Abstract
Increased circulation of enterovirus D68 in 2014 and 2016 temporally and geographically coincided with increases in cases of acute flaccid myelitis, an uncommon condition of paralysis due to lesions in the anterior horn of the spinal cord. The identification of enterovirus D68 in respiratory specimens from cases of acute flaccid myelitis worldwide further supports an association, yet the absence of direct virus isolation from affected tissues, infrequent detection in cerebrospinal fluid, and the absence, until recently, of an animal model has left the causal nature of the relationship unproven. In this Personal View we evaluate epidemiological and biological evidence linking enterovirus D68 and acute flaccid myelitis. We applied the Bradford Hill criteria to investigate the evidence for a causal relationship and highlight the importance of comprehensive surveillance and research to further characterise the role of enterovirus D68 in acute flaccid myelitis and pursue effective therapies and prevention strategies.
Introduction
In 2012, cases of a polio-like neurological disease, now designated acute flaccid myelitis (AFM), occurred in California, with enterovirus D68 identified in respiratory tract specimens.1 Subsequently, temporal and geographic correlations of increased enterovirus D68 circulation with clusters of AFM cases worldwide have further suggested a possible causal relationship.2 However, inability to identify enterovirus D68 in the CNS in most cases of AFM leaves the association without direct proof of causality. Thus, the relationship between the emergence of enterovirus D68 and the rise in AFM cases is controversial.
Enterovirus D68 and acute flaccid myelitis
Enterovirus D68 was discovered in 1962 after being isolated from respiratory specimens of children with pneumonia in California.3 Enterovirus D68 is a non-polio enterovirus with biological and clinical properties so similar to those of human rhinoviruses that it was initially classified as rhinovirus 87.4,5 Enterovirus D68 grows optimally at 33°C and primarily binds sialic acid receptors in the upper and, less commonly, lower respiratory tract.4,6,7 Enterovirus D68 is transmitted mainly via the respiratory route and detected in respiratory specimens early in the course of disease. Unlike acid-stable and heat-stable enteroviruses, enterovirus D68 is uncommonly detectable in stool.4
Only 26 cases of enterovirus D68 infection were reported through the passive US National Enterovirus Surveillance System from 1970 to 2005.8 Numbers of small clusters of enterovirus D68 respiratory illness increased in Europe, Asia, and the USA from 2008 to 2010.9 In 2014, the USA experienced a large enterovirus D68 outbreak, with 1153 confirmed infections coinciding with a surge in respiratory illnesses detected by syndromic surveillance that suggested millions of cases.10 Retrospective analysis showed enterovirus D68 circulation in Europe during the same period.11 In 2015, no enterovirus D68 isolates were reported in the USA, suggesting little to no circulation12–14 but in the late summer to autumn of 2016, enterovirus D68 was again detected at sites doing active surveillance in the USA and Europe.13–17
AFM has recently been described as acute onset of flaccid limb weakness, with imaging showing spinal cord grey matter lesions suggestive of anterior myelitis.18 The inclusion of imaging criteria in the case definition for AFM was intended to provide more specificity to the broader epidemiological case definition of acute flaccid paralysis (AFP), which is primarily used for global poliovirus surveillance. Although use of the term AFM is new, the clinical condition it describes is not, encompassing cases previously described as poliomyelitis, polio-like illness, and acute flaccid paralysis with anterior myelitis.19 Throughout this paper, AFP refers to cases of acute flaccid limb weakness without further characterisation, whereas AFM is used to refer to the subset of cases of AFP with additional imaging findings suggestive of myelitis. The asymmetric lower motor-neuron-specific deficits and characteristic longitudinal anterior horn predominant spinal cord grey matter lesions can help distinguish AFM from other causes of AFP, including Guillain-Barré syndrome, acute disseminated encephalomyelitis, and transverse myelitis, although some overlap in epidemiological case definitions and clinical findings exists.2 Most commonly associated with poliovirus and other non-polio enteroviruses, AFM has also been described in association with endemic and epidemic neurotropic flaviviruses, most notably West Nile virus and Japanese encephalitis virus.20–22 Although AFM is now rare in countries with adequate poliovirus vaccination, several AFM clusters occurred in the USA, Canada, and Europe in 2014 and 2016,23–30 coincident with emergence of enterovirus D68.
Criteria for causality
Koch’s postulates, developed in the 19th century, are the traditional criteria used to establish a causal relationship between an infectious agent and disease.31 However, in 1965, Bradford Hill provided a set of fundamental tenets of causal inference in epidemiology to move from an observed association to a verdict of causation.32 The Bradford Hill criteria provide a rigorous method to assess what is known and unknown about a potential causal relationship, but they do not require fulfilment of all criteria to establish causality. Fredricks and Relman33 and Lipkin34 highlighted the role of molecular diagnostics and the strength of epidemiological association and biological plausibility as important components for consideration of causality particular to pathogens, such as viruses, to which Koch’s postulates may not readily apply.
The establishment of a causal relationship between enteroviruses, such as polioviruses and enterovirus A71, and neurological manifestations evolved from initial epidemiological observations to animal experimentation and descriptions of pathological changes in and viral isolation from human CNS tissue. Epidemiological descriptions of paralytic outbreaks in the 19th century were followed by experimental evidence of a causal link for polioviruses in primate models and subsequent demonstration of pathological changes in and isolation of poliovirus from human spinal cord tissue.35–38 Similarly, epidemiological descriptions of brainstem encephalitis and paralytic disease associated with outbreaks of enterovirus A71 hand-foot-mouth disease in the 1970s to 1990s were followed by confirmation of a causal link to the virus in mouse models and by demonstration of pathological changes in and viral isolation from human CNS tissues.20,39,40 In the absence of consistent detection of enterovirus D68 in the cerebrospinal fluid (CSF) of patients with AFM or sufficient CNS tissues available for pathological evaluation to prove causality, we applied the Bradford Hill criteria to examine current epidemiological and biological evidence for a causal relationship between enterovirus D68 and AFM (panel) and highlight areas where further investigation is needed.
Panel: Bradford Hill criteria for evidence of causality applied to the relationship between enterovirus D68 infection and acute flaccid myelitis.
Strength of association
Supportive evidence exists:
Increased acute flaccid myelitis (AFM) cases clustering during periods of enterovirus D68 circulation in summer–autumn 2014 and 2016; sporadic AFM cases with no clustering in 2015 when enterovirus D68 was not circulating.10,13,14,23,26,27,29
Enterovirus D68 was the most commonly identified pathogen in 2014 and 2016 US cases of AFM; enterovirus D68 was found in respiratory specimens, not cerebrospinal fluid (CSF).23,27,30
Increased AFM incidence in California during 2014 enterovirus D68 outbreak using longitudinal surveillance in place since 2012.1
4·5–10·3 greater odds of enterovirus D68 detection in AFM patients than respiratory controls in Colorado case control study.41
Assessment limited by scarcity of prospective longitudinal active surveillance.
Consistency
Supportive evidence exists:
Cases of paralysis with enterovirus D68 detection reported from 14 countries on six continents with consistent clinical presentation.
Clustering of enterovirus D68-associated AFM cases in Europe in 2016.25
Specificity
Evidence does not support specificity.
Temporality
Supportive evidence exists:
Biological gradient
Evidence does not support biological gradient.
No dose-response relationship noted and low level detection of enterovirus D68 in respiratory specimens of some severe AFM cases.43
Assessment limited by timing, dilution of respiratory specimens, and scarcity of CNS tissue.
Plausibility
Supportive evidence exists:
Five case reports of accute flaccid paralysis or AFM with enterovirus D68 in CSF. Most AFM cases with no enterovirus D68 and no alternative pathogens detected in CSF.8,23,44,45,46
One case with autopsy histopathology consistent with enterovirus encephalitis and myelitis and enterovirus D68 in CSF.44
Assessment limited by scarcity of CNS tissue for testing.
Coherence
Supportive evidence exists:
Experiment
Supportive evidence exists:
Recent enterovirus D68 strains cause paralytic myelitis in mouse model, whereas historical strains do not.49
Enterovirus D68 infects and causes loss of motor neurons in anterior horn of spinal cord in mice.49
Enterovirus D68 isolated from spinal cord of paralysed mice transmits paralytic disease to naive mice.49
Enterovirus D68 antibodies protect against paralytic disease in mice,49 whereas immunosuppression leads to increased paralysis and mortality.50
Analogy
Supportive evidence exists:
Clinical presentation, neuroimaging, electrophysiological findings in recent enterovirus D68-associated AFM cases similar to paralytic disease due to poliovirus and enterovirus A71.2
Enterovirus D68 found less commonly in CSF than poliovirus or enterovirus A71.23
Detection of poliovirus or enterovirus A71 from stool when absent in CSF analogous to enterovirus D68 detection in respiratory specimens.4,38,51,52
Weighing the evidence for causality using Bradford Hill criteria
Strength of the association
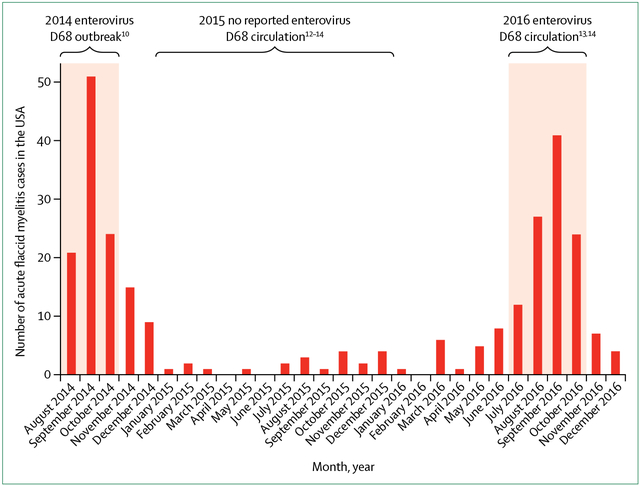
The strength of an epidemiological association helps to ascertain the likelihood of causal interdependence versus association by chance. At a national level, increases in AFM cases in the USA in 2014 and 2016 temporally correlated with enterovirus D68 circulation (figure 1).10,13,14,26 120 cases meeting the US Centers for Disease Control and Prevention (CDC) case definition for AFM were identified from August to December, 2014, during the enterovirus D68 outbreak, and enterovirus D68 was detected in 47% of respiratory tract specimens collected within 7 days of prodromal respiratory illness onset.23 By contrast, enterovirus D68 circulation was not detected in the USA in 2015,12–14 and only 21 cases of AFM without temporal or geographical clustering were reported sporadically throughout the year.26 In 2016, 144 AFM cases were noted in the USA,26 with a marked increase in cases noted from July to October correlating with a period of enterovirus D68 circulation detected at sites with active surveillance.13,14,29 Again, enterovirus D68 was the predominant pathogen identified from respiratory specimens in some reported clusters.27–30
Figure 1: Acute flaccid myelitis cases and enterovirus D68 circulation in the USA from 2014 to 2016.
Shaded areas represent periods in which enterovirus D68 circulation was identified in the USA during 2014 to 2016, although absence of active surveillance precludes quantification of prevalence.10,12–14 Bars represent the monthly number of confirmed acute flaccid myelitis cases in the USA reported to the Centers for Disease Control and Prevention with onset from August, 2014, to December, 2016 (adapted from Centers for Disease Control and Prevention26).
At a regional level, prospective AFM surveillance in California since 2012 detected an increase in baseline AFM incidence from 0·028 to 0·16 cases per 100 000 person-years from August, 2014, to January, 2015, (p<0·001) coinciding with the enterovirus D68 outbreak.1 In Colorado, the number of AFM cases from August to October, 2014, during the enterovirus D68 outbreak was 3 times higher than the number of cases retrospectively identified during any 3-month period in the previous 4 years (p=0·0009).41 The odds of having enterovirus D68 infection were 10·3 (95% CI 1·8–64·8) and 4·5 (1·0–21·2) times greater in patients with AFM than in the general patient population tested for respiratory infection and pertussis infection, respectively, after adjusting for age, time to specimen collection, and week of collection.41 The available data suggest a strong epidemiological association between enterovirus D68 and AFM, which is unlikely to be due solely to increased case finding, incidental detection, or chance, although more longitudinal and granular prospective surveillance is needed to confirm these findings.
Consistency
Consistent observations in diverse populations in different settings over time suggest that an association is more likely to be causal. AFP and AFM cases with enterovirus D68 detected in biological specimens have been published from 14 countries on six continents (table). Increasing awareness of and testing for enterovirus D68 in Europe led to 31 enterovirus D68-associated AFM cases reported in 2016, an increase of greater than 10 times compared with 2014.25 The clinical course, laboratory, imaging, and electrophysiology in these cases and clusters are consistent with those reported in the USA in 2014.
Table:
Acute flaccid paralysis or acute flaccid myelitis cases with enterovirus D68 identified from biological specimens*
| Number of cases with enterovirus D68 from any source (year of presentation) | Number of cases with enterovirus D68 in CSF | |
|---|---|---|
| Argentina45,53 | 4 (2016) | 1 |
| Australia54 | 2 (2010) | 0 |
| Canada24,55 | 7 (2014) | 0 |
| Democratic Republic of the Congo56 | 1 (year not reported) | 0 |
| France11,57,58–60 | 2 (2014), 4 (2016) | 0 |
| Germany61,62 | 2 (2016) | 0 |
| Italy46,63 | 2 (2016) | 1 |
| Japan64 | 3 (2013, 2015) | 0 |
| Netherlands16,65 | 2 (2016) | 0 |
| Norway11,66,67 | 2 (2014) | 0 |
| Spain68 | 1 (2015), 2 (2016) | 0 |
| Sweden15 | 3 (2016) | 0 |
| UK69–72 | 1 (2014), 2 (2015), 6 (2016) | 0 |
| USA1,8,23,27–30,42,44,73 | 1 (2005), 1 (2008), 3 (2012), 12 (2014), 15 (2016) | 3 |
| Total | 78 cases in 14 countries on six continents | 5 |
CSF=cerebrospinal fluid.
Includes cases of acute flaccid paralysis or acute flaccid myelitis with enterovirus D68 detected from respiratory, stool or rectal, blood, or CSF specimens.
Late or absent sampling of the respiratory tract remains an impediment to detection of enterovirus D68 in patients with AFM. Diagnostic testing for WHO poliovirus surveillance has traditionally involved sampling of CSF and serial stool or rectal swab specimens.74 Enteroviruses previously associated with paralytic disease, such as poliovirus and enterovirus A71, are uncommonly detected in CSF, but readily detectable in stool or rectal samples for weeks to months after infection.51,52 By contrast, enterovirus D68 is uncommonly detected in stool and is likely to be shed from the respiratory tract for a shorter period of time similar to human rhinoviruses.75 The delay in presentation of neurological symptoms in AFM following an initial prodromal respiratory illness decreases the potential to detect an infectious trigger.2 Despite this impediment, detection of enterovirus D68 in AFM cases since 2014 in diverse populations across the globe further supports a causal relationship.
Specificity
Associations are more likely to be causal if they are specific—ie, when an exposure causes only one disease and a disease is only caused by one exposure. Enterovirus D68 does not have one-to-one specificity with AFM because most of those infected with enterovirus D68 do not have a neurological disease. Many infected with enterovirus D68 have mild respiratory symptoms; fewer have more severe lower respiratory disease and asthma-like symptoms.12 If a causal relationship exists, AFM would appear to be an uncommon complication of enterovirus D68 infection. Similarly, less than 1% of those infected with poliovirus and enterovirus A71 develop severe neurological disease, including paralysis.38,40 Two siblings infected with identical enterovirus D68 strains were identified in California in 2014, one with AFM and one with respiratory illness, which suggests that enterovirus D68 infection with a neurotropic strain alone would not be sufficient to cause AFM.43 Investigations into host and environmental factors may help to explain why some individuals infected with the same virus may manifest neurological symptoms whereas others do not.
Additionally, AFM does not have one-to-one specificity with enterovirus D68, as there are other known infectious causes of this clinical syndrome. Notably, non-polio enteroviruses previously described as causing AFM, such as enterovirus C105, echoviruses, and coxsackieviruses, were detected in single cases of AFM during the 2014 and 2016 outbreaks in the USA, as would be expected from background seasonal circulation of these viruses.23,76 There are no unique phenotypic characteristics to distinguish enterovirus D68-associated AFM from other infectious causes.2 Nevertheless, despite considerable overlap with paralytic disease associated with poliovirus and enterovirus A71, AFM associated with enterovirus D68 appears to more commonly have a respiratory prodrome and predilection for areas high in the brainstem and spinal cord, causing more upper limb paralysis and cranial neuropathies.42 The long-term outcomes of enterovirus D68-associated AFM appear to be more similar to those of paralytic poliovirus disease than to those of enterovirus A71-associated paralysis, with muscle atrophy and long-term disability in the most affected muscle groups in most patients.77 Although there is no one-to-one specificity between enterovirus D68 and AFM, such specificity is uncommon for viruses which cause a wide spectrum of clinical presentations and clinically defined syndromes which can be attributed to a variety of pathogens.
Temporality
If a pathogen causes a disease, infection with that agent must precede the disease in time. In AFM, clinical symptoms of a febrile prodromal respiratory illness precede the acute onset of headache, meningeal signs, and pain in either the neck, back, or affected limb, with associated limb weakness by a median of 5 days.23 At least two published cases describe children with enterovirus D68 detected during hospitalisation for the initial prodromal presentation of respiratory illness who subsequently developed neurological illness meeting AFM criteria.42,57 Further evidence for a temporal relationship is the finding that delayed respiratory sampling following presentation with AFM decreases the frequency of enterovirus D68 detection. In the USA during 2014 enterovirus D68 was identified in 47% of respiratory specimens collected within 7 days, 20% of specimens collected between 7 and 14 days, and 0% of 17 specimens collected more than 14 days after onset of prodromal respiratory symptoms.23 Likewise, respiratory specimens that tested positive for enterovirus D68 in AFM cases in Canada in 2014 were more likely to be obtained earlier than those that tested negative (median 3·5 days’ difference).24 The pattern of respiratory symptoms and detection of enterovirus D68 before onset of neurological symptoms in AFM shows a temporal relationship between enterovirus D68 and AFM that suggests causality.
Biological gradient
An increase in pathogenicity or severity of disease with increasing exposure to a putative aetiological agent can support causality. However, for most viruses, dose-response curves are non-linear and vary depending on unique characteristics of the given population, exposure route, and molecular endpoints assessed. Further, virus quantification from respiratory samples is influenced by dilution from the method of collection (nasopharyngeal swab vs nasal wash vs bronchoalveolar lavage), viral replication within the host after exposure, and timing of specimen collection. Several samples from AFM patients demonstrated low concentrations of virus in respiratory specimens compared with samples from patients with isolated respiratory symptoms, but this result might have been confounded by longer intervals between infection and sampling in AFM cases.43 No study of virus quantification in human spinal cord or brain tissue to analyse an association of tissue virus concentration with AFM severity has been reported. Thus, although a dose-response relationship between enterovirus D68 and AFM has not yet been identified, demonstration of such a relationship might not be expected based on existing information.
Plausibility
Consistent identification of a virus in the CNS with associated pathology would suggest that the virus is a neuropathogen. However, obtaining spinal cord tissue to assess pathology is only feasible postmortem. Furthermore, CSF testing might not reliably detect infection of brain and spinal cord tissue, as shown by viruses proven to cause neuroinvasive infections, such as poliovirus, enterovirus A71, West Nile virus, and rabies virus, which are frequently absent in CSF at the time of clinical presentation.38,51,78,79 Despite these limitations, enterovirus D68 has been identified in the CNS, albeit uncommonly, in association with AFM with consistent pathology. In 2008, a previously healthy 5-year-old boy with preceding upper respiratory symptoms developed pneumonia, progressive bulbar and limb paralysis, and encephalopathy and subsequently died.44 Autopsy showed meningoencephalomyelitis with lymphocytic inflammation in the motor nuclei of the anterior spinal cord in a histopathological pattern consistent with CNS enterovirus infection. Enterovirus D68 was identified in the CSF by PCR. Four additional cases have been reported of AFP or AFM with enterovirus D68 identified in CSF by PCR: one in a young adult with AFP in the USA in 2005 identified through enteroviral surveillance,8 one in a child with AFM during the 2014 USA enterovirus D68 outbreak (noted to have a blood-contaminated CSF specimen and co-detection of Epstein-Barr virus nucleic acid),23 one in a child with AFM during a cluster of cases in Argentina in 2016,45,53 and a fatal case of AFM in an immunosuppressed adult in Italy in 2016.46 Enterovirus D68 has also been detected in the CSF of two children and a young adult with aseptic meningitis without AFM.57,80 These reports suggest that it is biologically plausible that enterovirus D68 can be neuroinvasive and cause AFM.
In most cases of AFM, even those associated with enterovirus D68 outbreaks or with enterovirus D68 identified from other sites, enterovirus D68 is not detected in CSF. Among the cases of AFM reported in the USA in 2014, enterovirus D68 was only identified in one of 54 CSF specimens collected a median of 7.5 days after onset of respiratory or febrile illness.23 Testing for a broad range of pathogens in CSF, including enteroviruses, adenoviruses, herpesviruses and arboviruses, and metagenomic next-generation sequencing, failed to identify alternative pathogenic organisms in any patient with AFM tested in 2014 or 2016.23,43 Scarcity of spinal cord specimens for evaluation precludes the ability to definitively assess whether virus isolation in CNS tissues or histopathological changes consistent with neuroinvasive destruction of anterior horn cells is present in most cases of enterovirus D68-associated AFM.
Coherence
A causal relationship should be consistent with the scientific knowledge regarding pathogen and disease. Enterovirus D68 appears to be primarily a respiratory virus that binds to sialic acid receptors on respiratory epithelium.6,81 However, there is emerging evidence that enterovirus D68 might have mechanisms to invade the CNS. Viraemia was detected in 43% of patients with enterovirus D68 pneumonia a median of 2 days after symptom onset, suggesting that haematogenous migration, similar to poliovirus, may be possible.47 Additionally, sialic acid-independent enterovirus D68 strains have been identified that bind to the neuron-specific receptor intracellular adhesion molecule 5, which is present only on neurons of the telencephalon, including cranial nerves I and II, and the cerebral cortex.48,81,82 The possibility of a functional enterovirus D68 receptor being located on olfactory nerve endings in the upper nasal cavity epithelium provides another potential portal of CNS entry through retrograde axonal transport. Thus, although enterovirus D68 is most commonly associated with respiratory disease, a causal role in AFM is consistent with potential mechanisms of neuroinvasion.
Experiment
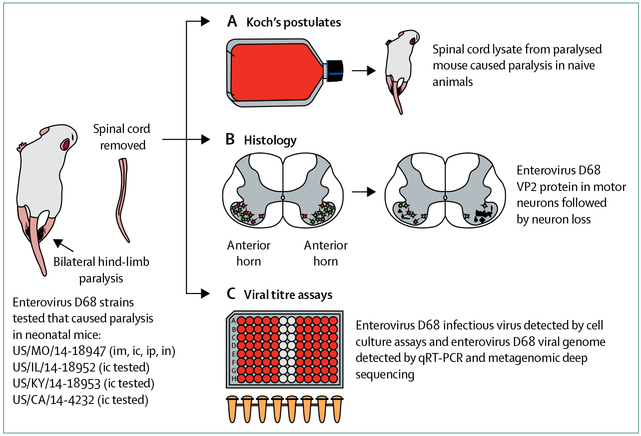
Controlled investigation in animal models can provide experimental evidence of the relationship between pathogen and disease, although it cannot prove causality in humans. Four enterovirus D68 strains from the 2014 US outbreak were found to cause paralytic myelitis in a neonatal mouse model following intracerebral infection (figure 2), whereas inoculation of equivalent titres of the 1962 prototype enterovirus D68 strains Fermon and Rhyne did not.49 The potential to cause paralysis via intramuscular, intraperitoneal, and intranasal routes of infection was also shown with a 2014 enterovirus D68 strain. Immunofluorescent and electron microscopy showed enterovirus D68 infection of neurons and motor neuron death within the anterior horns of spinal cord segments corresponding to paralysed limbs. Infectious virus and enterovirus D68 viral RNA increased over time in spinal cord, but not brain tissue, concordant with disease progression. In agreement with Koch’s postulates, enterovirus D68 isolated from spinal cords of paralysed mice transmitted paralytic disease when injected into naive mice not previously exposed to the virus. Enterovirus D68 antiserum samples and human intravenous immunoglobulin containing neutralising enterovirus D68 antibodies given before inoculation protected mice from development of paralysis and death, whereas mouse serum samples not containing enterovirus D68 antibodies failed to provide protection; conversely, corticosteroid treatment worsened motor outcomes and mortality, and increased spinal cord viral loads.50
Figure 2: Experimental mouse model of enterovirus D68 paralytic disease.
Several 2014 strains of enterovirus D68 caused permanent paralysis in neonatal mice by intracerebral (ic) inoculation. One strain tested in further detail, US/MO/14–18947, caused paralysis by multiple routes of inoculation, in inverse order of disease frequency: intramuscular (im, 100%), intracerebral (ic, about 50%), intraperitoneal (ip, about 5%), and intranasal (in, about 3%). During the course of enterovirus D68 infection, whole spinal cords were removed from paralysed animals. (A) Infected spinal cords inoculated into cell culture resulted in cytopathic effect. Media from cell culture inoculated into naive mice produced paralytic disease, as per Koch’s postulates. (B) Spinal cords taken over the course of infection showed enterovirus D68 VP2 protein within motor neurons of the anterior horn and subsequent loss of infected neurons. (C) Cell culture assays of affected spinal cords demonstrated presence of infectious virus. Spinal cords tested by qRT-PCR and metagenomic deep sequencing confirmed the presence of enterovirus D68.
This model suggests that enterovirus D68 is neuroinvasive, with tropism for spinal cord motor neurons, and causes direct viral injury that results in paralysis. These observations match the clinical findings and biological mechanisms associated with other enteroviruses that cause paralysis. Serological protection against paralysis conveyed by enterovirus D68 antibodies and worsening of outcomes after immunosuppression further strengthens the case for causality and has implications for therapeutic and preventive strategies. Data from studies in animals, however, must be interpreted cautiously, as there may be differing patterns of infectivity and disease between animal hosts and human beings. For example, Sabin poliovirus vaccine strains without reversion mutations that confer neurovirulence have been shown to cause paralysis in neonatal mice following intracerebral injection, but do not cause paralysis in humans.83 Nevertheless, these mouse model studies provide additional evidence of the neuropathogenicity of enterovirus D68.
Analogy
A causal relationship is more conceivable if similar exposures are known to cause analogous disease. Polioviruses and non-polio enteroviruses, which are among the most common infectious causes of AFM, are known to be neuroinvasive with tropism for spinal cord motor neurons. The clinical course seen with these viruses of febrile illness followed by acute-onset asymmetrical flaccid limb weakness and cranial nerve motor neuropathies, particularly bulbar paralysis, with infrequent sensory findings resembles the clinical pattern observed in AFM cases associated with enterovirus D68.2 Tropism for spinal cord motor neurons characteristic of polioviruses and other non-polio enteroviruses is analogous to the pattern of spinal cord involvement shown in enterovirus D68-associated AFM: longitudinal, anterior horn-specific lesions shown by MRI and pure motor neuron deficits shown by electrophysiological studies.66,77,84 Detection of poliovirus and enterovirus A71 in stool or rectal samples with negative CSF testing in most patients with paralytic disease is similar to detection of enterovirus D68 in respiratory specimens in many AFM cases, but rarely in CSF.38,51,52 The ability of enteroviruses, such as poliovirus and enterovirus A71, to cause anterior horn motor neuron disease reinforces the plausibility that enterovirus D68 might similarly cause paralytic disease.
Next steps in investigating a causal association
Prospective studies evaluating the epidemiological association between enterovirus D68 and AFM are needed. Because of the voluntary and passive nature of most enteroviruses surveillance systems in the USA and Europe, enterovirus D68 infections are probably under-recognised and under-reported. A global network of active enterovirus surveillance sites would provide real-time monitoring for resurgences of enterovirus D68 with collaborative genotyping efforts to allow for detection of strain changes and monitoring of molecular epidemiology.
Before 2014, AFP and AFM were not reportable conditions in the USA. In 2015, voluntary, passive surveillance for the clinical syndrome of AFM was adopted in the USA by the Council of State and Territorial Epidemiologists, with the CDC compiling nationwide US data.85 Mandated reporting of AFM cases in the USA and Europe would ensure more robust epidemiological data, improve outbreak investigations, and provide better estimates of disease burden for public health planning and response. Additionally, dedication of financial and logistical resources to expand WHO surveillance to include collection of respiratory samples with molecular testing for non-polio enteroviruses would facilitate worldwide detection of enterovirus D68-associated AFM, strengthening virological investigation and research. Essential to these efforts would be international sharing of epidemiological data, virus strains, and outcomes data with cross-specialty collaboration among the neurology, physiatry, infectious diseases, virology, and epidemiology communities.
Awareness of AFM amongst health-care providers, leading to earlier recognition and earlier collection of biological specimens, would improve the potential for detecting enterovirus D68. Spinal cord tissue from any fatal AFM case should be tested for enterovirus D68 and evaluated for anterior horn motor neuron pathology. An enterovirus D68-specific neutralising antibody test would enable intrathecal antibody testing to detect host response to CNS infection when virus is not present in CSF at the time of testing, a diagnostic standard employed for West Nile virus and other known neuroinvasive pathogens. Additional laboratory investigation, including animal and cell culture models, is needed to identify pathophysiological mechanisms and inform approaches to prevention and treatment.
Conclusion
Application of the Bradford Hill criteria to the putative association between enterovirus D68 and AFM supports a causal relationship, specifically through the fulfilment of the strength, consistency, temporality, plausibility, coherence, experiment, and analogy criteria. Of the remaining Bradford Hill criteria, evidence of specificity or biologic gradient is insufficient but these criteria are not met for many infectious conditions, including enterovirus A71 and West Nile virus-associated AFM. Importantly, the lack of specificity suggests that, if indeed a causal relationship exists, AFM is an infrequent manifestation of enterovirus D68 infection and enterovirus D68 is only one of several pathogens with the capacity to cause the condition. Prospective epidemiological studies with collection of appropriately timed specimens from all relevant anatomical sites (respiratory, stool, rectal, blood, CSF, and CNS tissue when available) for virological, serological, and immunological analyses are needed to further define the role of enterovirus D68 in AFM, identify other potential causes, and define disease pathogenesis. Currently available evidence supporting a causal role of enterovirus D68 in AFM and the potential for future disease outbreaks highlights the need for comprehensive surveillance of enterovirus D68 and AFM and development of preventive and therapeutic strategies.
Search strategy and selection criteria.
We searched PubMed for articles published in English and Japanese before Sept 9, 2017, using the terms “acute flaccid paralysis” or “acute flaccid myelitis” and “enterovirus 68” or “enterovirus D68” as well as relevant articles identified through searches in the authors’ personal files, in Google Scholar, and conference proceedings. We reviewed relevant articles resulting from these searches and references cited in those articles. We included articles pertinent to the relationship between enterovirus D68 and acute flaccid myelitis, including case reports, case series, epidemiological studies, research articles, and review articles.
Acknowledgments
Authors received support from US NIH grants R561NS101208 and 1K23AI128069-01. The funding sources had no role in the writing of the manuscript. The views expressed are those of the authors and do not reflect those of the National Institutes of Health. All authors had full access to the data presented and accept responsibility for the decision to submit for publication. We thank Satoshi Kamidani MD for his assistance in obtaining and translating references in Japanese.
Footnotes
Declaration of interests
We declare no competing interests.
References
- 1.Van Haren K, Ayscue P, Waubant E, et al. Acute flaccid myelitis of unknown etiology in California, 2012–2015. JAMA 2015;314: 2663–71. [DOI] [PubMed] [Google Scholar]
- 2.Messacar K, Schreiner TL, Van Haren K, et al. Acute flaccid myelitis: a clinical review of US cases 2012–2015. Ann Neurol 2016; 80: 326–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Schieble JH, Fox VL, Lennette EH. A probable new human picornavirus associated with respiratory diseases.Am J Epidemiol 1967; 85: 297–310. [DOI] [PubMed] [Google Scholar]
- 4.Oberste MS, Maher K, Schnurr D, et al. Enterovirus 68 is associated with respiratory illness and shares biological features with both the enteroviruses and the rhinoviruses. J Gen Virol 2004; 85: 2577–84. [DOI] [PubMed] [Google Scholar]
- 5.Blomqvist S, Savolainen C, Raman L, Roivainen M, Hovi T. Human rhinovirus 87 and enterovirus 68 represent a unique serotype with rhinovirus and enterovirus features. J Clin Microbiol 2002; 40: 4218–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Imamura T, Okamoto M, Nakakita S, et al. Antigenic and receptor binding properties of enterovirus 68. J Virol 2014; 88: 2374–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Liu Y, Sheng J, Baggen J, et al. Sialic acid-dependent cell entry of human enterovirus D68. Nat Commun 2015; 6: 8865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Khetsuriani N, Lamonte-Fowlkes A, Oberst S, Pallansch MA. Enterovirus surveillance—United States, 1970–2005. MMWR Surveill Summ 2006; 55: 1–20. [PubMed] [Google Scholar]
- 9.Centers for Disease Control and Prevention. Clusters of acute respiratory illness associated with human enterovirus 68—Asia, Europe, and United States, 2008–2010. MMWR Morb Mortal Wkly Rep 2011; 60: 1301–04. [PubMed] [Google Scholar]
- 10.Midgley CM, Watson JT, Nix WA, et al. Severe respiratory illness associated with a nationwide outbreak of enterovirus D68 in the USA (2014): a descriptive epidemiological investigation. Lancet Respir Med 2015; 3: 879–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Poelman R, Schuffenecker I, Van Leer-Buter C, et al. European surveillance for enterovirus D68 during the emerging North-American outbreak in 2014. J Clin Virol 2015; 71: 1–9. [DOI] [PubMed] [Google Scholar]
- 12.Centers for Disease Control and Prevention. Enterovirus D68. 2017. https://www.cdc.gov/non-polio-enterovirus/about/ev-d68.html (accessed April 21, 2017).
- 13.Wang G, Zhuge J, Huang W, et al. Enterovirus D68 subclade B3 strain circulating and causing an outbreak in the United States in 2016. Sci Rep 2017; 7: 1242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Messacar K, Robinson CC, Pretty K, Yuan J, Dominguez SR. Surveillance for enterovirus D68 in colorado children reveals continued circulation. J Clin Virol 2017; 92: 39–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dyrdak R, Grabbe M, Hammas B, et al. Outbreak of enterovirus D68 of the new B3 lineage in Stockholm, Sweden, August to September 2016. Euro Surveill 2016; 21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Knoester M, Scholvinck EH, Poelman R, et al. Upsurge of enterovirus D68, the Netherlands, 2016. Emerg Infect Dis 2017; 23: 140–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Barnadas C, Midgley SE, Skov MN, Jensen L, Poulsen MW, Fischer TK. An enhanced enterovirus surveillance system allows identification and characterization of rare and emerging respiratory enteroviruses in Denmark, 2015–16. J Clin Virol 2017; 93: 40–44. [DOI] [PubMed] [Google Scholar]
- 18.Centers for Disease Control and Prevention. Notes from the field: acute flaccid myelitis among persons aged ≤21 years—United States, August 1–November 13, 2014. MMWR Morb Mortal Wkly Rep 2015; 63: 1243–44. [PMC free article] [PubMed] [Google Scholar]
- 19.Sejvar JJ. West Nile virus and “poliomyelitis”. Neurology 2004; 63: 206–07. [DOI] [PubMed] [Google Scholar]
- 20.Huang CC, Liu CC, Chang YC, Chen CY, Wang ST, Yeh TF. Neurologic complications in children with enterovirus 71 infection. N Engl J Med 1999; 341: 936–42. [DOI] [PubMed] [Google Scholar]
- 21.Solomon T, Kneen R, Dung NM, et al. Poliomyelitis-like illness due to Japanese encephalitis virus. Lancet 1998; 351: 1094–97. [DOI] [PubMed] [Google Scholar]
- 22.Sejvar JJ, Bode AV, Marfin AA, et al. West Nile virus-associated flaccid paralysis. Emerg Infect Dis 2006; 11: 1021–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sejvar JJ, Lopez AS, Cortese MM, et al. Acute flaccid myelitis in the United States, August–December 2014: results of nationwide surveillance. Clin Infect Dis 2016; 63: 737–45 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yea C, Bitnun A, Robinson J, et al. Longitudinal outcomes in the 2014 accute flaccid paralysis cluster in Canada. J Child Neurol 2017; 32: 301–07. [DOI] [PubMed] [Google Scholar]
- 25.Hurley D. Rise in acute flaccid myelitis cases reported in the US and Europe. Neurol Today 2017; 17: 8–9. [Google Scholar]
- 26.Centers for Disease Control and Prevention. Acute flaccid myelitis surveillance. 2017. https://www.cdc.gov/acute-flaccid-myelitis/afmsurveillance.html (accessed Sept 19, 2017).
- 27.Iverson SA, Ostdiek S, Prasai S, et al. Notes from the field: cluster of acute flaccid myelitis in five pediatric patients—Maricopa County, Arizona, 2016. MMWR Morb Mortal Wkly Rep 2017;66: 758–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bonwitt J, Poel A, DeBolt C, et al. Acute flaccid myelitis among children—Washington, September–November 2016. MMWR Morb Mortal Wkly Rep 2017; 66: 826–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Naccache SN, Bender J, Desai J, et al. Acute flaccid myelitis cases presenting during a spike in respiratory enterovirus D68 circulation: case series from a single pediatric referral center. Open Forum Infect Dis 2017; 4 (suppl 1): s305–06. [Google Scholar]
- 30.Hopkins S, McGuire J, Swami S, Ulloa ER, Banwell B. Acute flaccid myelitis: characteristics and outcomes in 2014 and 2016 clusters. April 27, 2017. https://www.mdlinx.com/internal-medicine/conference-abstract.cfm/61305/?nonus=0&searchstring=&coverage_day=0&page=1 (accessed Feb 13, 2018).
- 31.Koch R. Uber bakteriologische Forschung Verhandlung des X Internationalen Medichinischen Congresses, Berlin In: Carter KC, ed. Hirschwald A, ed. Essays of Robert Koch. New York: Greenwood Press, 1987: 179–86. [Google Scholar]
- 32.Hill AB. The environment and disease: association or causation? Proc R Soc Med 1965; 58: 295–300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Fredricks DN, Relman DA. Sequence-based identification of microbial pathogens: a reconsideration of Koch’s postulates. Clin Microbiol Rev 1996; 9: 18–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lipkin WI. Pathogen discovery. PLoS Pathog 2008; 4: e1000002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wickman I. Acute poliomyelitis (Heine-Medin’s disease). New York, NY: The Journal of nervous and mental disease publishing company, 1913. [PubMed] [Google Scholar]
- 36.Landsteiner K, Popper E. Übertragung der Poliomyelitis acuta auf Affen. Zeitschrift für Immunitäts und exp Therapie 1909; 2: 377–90. [Google Scholar]
- 37.Sabin AB, Ward R. The natural history of human poliomyelitis—1. distribution of virus in nervous and non-nervous tissues. J Exp Med 1941; 73: 771–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Centers for Disease Control and Prevention. Poliomyelitis In: Hamborsky J, Kroger A, Wolfe S, eds. Epidemiology and prevention of vaccine-preventable diseases, 13th edn. Washington, DC: Public Health Foundation, 2015: 297–310. [Google Scholar]
- 39.Khong WX, Yan B, Yeo H, et al. A non-mouse-adapted enterovirus 71 (EV71) strain exhibits neurotropism, causing neurological manifestations in a novel mouse model of EV71 infection. J Virol 2012; 86: 2121–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.McMinn PC. An overview of the evolution of enterovirus 71 and its clinical and public health significance. FEMS Microbiol Rev 2002; 26: 91–107. [DOI] [PubMed] [Google Scholar]
- 41.Aliabadi N, Messacar K, Pastula DM, et al. Enterovirus D68 infection in children with acute flaccid myelitis, Colorado, USA, 2014. Emerg Infect Dis 2016; 22: 1387–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Messacar K, Schreiner TL, Maloney JA, et al. A cluster of acute flaccid paralysis and cranial nerve dysfunction temporally associated with an outbreak of enterovirus D68 in children in Colorado, USA. Lancet 2015; 385: 1662–71. [DOI] [PubMed] [Google Scholar]
- 43.Greninger AL, Naccache SN, Messacar K, et al. A novel outbreak enterovirus D68 strain associated with acute flaccid myelitis cases in the USA (2012–14): a retrospective cohort study. Lancet Infect Dis 2015; 15: 671–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kreuter JD, Barnes A, McCarthy JE, et al. A fatal central nervous system enterovirus 68 infection. Arch Pathol Lab Med 2011; 135: 793–96. [DOI] [PubMed] [Google Scholar]
- 45.Perez G, Rosanova MT, Freire MC, et al. Unusual increase of cases of myelitis in a pediatric hospital in Argentina. Arch Argent Pediatr 2017; 115: 364–69. [DOI] [PubMed] [Google Scholar]
- 46.Giombini E, Rueca M, Barberi W, et al. Enterovirus D68–associated acute flaccid myelitis in immunocompromised woman, Italy. Emerg Infect Dis 2017; 23: 1690–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Imamura T, Suzuki A, Lupisan S, et al. Detection of enterovirus 68 in serum from pediatric patients with pneumonia and their clinical outcomes. Influenza Other Respir Viruses 2014; 8: 21–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wei W, Guo H, Chang J, et al. ICAM-5/telencephalin is a functional entry receptor for enterovirus D68. Cell Host Microbe 2016; 20: 63–41. [DOI] [PubMed] [Google Scholar]
- 49.Hixon AM, Yu G, Leser JS, et al. A mouse model of paralytic myelitis caused by enterovirus D68. PLoS Pathog 2017; 13: e1006199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hixon AM, Clarke P, Tyler KL. Evaluating treatment efficacy in a mouse model of enterovirus D68 paralytic myelitis. J Infect Dis 2017; 216: 1245–1253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Perez-Velez CM, Anderson MS, Robinson CC, et al. Outbreak of neurologic enterovirus type 71 disease: a diagnostic challenge. Clin Infect Dis 2007; 45: 950–57. [DOI] [PubMed] [Google Scholar]
- 52.Alexander JP Jr, Gary HE Jr, Pallansch MA. Duration of poliovirus excretion and its implications for acute flaccid paralysis surveillance: a review of the literature. J Infect Dis 1997;175 (suppl 1): S176–82. [DOI] [PubMed] [Google Scholar]
- 53.Ruggieri V, Paz MI, Peretti MG, et al. Enterovirus D68 infection in a cluster of children with acute flaccid myelitis, Buenos Aires, Argentina, 2016. Eur J Paediatr Neurol 2017; 21: 884–90. [DOI] [PubMed] [Google Scholar]
- 54.Levy A, Roberts J, Lang J, et al. Enterovirus D68 disease and molecular epidemiology in Australia. J Clin Virol 2015; 69: 117–21. [DOI] [PubMed] [Google Scholar]
- 55.Crone M, Tellier R, Wei XC, et al. Polio-like Illness associated with outbreak of upper respiratory tract infection in children. J Child Neurol 2016; 31: 409–14. [DOI] [PubMed] [Google Scholar]
- 56.Smura TP, Junttila N, Blomqvist S, et al. Enterovirus 94, a proposed new serotype in human enterovirus species D. J Gen Virol 2007;88: 849–58. [DOI] [PubMed] [Google Scholar]
- 57.Lang M, Mirand A, Savy N, et al. Acute flaccid paralysis following enterovirus D68 associated pneumonia, France, 2014. Euro Surveill 2014; 19: 20952. [DOI] [PubMed] [Google Scholar]
- 58.Schuffenecker I, Mirand A, Josset L, et al. Epidemiological and clinical characteristics of patients infected with enterovirus D68, France, July to December 2014. Euro Surveill 2016; 21: 30226. [DOI] [PubMed] [Google Scholar]
- 59.Engelmann I, Fatoux M, Lazrek M, et al. Enterovirus D68 detection in respiratory specimens: association with severe disease. J Med Virol 2017; 89: 1201–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Antona D, Kossorotoff M, Schuffenecker I, et al. Severe paediatric conditions linked with EV-A71 and EV-D68, France, May to October 2016. Euro Surveill 2016; 21: 30402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Oster I, Horneff G, Stucklin A, Muller K. Acute flaccid myelitis (AFM) associated with enterovirus: a distinct clinical entity? Neuropediatrics 2017; 48: S1–45. [Google Scholar]
- 62.Hubner J, Kruse B, Christen HJ, et al. Acute flaccid myelitis in German children in 2016—the return of polio? Dtsch Arztebl Int 2017; 114: 551–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Esposito S, Chidini G, Cinnante C, et al. Acute flaccid myelitis associated with enterovirus-D68 infection in an otherwise healthy child. Virol J 2017; 14: 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Horikoshi Y. Enterovirus D68 infections. J Jpn Pediatr Soc 2016; 120: 1330–37. [Google Scholar]
- 65.Helfferich J, Meiners LC, Brouwer OF. Acute flaccid weakness associated with enterovirus D68. Eur J Paediatr Neurol 2017; 21: 594–95. [DOI] [PubMed] [Google Scholar]
- 66.Hynas Hovden IA, Pfeiffer HC. Electrodiagnostic findings in acute flaccid myelitis related to enterovirus D68. Muscle Nerve 2015;52: 909–10. [DOI] [PubMed] [Google Scholar]
- 67.Pfeiffer HC, Bragstad K, Skram MK, et al. Two cases of acute severe flaccid myelitis associated with enterovirus D68 infection in children, Norway, autumn 2014. Euro Surveill 2015; 20: 21062. [DOI] [PubMed] [Google Scholar]
- 68.Cabrerizo M, Garcia-Iniguez JP, Munell F, et al. First cases of severe flaccid paralysis associated with enterovirus D68 infection in spain, 2015–2016. Pediatr Infect Dis J 2017; 36: 1214–16. [DOI] [PubMed] [Google Scholar]
- 69.Varghese R, Iyer A, Hunter K, Cargill JS, Cooke RP. Sampling the upper respiratory tract for enteroviral infection is important in the investigation of an acute neurological illness in children. Eur J Paediatr Neurol 2015; 19: 494–95. [DOI] [PubMed] [Google Scholar]
- 70.Williams CJ, Thomas RH, Pickersgill TP, et al. Cluster of atypical adult Guillain-Barre syndrome temporally associated with neurological illness due to EV-D68 in children, South Wales, United Kingdom, October 2015 to January 2016. Euro Surveill 2016; 21: 30119. [DOI] [PubMed] [Google Scholar]
- 71.Pilley ES, Chin E, Freeman J, et al. Case series of acute flaccid paralysis attributable to enterovirus D68—the beginning of a new epidemic? Dev Med Child Neurol 2017; 59 (suppl 1): 76. [Google Scholar]
- 72.Stacpoole SRL, Molyneux A, Baumer D. Acute segmental poliomyelitis-like flaccid paralysis in an adult in the UK, associated with enterovirus D68. Pract Neurol 2017; 17: 297–301. [DOI] [PubMed] [Google Scholar]
- 73.Yoder JA, Lloyd M, Zabrocki L, Auten J. Pediatric acute flaccid paralysis: enterovirus D68-associated anterior myelitis. J Emerg Med 2017; 53: e19–23. [DOI] [PubMed] [Google Scholar]
- 74.World Health Organization. WHO-recommended surveillance standard of poliomyelitis. 2017. http://www.who.int/immunization/monitoring_surveillance/burden/vpd/surveillance_type/active/poliomyelitis_standards/en/ (accessed April 4, 2017).
- 75.Peltola V, Waris M, Kainulainen L, Kero J, Ruuskanen O. Virus shedding after human rhinovirus infection in children, adults and patients with hypogammaglobulinaemia. Clin Microbiol Infect 2013; 19: E322–27. [DOI] [PubMed] [Google Scholar]
- 76.Horner L, Poulter M, Brenton J, Turner R. Acute flaccid paralysis associated with novel enterovirus C105. Emerg Infect Dis 2015;21: 1858–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Martin JA, Messacar K, Yang ML, et al. Outcomes of Colorado children with acute flaccid myelitis at 1 year. Neurology 2017; 89: 129–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Lanciotti RS, Kerst AJ. Nucleic acid sequence-based amplification assays for rapid detection of West Nile and St Louis encephalitis viruses. J Clin Microbiol 2001; 39: 4506–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Crepin P, Audry L, Rotivel Y, Gacoin A, Caroff C, Bourhy H. Intravitam diagnosis of human rabies by PCR using saliva and cerebrospinal fluid. J Clin Microbiol 1998; 36: 1117–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Esposito S, Lunghi G, Zampiero A, et al. Enterovirus-D68 in the cerebrospinal fluid of two children with aseptic meningitis. Pediatr Infect Dis J 2016; 35: 589–91. [DOI] [PubMed] [Google Scholar]
- 81.Baggen J, Thibaut HJ, Staring J, et al. Enterovirus D68 receptor requirements unveiled by haploid genetics. Proc Natl Acad Sci USA 2016; 113: 1399–404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Structure Yang H., expression, and function of ICAM-5. Comp Funct Genomics 2012; 2012: 368938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Kauder SE, Racaniello VR. Poliovirus tropism and attenuation are determined after internal ribosome entry. J Clin Invest 2004;113: 1743–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Maloney JA, Mirsky DM, Messacar K, Dominguez SR, Schreiner T, Stence NV. MRI findings in children with acute flaccid paralysis and cranial nerve dysfunction occurring during the 2014 enterovirus D68 outbreak. AJNR Am J Neuroradiol 2015; 36: 245–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Miller L. Standardized case definition for acute flaccid myelitis. 2015. http://c.ymcdn.com/sites/www.cste.org/resource/resmgr/2015PS/2015PSFinal/15-ID-01.pdf (accessed Oct 30, 2015).