd, Ciunci C, Gabriel P, Lin H, Both S, Langer C, Lelionis K, Rengan R, Hahn SM, Prabhu K, Fagundes M, Hartsell W, Mick R and Plastaras JP (2017) Multi-institutional prospective study of reirradiation with proton beam radiotherapy for locoregionally recurrent non-small cell lung cancer. J. Thorac. Oncol. 12, 281–292. [DOI] [PubMed] [Google Scholar]
- 60.Mole RH (1953) Whole body irradiation; radiobiology or medicine? Br. J. Radiol. 26(305), 234–241. [DOI] [PubMed] [Google Scholar]
- 61.Postow MA, Callahan MK, Barker CA, Yamada Y, Yuan J, Kitano S, Mu Z, Rasalan T, Adamow M, Ritter E, Sedrak C, Jungbluth AA, Chua R, Yang AS, Roman RA, Rosner S, Benson B, Allison JP, Lesokhin AM, Gnjatic S and Wolchok JD (2012) Immunologic correlates of the abscopal effect in a patient with melanoma. N. Engl. J. Med. 366(10), 925–931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Gameiro SR, Malamas AS, Bernstein MB, Tsang KY, Vassantachart A, Sahoo N, Tailor R, Pidikiti R, Guha CP, Hahn SM, Krishnan S and Hodge JW (2016) Tumor cells surviving exposure to proton or photon radiation share a common immunogenic modulation signature, rendering them more sensitive to T cell-mediated killing. Int. J. Radiat. Oncol. Biol. Phys. 95(1), 120–130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Canti G, Lattuada D, Nicolin A, Taroni P, Valentini G and Cubeddu R. (1994) Antitumor immunity induced by photodynamic therapy with aluminum disulfonated phthalocyanines and laser light. Anticancer Drugs, 5(4), 443–447. [DOI] [PubMed] [Google Scholar]
- 64.Kabingu E, Oseroff AR, Wilding GE and Gollnick SO (2009) Enhanced systemic immune reactivity to a basal cell carcinoma associated antigen following photodynamic therapy. Clin. Cancer Res. 15(13), 4460–4466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Brackett CM and Gollnick SO (2011) Photodynamic therapy enhancement of anti-tumor immunity. Photochem. Photobiol Sci. 10 (5), 649–652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Veiga C, Janssens G, Teng CL, Baudier T, Hotoiu L, McClelland JR, Royle G, Lin L, Yin L, Metz J, Solberg TD, Tochner Z, Simone CB 2nd, McDonough J and Teo BK (2016) First clinical investigation of cone beam computed tomography and deformable registration for adaptive proton therapy for lung cancer. Int. J. Radiat. Oncol. Biol. Phys. 95(1), 549–559. [DOI] [PubMed] [Google Scholar]
- 67.Zhu TC, Kim MM, Jacques SL, Penjweini R, Dimofte A, Finlay JC, Simone CB 2nd, Cengel KA and Friedberg J (2015b) Real-time treatment light dose guidance of Pleural PDT: An update. Proc. SPIE Int. Soc. Opt. Eng. 9308, 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Vulcan TG, Zhu TC, Rodriguez CE, Hsi A, Fraker DL, Baas P, Murrer LH, Star WM, Glatstein E, Yodh AG and Hahn SM (2000) Comparison between isotropic and nonisotropic dosimetry systems during intraperitoneal photodynamic therapy. Lasers Surg. Med. 26(3), 292–301. [DOI] [PubMed] [Google Scholar]
- 69.Zhu TC, Dimofte A, Hahn SM and Lustig RA (2003) Light dosimetry at tissue surfaces for small circular fields. Proc SPIE. 4952, 56–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Zhu TC, Dimofte A, Finlay JC, Glatstein E and Hahn SM (2005) Detector calibration factor for interstitial in-vivo light dosimetry using isotropic detectors with scattering tip. Proc. SPIE Int. Soc. Opt. Eng, 5689, 26113754. 10.1117/12.590330 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Dimofte A, Zhu TC, Finlay JC, Cullighan M, Edmonds CE, Friedberg JS, Cengel K and Hahn SM (2009) In-vivo Light dosimetry for pleural PDT. In Proceedings of SPIE, Vol. 7164 (Edited by Kessel D), pp. 71640A–71640A-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Rusch VW, Rosenzweig K, Venkatraman E, Leon L, Raben A, Harrison L, Bains MS, Downey RJ, Ginsberg RJ (2001) A phase II trial of surgical resection and adjuvant highdose hemitho-racic radiation for malignant pleural mesothelioma. J. Thorac. Cardiovasc. Surg. 122(4), 788–795. [DOI] [PubMed] [Google Scholar]
- 73.Li YR, Alley EW, Friedberg JS, Culligan MJ, Busch TM, Hahn SM, Cengel KA and Simone CB (2015) Prospective Assessment of Proton Therapy for Malignant Pleural Mesothelizoma. International Association for the Study of Lung Cancer. 16th World Conference on Lung Cancer. [Google Scholar]
- 74.Zulaziz N, Azhim A, Himeno N, Tanaka M, Satoh Y, Kinoshita M, Miyazaki H, Saitoh D and Shinomiya N (2015) Photodynamic therapy mediates innate immune responses via fibroblast–macrophage interactions. Hum. Cell 28(4), 159–166. [DOI] [PubMed] [Google Scholar]
- 75.Garg AD and Agostinis P (2014) ER stress, autophagy and immunogenic cell death in photodynamic therapy-induced anti-cancer immune responses. Photochem. Photobiol. Sci. 13(3), 474–487. [DOI] [PubMed] [Google Scholar]
- 76.Simone CB 2nd, Friedberg JS, Glatstein E, Stevenson JP, Sterman DH, Hahn SM and Cengel KA (2012) Photodynamic therapy for the treatment of non-small cell lung cancer. J. Thorac. Dis. 4(1), 63–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Shimokawa T, Ma L, Ando K, Sato K and Imai T (2016) The future of combining carbon-ion radiotherapy with immunotherapy: Evidence and progress in mouse models. Int J Part Ther. 3(1), 61–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Palma DA, Senan S, Tsujino K, Barriger RB, Rengan R, Moreno M, Bradley JD, Kim TH, Ramella S, Marks LB, De Petris L, Stitt L and Rodrigues G (2013) Predicting radiation pneumonitis after chemoradiation therapy for lung cancer: An international individual patient data meta-analysis. Int J Radiat Oncol. 85 (2), 444–450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Verma V, Ahern CA, Berlind CG, Lindsay WD, Grover S, Culligan MJ, Friedberg JS and Simone CB 2nd (2018). Facility volume and postoperative outcomes for malignant pleural mesothelioma: A National Cancer Data Base analysis. Lung Cancer. 120, 7–13. [DOI] [PubMed] [Google Scholar]
- 80.Alley EW, Katz SI, Cengel KA and Simone CB 2nd (2017) Immunotherapy and radiation therapy for malignant pleural mesothelioma. Transl Lung Cancer Res. 6(2), 212–219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Davis RW, Papasavvas E, Klampatsa A, Putt M, Montaner LJ, Culligan MJ, McNulty S, Friedberg JS, Simone CB 2nd, Singhal S, Albelda SM, Cengel KA and Busch TM (2018) A preclinical model to investigate the role of surgically-induced inflammation in tumor responses to intraoperative photodynamic therapy. Lasers Surg. Med. 50, 440–450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Scherpereel A, Mazieres J, Greillier L, Dô P, Bylicki O, Monnet I, Corre R, Audigier-Valette C, Locatelli-Sanchez M, Molinier O and Thiberville L (2017) Second-or third-line nivolumab (Nivo) versus nivo plus ipilimumab (Ipi) in malignant pleural mesothelioma (MPM) patients: Results of the IFCT-1501 MAPS2 randomized phase II trial. J. Clin. Oncol. 35(Suppl 18), LBA8507–LBA8507. [Google Scholar]


