Abstract
Obesity is demonstrated to be a risk factor in the development of cancers of various organs, such as colon, prostate, pancreas and so on. Leptine (LEP) is the most renowned of the adipokines. As a hormone, it mediates its effect through leptin receptor (LEPR), which is widely expressed in various tissues including colon mucosa. In this study, we have investigated the degree of expression of LEP and LEPR in colorectal cancer (CRC). We collected 44 surgically resected colon cancer tissues along with normal adjacent colon tissue (NACT) from a sample of CRC patients from the Malaysian population and looked for leptin and leptin receptors using immunohistochemistry (IHC). All the samples showed low presence of both LEP and LEPR in NACT, while both LEP and LEPR were present at high intensity in the cancerous tissues with 100% and 97.7% prevalence, respectively. Both were sparsed in the cytoplasm and were concentrated beneath the cell membrane. However, we did not find any significant correlation between their expression and pathological parameters like grade, tumor size, and lymph node involvement. Our study further emphasizes the possible causal role of LEP and LEPR with CRC, and also the prospect of using LEPR as a possible therapeutic target.
Keywords: Colorectal cancer, Immunohistochemistry, Leptin receptor, Leptin, Leptin receptor phenotype, LEP, LEPR
Introduction
Adiposity is considered a major health problem of pandemic dimension and involves both developed and developing countries (WHO, 2016; Popkin, Adair & Ng, 2012; Misra & Khurana, 2011). Much surpassing its physical and mechanical burden, it comes out as a significant metabolic player in various endocrine and cardiovascular disorders, and has also been demonstrated to be a risk factor in the development of cancers of various organs, such as colon, esophagus, gall bladder, pancreas, kidney, thyroid, prostate, uterus and breast (WHO, 2016; Bhaskaran et al., 2014; NCI, 2016).
Colorectal cancer (CRC) is one of the most common causes of cancer-related death worldwide (Yu et al., 2014). The epidemiological evidence shows a clear link between colon cancer and obesity (Robsahm et al., 2013; Harriss et al., 2009; Potter, 1999). The two are associated with a sedentary lifestyle, high-energy diets, and limited consumption of vegetables, fruits, and fibers (Robsahm et al., 2013; Harriss et al., 2009; Potter, 1999). The relationship between the two, if any, needs to be studied through deeper investigations.
Adipose tissue synthesizes and secretes a number of hormones and cytokines, also called adipokines, such as leptin (LEP), adiponectin, resistin, apelin, omentin, tumor necrosis factor-a, IL-6, etc (Kuryszko, Sławuta & Sapikowski, 2016; Booth et al., 2016; Goodwin & Stambolic, 2015). The tissue acts upon other organs through the adipokines. The most widely researched and most significant obesity-related adipokine is leptin (LEP) (Friedman & Halaas, 1998; Guo et al., 2012; Friedman, 2015; Friedman & Mantzoros, 2015; Lipsey et al., 2016).
It is established that LEP mediates its action through its receptor (LEPR) (Schwartz et al., 2000; Ha et al., 2013; Allison & Myers, 2014). Therefore, LEPR also has become an important target of research as part of the LEP-LEPR system for humoral control of organs and their functions.
A baffling feature of LEP is that although its most abundant source is white adipose tissue (WAT), it is also secreted by many other tissues; for example, placenta (Hoggard et al., 1997; Masuzaki et al., 1997), stomach (Bado et al., 1998), mammary gland (Smith-Kirwin et al., 1998), brain and pituitary, (Morash et al., 1999), colon (Hardwick et al., 2001), ovaries (Löffler et al., 2001), bone and cartilage cells (Morroni et al., 2004), testicles (Soyupek et al., 2005), skeletal muscle (Solberg et al., 2005), and so on.
Likewise, the leptin receptor (LEPR) is found in many tissues other than its canonical target organ: the hypothalamus. The receptor is expressed in placenta (Amico et al., 1998; Ebenbichler et al., 2002), gastric mucosa (Mix et al., 2000; Sobhani et al., 2000; Breidert et al., 1999), lung (Tsuchiya et al., 1999), endometrium (Kitawaki et al., 2000), immune cells (Caldefie-Chezet et al., 2001), liver (Otte et al., 2004), and pancreas (Tudurí et al., 2013). Adipose tissue and gastric mucosa also produce forms of LEPR that are soluble in plasma and tissue fluids and remain bound to circulating leptin increasing its half-life (Cammisotto & Bendayan, 2007).
Consistent with the wide occurrence of LEP and LEPR, the system besides its canonical function of balancing food intake and body mass (Friedman & Halaas, 1998; Guo et al., 2012; Friedman & Mantzoros, 2015; Lipsey et al., 2016; Schwartz et al., 2000; Ha et al., 2013; Allison & Myers, 2014; Ahima, 2008; Bjørbaek & Kahn, 2004) is found to regulate a plethora of processes and pathways, including growth of and secretion from gastric epithelial cells (Mix et al., 2000), secretion of mucin from colonic goblet cells (Plaisancie et al., 2006); reproduction, bone remodeling, insulin signaling, neuroendocrine function (Slattery et al., 2008) inflammatory response (Aloulou et al., 2008; Shamsuzzaman et al., 2004), regulation of blood pressure, thyroid hormone release, immune factors and cells (Bado et al., 1998; Barrachina et al., 1997; Adeyemi et al., 2005), pancreatic beta cells, regulation of fat and glucose metabolism, insulin sensitivity, and so on Bjørbaek & Kahn (2004).
The most important action of the LEP-LEPR system in relation to tumorigenesis is its growth effects. It has been found to be required as a growth factor for mammary gland (Hu et al., 2002; Esper et al., 2015; Cleary et al., 2004), fetal and neonatal growth (Masuzaki et al., 1997; Heiman et al., 1997), lung (Tsuchiya et al., 1999), hepatic cells (Chen et al., 2007), pancreatic β cell growth and function (Morioka et al., 2007), colonic epithelial cells (Hardwick et al., 2001), and so on. Bone growth and bone mass are severely reduced in LEP deficient (ob/ob) animals, but it can be restored with the administration of LEP (Steppan et al., 2000). More significantly, the system can interact with a number of other hormonal mediators including insulin, glucagon, the insulin-like growth factors, estrogen, progesterone, growth hormone and glucocorticoids (Margetic et al., 2002). Notably, to execute its growth effects, it has been demonstrated that the system promotes cell proliferation, angiogenesis, mesenchymal transformation, and exerts anti-apoptotic effect (Lipsey et al., 2016; Russo et al., 2004; Endo et al., 2011; Mencarelli et al., 2011; Guo, Liu & Gonzalez-Perez, 2011; Mullen & Gonzalez-Perez, 2016; Ghasemi et al., 2019), which also are essential requirements of tumorigenesis (Guo et al., 2012; Mullen & Gonzalez-Perez, 2016; Surmacz, 2013).
As evidence to the above hypothesis, LEP and LEPR have been demonstrated in unusually high concentration in various cancerous tissues by many authors (Koda et al., 2007a). They are found in high concentration in breast carcinoma (O’brien, Welter & Price, 1999; Ishikawa, Kitayama & Nagawa, 2004; Al-Shibli et al., 2017), leukemia (Konopleva et al., 1999), as well as prostate (Saglam et al., 2003), esophagus (Somasundar et al., 2003), gastric (Hong et al., 2006), lung (Ribeiro et al., 2006), adenocarcinomas, etc.
Many authors have reported high presence of LEP in colorectal cancerous cells (Koda et al., 2007b; Paik et al., 2009; Liu et al., 2011; Wang et al., 2012; Yoon et al., 2014; Jeong et al., 2015). Recently, a study in Saudi Arabia on colorectal tumors has found LEP in a very high percentage (93%) of the samples on immunostaining (Al-Maghrabi, Qureshi & Khabaz, 2018). Nevertheless, some authors reported that in advanced cancers LEP expression diminishes (Hong et al., 2006; Koda et al., 2007b), suggesting silencing of LEP expression in an advanced stage, which indicates the anti-tumorigenic role of the LEP. Again, Aparicio et al. (2005) have reported that LEP acts as an in vitro growth factor for colon cancer cells, but does not promote tumor growth in vivo. Such conflicting reports make the case of LEP in CRC all the more interesting.
Many studies have also demonstrated over-expression of LEPR in colon cancer (Hardwick et al., 2001; Aparicio et al., 2005; Hoda et al., 2007; Drew, 2012; Erkasap et al., 2013; Mu et al., 2014). Altered patterns of LEPR expression have been reported by a number of authors (Aloulou et al., 2008; Uchiyama et al., 2011; Uddin et al., 2009b; Stachowicz et al., 2010). It has been proposed that phenotypic variation of LEPR expression may give variants with better prognosis (Aloulou et al., 2008; Uddin et al., 2009b). Some other authors have reported that in CRC patients, tissue LEP and LEPR are related significantly to the grade of tumor differentiation, depth of bowel wall invasion, and distant metastasis (Joshi & Lee, 2014). The confounding complexity of LEP-LEPR system in human colon cancer patients obviously demands wider studies.
In this paper, we report an investigation of the degree of expression/presence of both LEP and LEPR using immunohistochemistry in colorectal mucosa in surgically resected CRC specimens from a sample of the Malaysian population.
Materials & Methods
Tissue samples
A total of 44 paraffin blocks of colorectal cancer (CRC) were taken from the histopathology laboratory in Hospital Tengku Ampuan Afzan (HTAA), Kuantan, Pahang, Malaysia. Colon samples were taken from resected tumors along with the adjacent normal tissue, which was used as controls. Selection of patients and clinical diagnosis were done in collaboration with the Department of Histopathology at HTAA from January 2017 to May 2018.
The age distribution of the patients was as such: 4 (9.1%), 5 (11.4%), 8 (18.2%), 19 (43.2%), 6 (13.6%) and 2 (4.5%) were in their 30s, 40s, 50s, 60s, 70s, and 80s respectively. Most of the samples were from males 26 (59.1%). Most 40 (91%) were moderately differentiated, only 2 (4.5%) of samples were well differentiated and only 2 (4.5%) undifferentiated. Age, sex and tumor grade distribution of the patients are shown in Tables 1–3.
Table 1. Age distribution of patients with colorectal carcinoma.
| Age groups: (years) |
30 s | 40 s | 50 s | 60 s | 70 s | 80 s |
|---|---|---|---|---|---|---|
| n = 44 | 4 | 5 | 8 | 19 | 6 | 2 |
| (in %) | (9.1) | (11.4) | (18.2) | (43.2) | (13.6) | (4.5) |
Table 3. Tumor grade distribution of the patients with colorectal carcinoma.
| Tumor grade | Well differentiated | Moderately differentiated | Undifferentiated |
|---|---|---|---|
| n = 44 | 2(4.5%) | 40(91%) | 2(4.5%) |
Table 2. Sex distribution of patients with colorectal carcinoma.
| Sex: | Female | Male |
|---|---|---|
| n = 44 | 18(40.9%) | 26(59.1%) |
Tissue collection and preparation
The study protocol will include studying of histopathological samples from 44 patients with colorectal cancer. From the patients’ forty-four pairs of histopathological samples, each pair consisting of a sample from the cancer tissue and another from adjacent normal colon tissue (ANCT) were obtained by the histopathological laboratory (HTAA). The tissue samples taken from ANCT of the same patient were considered as controls. All tissue samples were subjected to histopathological examination using immunohistochemistry procedure detailed below.
A rotatory microtome machine was used for sectioning. Trimming and sectioning were done with about 4–5 µm thick. All slides were stained with H&E and stored until the histopathological examination was achieved.
All histopathological procedures were conducted at the research laboratories in the Department of Basic Medical Sciences, Faculty of Medicine, International Islamic University Malaysia (IIUM), Kuantan, Pahang, Malaysia in collaboration with the Department of Pathology at HTAA.
Immunohistochemistry (IHC)
IHC for Leptin and Leptin receptors were done using a Dako Autostainer (Dako, Glostrup, Denmark), utilizing the REAL™ EnVision™ Detection System, Peroxidase/Dab+, Rabbit protocols from Dako, Denmark. Tissue sections were made on saline coated glass slides for IHC staining. AB9749 Abcam Rabbit polyclonal to Leptin and AB104403 Abcam Rabbit polyclonal to Leptin Receptor were used.
Anti-leptin antibody and anti-leptin receptor antibody were diluted to 1:1000 and 1:100 respectively by adding the appropriate amount of Dako antibody diluent.
IHC staining was performed using a Dako Autostainer according to the manufacturer’s protocol. The IHC-stained tissue sections were counterstained in hematoxylin solution for 15 s and were rinsed in running tap water for 5 min before differentiating in 1% acid alcohol for 2 dips. The sections were then rinsed in running tap water for 5 min. The tissue sections were dehydrated in 70% alcohol for 3 min, followed by 80%, 90% and absolute alcohol 2 and 1.5 min respectively. Then, the tissue sections were dried in an oven for 10 min at 37 °C, followed by clearing in xylene twice for 2 min. The slides were finally cover-slipped with DPX mounting medium.
IHC stained slides were examined under low and high power magnification using an Olympus BX15 (Tokyo, Japan) light microscope. The result was then analyzed for the expression of leptin and Leptin receptors.
In our study, the immunoreactivity of Leptin and leptin receptor has been examined, by two pathologists, for staining intensity and positively stained cells percentage. The frequency of positive cells was evaluated by applying a semiquantitative method. Staining intensity has been given scores 0, 1, 2, and 3 demonstrating negative, faint, moderate, and strong staining respectively. For simplification of analysis, scores of staining intensity have been grouped as negative, low (1+) and high (2+ and 3+), as was reported previously (Koda et al., 2007b; Al-Maghrabi, Qureshi & Khabaz, 2018).
Statistical analysis
SPSS software (version 22.0) was used for all statistical calculations. Spearman’s rho coefficient was used to determine correlations between the variables.
Ethical approvals
The full research protocol was approved by the International Islamic University Malaysia Research Ethical Committee (IREC). The approval reference number is IIUM/305/20/4/1/7. Since already recorded data and stored surgical tissue samples were issued no consent was thought necessary or feasible.
Results
Presence of leptin (LEP) and leptin receptor (LEPR) in the cancerous and the normal adjacent colon tissue samples
We selected 44 out of 56 CRC tissue samples as qualitatively acceptable for the purpose of our study. Among the 44 CRC samples, moderately differentiated (M) tumors were significantly higher compared to well differentiated (W) and undifferentiated (U) samples, 40, two and two, respectively (P < 0.01, Chi-square value = 61.714). Among the cancerous tissues, all of the 44 (100%) samples stained strongly (2+ or 3+) for LEP and all but one or 43(97.7%) samples stained strongly for LEPR with the IHC technique. In contrast, all of the 44 normal adjacent colon tissue (NACT) took low stain for both LEP and LEPR. The result is tabulated in Table 4.
Table 4. Percentage of positive (low and high) cases of LEP and LEPR in IHC stained of colorectal tissue.
| Immunoreactivity for LEP | Immunoreactivity for LEPR | |||
|---|---|---|---|---|
| Low | High | Low | High | |
| Cancer tissue N = 44 |
0 | 44 (100%) | 1(2.3%) | 43 (97.7%) |
| Adjacent normal colon tissue N = 44 |
44 (100%) | 0 | 44 (100%) | 0 |
| P value* | <0.01 | <0.01 | ||
Notes.
P values for significant difference in expression of LEP and LEPR between NACT and cancerous colon tissue were calculated by applying Wilcoxon signed rank test.
- IHC
- immunihistochemistry
- LEP
- leptin
- LEPR
- leptin receptor
- NACT
- normal adjacent colon tissues
Results of the statistical analysis
The difference in the expressions of LEP and LEPR between the cancerous and NACT were very significant (P < 0.01, Wilcoxon signed rank test). The statistical values are summarized in Table 5.
Table 5. Results of the statistical analysis of the correlation of LEP and LEPR with the common pathological parameters.
| Tumor size | Lymph node involvement | Grade (W,M,U)* | |
|---|---|---|---|
| LEP correlation coefficient (Spearman’s rho, P value) |
−0.054, P = 0.73 | −0.205, P = 0.181 | −0.000, P = 1.00 |
| LEPR correlation coefficient (Spearman’s rho, P value) |
−0.018, P = 0.907 | −0.206, P = 0.179 | −0.285, P = 0.06 |
Notes.
W: Well differentiated cells; M: Moderately differentiated cells; U: Undifferentiated cells.
- LEP
- leptin
- LEPR
- leptin receptor
However, we did not find any significant correlation between their expression and pathological parameters like grade, tumor size, and lymph node involvement (Table 5).
Discussion
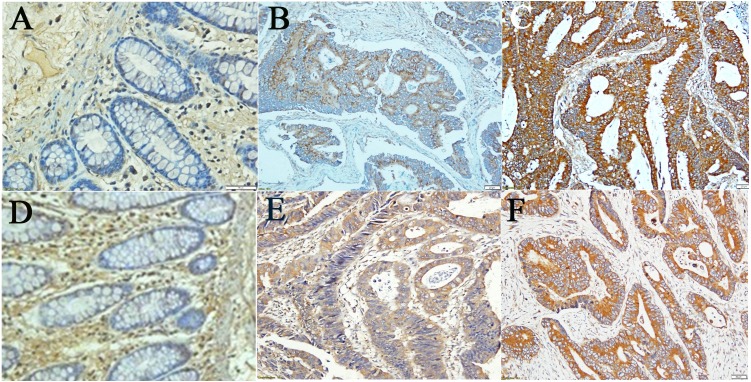
Definite association of obesity with various types of tumors readily drew attention towards its flag bearer hormone leptin (LEP), as it is the humoral mediator through which adipose tissue could exert a direct effect on other organs and processes. An indispensable part of LEP action is its receptor (LEPR). In this study, we graded staining as ‘negative,’ ’low,’ and ’high’ (as elaborated in methodology) and observed presence of both LEP and LEPR in colorectal cancer (CRC) tissue samples in high intensity with high prevalence, 100% and 97.7% respectively (p < 0.01) (Table 4). However, adjacent normal colon tissue (ANCT) were weakly and invariably immunostained for both LEP and LEPR (100%). In contrast to the previous report by Koda et al. (2007b), the distribution of stain in the ANCT was comparatively uniform too (Fig. 1).
Figure 1. Results from Immunohistochemical (IHC) staining.
Immunohistochemical (IHC) staining for LEP (A–C), and LEPR (D–F): (A,D) Adjacent Normal Colon Tissue (ANCT) with low staining (intensity 1+), (B,E) Moderately differentiated CRC with high staining (intensity (2+), (C,F) Moderately differentiated CRC with high staining (intensity 3+); (all ×200 magnification).
We do not know of any other report so far that has found such a high occurrence of LEP and LEPR in colonic or any other types of tumors. The numerically nearest prevalence of LEP (not LEPR) has been reported by Al-Maghrabi, Qureshi & Khabaz (2018), where LEP has been observed to be present in 93.5% of the CRC cases in the Western Province of Saudi Arabia, but moderate to strong staining in only 22.75% of the cases. While findings of Al-Maghrabi, Qureshi & Khabaz (2018) match with those of Jeong et al. (2015); previous reports by Koda et al. (2007b) from Europe, Paik et al. (2009) from Korea, Liu et al. (2011) and Wang et al. (2012) from China showed further lower values (LEP was just present only in 51.2%, 73.5%, 72.1, and 71.3%, respectively). Although the presence of both LEP and LEPR in CRC tissues in this Malaysian sample of the population is strikingly high and interesting, it would be premature to draw any conclusion about whether the differences are due to regional, temporal or racial factors, before further independent studies.
Koda et al. (2007b) have reported that LEP is overexpressed in human CRCs relative to normal colorectal mucosa. While in normal mucosa it is low or undetectable, its level is higher in tissues adjacent to CRCs. But LEP concentration is the highest in moderately differentiated (G2) cancers compared to poorly differentiated (G3) cancers. Similarly, Al-Maghrabi, Qureshi & Khabaz (2018) reported that larger size tumors gave a significantly higher proportion of negative immunostaining. Lower LEP occurrence has also been reported with less differentiated gastric adenocarcinomas, and the authors suggested silencing of LEP/LEPR expression in advanced stages of cancers (Hong et al., 2006). It is possible that in advanced stages of cancer strong oncogenes takes over the processes and the LEP system is overwhelmed at least in some cases. However, we did not find any negative correlation of LEP with advanced CRC. We had only two undifferentiated cases out of 44, but both the undifferentiated samples immunostained strongly for LEP. However, the number of cases of undifferentiated CRCs are statistically inadequate in our study to draw any conclusion. We did not find any significant correlation of LEP or LEPR staining with tumor grade. Our samples also failed to show any significant correlation either with lymph node involvement or invasion (Table 5). These findings match with those of Al-Maghrabi, Qureshi & Khabaz (2018) and Jeong et al. (2015), however, they contrast with Koda et al. (2007b) and Liu et al. (2011).
Concomitant occurrence of high concentration LEP and LEPR in various cancerous cells are so far explained by co-expression of both in the same cell (Ishikawa, Kitayama & Nagawa, 2004; Jardé et al., 2008; Garofalo et al., 2006). However, an alternative explanation might be that overexpression of only LEPR is followed by binding and trapping of the equivalent amount of LEP from circulation. Such a situation would result in overstaining of both in the respective cells. This hypothesis is supported by the findings of Stachowicz et al. (2010), who did not find mRNA for LEP in human CRC tissue samples. Interestingly, Erkasap et al. (2013) reported over-expression of LEPR mRNA in human metastatic CRCs, but not in CRCs of local origin. On the other hand, some authors reported expression of LEP mRNA in normal colonic cells (Attoub et al., 2000). These controversies demand much more detailed study of the expression of LEP and LEPR through mRNA studies instead of protein staining.
The very high presence of LEPR, as is found in our study, must be very significant as it is the receptor of LEP. The presence of only high level of circulating LEP cannot be sufficient to produce excessive growth promotion leading to cancer; it must need the receptor. The over-expression of LEPR, as we found in one of our previous studies with breast cancer (Al-Shibli et al., 2017), and by many authors with various cancers (Aloulou et al., 2008; Ishikawa, Kitayama & Nagawa, 2004; Mu et al., 2014; Uchiyama et al., 2011; Uddin et al., 2009b; Jardé et al., 2008; Miyoshi et al., 2006; Uddin et al., 2009a; Hoon Kim et al., 2008) must be significant in the carcinogenic influence of obesity or LEP. However, the claims by various authors about the effect of LEP-LEPR system in colon cancer are confounding, and maybe even contradictory (Hoda et al., 2007; Uchiyama et al., 2011; Stattin et al., 2003; Stattin et al., 2004; Bolukbas et al., 2004; Kumor et al., 2009; Sălăgeanu et al., 2010; Kosova et al., 2013). Some authors reported increased LEP levels in male but not in female CRC patients (Stattin et al., 2003; Stattin et al., 2004). Other showed that LEP expression in cancer tissue rises as carcinogenesis progresses (Koda et al., 2007b; Paik et al., 2009) and that LEP expression in cancer tissue may be positively correlated with survival of colorectal cancer patients (Paik et al., 2009) or LEPR over-expression is associated with anti-tumor response and better prognosis (Aloulou et al., 2008); while some claim that serum LEP values are lower in patients with colon cancer (Bolukbas et al., 2004; Kumor et al., 2009), serum LEP levels decreases with the progress and aggressiveness of tumor (Sălăgeanu et al., 2010); while others even fail to determine any significant difference in serum LEP between colon cancer patients and controls (Soyupek et al., 2005). An explanation to such contradictory report might lie in the fact that LEPR shows polymorphism and phenotypic variants (Aloulou et al., 2008; Mu et al., 2014; Uchiyama et al., 2011; Uddin et al., 2009b) and it is very much possible that different variants respond differently to LEP stimulation—one variant might over-stimulate cells towards carcinogenesis, while another might be less sensitive to circulating LEP concentration or even act against the progress of the tumor.
Conclusions
Over-expression of both LEP and LEPR in CRC, along with similar findings in our previous studies with breast carcinoma and related works of other authors, as mentioned above, suggest strong association of LEP/LEPR system with carcinogenesis. Given the already established role of LEP/LEPR system in growth promotion, it may be assumed to be causally related to carcinogenesis, where its growth effect may go out of control and result in tumorous growths or its hyperactivity may help the tumor to grow faster.
Since LEP/LEPR is over-expressed in so many processes, they could not be generally used as cancer markers; however, if specific phenotypic variants of LEPR are involved with cancers, which is yet to be studied, then such phenotypes could be used as cancer markers. Apparent contradictions about the role of LEPR may be due to phenotypic variants of LEPR, which may respond differently to LEP stimulation. It seems that while the LEPR pathway has much potential to be a target for anti-cancer drug therapy, drugs might be better targeted to particular variants of LEPR. Therefore, elaborate characterizations of phenotypic variants of LEPR are essential for various reasons.
Supplemental Information
Acknowledgments
Our thankfulness would be directed to all the faculties, friends and staffs in the Department Basic Medical Sciences, Faculty of Medicine, International Islamic University Malaysia; and to the Department of Pathology, Hospital Tengku Ampuan Afzan, Kuantan, Pahang, Malaysia for their extraordinary assistance.
Funding Statement
This work was supported by International Islamic University Malaysia. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Additional Information and Declarations
Competing Interests
The authors declare there are no competing interests.
Author Contributions
Saad M. Al-Shibli conceived and designed the experiments, performed the experiments, analyzed the data, contributed reagents/materials/analysis tools, prepared figures and/or tables, authored or reviewed drafts of the paper, approved the final draft, coordinated between labs.
Norra Harun conceived and designed the experiments, performed the experiments, analyzed the data, contributed reagents/materials/analysis tools, prepared figures and/or tables, approved the final draft.
Abdelkader E. Ashour conceived and designed the experiments, analyzed the data, prepared figures and/or tables, authored or reviewed drafts of the paper, approved the final draft.
Mohd Hanif B. Mohd Kasmuri performed the experiments, contributed reagents/materials/analysis tools, approved the final draft, did a lot of bench work.
Shaikh Mizan conceived and designed the experiments, performed the experiments, analyzed the data, prepared figures and/or tables, authored or reviewed drafts of the paper, approved the final draft.
Human Ethics
The following information was supplied relating to ethical approvals (i.e., approving body and any reference numbers):
The full research protocol was approved by the International Islamic University Malaysia Research Ethical Committee (IREC; approval reference number: IIUM/ 305/20/4/1/7.).
Data Availability
The following information was supplied regarding data availability:
The raw data is available as a Supplemental File.
References
- Adeyemi et al. (2005).Adeyemi EO, Bastaki SA, Chandranath IS, Hasan MY, Fahim M, Adem A. Mechanisms of action of leptin in preventing gastric ulcer. World Journal of Gastroenterology. 2005;11(27):4154–4160. doi: 10.3748/wjg.v11.i27.4154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahima (2008).Ahima RS. Revisiting leptin’s role in obesity and weight loss. Journal of Clinical Investigation. 2008;118(7):2380–2383. doi: 10.1172/JCI36284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al-Maghrabi, Qureshi & Khabaz (2018).Al-Maghrabi JA, Qureshi IA, Khabaz MN. Expression of leptin in colorectal adenocarcinoma showed significant different survival patterns associated with tumor size, lymphovascular invasion, distant metastasis, local recurrence, and relapse of disease in the western province of Saudi Arabia. Medicine. 2018;97(34):e12052. doi: 10.1097/MD.0000000000012052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al-Shibli et al. (2017).Al-Shibli SM, Amjad NM, Al-Kubaisi MK, Mizan S. Subcellular localization of leptin and leptin receptor in breast cancer detected in an electron microscopic study. Biochemical and Biophysical Research Communications. 2017;482(4):1102–1106. doi: 10.1016/j.bbrc.2016.11.165. [DOI] [PubMed] [Google Scholar]
- Allison & Myers (2014).Allison MB, Myers MG. 20 years of leptin: connecting leptin signaling to biological function. Journal of Endocrinology. 2014;223(1):T25–T35. doi: 10.1530/JOE-14-0404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aloulou et al. (2008).Aloulou N, Bastuji-Garin S, Le Gouvello S, Abolhassani M, Chaumette MT, Charachon A, Leroy K, Sobhani I. Involvement of the leptin receptor in the immune response in intestinal cancer. Cancer Research. 2008;68(22):9413–9422. doi: 10.1158/0008-5472.CAN-08-0909. [DOI] [PubMed] [Google Scholar]
- Amico et al. (1998).Amico JA, Thomas A, Crowley RS, Burmeister LA. Concentrations of leptin in the serum of pregnant, lactating, and cycling rats and of leptin messenger ribonucleic acid in rat placental tissue. Life Sciences. 1998;63(16):1387–1395. doi: 10.1016/S0024-3205(98)00405-6. [DOI] [PubMed] [Google Scholar]
- Aparicio et al. (2005).Aparicio T, Kotelevets L, Tsocas A, Laigneau J-P, Sobhani I, Chastre E, Lehy T. Leptin stimulates the proliferation of human colon cancer cells in vitro but does not promote the growth of colon cancer xenografts in nude mice or intestinal tumorigenesis in ApcMin/+ mice. Gut. 2005;54(8):1136–1145. doi: 10.1136/gut.2004.060533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Attoub et al. (2000).Attoub S, Noe V, Pirola L, Bruyneel E, Chastre E, Mareel M, Wymann MP, Gespach C. Leptin promotes invasiveness of kidney and colonic epithelial cells via phosphoinositide 3-kinase-, Rho-, and Rac-dependent signaling pathways. FASEB Journal. 2000;14(14):2329–2338. doi: 10.1096/fj.00-0162. [DOI] [PubMed] [Google Scholar]
- Bado et al. (1998).Bado A, Levasseur S, Attoub S, Kermorgant S, Laigneau JP, Bortoluzzi MN, Moizo L, Lehy T, Guerre-Millo M, Le Marchand-Brustel Y, Lewin MJ. The stomach is a source of leptin. Nature. 1998;394(6695):790–793. doi: 10.1038/29547. [DOI] [PubMed] [Google Scholar]
- Barrachina et al. (1997).Barrachina MD, Martínez V, Wang L, Wei JY, Taché Y. Synergistic interaction between leptin and cholecystokinin to reduce short-term food intake in lean mice. Proceedings of the National Academy of Sciences of the United States of America. 1997;94(19):10455–10460. doi: 10.1073/pnas.94.19.10455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhaskaran et al. (2014).Bhaskaran K, Douglas I, Forbes H, dos Santos-Silva I, Leon DA, Smeeth L. Body-mass index and risk of 22 specific cancers: a population-based cohort study of 5.24 million UK adults. Lancet. 2014;384(9945):755–765. doi: 10.1016/S0140-6736(14)60892-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bjørbaek & Kahn (2004).Bjørbaek C, Kahn BB. Leptin signaling in the central nervous system and the periphery. Recent Progress in Hormone Research. 2004;59:305–331. doi: 10.1210/rp.59.1.305. [DOI] [PubMed] [Google Scholar]
- Bolukbas et al. (2004).Bolukbas FF, Kilic H, Bolukbas C, Gumus M, Horoz M, Turhal NS, Kavakli B. Serum leptin concentration and advanced gastrointestinal cancers: a case controlled study. BMC Cancer. 2004;4(1):29. doi: 10.1186/1471-2407-4-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Booth et al. (2016).Booth A, Magnuson A, Fouts J, Foster MT. Adipose tissue: an endocrine organ playing a role in metabolic regulation. Hormone Molecular Biology and Clinical Investigation. 2016;26(1):25–42. doi: 10.1515/hmbci-2015-0073. [DOI] [PubMed] [Google Scholar]
- Breidert et al. (1999).Breidert M, Miehlke S, Glasow A, Orban Z, Stolte M, Ehninger G, Bayerdörffer E, Nettesheim O, Halm U, Haidan A, Bornstein SR. Leptin and its receptor in normal human gastric mucosa and in Helicobacter pylori-associated gastritis. Scandinavian Journa of Gastroenterology. 1999;34(10):954–961. doi: 10.1080/003655299750025039. [DOI] [PubMed] [Google Scholar]
- Caldefie-Chezet et al. (2001).Caldefie-Chezet F, Poulin A, Tridon A, Sion B, Vasson MP. Leptin: a potential regulator of polymorphonuclear neutrophil bactericidal action? Journal of Leukocyte Biology. 2001;69(3):414–418. [PubMed] [Google Scholar]
- Cammisotto & Bendayan (2007).Cammisotto PG, Bendayan M. Leptin secretion by white adipose tissue and gastric mucosa. Histology and Histopathology. 2007;22(1–3):199–210. doi: 10.14670/HH-22.199. [DOI] [PubMed] [Google Scholar]
- Chen et al. (2007).Chen C, Chang Y-C, Liu C-L, Liu T-P, Chang K-J, Guo I-C. Leptin induces proliferation and anti-apoptosis in human hepatocarcinoma cells by up-regulating cyclin D1 and down-regulating Bax via a Janus kinase 2-linked pathway. Endocrine Related Cancer. 2007;14(2):513–529. doi: 10.1677/ERC-06-0027. [DOI] [PubMed] [Google Scholar]
- Cleary et al. (2004).Cleary MP, Juneja SC, Phillips FC, Hu X, Grande JP, Maihle NJ. Leptin receptor-deficient MMTV-TGF-alpha/Lepr(db)Lepr(db) female mice do not develop oncogene-induced mammary tumors. Experimental Biology and Medicine. 2004;229(2):182–193. doi: 10.1177/153537020422900207. [DOI] [PubMed] [Google Scholar]
- Drew (2012).Drew JE. Molecular mechanisms linking adipokines to obesity-related colon cancer: focus on leptin. Proceedings of the Nutrition Society. 2012;71(1):175–180. doi: 10.1017/S0029665111003259. [DOI] [PubMed] [Google Scholar]
- Ebenbichler et al. (2002).Ebenbichler CF, Kaser S, Laimer M, Wolf HJ, Patsch JR, Illsley NP. Polar expression and phosphorylation of human leptin receptor isoforms in paired, syncytial, microvillous and basal membranes from human term placenta. Placenta. 2002;23(6):516–521. doi: 10.1053/plac.2002.0836. [DOI] [PubMed] [Google Scholar]
- Endo et al. (2011).Endo H, Hosono K, Uchiyama T, Sakai E, Sugiyama M, Takahashi H, Nakajima N, Wada K, Takeda K, Nakagama H, Nakajima A. Leptin acts as a growth factor for colorectal tumours at stages subsequent to tumour initiation in murine colon carcinogenesis. Gut. 2011;60(10):1363–1371. doi: 10.1136/gut.2010.235754. [DOI] [PubMed] [Google Scholar]
- Erkasap et al. (2013).Erkasap N, Ozkurt M, Erkasap S, Yasar F, Uzuner K, Ihtiyar E, Uslu S, Kara M, Bolluk O. Leptin receptor (Ob-R) mRNA expression and serum leptin concentration in patients with colorectal and metastatic colorectal cancer. Brazilian Journal of Medical and Biological Research = Rev Bras Pesqui médicas e biológicas / Soc Bras Biofísica. 2013;46(3):306–310. doi: 10.1590/1414-431X20122559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esper et al. (2015).Esper RM, Dame M, McClintock S, Holt PR, Dannenberg AJ, Wicha MS, Brenner DE. Leptin and adiponectin modulate the self-renewal of normal human breast epithelial stem cells. Cancer Prevention Research. 2015;8(12):1174–1183. doi: 10.1158/1940-6207.CAPR-14-0334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedman (2015).Friedman J. Leptin and the regulation of food intake and body weight. Journal of Nutritional Science and Vitaminology. 2015;61(Suppl):S202. doi: 10.3177/jnsv.61.S202. [DOI] [PubMed] [Google Scholar]
- Friedman & Halaas (1998).Friedman JM, Halaas JL. Leptin and the regulation of body weight in mammals. Nature. 1998;395(6704):763–770. doi: 10.1038/27376. [DOI] [PubMed] [Google Scholar]
- Friedman & Mantzoros (2015).Friedman JM, Mantzoros CS. 20 years of leptin: from the discovery of the leptin gene to leptin in our therapeutic armamentarium. Metabolism: Clinical and Experimental. 2015;64(1):1–4. doi: 10.1016/j.metabol.2014.10.023. [DOI] [PubMed] [Google Scholar]
- Garofalo et al. (2006).Garofalo C, Koda M, Cascio S, Sulkowska M, Kanczuga-Koda L, Golaszewska J, Russo A, Sulkowski S, Surmacz E. Increased expression of leptin and the leptin receptor as a marker of breast cancer progression: possible role of obesity-related stimuli. Clinical Cancer Research. 2006;12(5):1447–1453. doi: 10.1158/1078-0432.CCR-05-1913. [DOI] [PubMed] [Google Scholar]
- Ghasemi et al. (2019).Ghasemi A, Saeidi J, Azimi-Nejad M, Hashemy SI. Leptin-induced signaling pathways in cancer cell migration and invasion. Cellular Oncology. 2019 doi: 10.1007/s13402-019-00428-0. [DOI] [PubMed] [Google Scholar]
- Goodwin & Stambolic (2015).Goodwin PJ, Stambolic V. Impact of the obesity epidemic on cancer. Annual Review of Medicine. 2015;66:281–296. doi: 10.1146/annurev-med-051613-012328. [DOI] [PubMed] [Google Scholar]
- Guo, Liu & Gonzalez-Perez (2011).Guo S, Liu M, Gonzalez-Perez RR. Role of Notch and its oncogenic signaling crosstalk in breast cancer. Biochimica et Biophysica Acta. 2011;1815(2):197–213. doi: 10.1016/j.bbcan.2010.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo et al. (2012).Guo S, Liu M, Wang G, Torroella-Kouri M, Gonzalez-Perez RR. Oncogenic role and therapeutic target of leptin signaling in breast cancer and cancer stem cells. Biochimica et Biophysica Acta. 2012;1825(2):207–222. doi: 10.1016/j.bbcan.2012.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ha et al. (2013).Ha S, Baver S, Huo L, Gata A, Hairston J, Huntoon N, Li W, Zhang T, Benecchi EJ, Ericsson M, Hentges ST, Bjørbæk C. Somato-dendritic localization and signaling by leptin receptors in hypothalamic POMC and AgRP neurons. PLOS ONE. 2013;8(10):e77622. doi: 10.1371/journal.pone.0077622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardwick et al. (2001).Hardwick JC, Van Den Brink GR, Offerhaus GJ, Van Deventer SJ, Peppelenbosch MP. Leptin is a growth factor for colonic epithelial cells. Gastroenterology. 2001;121(1):79–90. doi: 10.1053/gast.2001.25490. [DOI] [PubMed] [Google Scholar]
- Harriss et al. (2009).Harriss DJ, Atkinson G, George K, Tim Cable N, Reilly T, Haboubi N, Zwahlen M, Egger M, Renehan AG, C-CLEAR group Lifestyle factors and colorectal cancer risk (1): systematic review and meta-analysis of associations with body mass index. Colorectal Disease. 2009;11(6):547–563. doi: 10.1111/j.1463-1318.2009.01766.x. [DOI] [PubMed] [Google Scholar]
- Heiman et al. (1997).Heiman ML, Ahima RS, Craft LS, Schoner B, Stephens TW, Flier JS. Leptin inhibition of the hypothalamic-pituitary-adrenal axis in response to stress1. Endocrinology. 1997;138(9):3859–3863. doi: 10.1210/endo.138.9.5366. [DOI] [PubMed] [Google Scholar]
- Hoda et al. (2007).Hoda MR, Keely SJ, Bertelsen LS, Junger WG, Dharmasena D, Barrett KE. Leptin acts as a mitogenic and antiapoptotic factor for colonic cancer cells. British Journal of Surgery. 2007;94(3):346–354. doi: 10.1002/bjs.5530. [DOI] [PubMed] [Google Scholar]
- Hoggard et al. (1997).Hoggard N, Hunter L, Duncan JS, Williams LM, Trayhurn P, Mercer JG. Leptin and leptin receptor mRNA and protein expression in the murine fetus and placenta. Proceedings of the National Academy of Sciences of the United States of America. 1997;94(20):11073–8. doi: 10.1073/pnas.94.20.11073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong et al. (2006).Hong S, Kwon K, Kim S, Ko B, Ryu C, Kim Y, Moon JH, Cho JY, Lee JS, Lee MS, Shim CS, Kim BS. Variation in expression of gastric leptin according to differentiation and growth pattern in gastric adenocarcinoma. Cytokine. 2006;33(2):66–71. doi: 10.1016/j.cyto.2005.11.015. [DOI] [PubMed] [Google Scholar]
- Hoon Kim et al. (2008).Hoon Kim J, Lee SY, Myung SC, Kim YS, Kim T-H, Kim MK. Clinical significance of the leptin and leptin receptor expressions in prostate tissues. Asian Journal of Andrology. 2008;10(6):923–928. doi: 10.1111/j.1745-7262.2008.00438.x. [DOI] [PubMed] [Google Scholar]
- Hu et al. (2002).Hu X, Juneja SC, Maihle NJ, Cleary MP. Leptin–a growth factor in normal and malignant breast cells and for normal mammary gland development. Journal of the National Cancer Institute. 2002;94(22):1704–1711. doi: 10.1093/jnci/94.22.1704. [DOI] [PubMed] [Google Scholar]
- Ishikawa, Kitayama & Nagawa (2004).Ishikawa M, Kitayama J, Nagawa H. Enhanced expression of leptin and leptin receptor (OB-R) in human breast cancer. Clinical Cancer Research. 2004;10(13):4325–4331. doi: 10.1158/1078-0432.CCR-03-0749. [DOI] [PubMed] [Google Scholar]
- Jardé et al. (2008).Jardé T, Caldefie-Chézet F, Damez M, Mishellany F, Penault-Llorca F, Guillot J, Vasson MP. Leptin and leptin receptor involvement in cancer development: a study on human primary breast carcinoma. Oncology Reports. 2008;19(4):905–911. [PubMed] [Google Scholar]
- Jeong et al. (2015).Jeong WK, Baek SK, Kim MK, Kwon SY, Kim HS. Prognostic significance of tissue leptin expression in colorectal cancer patients. Annals of Coloproctology. 2015;31(6):222. doi: 10.3393/ac.2015.31.6.222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joshi & Lee (2014).Joshi RK, Lee S-A. Obesity related adipokines and colorectal cancer: a review and meta-analysis. Asian Pacific Journal of Cancer Prevention. 2014;15(1):397–405. doi: 10.7314/APJCP.2014.15.1.397. [DOI] [PubMed] [Google Scholar]
- Kitawaki et al. (2000).Kitawaki J, Koshiba H, Ishihara H, Kusuki I, Tsukamoto K, Honjo H. Expression of leptin receptor in human endometrium and fluctuation during the menstrual cycle. Journal of Clinical Endocrinology and Metabolism. 2000;85(5):1946–1950. doi: 10.1210/jcem.85.5.6567. [DOI] [PubMed] [Google Scholar]
- Koda et al. (2007a).Koda M, Sulkowska M, Kanczuga-Koda L, Cascio S, Colucci G, Russo A, Surmacz E, Sulkowski S. Expression of the obesity hormone leptin and its receptor correlates with hypoxia-inducible factor-1 α in human colorectal cancer. Annals of Oncology. 2007a;18(SUPPL. 6):116–119. doi: 10.1093/annonc/mdm238. [DOI] [PubMed] [Google Scholar]
- Koda et al. (2007b).Koda M, Sulkowska M, Kanczuga-Koda L, Surmacz E, Sulkowski S. Overexpression of the obesity hormone leptin in human colorectal cancer. Journal of Clinical Pathology. 2007b;60(8):902–906. doi: 10.1136/jcp.2006.041004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konopleva et al. (1999).Konopleva M, Mikhail A, Estrov Z, Zhao S, Harris D, Sanchez-Williams G, Kornblau SM, Dong J, Kliche KO, Jiang S, Snodgrass HR, Estey EH, Andreeff M. Expression and function of leptin receptor isoforms in myeloid leukemia and myelodysplastic syndromes: proliferative and anti-apoptotic activities. Blood. 1999;93(5):1668–1676. [PubMed] [Google Scholar]
- Kosova et al. (2013).Kosova F, Coskun T, Kaya Y, Kara E, Ari Z. Adipocytokine levels of colon cancer patients before and after treatment. Bratislavske Lekarske Listy. 2013;114(7):394–397. doi: 10.4149/bll_2013_083. [DOI] [PubMed] [Google Scholar]
- Kumor et al. (2009).Kumor A, Daniel P, Pietruczuk M, Małecka-Panas E. Serum leptin, adiponectin, and resistin concentration in colorectal adenoma and carcinoma (CC) patients. International Journal of Colorectal Disease. 2009;24(3):275–281. doi: 10.1007/s00384-008-0605-y. [DOI] [PubMed] [Google Scholar]
- Kuryszko, Sławuta & Sapikowski (2016).Kuryszko J, Sławuta P, Sapikowski G. Secretory function of adipose tissue. Polish Journal of Veterinary Sciences. 2016;19(2):441–446. doi: 10.1515/pjvs-2016-0056. [DOI] [PubMed] [Google Scholar]
- Lipsey et al. (2016).Lipsey CC, Harbuzariu A, Daley-Brown D, Gonzalez-Perez RR. Oncogenic role of leptin and Notch interleukin-1 leptin crosstalk outcome in cancer. World Journal of Methodology. 2016;6(1):43–55. doi: 10.5662/wjm.v6.i1.43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu et al. (2011).Liu H, Wan D, Pan Z, Cao L, Wu X, Lu Z, Kang T. Expression and Biological Significance of Leptin, Leptin Receptor, VEGF, and CD34 in Colorectal Carcinoma. Cell Biochemistry and Biophysics. 2011;60(3):241–244. doi: 10.1007/s12013-010-9145-5. [DOI] [PubMed] [Google Scholar]
- Löffler et al. (2001).Löffler S, Aust G, Köhler U, Spanel-Borowski K. Evidence of leptin expression in normal and polycystic human ovaries. Molecular Human Reproduction. 2001;7(12):1143–1149. doi: 10.1093/molehr/7.12.1143. [DOI] [PubMed] [Google Scholar]
- Margetic et al. (2002).Margetic S, Gazzola C, Pegg GG, Hill RA. Leptin: a review of its peripheral actions and interactions. International Journal of Obesity and Related Metabolic Disorders. 2002;26(11):1407–1433. doi: 10.1038/sj.ijo.0802142. [DOI] [PubMed] [Google Scholar]
- Masuzaki et al. (1997).Masuzaki H, Ogawa Y, Sagawa N, Hosoda K, Matsumoto T, Mise H, Nishimura H, Yoshimasa Y, Tanaka I, Mori T, Nakao K. Nonadipose tissue production of leptin: leptin as a novel placenta-derived hormone in humans. Nature Medicine. 1997;3(9):1029–1033. doi: 10.1038/nm0997-1029. [DOI] [PubMed] [Google Scholar]
- Mencarelli et al. (2011).Mencarelli A, Distrutti E, Renga B, D’Amore C, Cipriani S, Palladino G, Donini A, Ricci P, Fiorucci S. Probiotics modulate intestinal expression of nuclear receptor and provide counter-regulatory signals to inflammation-driven adipose tissue activation. Bonecchi R. editor. PLOS ONE. 2011;6(7):e22978. doi: 10.1371/journal.pone.0022978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Misra & Khurana (2011).Misra A, Khurana L. Obesity-related non-communicable diseases: South Asians vs White Caucasians. International Journal of Obesity. 2011;35(2):167–187. doi: 10.1038/ijo.2010.135. [DOI] [PubMed] [Google Scholar]
- Mix et al. (2000).Mix H, Widjaja A, Jandl O, Cornberg M, Kaul A, Göke M, Beil W, Kuske M, Brabant G, Manns MP, Wagner S. Expression of leptin and leptin receptor isoforms in the human stomach. Gut. 2000;47(4):481–486. doi: 10.1136/gut.47.4.481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyoshi et al. (2006).Miyoshi Y, Funahashi T, Tanaka S, Taguchi T, Tamaki Y, Shimomura I, Noguchi S. High expression of leptin receptor mRNA in breast cancer tissue predicts poor prognosis for patients with high, but not low, serum leptin levels. International Journal of Cancer. 2006;118(6):1414–1419. doi: 10.1002/ijc.21543. [DOI] [PubMed] [Google Scholar]
- Morash et al. (1999).Morash B, Li A, Murphy PR, Wilkinson M, Ur E. Leptin gene expression in the brain and pituitary gland. Endocrinology. 1999;140(12):5995–5998. doi: 10.1210/endo.140.12.7288. [DOI] [PubMed] [Google Scholar]
- Morioka et al. (2007).Morioka T, Asilmaz E, Hu J, Dishinger JF, Kurpad AJ, Elias CF, Li H, Elmquist JK, Kennedy RT, Kulkarni RN. Disruption of leptin receptor expression in the pancreas directly affects beta cell growth and function in mice. Journal of Clinical Investigation. 2007;117(10):2860–2868. doi: 10.1172/JCI30910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morroni et al. (2004).Morroni M, De Matteis R, Palumbo C, Ferretti M, Villa I, Rubinacci A, Cinti S, Marotti G. In vivo leptin expression in cartilage and bone cells of growing rats and adult humans. Journal of Anatomy. 2004;205(4):291–296. doi: 10.1111/j.0021-8782.2004.00333.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mu et al. (2014).Mu HJ, Zou J, Xie P, Xu ZQ, Ruan J, Yang SD, Yin Y. Association of leptin receptor Lys109Arg and Gln223Arg polymorphisms with increased risk of clear cell renal cell carcinoma. Asian Pacific Journal of Cancer Prevention. 2014;15(10):4211–4215. doi: 10.7314/APJCP.2014.15.10.4211. [DOI] [PubMed] [Google Scholar]
- Mullen & Gonzalez-Perez (2016).Mullen M, Gonzalez-Perez R. Leptin-induced JAK/STAT signaling and cancer growth. Vaccine. 2016;4(3):26. doi: 10.3390/vaccines4030026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NCI (2016).NCI Obesity and cancer risk. National Cancer Institute NIH USA. 2016. https://www.cancer.gov/about-cancer/causes-prevention/risk/obesity/obesity-fact-sheet. [18 September 2016]. https://www.cancer.gov/about-cancer/causes-prevention/risk/obesity/obesity-fact-sheet
- O’brien, Welter & Price (1999).O’brien SN, Welter BH, Price TM. Presence of leptin in breast cell lines and breast tumors. Biochemical and Biophysical Research Communications. 1999;259(3):695–698. doi: 10.1006/bbrc.1999.0843. [DOI] [PubMed] [Google Scholar]
- Otte et al. (2004).Otte C, Otte J-M, Strodthoff D, Bornstein SR, Fölsch UR, Mönig H, Kloehn S. Expression of leptin and leptin receptor during the development of liver fibrosis and cirrhosis. Experimental and Clinical Endocrinology & Diabetes. 2004;112(1):10–17. doi: 10.1055/s-2004-815720. [DOI] [PubMed] [Google Scholar]
- Paik et al. (2009).Paik SS, Jang S-M, Jang K-S, Lee KH, Choi D, Jang SJ. Leptin expression correlates with favorable clinicopathologic phenotype and better prognosis in colorectal adenocarcinoma. Annals of Surgical Oncology. 2009;16(2):297–303. doi: 10.1245/s10434-008-0221-7. [DOI] [PubMed] [Google Scholar]
- Plaisancie et al. (2006).Plaisancie P, Ducroc R, Homsi M El, Tsocas A, Guilmeau S, Zoghbi S, Thibaudeau O, Bado A. Luminal leptin activates mucin-secreting goblet cells in the large bowel. American Journal of Physiology-Gastrointestinal and Liver Physiology. 2006;290(4):G805–12. doi: 10.1152/ajpgi.00433.2005. [DOI] [PubMed] [Google Scholar]
- Popkin, Adair & Ng (2012).Popkin BM, Adair LS, Ng SW. Global nutrition transition and the pandemic of obesity in developing countries. Nutrition Reviews. 2012;70(1):3–21. doi: 10.1111/j.1753-4887.2011.00456.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Potter (1999).Potter JD. Colorectal cancer: molecules and populations. Journal of the National Cancer Institute. 1999;91(11):916–932. doi: 10.1093/jnci/91.11.916. [DOI] [PubMed] [Google Scholar]
- Ribeiro et al. (2006).Ribeiro R, Araújo AP, Coelho A, Catarino R, Pinto D, Araújo A, Calçada C, Lopes C, Medeiros R. A functional polymorphism in the promoter region of leptin gene increases susceptibility for non-small cell lung cancer. European Journal of Cancer. 2006;42(8):1188–1193. doi: 10.1016/j.ejca.2006.02.004. [DOI] [PubMed] [Google Scholar]
- Robsahm et al. (2013).Robsahm TE, Aagnes B, Hjartåker A, Langseth H, Bray FI, Larsen IK. Body mass index, physical activity, and colorectal cancer by anatomical subsites. European Journal of Cancer Prevention. 2013;22(6):492–505. doi: 10.1097/CEJ.0b013e328360f434. [DOI] [PubMed] [Google Scholar]
- Russo et al. (2004).Russo VC, Metaxas S, Kobayashi K, Harris M, Werther GA. Antiapoptotic effects of leptin in human neuroblastoma cells. Endocrinology. 2004;145(9):4103–4112. doi: 10.1210/en.2003-1767. [DOI] [PubMed] [Google Scholar]
- Saglam et al. (2003).Saglam K, Aydur E, Yilmaz M, Göktaş S. Leptin influences cellular differentiation and progression in prostate cancer. Journal of Urology. 2003;169(4):1308–1311. doi: 10.1097/01.ju.0000055903.18400.25. [DOI] [PubMed] [Google Scholar]
- Sălăgeanu et al. (2010).Sălăgeanu A, Tucureanu C, Lerescu L, Caraş I, Pitica R, Gangurà G, Costea R, Neagu S. Serum levels of adipokines resistin and leptin in patients with colon cancer. Journal of Medicine and Life. 2010;3(4):416–420. [PMC free article] [PubMed] [Google Scholar]
- Schwartz et al. (2000).Schwartz MW, Woods SC, Porte D, Seeley RJ, Baskin DG. Central nervous system control of food intake. Nature. 2000;404(6778):661–671. doi: 10.1038/35007534. [DOI] [PubMed] [Google Scholar]
- Shamsuzzaman et al. (2004).Shamsuzzaman ASM, Winnicki M, Wolk R, Svatikova A, Phillips BG, Davison DE, Berger PB, Somers VK. Independent association between plasma leptin and C-reactive protein in healthy humans. Circulation. 2004;109(18):2181–2185. doi: 10.1161/01.CIR.0000127960.28627.75. [DOI] [PubMed] [Google Scholar]
- Slattery et al. (2008).Slattery ML, Wolff RK, Herrick J, Caan BJ, Potter JD. Leptin and leptin receptor genotypes and colon cancer: gene-gene and gene-lifestyle interactions. International Journal of Cancer. 2008;122(7):1611–1617. doi: 10.1002/ijc.23135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith-Kirwin et al. (1998).Smith-Kirwin SM, O’Connor DM, De Johnston J, Lancey ED, Hassink SG, Funanage VL. Leptin expression in human mammary epithelial cells and breast milk. Journal of Clinical Endocrinology and Metabolism. 1998;83(5):1810–1813. doi: 10.1210/jcem.83.5.4952. [DOI] [PubMed] [Google Scholar]
- Sobhani et al. (2000).Sobhani I, Bado A, Vissuzaine C, Buyse M, Kermorgant S, Laigneau JP, Attoub S, Lehy T, Henin D, Mignon M, Lewin MJ. Leptin secretion and leptin receptor in the human stomach. Gut. 2000;47(2):178–183. doi: 10.1136/gut.47.2.178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solberg et al. (2005).Solberg R, Aas V, Thoresen GH, Kase ET, Drevon CA, Rustan AC, Resel JE. Leptin expression in human primary skeletal muscle cells is reduced during differentiation. Journal of Cellular Biochemistry. 2005;96(1):89–96. doi: 10.1002/jcb.20521. [DOI] [PubMed] [Google Scholar]
- Somasundar et al. (2003).Somasundar P, Riggs D, Jackson B, Vona-Davis L, McFadden DW. Leptin stimulates esophageal adenocarcinoma growth by nonapoptotic mechanisms. American Journal of Surgery. 2003;186(5):575–578. doi: 10.1016/j.amjsurg.2003.07.017. [DOI] [PubMed] [Google Scholar]
- Soyupek et al. (2005).Soyupek S, Armağan A, Serel TA, Hoşcan MB, Perk H, Karaöz E, Candir O. Leptin expression in the testicular tissue of fertile and infertile men. Archives of Andrology. 2005;51(3):239–246. doi: 10.1080/01485010590919666. [DOI] [PubMed] [Google Scholar]
- Stachowicz et al. (2010).Stachowicz M, Mazurek U, Nowakowska-Zajdel E, Niedworok E, Fatyga E, Muc-Wierzgon M. Leptin and its receptors in obese patients with colorectal cancer. Journal of Biological Regulators & Homeostatic Agents. 2010;24(3):287–295. [PubMed] [Google Scholar]
- Stattin et al. (2004).Stattin P, Lukanova A, Biessy C, Söderberg S, Palmqvist R, Kaaks R, Olsson T, Jellum E. Obesity and colon cancer: does leptin provide a link? International Journal of Cancer. 2004;109(1):149–152. doi: 10.1002/ijc.11668. [DOI] [PubMed] [Google Scholar]
- Stattin et al. (2003).Stattin P, Palmqvist R, Söderberg S, Biessy C, Ardnor B, Hallmans G, Kaaks R, Olsson T. Plasma leptin and colorectal cancer risk: a prospective study in Northern Sweden. Oncology Reports. 2003;10(6):2015–2021. [PubMed] [Google Scholar]
- Steppan et al. (2000).Steppan CM, Crawford DT, Chidsey-Frink KL, Ke H, Swick AG. Leptin is a potent stimulator of bone growth in ob/ob mice. Regulatory Peptides. 2000;92(1–3):73–78. doi: 10.1016/S0167-0115(00)00152-X. [DOI] [PubMed] [Google Scholar]
- Surmacz (2013).Surmacz E. Leptin and adiponectin: emerging therapeutic targets in breast cancer. Journal of Mammary Gland Biology and Neoplasia. 2013;18(3–4):321–332. doi: 10.1007/s10911-013-9302-8. [DOI] [PubMed] [Google Scholar]
- Tsuchiya et al. (1999).Tsuchiya T, Shimizu H, Horie T, Mori M. Expression of leptin receptor in lung: leptin as a growth factor. European Journal of Pharmacology. 1999;365(2–3):273–279. doi: 10.1016/S0014-2999(98)00884-X. [DOI] [PubMed] [Google Scholar]
- Tudurí et al. (2013).Tudurí E, Bruin JE, Denroche HC, Fox JK, Johnson JD, Kieffer TJ. Impaired Ca(2+) signaling in β-cells lacking leptin receptors by Cre-loxP recombination. PLOS ONE. 2013;8(8):e71075. doi: 10.1371/journal.pone.0071075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uchiyama et al. (2011).Uchiyama T, Takahashi H, Endo H, Sugiyama M, Sakai E, Hosono K, Nagashima Y, Inayama Y, Wada K, Hippo Y, Nakajima A. Role of the long form leptin receptor and of the STAT3 signaling pathway in colorectal cancer progression. International Journal of Oncology. 2011;39(4):935–940. doi: 10.3892/ijo.2011.1105. [DOI] [PubMed] [Google Scholar]
- Uddin et al. (2009a).Uddin S, Bu R, Ahmed M, Abubaker J, Al-Dayel F, Bavi P, Al-Kuraya KS. Overexpression of leptin receptor predicts an unfavorable outcome in Middle Eastern ovarian cancer. Molecular Cancer. 2009a;8:74. doi: 10.1186/1476-4598-8-74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uddin et al. (2009b).Uddin S, Bavi PP, Hussain AR, Alsbeih G, Al-Sanea N, AbdulJabbar A, Ashari LH, Alhomoud S, Al-Dayel F, Ahmed M, Al-Kuraya KS. Leptin receptor expression in Middle Eastern colorectal cancer and its potential clinical implication. Carcinogenesis. 2009b;30(11):1832–1840. doi: 10.1093/carcin/bgp145. [DOI] [PubMed] [Google Scholar]
- Wang et al. (2012).Wang D, Chen J, Chen H, Duan Z, Xu Q, Wei M, Wang L, Zhong M. Leptin regulates proliferation and apoptosis of colorectal carcinoma through PI3K/Akt/mTOR signalling pathway. Journal of Biosciences. 2012;37(1):91–101. doi: 10.1007/s12038-011-9172-4. [DOI] [PubMed] [Google Scholar]
- WHO (2016).World Health Organization (WHO) Obesity and overweight. Media centre. 2016. [19 September 2016]. http://www.who.int/mediacentre/factsheets/fs311/en/ http://www.who.int/mediacentre/factsheets/fs311/en/
- Yoon et al. (2014).Yoon KW, Park SY, Kim JY, Lee SM, Park CH, Cho SB, Lee WS, Joo YE, Lee JH, Kim HS, Choi SK, Rew JS. Leptin-induced adhesion and invasion in colorectal cancer cell lines. Oncology Reports. 2014;31(6):2493–2498. doi: 10.3892/or.2014.3128. [DOI] [PubMed] [Google Scholar]
- Yu et al. (2014).Yu K, Yang J, Jiang Y, Song R, Lu Q. Vitamin D receptor BsmI polymorphism and colorectal cancer risk: an updated analysis. Asian Pacific Journal of Cancer Prevention. 2014;15(12):4801–4807. doi: 10.7314/APJCP.2014.15.12.4801. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The following information was supplied regarding data availability:
The raw data is available as a Supplemental File.