Abstract
Objective
The impact of the M184V/I mutation on the virological failure (VF) rate in HIV-positive patients with suppressed viremia switching to an abacavir/lamivudine/dolutegravir regimen has been poorly evaluated.
Method
This is an observational study from 5 European HIV cohorts among treatment-experienced adults with ≤50 copies/mL of HIV-1 RNA who switched to abacavir/lamivudine/dolutegravir. Primary outcome was the time to first VF (2 consecutive HIV-1 RNA >50 copies/mL or single HIV-1 RNA >50 copies/mL accompanied by change in antiretroviral therapy [ART]). We also analyzed a composite outcome considering the presence of VF and/or virological blips. We report also the results of an inverse probability weighting analysis on a restricted population with a prior history of VF on any ART regimen to calculate statistics standardized to the disparate sampling population.
Results
We included 1626 patients (median follow-up, 288.5 days; interquartile range, 154–441). Patients with a genotypically documented M184V/I mutation (n = 137) had a lower CD4 nadir and a longer history of antiviral treatment. The incidence of VF was 29.8 cases (11.2–79.4) per 1000 person-years in those with a previously documented M184V/I, and 13.6 cases (8.4–21.8) in patients without documented M184V/I. Propensity score weighting in a restricted population (n = 580) showed that M184V/I was not associated with VF or the composite endpoint (hazard ratio [HR], 1.27; 95% confidence interval [CI], 0.35–4.59 and HR 1.66; 95% CI, 0.81–3.43, respectively).
Conclusions
In ART-experienced patients switching to an abacavir/lamivudine/dolutegravir treatment, we observed few VFs and found no evidence for an impact of previously-acquired M184V/I mutation on this outcome. Additional analyses are required to demonstrate whether these findings will remain robust during a longer follow-up.
Keywords: ABC/3TC/DTG, M184V/I, treatment-experienced patients, virological failure
In antiretroviral therapy-experienced patients on abacavir/lamivudine/dolutegravir, we observed few virological failures and found no evidence for an impact of previously-acquired M184V/I mutation on this outcome. Additional analyses are required to demonstrate whether these findings will remain robust during a longer follow-up.
Introduction
Integrase strand transfer inhibitors have been widely prescribed and represent the preferred first-line antiretroviral regimen in most major guidelines for HIV-positive patients [1, 2]. In combination with 2 nucleoside reverse transcriptase inhibitors (NRTIs), dolutegravir (DTG) was found to be superior to boosted protease inhibitors (PI/r) or a nonnucleoside NRTI-based regimen in large clinical trials [3, 4]. DTG also has been very effective in treatment-experienced patients due to its high resistance barrier [5, 6]. More recently, a randomized trial of rilpivirine-DTG dual therapy in treatment-experienced patients was shown to be noninferior for the maintenance of viral suppression when compared to continuing conventional 3-drug combination antiretroviral regimens [7]. However, the initial enthusiasm regarding the widespread use of DTG has been tempered by a number of factors, including side effects such as neuropsychiatric disturbances, emergent resistance when given in monotherapy, and possible teratogenicity in early pregnancy [8–10]. Although DTG monotherapy was found to be noninferior to combination antiretroviral therapy (ART) in the first 24 weeks, virological failure (VF) occurred thereafter and led to the emergence of DTG resistance [9]. Similar results have been found in the DOLAM trial where a higher risk of VF was observed with DTG monotherapy compared with dual lamivudine (3TC)/DTG and triple ART [11]. At present, DTG is not recommended as maintenance monotherapy [12]. However, its use in monotherapy was shown to be effective in patients conventionally treated during primary HIV infection and in whom treatment was thereafter simplified to DTG monotherapy [13].
Although DTG uptake has occurred at an unprecedented pace, the impact of past NRTI mutations in treatment-experienced patients switching to an abacavir (ABC)/3TC/DTG regimen has been only partially explored. Furthermore, the 2015 changes in the French National Agency for AIDS Research resistance algorithms [14] highlighted the risk of the impaired efficacy of ABC in the presence of the M184V/I mutation, as previously observed for 3TC. Thus, the clinical decision to switch to an ABC/3TC/DTG regimen could be influenced by a patient having evidence of harboring a M184V/I mutation, which leads to 3TC resistance and may potentially impair the effectiveness of both 3TC and, to a lesser extent ABC, thus resulting in a treatment representing functional DTG monotherapy.
We conducted a prospective study using data from 5 large HIV cohorts in four European countries (France, Italy, the Netherlands and Switzerland) to assess the efficacy of the ABC/3TC/DTG regimen in virologically-suppressed, ART-experienced patients, with or without a previously documented M184V/I mutation.
METHODS
Study Design
We conducted an observational longitudinal analysis of prospectively collected data from 5 different HIV European cohorts: (1) ANRS-CO3 Aquitaine for HIV-positive French patients [15]; (2) Antiviral Response Cohort Analysis (ARCA) containing data on HIV resistance in Italy [16]; (3) AIDS Therapy Evaluation in the Netherlands (ATHENA) [17]; (4) the Italian Cohort of Antiretroviral-Naïve Patients (ICONA) [18]; and (5) the Swiss HIV Cohort Study (SHCS) [19]. All patients provided informed consent for the use of their clinical and laboratory data for research purposes according to country-specific requirements. Data from the different sources were exchanged and pooled according to the HIV Cohorts Data Exchange Protocol standard in a pseudonymized form in compliance with national ethical principles and privacy legislation governing the individual cohorts [20].
Patient Selection
Observations spanned from when DTG became available in each cohort country (January 16, 2014, in the Netherlands; May 1, 2014, in France; May 8, 2014, in Switzerland; and October 10, 2014, in Italy) until February 2018. Eligible patients were 18 years of age or older, treatment-experienced HIV patients with least 1 resistance profile available before the start of the ABC/3TC/DTG regimen, a plasma HIV-1 RNA level ≤50 copies/mL at the time of the switch, and at least 1 HIV RNA assessment following the start of the ABC/3TC/DTG regimen. The selection process was conducted by each cohort according to the inclusion criteria, and the cohort-specific data were then transferred to the SHCS to be merged and analyzed. Patients with a follow-up of less than 30 days and those with missing data for the primary outcome were excluded from the analysis.
Virological Characteristics
The presence of M184V/I mutations was established by the cumulative resistance profile available before the switch to ABC/3TC/DTG, including resistance tests performed before any treatment was started for a fraction of patients. Information on thymidine analogue mutations (TAMs; M41L, D67N, K70R, L210W, T215Y/F, and K219Q/E) before the switch to ABC/3TC/DTG also was obtained.
Endpoints
The primary objective was to explore the effectiveness of ABC/3TC/DTG among treatment-experienced patients with or without a documented M184V/I mutation. VF was defined as 2 consecutive HIV-1 RNA measurements >50 copies/mL or a single HIV-RNA value >50 copies/mL accompanied by a change in ART or censoring, whichever occurred first.
As a secondary objective, we considered a composite outcome combining VF with virological blips (VBs), defined as isolated detectable HIV-1 RNA measurements >50 copies/mL followed by a viral load (VL) <50 copies/ml.
The following factors were explored as predictors of VF and/or VBs: age; sex; body mass index; ethnicity; VL before the first ART regimen above or below 100 000 copies/mL; CD4 count nadir; CD4 count at the time of the switch to ABC/3TC/DTG; number of TAMs from pre-ABC/3TC/DTG genotypes; prior VFs; years of HIV infection; HIV transmission route; intravenous drug use; HIV infection stage; duration of viral suppression before the switch to ABC/3TC/DTG; and previous PI or integrase inhibitor exposure.
Data Analysis
Kaplan Meier plots/estimators and unweighted and inverse probability weighted (IPW) univariate Cox proportional-hazard models were used to assess the primary and secondary outcomes [21, 22]. To calculate the weights, we used multivariate logistic regression modeling of the likelihood of a patient having the M184V/I mutation to calculate the propensity score (ê(x)), which indicates the calculated probability of each patient having the M184V/I mutation based on the following confounders: age; sex; body mass index; race; VL before the first antiretroviral regimen above or below 100 000 copies/mL; CD4 nadir; number of TAMs from pre-ABC/3TC/DTG genotypes; transmission route; years of HIV infection; months of viral suppression before the switch to ABC/3TC/DTG; and previous protease or integrase inhibitor exposure. The propensity scores were then converted to the inverse proportional weights where the weight was set as equal to 1/ê(x) for individuals with M184V/I and 1/[1- ê(x)] for individuals without the mutation [22]. As initial attempts at the weighting procedure yielded 2 groups that were exceedingly heterogenous (Supplementary Figure 1S), we performed the final weighted analysis only on the subpopulation of the 580 patients who had failed on an ART regimen prior to ABC/3TC/DTG initiation, with and without a documented M184V/I mutation.
With regards to the propensity score distributions, we observed an improved overlap between the M184V/I and non-M184V/I population when restricted to those with previous VF, which led to a convergence in their characteristics (Supplementary Figure 1S, Supplementary Table S1).
As recommended for weighted survival analyses, the confidence intervals were calculated using robust variance estimations [23, 24].
The secondary objectives were assessed with categorical variables analyzed by the χ 2 or Fisher exact test, as appropriate, and continuous variables by the t or Mann-Whitney test, depending on data distribution. Time-at-risk started with the switch to ABC/3TC/DTG and ended at the time of the penultimate VL measurement where the second reading may occur up to 6 months after discontinuation of the ABC/3TC/DTG regimen or first VF.
A sensitivity analysis was performed by excluding individuals who modified ABC/3TC/DTG treatment because of drug-related adverse events. Statistical significance was set at a P value <.05. Statistical analysis was performed using R version 3.3.1 on June 21, 2016 (R Studio, Inc, Boston, MA).
RESULTS
Baseline and Patient Characteristics
Table 1 shows the characteristics of 1626 patients who were included in the analysis: 778 (47.8%) from SHCS; 460 (28.3%) from ATHENA; 168 (10.3%) from the Aquitaine cohort; 132 (8.2%) from ICONA; and 88 (5.4%) from ARCA. The highest prevalence of the M184V/I mutation was found in the Aquitaine cohort (37 patients; 22.0%), followed by ARCA (14 patients; 15.9%), SHCS (56 patients; 7.2%), ATHENA (24 patients; 5.2%) and ICONA (6 patients; 4.5%). Overall, 137 patients had an M184V/I mutation (8.4%) with a ratio of the presence or absence of the M184V/I mutation of approximately 1:11. Median follow-up was 288.5 days (interquartile range, 154–441).
Table 1.
Baseline Characteristics of Patients With or Without an Archived M184V/I Mutation
| Baseline Characteristics | |||
|---|---|---|---|
| Variables | Without M184V/I (N = 1489) | With M184V/I (N = 137) | P value |
| Follow up, daysa | 307 (95% CI: 298–317) | 358 (95% CI: 321–394) | .010 |
| Age, yearsa | 48.5 (95% CI: 47.9–49.0) | 53.3 (95% CI: 51.6–55.0) | <.001 |
| Sex, female | 319 (21.4%) | 42 (30.7%) | .017 |
| BMI, kg/m2,a | 24.0 (95% CI: 23.6–24.3) | 23.5 (95% CI: 22.7–24.2) | .230 |
| Time since documented HIV infection, yearsa | 10.6 (95% CI: 10.2–10.9) | 20.2 (95% CI: 19.2–21.3) | <.001 |
| Time since ART initiation, yearsa | 8.5 (95% CI: 8.2–8.8) | 17.3 (95% CI: 16.5–18.2) | <.001 |
| Viral suppression before the switch to ABC/3TC/DTG, monthsa | 83.5 (95% CI: 82.5–88.1) | 134.0 (95% CI: 126.5–141.5) | <.001 |
| Patients with a previous documented VF | 453 (30.4%) | 127 (92.7%) | <.001 |
| CD4 T-cell count nadir, mm3,a | 232 (95% CI: 224–241) | 178 (95% CI: 155–201) | <.001 |
| CD4 T-cell count at the time of the switch to ABC/3TC/DTG, mm3,a | 665 (95% CI: 649–680) | 667 (95% CI: 612–722) | .929 |
| Viral load before 1st ART regimen, copies/ml, log10 transformeda | 4.84 (95% CI: 4.79–4.89) | 4.77 (95% CI: 4.54–5.00) | .576 |
| Prior AIDS diagnosis | 277 (18.6%) | 38 (27.7%) | .005 |
| At least 2 TAMs | 49 (3.3%) | 58 (42.3%) | <.001 |
| At least 3 TAMs | 29 (1.9%) | 41 (29.9%) | <.001 |
| Distribution of M184V/I by cohort | <.001 | ||
| Aquitaine | 131 (8.8%) | 37 (27.0%) | |
| Antiviral Response Cohort Analysis (ARCA) | 74 (5.0%) | 14 (10.2%) | |
| AIDS Therapy Evaluation in the Netherlands (ATHENA) | 436 (29.3%) | 24 (17.5%) | |
| Italian Cohort of Antiretroviral-Naïve Patients (ICONA) | 126 (8.5%) | 6 (4.4%) | |
| Swiss HIV Cohort Study (SHCS) | 722 (48.5%) | 56 (40.9%) | |
| Treatment class before the switch to ABC/3TC/DTG (at any time) | |||
| NNRTI | 897 (60.2%) | 111 (81.0%) | <.001 |
| INSTI | 282 (18.9%) | 25 (18.2%) | .933 |
| PI | 1082 (72.7%) | 133 (97.1%) | <.001 |
Abbreviations: ABC/3TC/DTG, abacavir/lamivudine/dolutegravir; ART, antiretroviral therapy; BMI, body mass index; CI, confidence interval; INSTI, integrase strand transfer inhibitors; IPW, inverse probability weighting analysis; NNRTI, nonnucleoside reverse transcriptase inhibitors; PI, protease inhibitor; TAM, thymidine analogue mutations.
a Mean.
Patients with M184V/I were predominantly male, with a mean age of 53.3 years. They had a longer duration of HIV infection, ART treatment, and a longer virological suppression before the switch to ABC/3TC/DTG compared with patients without the M184V/I mutation. Most patients with a M184V/I mutation had experienced VF during their previous treatment history (127; 92.7%) compared with those without the mutation (453; 30.4%).
VFs
We observed 21 (1.29%) VFs among the 1626 patients included in the study. Among these, 17 (1.21%) patients had no previously detected M184V/I mutation and 4 (3%) did (Table 2).
Table 2.
Virological Primary Outcomes of Patients With or Without M184V/I Mutations Archived in Overall Population
| Primary Outcomes (Overall Population) | |||
|---|---|---|---|
| Outcomes | Without M184V/I (N = 1489) | With M184V/I (N = 137) | P value |
| VF | 17 (1.21%) | 4 (3%) | .09 |
| - 2x HIV-RNA >50 copies/mL | 10 | 1 | — |
| - 1x HIV-RNA >50 copies/mL + ABC/3TC/DTG stop | 7 | 3 | — |
| Treatment (ABC/3TC/DTG) discontinued for reasons other than VF | 232 (15.6%) | 14 (10.2%) | .12 |
| VB | |||
| - at least 1 VB during ABC/3TC/DTG treatment | 63 (4.2%) | 12 (8.8%) | .03 |
| - mean copies/ml (95% CI) | 181 (92–269) | 267 (-28–563) | .55 |
| Incidence of VF per 1000 person-years | 13.6 (8.4–21.8) | 29.8 (11.2–79.4) | .09 |
Abbreviations: ABC/3TC/DTG, abacavir/lamivudine/dolutegravir; CI, confidence interval; VB, virological blips; VF, virological failure.
Seventy-five patients in total had a VB, of whom 63 (4.2%) were without and 12 (8.8%) had a M184V/I mutation (mean HIV-RNA level, 181 [92–269] and 267 [28–563], respectively). The incidence of VF after the switch to ABC/3TC/DTG was 29.8 (11.2–79.4) and 13.6 (8.4–21.8) per 1000 person-years in patients with and without M184V/I, respectively. The rate difference was 16.4 (-13.7–46.2; P = .09). Among the 6 patients with a genotype available after the VF, no new mutations were observed. Genotypic testing was not performed in 13 patients, because the low VL was followed by an undetectable VL and information was missing for the remaining 2 patients (Supplementary Table S2).
Risk Factor Analysis (VF)
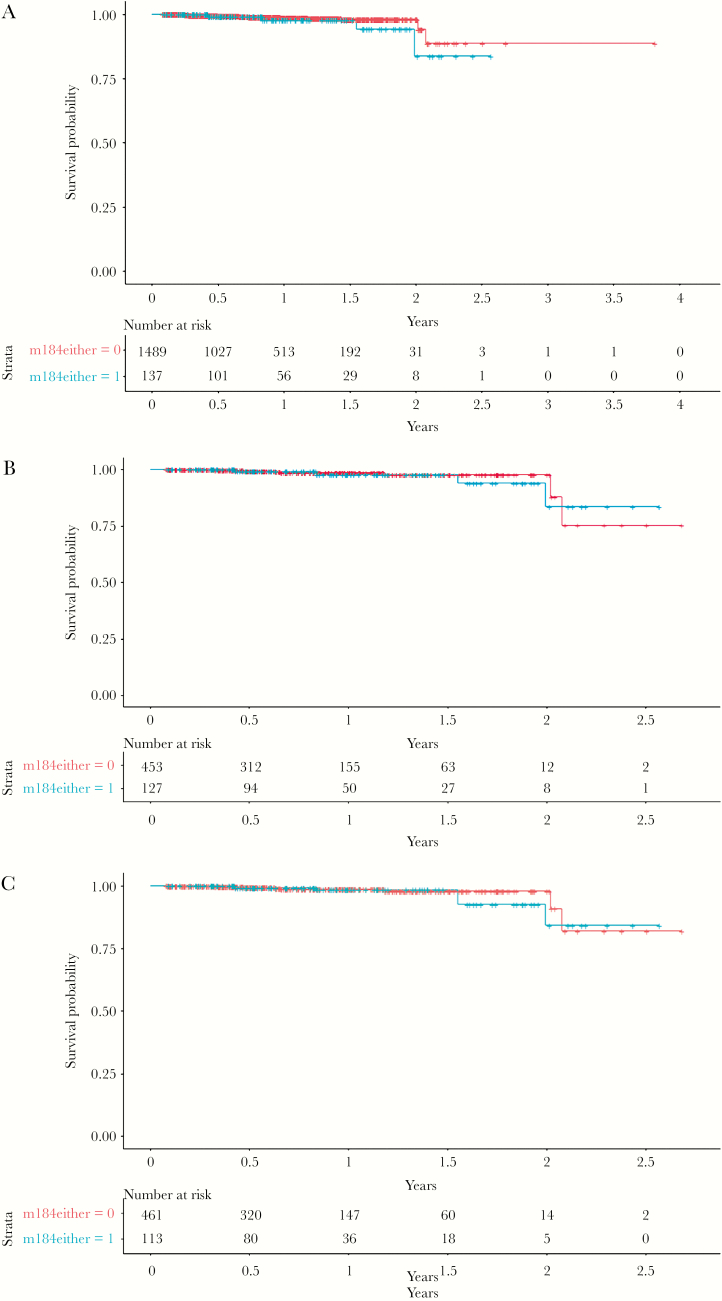
In the univariate Cox proportional-hazards analyses, the presence of the M184V/I mutation was not significantly associated with a higher risk of failure (hazard ratio [HR], 1.81; 95% confidence interval [CI], 0.60–5.49; P = .29; Figure 1A).
Figure 1.
Estimated probability of remaining free from virological failure (VF) with (blue) and without (red) the presence of the M184V/I mutation in (A) the overall population (P = .295) and in the subgroup population (at least 1 VF before the switch to ABC/3TC/DTG), (B) before (P = .781) and (C) after (e = 0.733) inverse probability weighted adjustment.
Composite Outcome: Occurrence of VF/VBs
VF or VBs, or both, were observed in 15 of 137 (10.95%) patients with a documented M184V/I mutation compared to 73 of 1489 (4.9%) patients in the group with a wild type at this position.
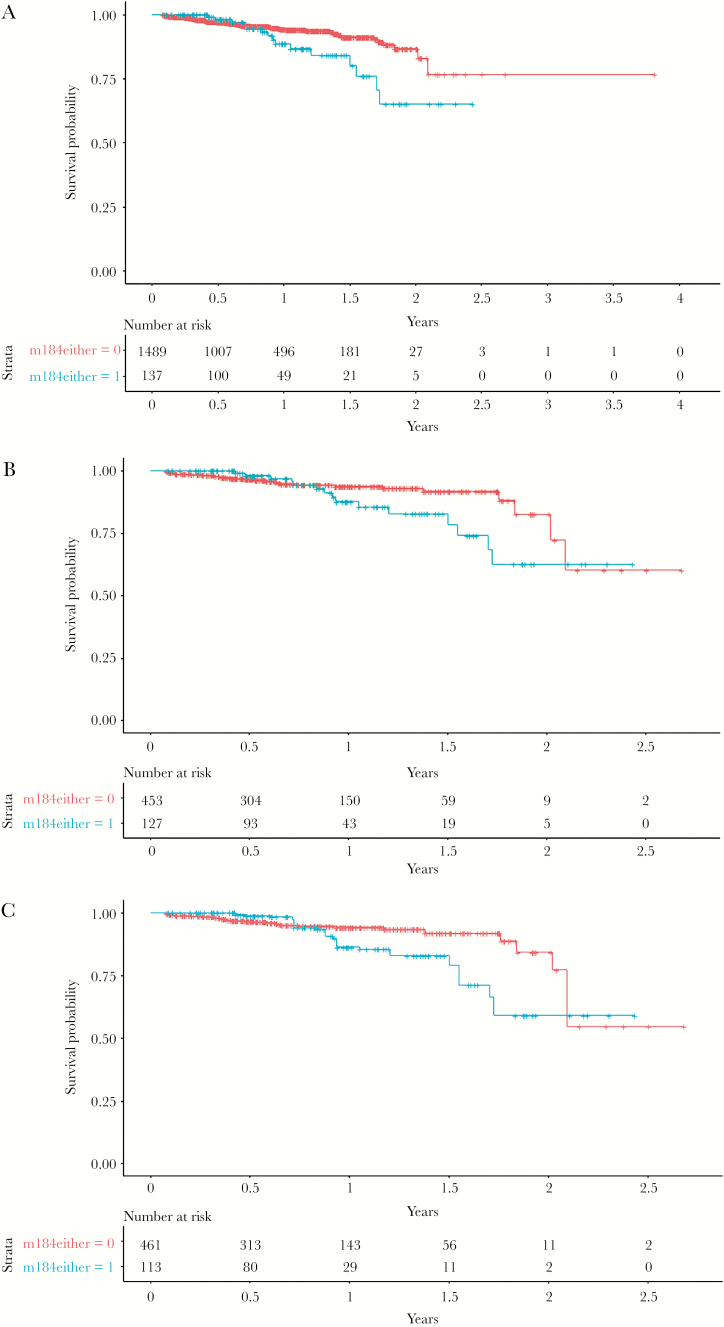
The unweighted univariate analysis showed that M184V/I had an effect on the increase of the risk of the composite outcome (HR, 1.92; 95% CI, 1.09–3.35; P = .022; Figure 2A). However, the propensity score on the total study population was not statistically significant (HR, 1.66; 95% CI, 0.93–2.96).
Figure 2.
Kaplan Meier plots showing probability of remaining free from virological failure (VF) or virological blips with (blue) and without (red) the presence of the M184V/I mutation in (A) the overall population (P = .022) and in the subgroup population (at least 1 VF before the switch to ABC/3TC/DTG), (B) before (P = .106) and (C) after (P = .160) inverse probability weighted analysis adjustment.
IPW-Adjusted Analysis on a Restricted Population (Patients with Prior VF)
To fulfil the prerequisites for the IPW-adjusted analysis, we only performed this particular analysis on the restricted population of 580 individuals who had experienced VF on an ART regimen prior to ABC/3TC/DTG, which led to a convergence of the population characteristics after IPW adjustment (Table 3). We observed 12 (2.1%) VFs among those with a previous VF before the switch to the ABC/3TC/DTG regimen. In this subpopulation, 8 (1.8%) patients had no previous archived M184V/I mutation before IPW adjustment, while 4 (3.2%) did (Table 4). VF incidence was 32.0 (0.64–63.4) and 10.4 (0.21–20.60) for those with and without the M184V/I mutation, respectively. The rate difference was 21.7 (-11.4–54.6; P = .199). The unweighted univariate analysis for the patients with prior VF also was similar (HR, 1.19; 95% CI, 0.35–4.06; P = .781; Figure 1B).
Table 3.
Baseline Characteristics of Patients with Virological Failure on Prior Treatment, With or Without Archived M184V/I Mutations, Before and After Inverse Probability Treatment Weighted Adjustment
| Baseline Characteristics | |||||
|---|---|---|---|---|---|
| Before IPW Adjustment | After IPW Adjustment | ||||
| Variables | Without M184V/I (N = 453) | With M184V/I (N = 127) | P value | Without M184V/I (N = 466)b | With M184V/I (N = 114)b |
| Follow-up, daysa | 310 (95% CI: 292–328) | 359 (95% CI: 320–398) | .023 | 304 (95% CI: 284–325) | 316 (95% CI: 261–371) |
| Age, years a | 51.4 (95% CI: 50.4–52.4) | 53.4 (95% CI: 51.7–55.1) | .048 | 51.6 (95% CI: 49.0–54.1) | 51.0 (95% CI: 43.1–59.0) |
| Sex, female | 114 (25.2%) | 38 (29.9%) | .336 | 130 (27.9%) | 37 (32.5%) |
| BMI, kg/m 2,a | 23.9 (95% CI: 23.3–24.6) | 23.5 (95% CI: 22.8–24.3) | .568 | 24.8 (95% CI: 23.5–26.2) | 23.0 (95% CI: 18.5–27.5) |
| Time since documented HIV infection, years a | 15.0 (95% CI: 14.4–15.7) | 20.6 (95% CI: 19.6–21.6) | <.001 | 16.5 (95% CI: 15.0–17.9) | 17.4 (95% CI: 15.4–19.4) |
| Time since antiretroviral therapy initiation, yearsa | 13.1 (95% CI: 12.6–13.7) | 17.7 (95% CI: 16.9–18.6) | <.001 | 14.3 (95% CI: 13.0–15.5) | 15.5 (95% CI: 13.5–17.5) |
| Viral suppression before the switch to ABC/3TC/DTG, months a | 115.8 (95% CI: 110.6–121.0) | 134.3 (95% CI: 126.5–142.1) | <.001 | 122.4 (95% CI: 112.2–132.7) | 128.2 (95% CI: 108.1–148.3) |
|
CD4 T-cell count nadir,
mm 3,a |
195 (95% CI:183–208) | 176 (95% CI: 152–200) | .151 | 187 (95% CI: 174–201) | 174 (95% CI: 136–213) |
| CD4 T-cell count at the time of the switch to ABC/3TC/DTG, mm3,a | 647 (95% CI: 621–673) | 665 (95% CI: 606–724) | .578 | 669 (95% CI: 616–723) | 694 (95% CI: 535–854) |
| Viral load before 1 st ART regimen, copies/ml, log10 transformed a | 4.91 (95% CI: 4.82–5.01) | 4.78 (95% CI: 4.53–5.02) | .303 | 4.86 (95% CI: 4.77–4.96) | 4.81 (95% CI: 4.54–5.07) |
| Prior AIDS diagnosis | 101 (22.3%) | 37 (29.1%) | .086 | 112 (24.0%) | 22 (19.3%) |
| At least 2 TAMs | 41 (9.1%) | 54 (42.5%) | <.001 | 38 (8.2%) | 5 (4.4%) |
| At least 3 TAMs | 24 (5.3%) | 40 (31.5%) | <.001 | 27 (5.8%) | 2 (1.8%) |
| Distribution of M184V/I by cohort | |||||
| Aquitaine | 51 (11.3%) | 33 (26.0%) | <.001 | 53 (11.4%) | 20 (17.5%) |
| Antiviral Response Cohort Analysis (ARCA) | 26 (5.4%) | 13 (10.2%) | 35 (7.51%) | 12 (10.5%) | |
| AIDS Therapy Evaluation in the Netherlands (ATHENA) | 100 (22.1%) | 24 (18.9%) | 98 (21.0%) | 36 (31.6%) | |
| Italian Cohort of Antiretroviral-Naïve Patients (ICONA) | 21 (4.6%) | 5 (3.9%) | 19 (4.1%) | 5 (4.4%) | |
| Swiss HIV Cohort Study (SHCS) | 255 (56.3%) | 52 (40.9%) | 261 (56.0%) | 41 (36.0%) | |
| Treatment class before the switch to ABC/3TC/DTG (at any time) | |||||
| NNRTI | 318 (70.2%) | 103 (81.1%) | .020 | 327 (70.2%) | 92 (80.7%) |
| INSTI | 81 (17.9%) | 24 (18.9%) | .895 | 88 (18.9%) | 22 (19.3%) |
| PI | 417 (92.1%) | 126 (99.2%) | .007 | 437 (93.8%) | 112 (98.2%) |
Abbreviations: ABC/3TC/DTG, abacavir/lamivudine/dolutegravir; ART, antiretroviral therapy; BMI, body mass index; CI, confidence interval; INSTI, integrase strand transfer inhibitors; IPW, inverse probability weighting analysis; NNRTI, nonnucleoside reverse transcriptase inhibitors; PI, protease inhibitor; TAM, thymidine analogue mutations.
a Mean.
b Synthetic n values derived from weights. The mean of weights was 1.02 in the group without M184V/I and 0.89 in the group with M184V/I.
Bold indicates all the confounders used in the multivariate logistic regression.
Table 4.
Virological Primary Outcomes of Patients With or Without Archived M184V/I Mutations in the Subgroup Population (At Least 1 Virological Failure Before the Switch to Abacavir/Lamivudine/Dolutegravir), Before and After Inverse Probability of Treatment Weighting Adjustment
| Primary Outcomes | |||||
|---|---|---|---|---|---|
| Population with Prior VF | Population with Prior VF | ||||
| Before IPW Adjustment | After IPW Adjustment | ||||
| Outcomes | Without M184V/I (N = 453) |
With M184V/I (N = 127) |
P value | Without M184V/I (N = 466)a |
With M184V/I (N = 114)a |
| VF: | 8 (1.8%) | 4 (3.2%) | .306 | 7 (1.5%) | 3 (2.6%) |
| 2x HIV-RNA >50 copies/mL | 5 | 1 | 4 | 1 | |
| 1x HIV-RNA >50 copies/mL + ABC/3TC/DTG stop | 3 | 3 | 3 | 2 | |
| Treatment (ABC/3TC/DTG) discontinued for reasons other than VF | 7 (17.22%) | 13 (10.24%) | .08 | 75 (16.1%) | 20 (17.5%) |
| VB | |||||
| at least 1 VB during ABC/3TC/DTG treatment | 22 (4.9%) | 12 (9.5%) | .083 | 23 (4.9%) | 9 (7.9%) |
| mean copies/ml (95% CI) | 120 (63–177) | 267 (-28–563) | .303 | 117 (65–169) | 307 (-157–772) |
| Incidence of VF per 1000 person-years | 10.4 (0.21–20.60) | 32.0 (0.64–63.4) | .199 | 18.5 (4.8–32.3) | 27.3 (-3.6–58.2) |
Abbreviations: ABC/3TC/DTG, abacavir/lamivudine/dolutegravir; CI, confidence interval; IPW, inverse probability weighting (analysis); VB, virological blips; VF, virological failure.
aSynthetic n values derived from weights. The mean of weights was 1.02 in the group without M184V/I and 0.89 in the group with M184V/I.
In the IPW-adjusted analysis of 580 patients with prior VF, results for the primary and secondary outcomes were essentially unchanged, For the primary outcome, the estimated HR for patients with the M184V/I mutation was 1.27 (95% CI, 0.35–4.59; P = .733; Figure 1C) and for the composite outcome the estimated HR was 1.66 (95% CI, 0.81–3.43; P = .17).
TAMs
At least 2 TAMs were documented in 42.3% of patients with M184V/I compared to 3.3% of those without the mutation (Supplementary Table S3). Among the 21 patients who experienced VF, 2 had 3 archived TAMs (D67N, K70R, and K219Q/E and M41L, K70R, and K219Q/E), including the M184V/I mutation. One patient had 2 TAMs (D67N and K219Q/E) without an archived M184V/I mutation. The remaining 18 patients had no archived TAMs.
Sensitivity Analysis
The sensitivity analysis was performed on 489 patients who had prior VF after excluding all those who stopped ABC/3TC/DTG due to drug-related adverse events. We did not observe any significant association between VF and the M184V/I mutation (data not shown). In the propensity weighted analysis, the HR was 1.61 (95% CI, 0.76–3.39; P = .22).
Discussion
To the best of our knowledge, this study is the largest observational study to focus on the impact of the M184V/I mutation on the risk of VF in virologically-suppressed patients switching to ABC/3TC/DTG. Overall, we observed a very low VF rate (1.3%) after the switch to ABC/3TC/DTG, with no statistically significant difference in the VF incidence among patients with or without M184V/I after correction for differences in patient characteristics. Furthermore, no new mutations were observed in reverse transcriptase or integrase after VF in any of the patients in which genotyping was successfully performed, with the caveat that the genotypic resistance results were available for only a minority of patients (6 of 21).
One of the main strengths of our study is the use of an IPW-adjusted analysis for the population restricted to those with VF on a prior regimen [25]. In this multi-cohort study, the characteristics of the groups of patients with or without M184V/I were too divergent to allow the use of a standard multivariate Cox proportional-hazards analysis. We took into account several predictors of VF. Among these, the presence of TAMs was considered as one of the most relevant. We showed with the IPW analysis that the M184V/I mutation had no statistically significant effect on VF, including when using the composite outcome (although a trend could not be excluded). However, the wide confidence intervals observed and the limited follow-up should be taken into consideration when interpreting the clinical relevance of our findings. Furthermore, we cannot exclude residual confounding given the absence of data on treatment adherence. Indeed, precise information about adherence to ART was only available for a small part of the data set and patients with or without emergence of the M184V/I mutation after VF may have had a different behavior with regards to treatment adherence.
Previous studies have addressed the effect of the M184V/I mutation on the efficacy of a DTG-containing regimen. Although most were small trials (less than 500 patients) originating from single cohorts, they all described low VF rates, irrespective of the presence of the M184V/I mutation. In the study by Gagliardini et al [26], the 3-year probability to remain VF-free among treatment-experienced patients on PI- or DTG-based dual therapy containing lamivudine in the presence or absence of the M184V/I mutation was 87.8% and 91.9%, respectively (P = .32). Furthermore, among 126 patients treated with a 3TC/DTG-containing regimen and followed for a median of 1.3 years, VF was detected in none of 21 patients with the M184V/I mutation and in 2 of 105 patients without the mutation. Marcelin et al [27] and Reynes et al [28] reported similar results. In both studies, no VF was detected in 59 and 27 patients, respectively, who switched to a DTG-based regimen, despite a high M184V/I prevalence (100% in the first study, 63% in the latter).
In a recent phase 3b, open-label, noninferiority, randomized clinical trial of 627 patients, DTG was shown to be superior at 48 weeks compared to ritonavir-boosted lopinavir, plus 2 NRTIs in adults in whom previous first-line ART with a non-NRTI plus 2 NRTIs had failed [29]. Of note, M184V/I was present at baseline in more than 80% of patients and 3TC or emtricitabine (FTC) was included as part of the backbone in both arms of the trial. A recent subgroup analysis [30] of this study showed that the response rate of DTG also was high when 3TC or FTC was used in the presence of a M184V/I mutation. Similar results were found in more recent studies where DTG plus 1 to 2 NRTIs were noninferior to PI-based therapy, regardless of the presence of the M184V/I mutation and other NRTI mutations at 48 [31] and 78 weeks [32].
Other integrase strand transfer inhibitors, such as elvitegravir in the co-formulation elvitegravir/cobicistat/emtricitabine/tenofovir alafenamide, showed in a prospective open-label study to be effective in maintaining viral suppression at week 12, despite archived M184V/I mutations [33].
An association between low-level viremia (considered as intermittent episodes of VBs) and subsequent VF, inadequate CD4 recovery, and the development of drug resistance have been demonstrated in a previous study [34–36]. More recently, Hermans et al [37] showed that VBs (defined as the occurrence of 1 viral load measurement of 51–999 copies per mL during ART, followed by virological suppression) might be associated with a 2.6-fold higher HR for VF. Similar results were shown by Young et al where an increase of HR 1.09 of viral rebound was detected for every magnitude blip increase of 100 copies/ml in naïve patients [38]. Our study is more comparable to the latter 1 in terms of the VF definition. Although the unweighted univariate analysis showed that M184V/I had a significant effect on the increase of the risk of the composite outcome, including both VBs and VF, this effect was no longer statistically significant in the IPW analysis.
Our study has several limitations. First, despite the fact that our study used data from 5 cohorts, the number of patients who developed VF during follow-up was small overall. Of note, our VF definition was particularly conservative, considering that for 13 patients a genotypic testing was not performed due to a limited VL, followed by an undetectable VL. In these cases, we probably identified a low-level viremia more than a clinically relevant true VF. A longer follow-up and additional data from more patients are needed before more definite conclusions can be drawn. It is also likely that patients with NRTI mutations in these cohorts are those who were treated with less potent regimens in the early days of highly active ART and perhaps were less compliant. Finally, a so-called “indication bias” may have occurred as physicians tended to prescribe a single pill regimen in patients with documented resistant mutations only if they were confident about patient adherence.
In conclusion, in this large international prospective study, we found an extremely low rate of VF among treatment-experienced patients receiving an ABC/3TC/DTG regimen, irrespective of the presence of a M184V/I mutation. Additional analyses are required to demonstrate whether these findings will remain robust during an extended observation period.
Supplementary Data
Supplementary materials are available at Open Forum Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Acknowledgments
We acknowledge all members of Aquitaine, including the following:
Scientific Committee of the ANRS CO3 Aquitaine Cohort
F. Bonnet (principal investigator), L. Wittkop (methodologist); C. Cazanave, V. Gaborieau, M. Hessamfar, E. Lazaro, G. Le Moal, D. Malvy, P. Mercié, D. Neau, M.O. Vareil, I. Pellegrin, P. Blanco, M.E. Lafon, P. Bellecave, S. Bouchet, D. Breilh, D. Lacoste, S. Lawson-Ayayi, A. Gimbert, S. Desjardin, L. Lacaze-Buzy, V. Petrov-Sanchez, L. Marchand, A. Perrier, F. Le Marec, and O. Leleux
Participating Clinical Centers
Hôpital Saint André, CHU de Bordeaux, Médecine Interne et Maladies Infectieuses: F. Bonnet, N. Bernard, D. Dondia, P. Duffau, I. Faure, M. Hessamfar, D. Lacoste, P. Mercié, P. Morlat, F. Paccalin, M.C. Pertusa, M.A. Vandenhende, E. Riebero, and C. Rivoisy
Hôpital Pellegrin, CHU de Bordeaux, Maladies Infectieuses et Tropicales: C. Cazanave, F.A. Dauchy, A. Desclaux, M. Dupon, H. Dutronc, D. Neau, D. Malvy, A. Ochoa, T. Pistone, M.C. Receveur, and G. Wirth
Hôpital Haut-Lévêque, CHU de Bordeaux, Médecine Interne et Maladies Infectieuses: C. Greib, E. Lazaro, J.L. Pellegrin, and J.F. Viallard
Hôpital d’Agen, Médecine Interne: Y. Imbert, M. Thierry-Mieg, and P. Rispal
Hôpital de Libourne, Médecine Interne: O. Caubet, H. Ferrand, and S. Tchamgoué
Hôpital de Bayonne, Maladies Infectieuses: S. Farbos, M.O. Vareil, and H. Wille
Hôpital de Dax, Médecine Interne et Maladies Infectieuses: K. Andre, L. Caunegre, Y. Gerard, and F. Osorio-Perez
Hôpital Saint-Cyr/Villeneuve sur Lot, Maladies Infectieuses: I. Chossat
Hôpital de Mont de Marsan, Médecine Interne et Maladies Infectieuses: G. Iles, Y. Gerard, M. Labasse-Depis, and F. Lacassin
Hôpital d’Arcachon, Médecine Interne: A. Barret and C. Courtault
Hôpital de Périgueux, Médecine Interne et Maladies Infectieuses: N. Berthol, B. Cougoul, P. Lataste, J. Marie, and N. Rouanes
Hôpital de Pau, Médecine Interne et Maladies Infectieuses: G. Dumondin and V. Gaborieau
Hôpital d’Orthez, Médecine Interne: Y. Gerard
Data Collection
S. Delveaux, B. Uwamaliya, K. Zara, A. Pougetoux, F. Diarra, C. Hanapier, M.J. Blaizeau, M. Decoin, E. Lenaud, and S. Lawson-Ayayi
Project Team CMG-EC
A. Perrier (data manager), F. Le Marec (statistician), and O. Leleux (project leader)
We thank the patients enrolled in the ARCA cohort as well as the ARCA scientific board and the companies supporting the initiative.
We acknowledge all members of ATHENA, including the following:
Academic Medical Centre of the University of Amsterdam (AMC-UvA)
HIV treating physicians: M. van der Valk, S.E. Geerlings, M.H. Godfried, A. Goorhuis, J.W. Hovius, T.W. Kuijpers, F.J.B. Nellen, D.T. van der Poll, J.M. Prins, P. Reiss, H.J. M. van Vugt, W.J. Wiersinga, and F.W.M.N. Wit
HIV nurse consultants: M. van Duinen, J. van Eden, A.M.H. van Hes, F.J.J. Pijnappel, and A.M. Weijsenfeld
HIV clinical virologists/chemists: S. Jurriaans, N.K.T. Back, H.L. Zaaijer, B. Berkhout, M.T.E. Cornelissen, C.J. Schinkel, and K.C. Wolthers
Admiraal De Ruyter Ziekenhui, Goes
HIV treating physicians: M. van den Berge and A. Stegeman
HIV nurse consultants: S. Baas and L. Hage de Looff
HIV clinical virologists/chemists: B. Wintermans, J. Veenemans. and C. Ziekenhuis
Admiraal De Ruyter Ziekenhui, Eindhoven
HIV treating physicians: M.J.H. Pronk and H.S.M. Ammerlaan
HIV nurse consultants: E.S. de Munnik
HIV clinical virologists/chemists: A.R. Jansz, J. Tjhie, M.C.A. Wegdam, B. Deiman, and V. Scharnhorst
DC Klinieken Lairesse - HIV Focus Centrum
HIV treating physicians: A. van Eeden and M. van der Valk
HIV nurse consultants: W. Brokking, M. Groot, and L.J.M. Elsenburg
HIV clinical virologists/chemists: M. Damen and I.S. Kwa
ETZ (Elisabeth-TweeSteden Ziekenhuis), Tilburg
HIV treating physicians: M.E.E. van Kasteren and A.E. Brouwer
HIV nurse consultants: R. van Erve, B.A.F.M. de Kruijf-van de Wiel, S. Keelan-Pfaf, and B. van der Ven
Data collection: B.A.F.M. de Kruijf-van de Wiel and B. van de Ven
HIV clinical virologists/chemists: A.G.M. Buiting, P.J. Kabel, and D.Versteeg
Erasmus MC, Rotterdam
HIV treating physicians: M.E. van der Ende, H.I. Bax, E.C.M. van Gorp, J.L. Nouwen, B.J.A. Rijnders, C.A.M. Schurink, A. Verbon, T.E.M.S. de Vries-Sluijs, and N.C. de Jong-Peltenburg
HIV nurse consultants: N. Bassant, J.E.A. van Beek, M. Vriesde, and L.M. van Zonneveld
Data collection: H.J. van den Berg-Cameron, J. de Groot, and M. de Zeeuw-de Man
HIV clinical virologists/chemists: C.A.B. Boucher, M.P.G Koopmans, J.J.A van Kampen, and S.D. Pas
Flevoziekenhuis, Almere
HIV treating physicians: J. Branger and R.A. Douma
HIV nurse consultant: C.J.H.M. Duijf-van de Ven
Haga Ziekenhuis, Den Haag
HIV treating physicians: E.F. Schippers and C. van Nieuwkoop
HIV nurse consultants: J.M. van IJperen and J. Geilings
Data collection: G. van der Hut
HIV clinical virologist/chemist: N.D. van Burgel
HMC (Haaglanden Medisch Centrum), Den Haag
HIV treating physicians: E.M.S. Leyten and L.B.S. Gelinck
HIV nurse consultants: S. Davids-Veldhuis, A.Y. van Hartingsveld, C. Meerkerk, and G.S. Wildenbeest
HIV clinical virologists/chemists: E. Heikens
Isala, Zwolle
HIV treating physicians: P.H.P. Groeneveld, J.W. Bouwhuis, and A.J.J. Lammers
HIV nurse consultants: S. Kraan, A.G.W. van Hulzen, and M.S.M. Kruiper
Data collection: G.L. van der Bliek and P.C.J. Bor
HIV clinical virologists/chemists: P. Bloembergen, M.J.H.M. Wolfhagen, and G.J.H.M. Ruijs
Leids Universitair Medisch Centrum, Leiden
HIV treating physicians: F.P. Kroon, M.G.J. de Boer, H. Scheper, and H. Jolink
HIV nurse consultants: W. Dorama and N. van Holten
HIV clinical virologists/chemists: E.C.J. Claas and E. Wessels
Maasstad Ziekenhuis, Rotterdam
HIV treating physicians: J.G. den Hollander, K. Pogany, and A. Roukens
HIV nurse consultants: M. Kastelijns, J.V. Smit, E. Smit, D. Struik-Kalkman, and C. Tearno
Data collection: T. van Niekerk
HIV clinical virologists/chemists: O. Pontesilli
Maastricht UMC+, Maastricht
HIV treating physicians: S.H. Lowe, A.M.L. Oude Lashof, and D. Posthouwer
HIV nurse consultants: R.P. Ackens, K. Burgers, and J. Schippers
Data collection: B. Weijenberg-Maes
HIV clinical virologists/chemists: I.H.M. van Loo and T.R.A. Havenith
MC Slotervaart, Amsterdam
HIV treating physicians: J.W. Mulder, S.M.E. Vrouenraets, and F.N. Lauw
HIV nurse consultants: M.C. van Broekhuizen and D.J. Vlasblom
HIV clinical virologists/chemists: P.H.M. Smits
MC Zuiderzee, Lelystad
HIV treating physicians: S. Weijer, R. El Moussaoui
HIV nurse consultant: A.S. Bosma
Medisch Centrum Leeuwarden, Leeuwarden
HIV treating physicians: M.G.A.van Vonderen and L.M. Kampschreur
HIV nurse consultants: K. Dijkstra and S. Faber
HIV clinical virologists/chemists: J Weel
Medisch Spectrum Twente, Enschede
HIV treating physicians: G.J. Kootstra and C.E. Delsing
HIV nurse consultants: M. van der Burg-van de Plas and H. Heins
Noordwest Ziekenhuisgroep, Alkmaar
HIV treating physicians: W. Kortmann, G. van Twillert, and R. Renckens
HIV nurse consultant and data collection: D. Ruiter-Pronk and F.A. van Truijen-Oud
HIV clinical virologists/chemists: J.W.T. Cohen Stuart, E.P. IJzerman, R. Jansen, W. Rozemeijer, and W.A. van der Reijden
OLVG, Amsterdam HIV treating physicians: K. Brinkman, G.E.L. van den Berk, W.L. Blok, P.H.J. Frissen, K.D. Lettinga W.E.M. Schouten, and J. Veenstra HIV nurse consultants: C.J. Brouwer, G.F. Geerders, K. Hoeksema, M.J. Kleene, I.B. van der Meché, M. Spelbrink, A.J.M. Toonen, and S. Wijnands HIV clinical virologists: D. Kwa Data collection: R. Regez (coordinator) Radboudumc, Nijmegen HIV treating physicians: R. van Crevel, M. Keuter, A.J.A.M. van der Ven, H.J.M. ter Hofstede, A.S.M. Dofferhoff, and J. Hoogerwerf HIV nurse consultants: K.J.T. Grintjes-Huisman, M. de Haan, and M. Marneef HIV clinical virologists/chemists: J. Rahamat-Langendoen and F.F. Stelma HIV clinical pharmacology consultant: D. Burger Rijnstate, Arnhem HIV treating physicians: E.H. Gisolf, R.J. Hassing, and M. Claassen HIV nurse consultants: G. ter Beest, P.H.M. van Bentum, and N. Langebeek HIV clinical virologists/chemists: R. Tiemessen and C.M.A. Swanink Spaarne Gasthuis, Haarlem HIV treating physicians: S.F.L. van Lelyveld and R. Soetekouw HIV nurse consultants: L.M.M. van der Prijt and J. van der Swaluw Data collection: N. Bermon HIV clinical virologists/chemists: W.A. van der Reijden, R. Jansen, B.L. Herpers, and D. Veenendaal Medisch Centrum Jan van Goyen, Amsterdam HIV treating physicians: D.W.M. Verhagen HIV nurse consultant: M. van Wijk Universitair Medisch Centrum Groningen, Groningen HIV treating physicians: W.F.W. Bierman, M. Bakker, J. Kleinnijenhuis, E. Kloeze, Y. Stienstra, K.R. Wilting, and M. Wouthuyzen-Bakker HIV nurse consultants: A. Boonstra, P.A. van der Meulen, and D.A. de Weerd HIV clinical virologists/chemists: H.G.M. Niesters, C.C. van Leer-Buter, and M. Knoester Universitair Medisch Centrum Utrecht, Utrecht HIV treating physicians: A.I.M. Hoepelman, J.E. Arends, R.E. Barth, A.H.W. Bruns, P.M. Ellerbroek, T. Mudrikova, J.J. Oosterheert, E.M. Schadd, M.W.M. Wassenberg, and M.A.D. van Zoelen HIV nurse consultants: K. Aarsman, D.H.M. van Elst-Laurijssen, I. de Kroon, C.S.A.M. van Rooijen Data collection: M. van Berkel and C.S.A.M. van Rooijen HIV clinical virologists/chemists: R. Schuurman, F. VerduynLunel, and A.M.J. Wensing VUmc, Amsterdam HIV treating physicians: E.J.G. Peters, M.A. van Agtmael, and M. Bomers HIV nurse consultants: M. Heitmuller and L.M. Laan HIV clinical virologists/chemists: C.W. Ang, R. van Houdt, A.M. Pettersson, and C.M.J.E. Vandenbroucke Grauls Coordinating centre: P. Reiss (director) and S. Zaheri (deputy director) Data analysis: D.O. Bezemer, A.I. van Sighem, C. Smit, F.W.M.N. Wit, and T.S. Boender Data management and quality control: M. Hillebregt, A. de Jong, and T. Woudstra Data monitoring: D. Bergsma, S. Grivell, R. Meijering, M. Raethke, and T. Rutkens Data collection: L. de Groot, M. van den Akker, Y. Bakker, M. Bezemer, A. El Berkaoui, J. Geerlinks, J. Koops, E. Kruijne, C. Lodewijk, E. Lucas, R. van der Meer, L. Munjishvili, F. Paling, B. Peeck, C. Ree, R. Regtop, Y. Ruijs, L. van de Sande, M. Schoorl, E. Tuijn, L. Veenenberg, S. van der Vliet, A. Wisse, and E.C. Witte Patient registration: B. Tuk We acknowledge all members of the ICONA, including the following: Board directors: A. d’Arminio Monforte (President), A. Antinori (Vice-President), M. Andreoni, A. Castagna, F. Castelli, R. Cauda, G. Di Perri, M. Galli, R. Iardino, G. Ippolito, A. Lazzarin, G.C. Marchetti, G. Rezza, F. von Schloesser, and P. Viale Scientific secretary: A. d’Arminio Monforte, A. Antinori, A. Castagna, F. Ceccherini-Silberstein, A. Cozzi-Lepri, E. Girardi, S. Lo Caputo, C. Mussini, M. Puoti, and C.F. Perno Steering committee: A. Antinori, F. Bai, C. Balotta, A. Bandera, S. Bonora, M. Borderi, A. Calcagno, A. Capetti, M.R. Capobianchi, A. Castagna, F. Ceccherini-Silberstein, S. Cicalini, A. Cingolani, P. Cinque, A. Cozzi-Lepri, A. d’Arminio Monforte, A. De Luca, A. Di Biagio, E. Girardi, N. Gianotti, A. Gori, G. Guaraldi, G. Lapadula, M. Lichtner, S. Lo Caputo, G. Madeddu, F. Maggiolo, G. Marchetti, L. Monno, C. Mussini, S. Nozza, C.F. Perno, C. Pinnetti, M. Puoti, E. Quiros Roldan, R. Rossotti, S. Rusconi, M.M. Santoro, A. Saracino, and L. Sarmati Statistical and monitoring team: A. Cozzi-Lepri, I. Fanti, L. Galli, P. Lorenzini, A. Rodano’, M. Macchia, and A. Tavelli Biological bank INMI: F. Carletti, S. Carrara, A. Di Caro, S. Graziano, F. Petroni, G. Prota, and S. Truffa Participating physicians and centers: A. Giacometti, A. Costantini, V. Barocci (Ancona); G. Angarano, L. Monno, E. Milano (Bari); F. Maggiolo, C. Suardi (Bergamo); P. Viale, V. Donati, G. Verucchi (Bologna); F. Castelnuovo, C. Minardi, E. Quiros Roldan (Brescia); B. Menzaghi, C. Abeli (Busto Arsizio); B. Cacopardo, B. Celesia (Catania); J. Vecchiet, K. Falasca (Chieti); A. Pan, S. Lorenzotti (Cremona); L. Sighinolfi, D. Segala (Ferrara); P. Blanc, F. Vichi (Firenze); G. Cassola, C. Viscoli, A. Alessandrini, N. Bobbio, G. Mazzarello (Genova); M. Lichtner, S. Vita, (Latina); P. Bonfanti, C. Molteni (Lecco); A. Chiodera, P. Milini (Macerata); G. Nunnari, G. Pellicanò (Messina); A. d’Arminio Monforte, M. Galli, A. Lazzarin, G. Rizzardini, M. Puoti, A. Castagna, E.S. Cannizzo, M.C. Moioli, R. Piolini, D. Bernacchia, S. Salpietro, C. Tincati, (Milano); C. Mussini, C. Puzzolante (Modena); C. Migliorino, G. Lapadula (Monza); V. Sangiovanni, G. Borgia, V. Esposito, F. Di Martino, I. Gentile, V. Rizzo (Napoli); A.M. Cattelan, S. Marinello (Padova); A. Cascio, M. Trizzino (Palermo); F. Baldelli, E. Schiaroli (Perugia); G. Parruti, F. Sozio (Pescara); G. Magnani, M.A. Ursitti (Reggio Emilia); M. Andreoni, A. Antinori, R. Cauda, A. Cristaudo, V. Vullo, R. Acinapura, D. Moschese, M. Capozzi, A. Mondi, A. Cingolani, M. Rivano Capparuccia, G. Iaiani, A. Latini, R. Gagliardini, M.M. Plazzi, S. Savinelli, A. Vergori (Roma); M. Cecchetto, F. Viviani (Rovigo); G. Madeddu, A. De Vito (Sassari); B. Rossetti, F. Montagnani (Siena); A. Franco, R. Fontana Del Vecchio (Siracusa); D. Francisci, C. Di Giuli (Terni); P. Caramello, G. Di Perri, S. Bonora, G.C. Orofino, M. Sciandra (Torino); M. Bassetti, A. Londero (Udine); G. Pellizzer, V. Manfrin (Vicenza); G. Starnini, A. Ialungo (Viterbo) We acknowledge all members of the SHCS: V. Aubert, M. Battegay, E. Bernasconi, J. Boni (chair of the resistance group), D.L. Braun, H. Bucher, A. Calmy, M. Cavassini, A. Ciuffi, G. Dollenmaier, M. Egger, L. Elzi, J. Fehr, J. Fellay, H. Furrer (chairman of the clinical and laboratory committee), C.A. Fux, H.F. Gunthard (president of the SHCS), D. Haerry (deputy of Positive Council), B. Hasse, H.H. Hirsch, M. Hoffmann, I. Hosli, C. Kahlert, L. Kaiser, O. Keiser, T. Klimkait, R.D. Kouyos, H. Kovari, B. Ledergerber, G. Martinetti, B. Martinez de Tejada, C. Marzolini, K.J. Metzner, N. Muller, D. Nicca, G. Pantaleo, P. Paioni, A. Rauch (chairman of the scientific board), C. Rudin (chairman of the mother and child substudy), A.U. Scherrer (head of the data center), P. Schmid, R. Speck, M. Stockle, P. Tarr, A. Trkola, P. Vernazza, G. Wandeler, R. Weber, and S Yerly Data were gathered by the 5 Swiss university hospitals, 2 cantonal hospitals, 15 affiliated hospitals, and 36 private physicians (lhttp://www.shcs.ch/180-health-care-providers).
Financial support. This work was financed within the framework of the SHCS, supported by the Swiss National Science Foundation (grant #177499), by SHCS project # 816 of the SHCS Research Foundation, and by the Yvonne Jacob Foundation (to H.F.G.) and the Clinical Research Priority Program of the University of Zurich Viral Infectious Diseases Section (to H.F.G.).
This work was also supported by the University of Zurich’s University Research Priority Program, “Evolution in Action: From Genomes to Ecosystems” (grant #U-702-26-01) and by the Swiss National Science Foundation (grant #BSSGI0_155851; both to H.N.).
The ATHENA cohort is managed by Stichting HIV Monitoring and supported by a grant from the Dutch Ministry of Health, Welfare, and Sport through the Centre for Infectious Disease Control of the National Institute for Public Health and the Environment.
Potential conflicts of interest. All authors: No reported conflicts of interest. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. Saag MS, Benson CA, Gandhi RT, et al. Antiretroviral drugs for treatment and prevention of HIV infection in adults: 2018 recommendations of the international antiviral society-USA panel. JAMA 2018; 320:379–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Panel on Antiretroviral Guidelines for Adults and Adolescents. Guidelines for the use of antiretroviral agents in adults and adolescents living with HIV Department of Health and Human Services. http://www.aidsinfo.nih.gov/ContentFiles/AdultandAdolescentGL.pdf. Last published July 10, 2019. Accessed February 16, 2019.
- 3. Clotet B, Feinberg J, van Lunzen J, et al. ; et al. Once-daily dolutegravir versus darunavir plus ritonavir in antiretroviral-naive adults with HIV-1 infection (FLAMINGO): 48 week results from the randomised open-label phase 3b study. Lancet 2014; 383:2222–31. [DOI] [PubMed] [Google Scholar]
- 4. Walmsley SL, Antela A, Clumeck N, et al. ; et al. Dolutegravir plus abacavir–lamivudine for the treatment of HIV-1 infection. N Engl J Med 2013; 369:1807–18. [DOI] [PubMed] [Google Scholar]
- 5. Cahn P, Pozniak AL, Mingrone H, et al. ; et al. Dolutegravir versus raltegravir in antiretroviral-experienced, integrase-inhibitor-naive adults with HIV: week 48 results from the randomised, double-blind, non-inferiority SAILING study. Lancet 2013; 382:700–8. [DOI] [PubMed] [Google Scholar]
- 6. Fourati S, Charpentier C, Amiel C, et al. ; et al. Cross-resistance to elvitegravir and dolutegravir in 502 patients failing on raltegravir: a French national study of raltegravir-experienced HIV-1-infected patients. J Antimicrob Chemother 2015; 70:1507–12. [DOI] [PubMed] [Google Scholar]
- 7. Llibre JM, Hung CC, Brinson C, et al. Efficacy, safety, and tolerability of dolutegravir-rilpivirine for the maintenance of virological suppression in adults with HIV-1: phase 3, randomised, non-inferiority SWORD-1 and SWORD-2 studies. Lancet 2018; 391:839–49. [DOI] [PubMed] [Google Scholar]
- 8. Wijting I, Rokx C, Boucher C, et al. Dolutegravir as maintenance monotherapy for HIV (DOMONO): a phase 2, randomised non-inferiority trial. Lancet HIV 2017; 4:e547–54. [DOI] [PubMed] [Google Scholar]
- 9. Hocqueloux L, Raffi F, Prazuck T, et al. Dolutegravir monotherapy versus dolutegravir/abacavir/lamivudine for virologically suppressed people living with chronic HIV infection: the randomized non-inferiority MONCAY trial. Clin Infect Dis 2019; doi: 10.1093/cid/ciy1132 [DOI] [PubMed] [Google Scholar]
- 10. Black V, Schwartz SR. Issues about periconception use of dolutegravir are reminiscent of early concerns about efavirenz. Lancet HIV 2018; 5:e732–6. [DOI] [PubMed] [Google Scholar]
- 11. Blanco JL, Rojas J, Paredes R, et al. Dolutegravir-based maintenance monotherapy versus dual therapy with lamivudine: a planned 24 week analysis of the DOLAM randomized clinical trial. J Antimicrob Chemother 2018; 73:1965–71. [DOI] [PubMed] [Google Scholar]
- 12. Ryom L, Boesecke C, Bracchi M, et al. Highlights of the 2017 European AIDS Clinical Society (EACS) Guidelines for the treatment of adult HIV-positive persons version 9.0. HIV Med 2018; 19:309–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Braun DL, Turk T, Tschumi F, et al. Non-inferiority of simplified dolutegravir monotherapy compared to continued combination antiretroviral therapy that was initiated during primary HIV infection: a randomized, controlled, multi-site, open-label, non-inferiority trial. Clin Infect Dis 2019;. doi: 10.1093/cid/ciy1131. [DOI] [PubMed] [Google Scholar]
- 14. HIV French Resistance Available at: http://www.hivfrenchresistance.org/.
- 15. Collin A, Le Marec F, Vandenhende M-A, et al. Incidence and risk factors for severe bacterial infections in people living with HIV. ANRS CO3 Aquitaine Cohort, 2000–2012. PLOS ONE 2016; 11:e0152970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Modica S, Rossetti B, Lombardi F, et al. Prevalence and determinants of resistance mutations in HIV-1-infected patients exposed to integrase inhibitors in a large Italian cohort. HIV Med 2019; 20:137–46. [DOI] [PubMed] [Google Scholar]
- 17. Boender TS, Smit C, van Sighem A, et al. AIDS Therapy Evaluation in the Netherlands (ATHENA) national observational HIV cohort: cohort profile. BMJ Open 2018; 8 e022516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Fondazione Icona Italian Cohort Naïve Antiretrovirals. http://www.fondazioneicona.org/_new2/. Published September 8, 2016. Accessed July 28, 2019.
- 19. The Swiss HIV Cohort Study, Schoeni-Affolter F, Ledergerber B, et al. Cohort profile: the Swiss HIV Cohort Study. Int J Epidemiol 2010; 39:1179–89. [DOI] [PubMed] [Google Scholar]
- 20. Center of Excellence for Health, Immunity and Infections. HICEP (HIV Collaboration Data Exchange Protocol). https://chip.dk/Tools-Standards/HICDEP. Published March 30, 2003. Accessed July 28, 2019.
- 21. Potard V, Simon A, Lacombe JM, Parienti JJ, Costagliola D; French Hospital Database on HIV (FHDH-ANRS CO4) Switching to raltegravir from a virologically effective boosted protease inhibitor regimen: a comparative effectiveness analysis from the French Hospital Database on HIV (FHDH-ANRS CO4). Clin Infect Dis 2016; 63:1254–61. [DOI] [PubMed] [Google Scholar]
- 22. Olmos A, Govindasamy P. A practical guide for using propensity score weighting in R. Pract Assessment Res Eval 2015; 20:1–8. [Google Scholar]
- 23. Buchanan AL, Hudgens MG, Cole SR, Lau B, Adimora AA; for the Women’s Interagency HIV Study Worth the weight: using inverse probability weighted Cox models in AIDS research. AIDS Res Hum Retroviruses 2014; 30:1170–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Cole SR, Hernan MA. Constructing inverse probability weights for marginal structural models. Pract Epidemiol 2008; 168: 656–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Kurth T, Walker AM, Glynn RJ, et al. Results of multivariable logistic regression, propensity matching, propensity adjustment, and propensity-based weighting under conditions of nonuniform effect. Am J Epidemiol 2006; 163:262–70. [DOI] [PubMed] [Google Scholar]
- 26. Gagliardini R, Ciccullo A, Borghetti A, et al. Impact of the M184V resistance mutation on virological efficacy and durability of lamivudine-based dual antiretroviral regimens as maintenance therapy in individuals with suppressed HIV-1 RNA: a cohort study. Open Forum Infect Dis 2018; 5:ofy113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Marcelin A, Charpentier C, Wirden M, et al. M184V/I as unique NRTI resistance mutation does not impact the efficacy of an abacavir/lamivudine/dolutegravi r use as switch in patients with a fully suppressed viral load. In: 15th European Meeting on HIV & Hepatitis Treatment Strategies & Antiviral Drug Resistance; June 7–9, 2017; Rome, Italy. Abstract 50. [Google Scholar]
- 28. Reynes J, Meftah N, Montes B. Dual regimen with dolutegravir and lamivudine maintains virologic suppression even in heavily treatment-experienced HIV-infected patients: 48-week results from a pilot study (DOLULAM). In: Abstracts from the International Congress of Drug Therapy in HIV Infection; October 23–26, 2016; Glasgow, United Kingdom. Abstract 80. [Google Scholar]
- 29. Aboud M, Kaplan R, Lombaard J, et al. Dolutegravir versus ritonavir-boosted lopinavir both with dual nucleoside reverse transcriptase inhibitor therapy in adults with HIV-1 infection in whom first-line therapy has failed (DAWNING): an open-label, non-inferiority, phase 3b trial. Lancet Infect Dis 2019; 19:253–64. [DOI] [PubMed] [Google Scholar]
- 30. Brown D, Wang R, Underwood M, et al. DTG VS LPV/R (DAWNING): Efficacy by baseline NRTI resistance and second -line NRTI use. In: Conference on Retroviruses and Opportunistic Infections (CROI); March 4–7, 2019; Seattle, WA. Abstract 144. [Google Scholar]
- 31. Chen GJ, Sun HY, Chang SY, et al. Effectiveness of switching from protease inhibitors to dolutegravir in combination with nucleoside reverse transcriptase inhibitors as maintenance antiretroviral therapy among HIV-positive patients. Int J Antimicrob Agents 2019; 54:35–42. [DOI] [PubMed] [Google Scholar]
- 32. Sörstedt E, Carlander C, Flamholc L, et al. Effect of dolutegravir in combination with Nucleoside Reverse Transcriptase Inhibitors (NRTIs) on people living with HIV who have pre-existing NRTI mutations. Int J Antimicrob Agents 2018; 51:733–8. [DOI] [PubMed] [Google Scholar]
- 33. Valero IP, Llibre JM, Lazzarin A, et al. A phase 3b, open-label, pilot study to evaluate switching to elvitegravir/cobicistat/emtricitabine/tenofovir alafenamide (E/C/F/TAF) in virologically-suppressed HIV-1 infected adult subjects harboring the NRTI resistance mutation M184V and/or M184I (GS-US-292–1824). International AIDS Society; July 23–27, 2018; Amsterdam, the Netherlands. Abstract B38. [Google Scholar]
- 34. Swenson LC, Min JE, Woods CK, et al. HIV drug resistance detected during low-level viraemia is associated with subsequent virologic failure. AIDS 2014; 28:1125–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Sungkanuparph S, Groger RK, Overton ET, et al. Persistent low-level viraemia and virological failure in HIV-1-infected patients treated with highly active antiretroviral therapy. HIV Med 2006; 7:437–41. [DOI] [PubMed] [Google Scholar]
- 36. Karlsson AC, Younger SR, Martin JN, et al. Immunologic and virologic evolution during periods of intermittent and persistent low-level viremia. AIDS 2004; 18:981–9. [DOI] [PubMed] [Google Scholar]
- 37. Hermans LE, Moorhouse M, Carmona S, et al. Effect of HIV-1 low-level viraemia during antiretroviral therapy on treatment outcomes in WHO-guided South African treatment programmes: a multicentre cohort study. Lancet Infect Dis 2018; 18:188–97. [DOI] [PubMed] [Google Scholar]
- 38. Young J, Rickenbach M, Calmy A, et al. Transient detectable viremia and the risk of viral rebound in patients from the Swiss HIV Cohort Study. BMC Infect Dis 2015; 15:382. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.